Pressure Perturbation Studies of Noncanonical Viral Nucleic Acid Structures
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Temperature Stability of HepB1-3 Detected by FRET
3.2. Stabilizing Effect of Monovalent Cations
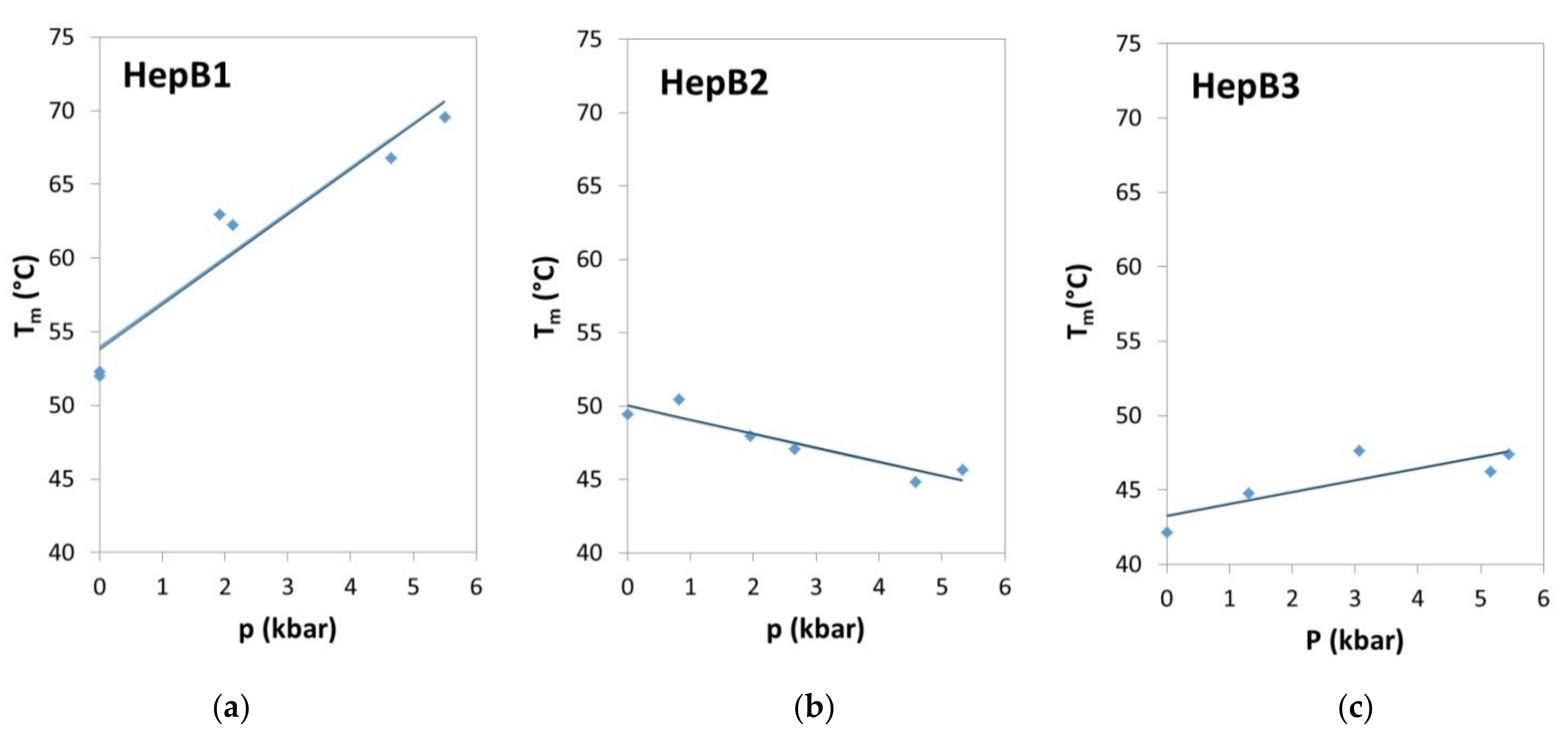
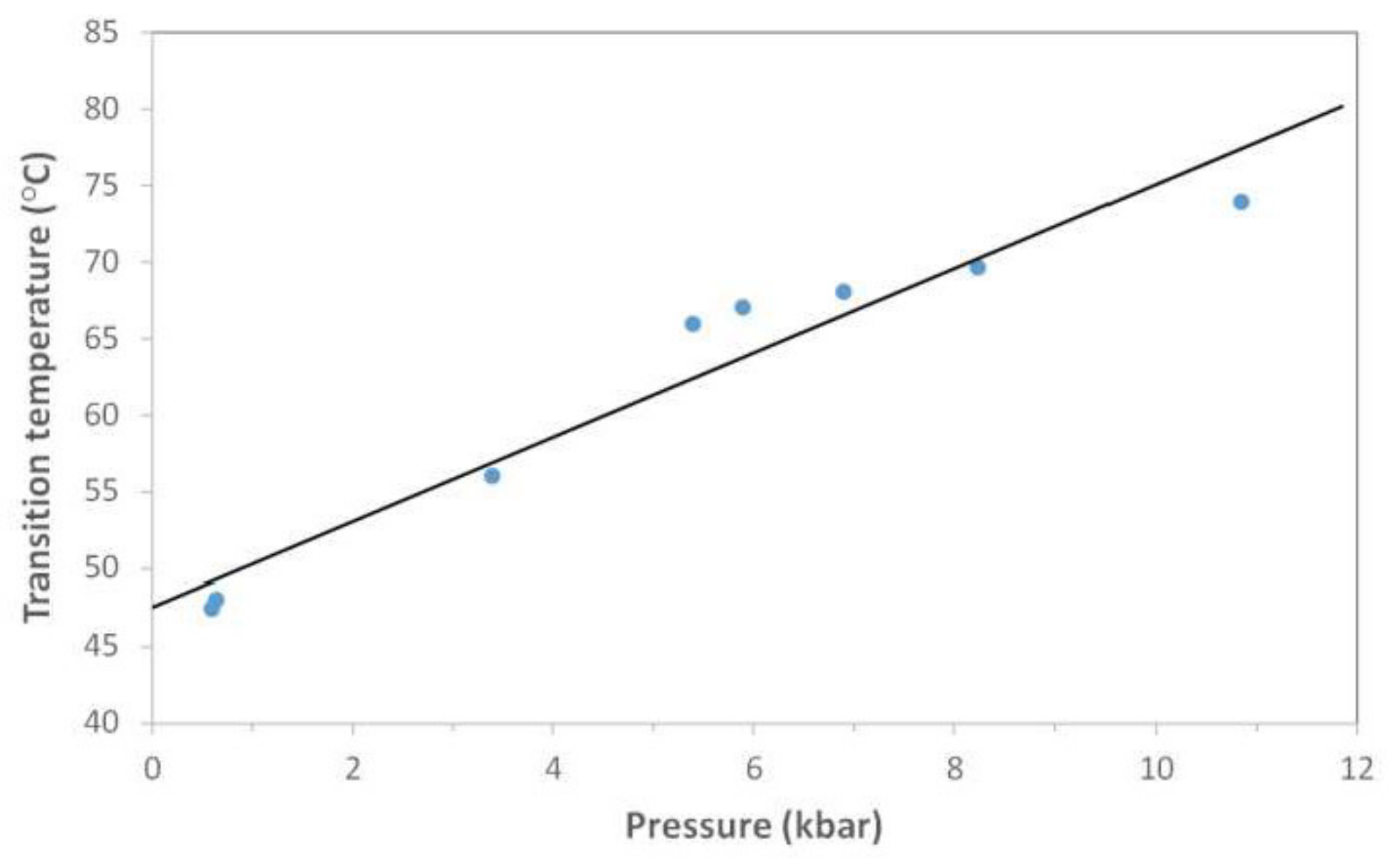
3.3. Pressure Experiments
3.3.1. FRET Experiments under Pressure
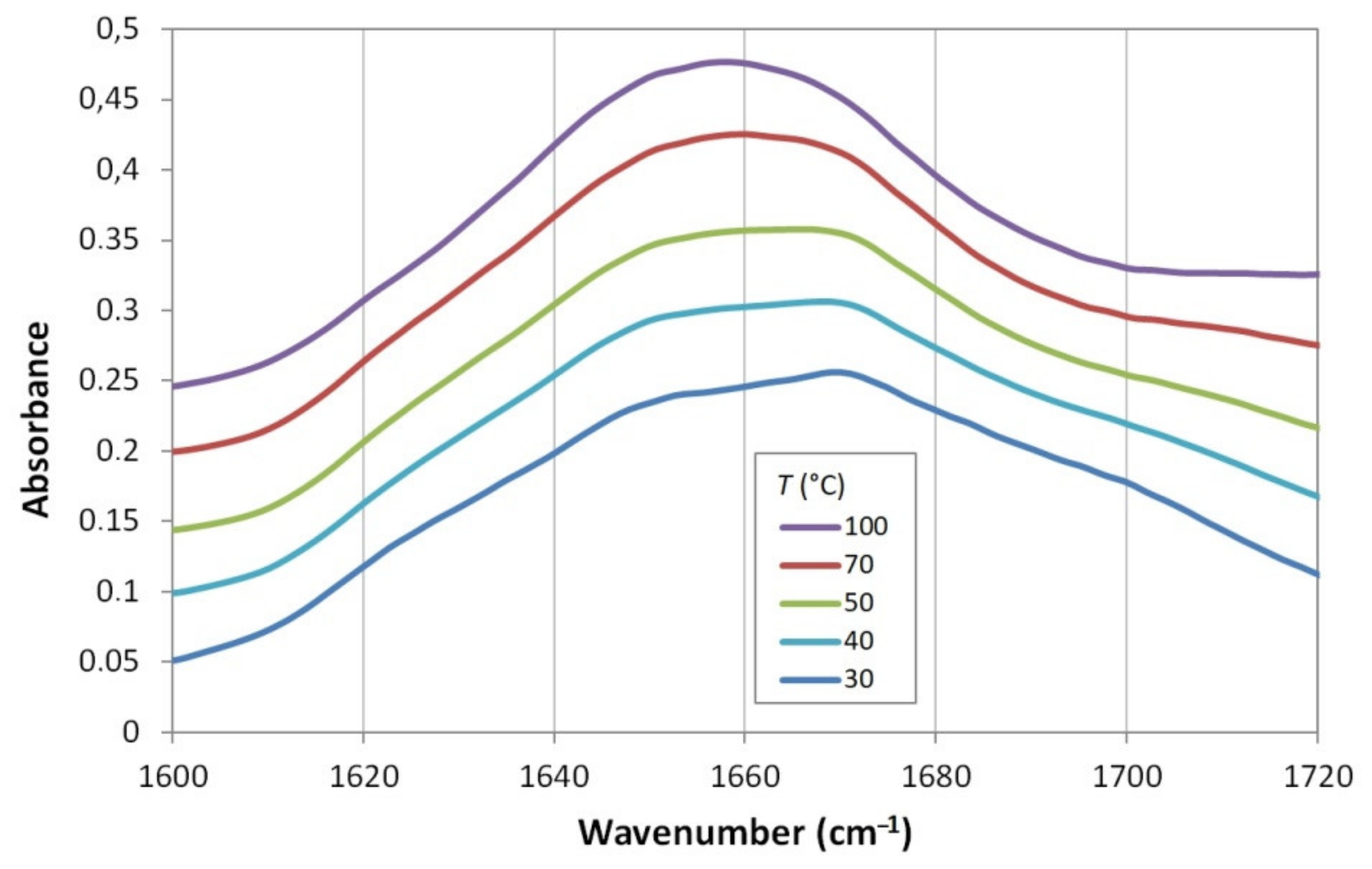
3.3.2. Unfolding of GQ as Seen from the Infrared Spectroscopy
3.3.3. Volume Changes at the Unfolding of HepB GQs
3.4. Stabilization of the HepB GQs with TMPyP4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fry, M. Tetraplex DNA and its interacting proteins. Front. Biosci. Landmark 2007, 12, 4336–4351. [Google Scholar] [CrossRef] [PubMed]
- Burge, S.; Parkinson, G.N.; Hazel, P.; Todd, A.K.; Neidle, S. Quadruplex DNA: Sequence, topology and structure. Nucleic Acids Res. 2006, 34, 5402–5415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosman, J.; Juskowiak, B. Peroxidase-mimicking DNAzymes for biosensing applications: A review. Anal. Chim. Acta 2011, 707, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Neidle, S. Human telomeric G-quadruplex: The current status of telomeric G-quadruplexes as therapeutic targets in human cancer. FEBS J. 2009, 277, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Neidle, S.; Parkinson, G.N. The structure of telomeric DNA. Curr. Opin. Struct. Biol. 2003, 13, 275–283. [Google Scholar] [CrossRef]
- Siddiqui-Jain, A.; Grand, C.L.; Bearss, D.; Hurley, L.H. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc. Natl. Acad. Sci. USA 2002, 99, 11593–11598. [Google Scholar] [CrossRef] [Green Version]
- Seenisamy, J.; Rezler, E.M.; Powell, T.J.; Tye, D.; Gokhale, V.; Joshi, C.S.; Siddiqui-Jain, A.; Hurley, L.H. The Dynamic Character of the G-Quadruplex Element in the c-MYC Promoter and Modification by TMPyP4. J. Am. Chem. Soc. 2004, 126, 8702–8709. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Hurley, L.H.; Neidle, S. Targeting G-quadruplexes in gene promoters: A novel anticancer strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Lavigne, M.; Helynck, O.; Rigolet, P.; Boudria-Souilah, R.; Nowakowski, M.; Baron, B.; Brülé, S.; Hoos, S.; Raynal, B.; Guittat, L.; et al. SARS-CoV-2 Nsp3 unique domain SUD interacts with guanine quadruplexes and G4-ligands inhibit this interaction. Nucleic Acids Res. 2021, 49, 7695–7712. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Chu, C.M. Hepatitis B virus infection. Lancet 2009, 373, 582–592. [Google Scholar] [CrossRef]
- Trepo, C.; Chan, H.L.Y.; Lok, A. Hepatitis B virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef]
- Biswas, B.; Kandpal, M.; Vivekanandan, P. A G-quadruplex motif in an envelope gene promoter regulates transcription and virion secretion in HBV genotype B. Nucleic Acids Res. 2017, 45, 11268–11280. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-L.; Kao, J.-H. The clinical implications of hepatitis B virus genotype: Recent advances. J. Gastroenterol. Hepatol. 2011, 26, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Seeger, C.; Mason, W.S. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 2000, 64, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavezzo, E.; Berselli, M.; Frasson, I.; Perrone, R.; Palù, G.; Brazzale, A.R.; Richter, S.N.; Toppo, S. G-quadruplex forming sequences in the genome of all known human viruses: A comprehensive guide. PLoS Comput. Biol. 2018, 14, e1006675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeller, L. Pressure-temperature phase diagrams of biomolecules. Biochim. Biophys. Acta 2002, 1595, 11–29. [Google Scholar]
- Meersman, F.; Dobson, C.M.; Heremans, K. Protein unfolding, amyloid fibril formation and configurational energy landscapes under high pressure conditions. Chem. Soc. Rev. 2006, 35, 908–917. [Google Scholar] [CrossRef]
- Sebert, P. (Ed.) Comparative High Pressure Biology; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 1–555. [Google Scholar]
- Smeller, L. Protein Denaturation on p-T Axes—Thermodynamics and Analysis. High Press. Biosci. 2015, 72, 19–39. [Google Scholar]
- Winter, R. Interrogating the Structural Dynamics and Energetics of Biomolecular Systems with Pressure Modulation. Annu. Rev. Biophys. 2019, 48, 441–463. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Royer, C.A. Lessons from pressure denaturation of proteins. J. R. Soc. Interface 2018, 15, 21. [Google Scholar] [CrossRef] [Green Version]
- Chalikian, T.V.; Macgregor, R.B. Volumetric Properties of Four-Stranded DNA Structures. Biology 2021, 10, 813. [Google Scholar] [CrossRef]
- Meersman, F.; McMillan, P.F. High hydrostatic pressure: A probing tool and a necessary parameter in biophysical chemistry. Chem. Commun. 2013, 50, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Somkuti, J.; Bublin, M.; Breiteneder, H.; Smeller, L. Pressure-Temperature Stability, Ca2+ Binding, and Pressure-Temperature Phase Diagram of Cod Parvalbumin: Gad m 1. Biochemistry 2012, 51, 5903–5911. [Google Scholar] [CrossRef] [PubMed]
- Molnár, O.R.; Somkuti, J.; Smeller, L. Negative volume changes of human G-quadruplexes at unfolding. Heliyon 2020, 6, e05702. [Google Scholar] [CrossRef]
- Somkuti, J.; Molnár, O.R.; Smeller, L. Revealing unfolding steps and volume changes of human telomeric i-motif DNA. Phys. Chem. Chem. Phys. 2020, 22, 23816–23823. [Google Scholar] [CrossRef]
- Meersman, F.; Dobson, C.M. Probing the pressure-temperature stability of amyloid fibrils provides new insights into their molecular properties. Biochim. Biophys. Acta 2006, 1764, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Jahmidi-Azizi, N.; Oliva, R.; Gault, S.; Cockell, C.; Winter, R. The Effects of Temperature and Pressure on Protein-Ligand Binding in the Presence of Mars-Relevant Salts. Biology 2021, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Dirix, C.; Meersman, F.; MacPhee, C.E.; Dobson, C.M.; Heremans, K. High hydrostatic pressure dissociates early aggregates of TTR105-115, but not the mature amyloid fibrils. J. Mol. Biol. 2005, 347, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Royer, C.A.; Roumestand, C. Exploring Protein Conformational Landscapes Using High-Pressure NMR. Methods Enzym. 2018, 614, 293–320. [Google Scholar]
- Williamson, M.P.; Kitahara, R. Characterization of low-lying excited states of proteins by high-pressure NMR. Biochim. Biophys. Acta 2018, 1867, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Somkuti, J.; Mártonfalvi, Z.; Kellermayer, M.; Smeller, L. Different pressure-temperature behavior of the structured and unstructured regions of titin. Biochim. Biophys. Acta 2013, 1834, 112–118. [Google Scholar] [CrossRef]
- Dubois, C.; Planelles-Herrero, V.; Tillatte-Tripodi, C.; Delbecq, S.; Mammri, L.; Sirkia, E.; Ropars, V.; Roumestand, C.; Barthe, P. Pressure and Chemical Unfolding of an α-Helical Bundle Protein: The GH2 Domain of the Protein Adaptor GIPC1. Int. J. Mol. Sci. 2021, 22, 3597. [Google Scholar] [CrossRef] [PubMed]
- Smeller, L.; Rubens, P.; Heremans, K. Pressure Effect on the Temperature-Induced Unfolding and Tendency to Aggregate of Myoglobin. Biochemistry 1999, 38, 3816–3820. [Google Scholar] [CrossRef]
- Scheyhing, C.H.; Meersman, F.; Ehrmann, M.A.; Heremans, K.; Vogel, R.F. Temperature-pressure stability of green fluorescent protein: A Fourier transform infrared spectroscopy study. Biopolymers 2002, 65, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ostermeier, L.; de Oliveira, G.A.; Dzwolak, W.; Silva, J.L.; Winter, R. Exploring the polymorphism, conformational dynamics and function of amyloidogenic peptides and proteins by temperature and pressure modulation. Biophys. Chem. 2020, 268, 106506. [Google Scholar] [CrossRef] [PubMed]
- Kriegler, S.; Herzog, M.; Oliva, R.; Gault, S.; Cockell, C.S.; Winter, R. Structural responses of model biomembranes to Mars-relevant salts. Phys. Chem. Chem. Phys. 2021, 23, 14212–14223. [Google Scholar] [CrossRef] [PubMed]
- Winter, R. Pressure Effects on Artificial and Cellular Membranes, in High Pressure Bioscience: Basic Concepts, Applications and Frontiers; Akasaka, K., Matsuki, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 345–370. [Google Scholar]
- Takahashi, S.; Sugimoto, N. Pressure-dependent formation of i-motif and G-quadruplex DNA structures. Phys. Chem. Chem. Phys. 2015, 17, 31004–31010. [Google Scholar] [CrossRef]
- Fan, H.Y.; Shek, Y.L.; Amiri, A.; Dubins, D.N.; Heerklotz, H.; MacGregor, R.B.; Chalikian, T.V. Volumetric Characterization of Sodium-Induced G-Quadruplex Formation. J. Am. Chem. Soc. 2011, 133, 4518–4526. [Google Scholar] [CrossRef]
- Takahashi, S.; Sugimoto, N. Effect of pressure on the stability of G-quadruplex DNA: Thermodynamics under crowding conditions. Angew. Chem. Int. Ed. Engl. 2013, 52, 13774–13778. [Google Scholar] [CrossRef] [PubMed]
- Somkuti, J.; Adányi, M.; Smeller, L. Self-crowding influences the temperature—Pressure stability of the human telomere G-quadruplex. Biophys. Chem. 2019, 254, 106248. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.T.T.; Moffat, D.J. A New Internal Pressure Calibrant for High-Pressure Infrared Spectroscopy of Aqueous Systems. Appl. Spectrosc. 1989, 43, 1279–1281. [Google Scholar] [CrossRef]
- Dai, J.; Punchihewa, C.; Ambrus, A.; Chen, D.; Jones, R.A.; Yang, D. Structure of the intramolecular human telomeric G-quadruplex in potassium solution: A novel adenine triple formation. Nucleic Acids Res. 2007, 35, 2440–2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva, R.; Mukherjee, S.; Winter, R. Unraveling the binding characteristics of small ligands to telomeric DNA by pressure modulation. Sci. Rep. 2021, 11, 9714. [Google Scholar] [CrossRef]
- Orsolya Réka Molnár, A.V.; Somkuti, J.; Smeller, L. Characterization of a G-quadruplex from hepatitis B virus and its stabilization by binding TMPyP4, BRACO19 and PhenDC3. Sci. Rep. 2021, in press. [Google Scholar]
- Largy, E.; Mergny, J.-L.; Gabelica, V. Role of Alkali Metal Ions in G-Quadruplex Nucleic Acid Structure and Stability. Alkali Metal Ions 2016, 16, 203–258. [Google Scholar]
- Ambrus, A.; Chen, D.; Dai, J.; Bialis, T.; Jones, R.A.; Yang, D. Human telomeric sequence forms a hybrid-type intramolecular G-quadruplex structure with mixed parallel/antiparallel strands in potassium solution. Nucleic Acids Res. 2006, 34, 2723–2735. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y.; Dubins, D.N.; Le, D.M.N.T.; Leung, K.; Macgregor, R.B. The role of loops and cation on the volume of unfolding of G-quadruplexes related to HTel. Biophys. Chem. 2017, 231, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Guédin, A.; Gros, J.; Alberti, P.; Mergny, J.-L. How long is too long? Effects of loop size on G-quadruplex stability. Nucleic Acids Res. 2010, 38, 7858–7868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, D.; Arachchilage, G.M.; Basu, S. Metal Cations in G-Quadruplex Folding and Stability. Front. Chem. 2016, 4, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venczel, E.A.; Sen, D. Parallel and antiparallel G-DNA structures from a complex telomeric sequence. Biochemistry 1993, 32, 6220–6228. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Gilbert, W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature 1990, 344, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Good, N.E.; Winget, G.D.; Winter, W.; Connolly, T.N.; Izawa, S.; Singh, R.M.M. Hydrogen Ion Buffers for Biological Research. Biochemistry 1966, 5, 467–477. [Google Scholar] [CrossRef]
- Banyay, M.; Sarkar, M.; Gräslund, A. A library of IR bands of nucleic acids in solution. Biophys. Chem. 2003, 104, 477–488. [Google Scholar] [CrossRef]
- Mondragón-Sánchez, J.A.; Liquier, J.; Shafer, R.H.; Taillandier, E. Tetraplex Structure Formation in the Thrombin-Binding DNA Aptamer by Metal Cations Measured by Vibrational Spectroscopy. J. Biomol. Struct. Dyn. 2004, 22, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Taillandier, E.; Liquier, J. Infrared-Spectroscopy of DNA. Methods Enzymol. 1992, 211, 307–335. [Google Scholar] [PubMed]
- Li, Y.Y.; Abu-Ghazalah, R.; Zamiri, B.; MacGregor, R.B., Jr. Concentration-dependent conformational changes in GQ-forming ODNs. Biophys. Chem. 2016, 211, 70–75. [Google Scholar] [CrossRef]
- Panick, G.; Vidugiris, G.J.A.; Malessa, R.; Rapp, G.; Winter, R.; Royer, C.A. Exploring the Temperature-Pressure Phase Diagram of Staphylococcal Nuclease. Biochemistry 1999, 38, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.S.; Kauzmann, W. The Structure and Properties of Water; Oxford University Press: Oxford, UK, 2005; p. 296. [Google Scholar]
- Basu, A.; Kumar, G.S. Calorimetric investigation on the interaction of proflavine with human telomeric G-quadruplex DNA. J. Chem. Thermodyn. 2016, 98, 208–213. [Google Scholar] [CrossRef]
- Takahashi, S.; Bhowmik, S.; Sugimoto, N. Volumetric analysis of formation of the complex of G-quadruplex DNA with hemin using high pressure. J. Inorg. Biochem. 2017, 166, 199–207. [Google Scholar] [CrossRef]
- Shek, Y.L.; Noudeh, G.D.; Nazari, M.; Heerklotz, H.; Abu-Ghazalah, R.M.; Dubins, D.N.; Chalikian, T.V. Folding thermodynamics of the hybrid-1 type intramolecular human telomeric G-quadruplex. Biopolymers 2013, 101, 216–227. [Google Scholar] [CrossRef]
- Luo, Y.; Granzhan, A.; Verga, D.; Mergny, J. FRET—MC: A fluorescence melting competition assay for studying G4 structures in vitro. Biopolymers 2020, 112, ebip23415. [Google Scholar] [CrossRef] [PubMed]
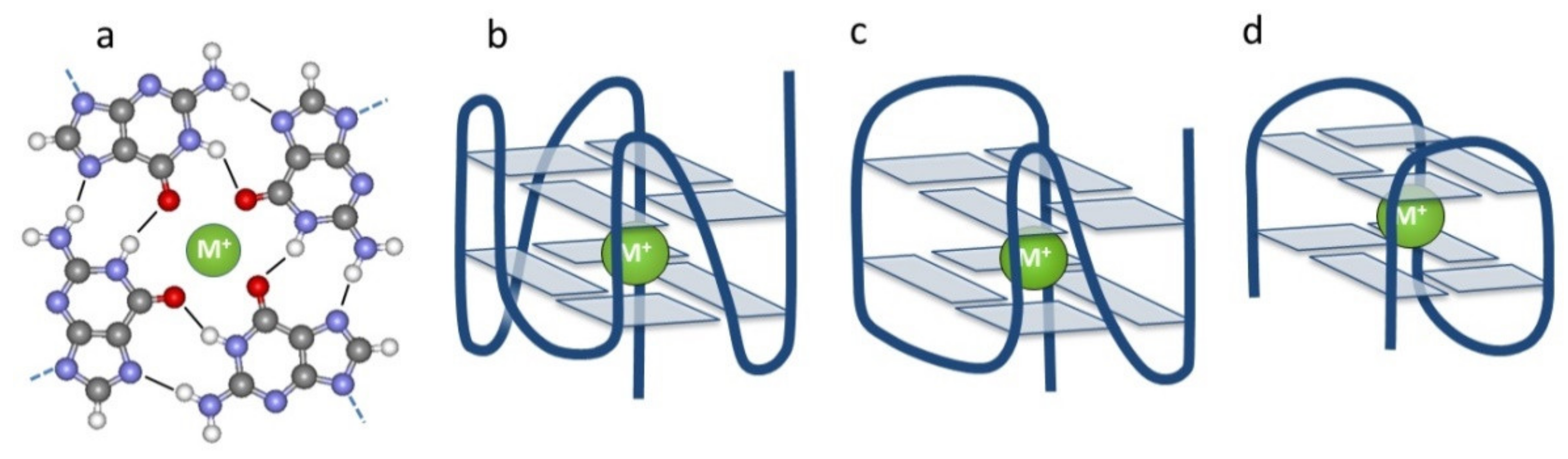
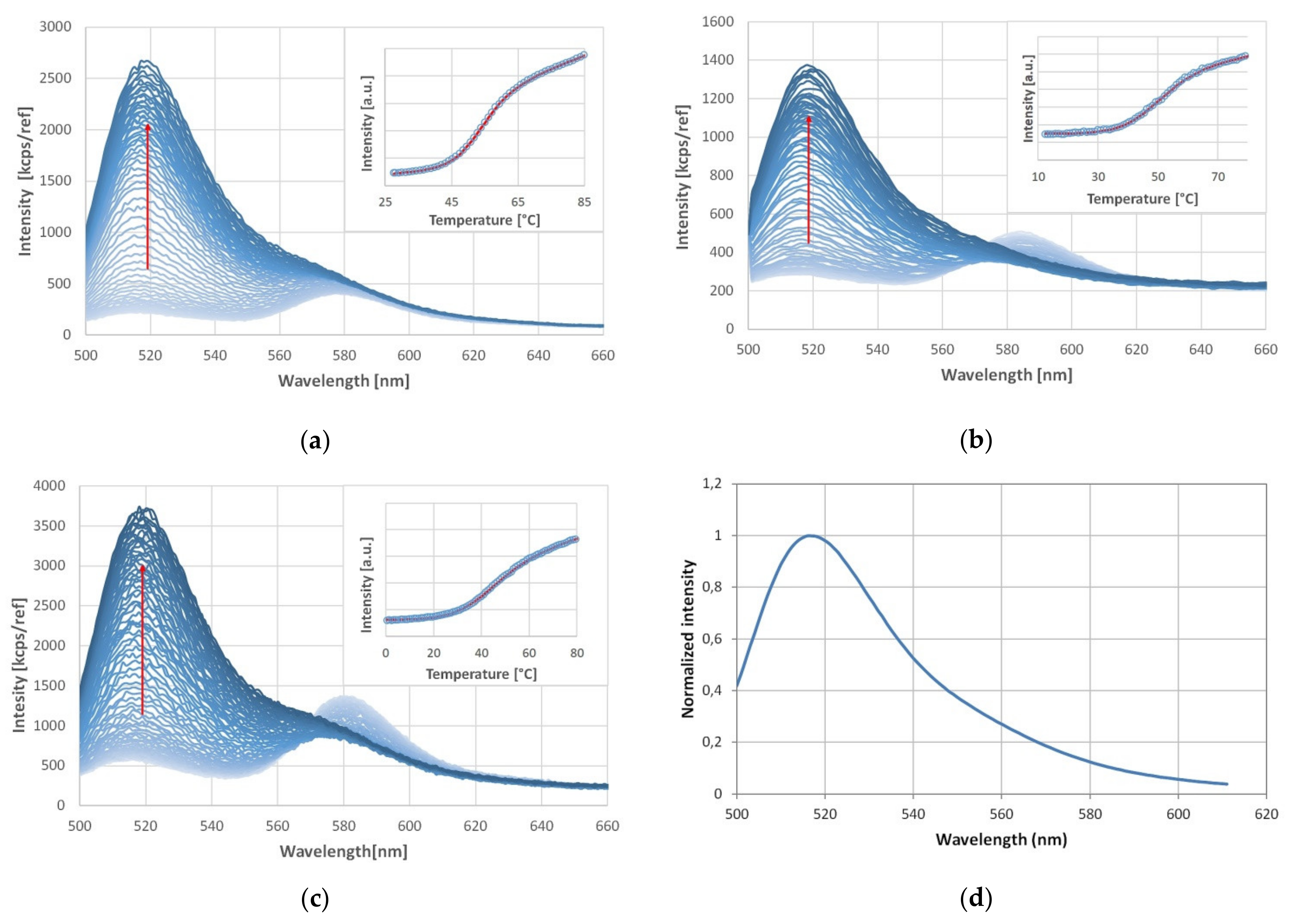

| Oligo Name | Relative FRET Efficiency Erel | |
|---|---|---|
| Folded | Unfolded | |
| HepB1 | 0.65 | 0.21 |
| HepB2 | 0.64 | 0.27 |
| HepB3 | 0.69 | 0.24 |
| Oligo | Li+ | Na+ | K+ | Rb+ | Stabilizing Order |
|---|---|---|---|---|---|
| HepB1 | 58.5 | 56.6 | 53.8 | 54.4 | Li+ > Na+ > Rb+ ≳ K+ |
| HepB2 | 46.9 | 51.0 | 51.9 | 45.6 | K+ > Na+ > Li+ ≳ Rb+ |
| HepB3 | 49.4 | 48.1 | 41.4 | 44.4 | Li+ > Na+ > Rb+ > K+ |
| Oligo Name | Technique | dTm/dp (°C/kbar) | dV (cm3/mol) |
|---|---|---|---|
| HepB1 | FRET | 3.04 ± 0.58 | 17.8 ± 3.8 |
| IR | 2.75 ± 0.28 | 16.1 ± 2.0 | |
| HepB2 | FRET | −0.96 ± 0.22 | −3.7 ± 0.6 |
| HepB3 | FRET | 0.79 ± 0.38 | 2.0 ± 1.1 |
| Oligo Name | |||
|---|---|---|---|
| HepB1 | HepB2 | HepB3 | |
| ΔTm (°C) = Tm,HepB+TMPyP4 − Tm,HepB | 23 | >36 | >47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somkuti, J.; Molnár, O.R.; Grád, A.; Smeller, L. Pressure Perturbation Studies of Noncanonical Viral Nucleic Acid Structures. Biology 2021, 10, 1173. https://doi.org/10.3390/biology10111173
Somkuti J, Molnár OR, Grád A, Smeller L. Pressure Perturbation Studies of Noncanonical Viral Nucleic Acid Structures. Biology. 2021; 10(11):1173. https://doi.org/10.3390/biology10111173
Chicago/Turabian StyleSomkuti, Judit, Orsolya Réka Molnár, Anna Grád, and László Smeller. 2021. "Pressure Perturbation Studies of Noncanonical Viral Nucleic Acid Structures" Biology 10, no. 11: 1173. https://doi.org/10.3390/biology10111173
APA StyleSomkuti, J., Molnár, O. R., Grád, A., & Smeller, L. (2021). Pressure Perturbation Studies of Noncanonical Viral Nucleic Acid Structures. Biology, 10(11), 1173. https://doi.org/10.3390/biology10111173






