Internalized Nanoceria Modify the Radiation-Sensitivity Profile of MDA MB231 Breast Carcinoma Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of MDA MB231 Cells
2.2. Cellular Uptake and Compartmental Localization of Nanoparticles
2.3. Determination of the Effect of CeO2 Nanoparticles on Native Cellular Heath and Redox Systems
2.4. Determination of the Effect of CeO2 Nanoparticles on Cellular Health following Irradiation with Ionizing Radiation
2.5. Determination of the Effect of CeO2 Nanoparticles on Intracellular Reactive Oxygen Species Levels following Irradiation with Ionizing Radiation
2.6. Statistical Analyses
3. Results
3.1. CeO2 NPs Localize within Cellular Compartments in MDA MB231 Cells
3.2. CeO2 NPs Modulate Cellular Health and Reactive Oxygen Species in a Concentration-Dependent Manner in MDA MB231 Cells
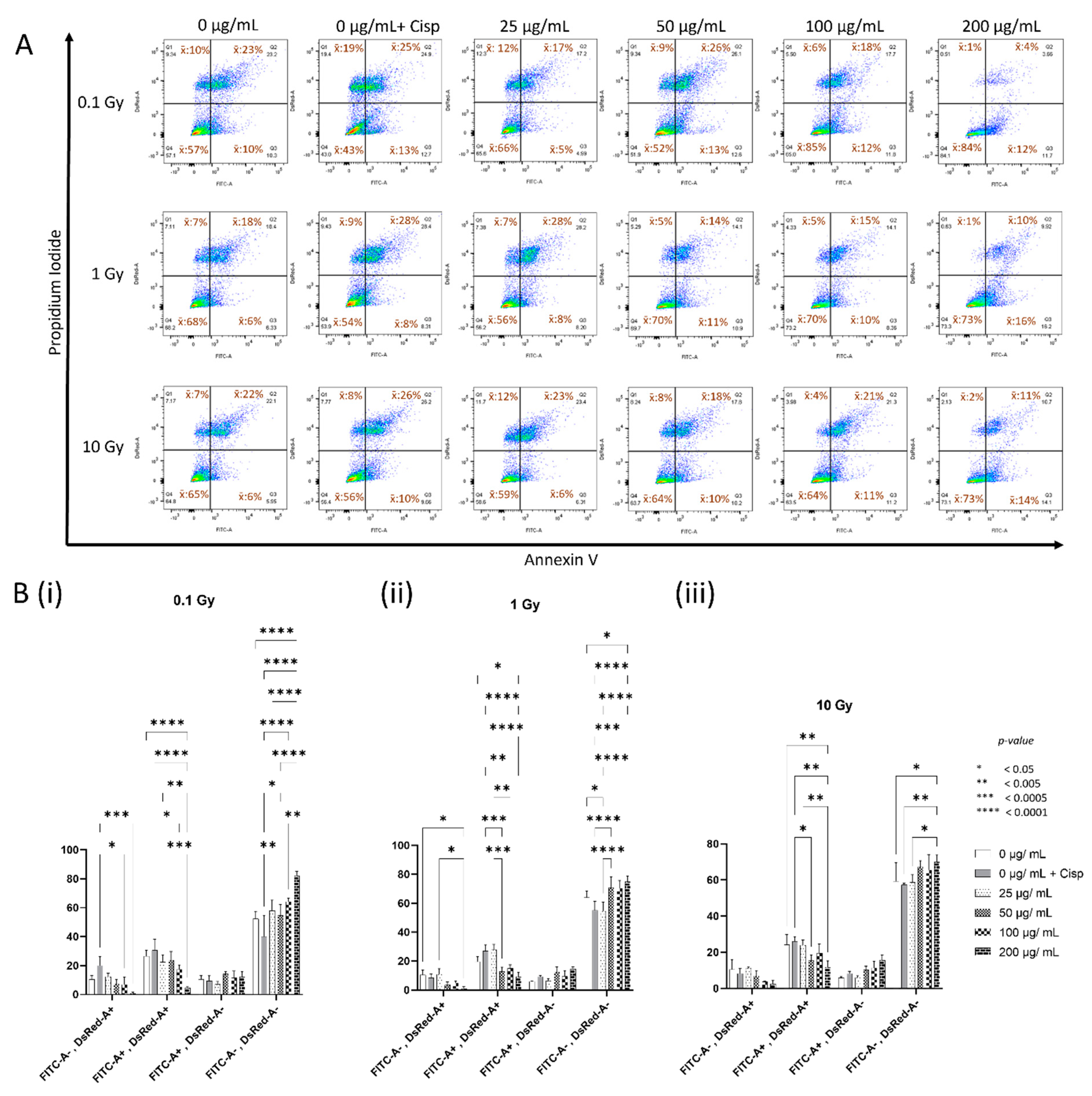
3.3. Concentration-Dependent Modulation of Cellular Health by CeO2 NPs in MDA MB231 Cells following Irradiation
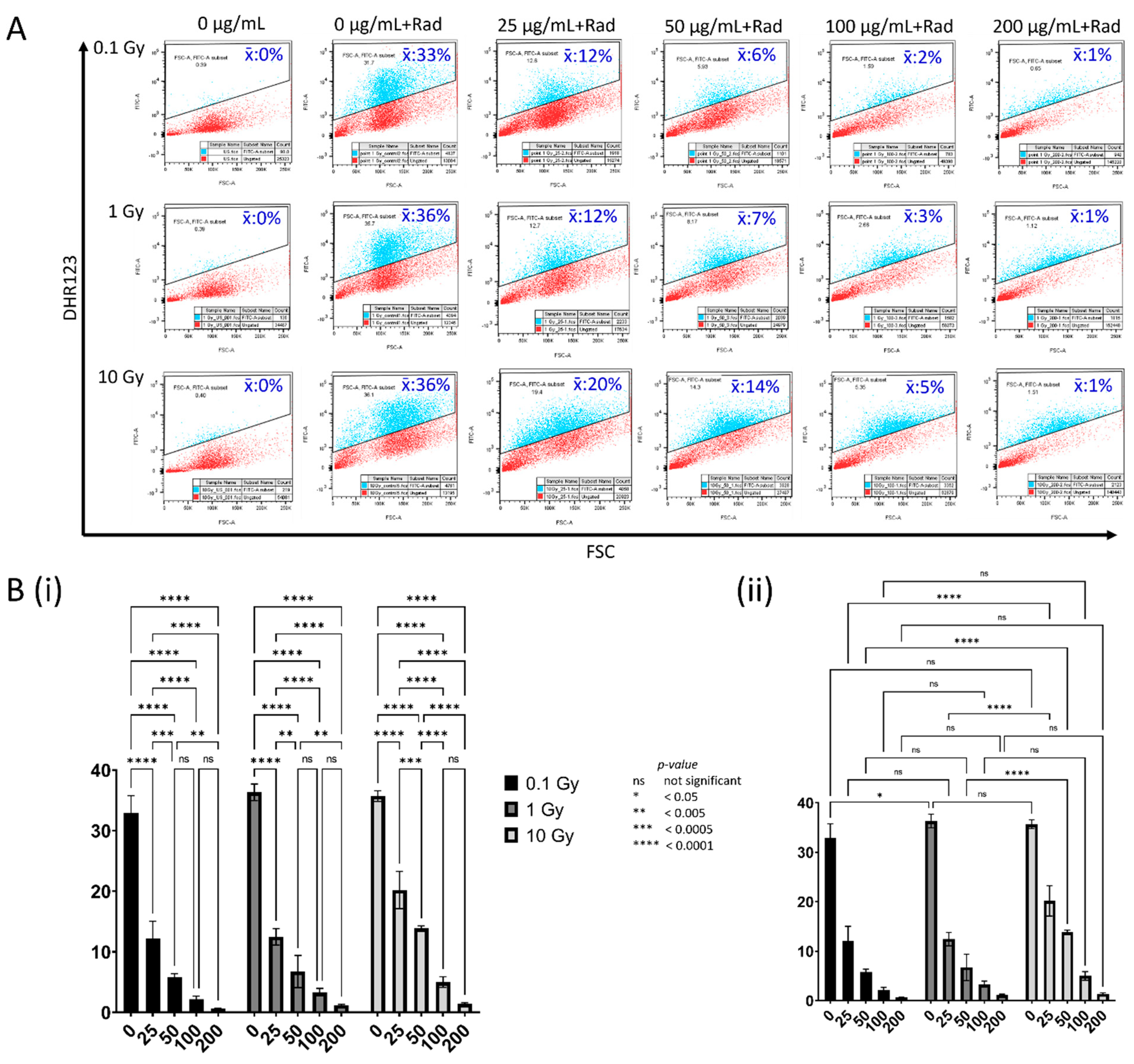
3.4. Concentration-Dependent Modulation of Reactive Oxygen Species by CeO2 NPs in MDA MB231 Cells following Irradiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brucer, M. A Chronology of Nuclear Medicine, 1600–1989; Heritage Publications: St. Louis, MO, USA, 1990; 496p. [Google Scholar]
- Quinn, R.A.; Sigl, C.C. Eastman Kodak Company. Radiography in Modern Industry, 4th ed.; Eastman Kodak: Rochester, NY, USA, 1980; 166p. [Google Scholar]
- Luckey, T.D. Radiation Hormesis; CRC Press: Boca Raton, FL, USA, 1991; 306p. [Google Scholar]
- Pawel, D.J.; Puskin, J.S. Us Environmental Protection Agency Radiogenic Risk Models and Projections for the Us Population. Health Phys. 2012, 102, 646–656. [Google Scholar] [CrossRef]
- Sykes, P.J. Until There Is a Resolution of the Pro-LNT/Anti-LNT Debate, We Should Head Toward a More Sensible Graded Approach for Protection From Low-Dose Ionizing Radiation. Dose Response 2020, 18, 1559325820921651. [Google Scholar] [CrossRef] [PubMed]
- Argonne National Laboratory. Radiation Chemistry; Advances in Chemistry Series; American Chemical Society: Washington, DC, USA, 1968. [Google Scholar]
- Desouky, O.; Ding, N.; Zhou, G.M. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Limoli, C.L.; Corcoran, J.J.; Milligan, J.R.; Ward, J.F.; Morgan, W.F. Critical target and dose and dose-rate responses for the induction of chromosomal instability by ionizing radiation. Radiat. Res. 1999, 151, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.P.; Georgakilas, A.G.; Ravanat, J.L. Targeted and Off-Target (Bystander and Abscopal) Effects of Radiation Therapy: Redox Mechanisms and Risk/Benefit Analysis. Antioxid Redox Signal 2018, 29, 1447–1487. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.; Matthews, R.H.; Lutz, P.; Ercal, N. Antioxidant role of N-acetyl cysteine isomers following high dose irradiation. Free Radic. Biol. Med. 2003, 34, 689–695. [Google Scholar] [CrossRef]
- Jin, W.; Wang, J.; Xu, S.; Xiao, L.; Chen, G.; Zhang, W.; Li, J. Radioprotective effect on HepG2 cells of low concentrations of cobalt chloride: Induction of hypoxia-inducible factor-1 alpha and clearance of reactive oxygen species. J. Radiat. Res. 2013, 54, 203–209. [Google Scholar] [CrossRef]
- Renson, J. Radioprotection of the mouse by 5-hydroxytryptamine and some related substances. Arch. Int. Physiol. Biochim. 1960, 68, 531–534. [Google Scholar] [PubMed]
- Das, B.; Bennett, P.V.; Cutter, N.C.; Sutherland, J.C.; Sutherland, B.M. Melatonin protects human cells from clustered DNA damages, killing and acquisition of soft agar growth induced by X-rays or 970 MeV/n Fe ions. Int. J. Radiat. Biol. 2011, 87, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Jelveh, S.; Kaspler, P.; Bhogal, N.; Mahmood, J.; Lindsay, P.E.; Okunieff, P.; Doctrow, S.R.; Bristow, R.G.; Hill, R.P. Investigations of antioxidant-mediated protection and mitigation of radiation-induced DNA damage and lipid peroxidation in murine skin. Int. J. Radiat. Biol. 2013, 89, 618–627. [Google Scholar] [CrossRef]
- Denissova, N.G.; Nasello, C.M.; Yeung, P.L.; Tischfield, J.A.; Brenneman, M.A. Resveratrol protects mouse embryonic stem cells from ionizing radiation by accelerating recovery from DNA strand breakage. Carcinogenesis 2012, 33, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, S.M.; Tochner, Z.; Krishna, C.M.; Glass, J.; Wilson, L.; Samuni, A.; Sprague, M.; Venzon, D.; Glatstein, E.; Mitchell, J.B.; et al. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992, 52, 1750–1753. [Google Scholar]
- Thotala, D.K.; Geng, L.; Dickey, A.K.; Hallahan, D.E.; Yazlovitskaya, E.M. A new class of molecular targeted radioprotectors: GSK-3beta inhibitors. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 557–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Chen, R.; Lin, Y.T.; Humayun, A.; Fornace, A.J., Jr.; Li, H.H. 3,3′-Diindolylmethane Enhances Tumor Regression after Radiation through Protecting Normal Cells to Modulate Antitumor Immunity. Adv. Radiat. Oncol. 2021, 6, 100601. [Google Scholar] [CrossRef] [PubMed]
- Burdelya, L.G.; Krivokrysenko, V.I.; Tallant, T.C.; Strom, E.; Gleiberman, A.S.; Gupta, D.; Kurnasov, O.V.; Fort, F.L.; Osterman, A.L.; Didonato, J.A.; et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008, 320, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Brizel, D.M.; Wasserman, T.H.; Henke, M.; Strnad, V.; Rudat, V.; Monnier, A.; Eschwege, F.; Zhang, J.; Russell, L.; Oster, W.; et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J. Clin. Oncol. 2000, 18, 3339–3345. [Google Scholar] [CrossRef] [PubMed]
- Meyn, R.E.; Krishnan, S.; Skinner, H.D. Everything Old Is New Again: Using Nelfinavir to Radiosensitize Rectal Cancer. Clin. Cancer Res. 2016, 22, 1834–1836. [Google Scholar] [CrossRef] [Green Version]
- Ai, D.; Chen, Y.; Liu, Q.; Zhang, J.; Deng, J.; Zhu, H.; Ren, W.; Zheng, X.; Li, Y.; Wei, S.; et al. Comparison of paclitaxel in combination with cisplatin (TP), carboplatin (TC) or fluorouracil (TF) concurrent with radiotherapy for patients with local advanced oesophageal squamous cell carcinoma: A three-arm phase III randomized trial (ESO-Shanghai 2). BMJ Open 2018, 8, e020785. [Google Scholar] [CrossRef] [PubMed]
- Larue, R.T.; Van De Voorde, L.; Berbee, M.; van Elmpt, W.J.; Dubois, L.J.; Panth, K.M.; Peeters, S.G.; Claessens, A.; Schreurs, W.M.; Nap, M.; et al. A phase 1 ‘window-of-opportunity’ trial testing evofosfamide (TH-302), a tumour-selective hypoxia-activated cytotoxic prodrug, with preoperative chemoradiotherapy in oesophageal adenocarcinoma patients. BMC Cancer 2016, 16, 644. [Google Scholar] [CrossRef] [Green Version]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.A.; Molana, S.H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, M.; Doyle, T.; Kumar, P.; McLeod, D.; Badger, W.; Prater, S.; Moran, M.; Rosenberg, S.; Bonatsos, C.; Kreitner, C.; et al. Phase I/II trial of docetaxel and concurrent radiation therapy in localized high risk prostate cancer (AGUSG 03-10). Urol. Oncol. 2008, 26, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Saito, A.I.; Mitsuhashi, T.; Inoue, T.; Ota, T.; Ujihira, T.; Yoshida, K.; Sasai, K. Radiosensitization using hydrogen peroxide in patients with cervical cancer. Mol. Clin. Oncol. 2021, 15, 142. [Google Scholar] [CrossRef] [PubMed]
- Duenas-Gonzalez, A.; Cetina-Perez, L.; Lopez-Graniel, C.; Gonzalez-Enciso, A.; Gomez-Gonzalez, E.; Rivera-Rubi, L.; Montalvo-Esquivel, G.; Munoz-Gonzalez, D.; Robles-Flores, J.; Vazquez-Govea, E.; et al. Pathologic response and toxicity assessment of chemoradiotherapy with cisplatin versus cisplatin plus gemcitabine in cervical cancer: A randomized Phase II study. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 817–823. [Google Scholar] [CrossRef]
- Oronsky, B.T.; Knox, S.J.; Scicinski, J. Six degrees of separation: The oxygen effect in the development of radiosensitizers. Transl Oncol. 2011, 4, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar] [CrossRef]
- Szatmari, T.; Hargitai, R.; Safrany, G.; Lumniczky, K. Extracellular Vesicles in Modifying the Effects of Ionizing Radiation. Int. J. Mol. Sci. 2019, 20, 5527. [Google Scholar] [CrossRef] [Green Version]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Zhu, A.; Sun, K.; Petty, H.R. Cerium oxide and platinum nanoparticles protect cells from oxidant-mediated apoptosis. J. Nanopart. Res. 2011, 13, 5547–5555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celardo, I.; De Nicola, M.; Mandoli, C.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Ce(3)+ ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticles. ACS Nano 2011, 5, 4537–4549. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Choi, J.; Park, Y.K.; Park, K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology 2008, 245, 90–100. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alili, L.; Sack, M.; Karakoti, A.S.; Teuber, S.; Puschmann, K.; Hirst, S.M.; Reilly, C.M.; Zanger, K.; Stahl, W.; Das, S.; et al. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor-stroma interactions. Biomaterials 2011, 32, 2918–2929. [Google Scholar] [CrossRef]
- Montazeri, A.; Zal, Z.; Ghasemi, A.; Yazdannejat, H.; Asgarian-Omran, H.; Hosseinimehr, S.J. Radiosensitizing Effect of Cerium Oxide Nanoparticles on Human Leukemia Cells. Pharm. Nanotechnol. 2018, 6, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Zal, Z.; Ghasemi, A.; Azizi, S.; Asgarian-Omran, H.; Montazeri, A.; Hosseinimehr, S.J. Radioprotective Effect of Cerium Oxide Nanoparticles Against Genotoxicity Induced by Ionizing Radiation on Human Lymphocytes. Curr. Radiopharm. 2018, 11, 109–115. [Google Scholar] [CrossRef]
- Rodea-Palomares, I.; Boltes, K.; Fernandez-Pinas, F.; Leganes, F.; Garcia-Calvo, E.; Santiago, J.; Rosal, R. Physicochemical characterization and ecotoxicological assessment of CeO2 nanoparticles using two aquatic microorganisms. Toxicol. Sci. 2011, 119, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Guo, C.; Robertson, S.; Weber, R.J.M.; Buckley, A.; Warren, J.; Hodgson, A.; Rappoport, J.Z.; Ignatyev, K.; Meldrum, K.; Romer, I.; et al. Pulmonary toxicity of inhaled nano-sized cerium oxide aerosols in Sprague-Dawley rats. Nanotoxicology 2019, 13, 733–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemmar, A.; Al-Salam, S.; Al Ansari, Z.; Alkharas, Z.A.; Al Ahbabi, R.M.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H. Impact of Pulmonary Exposure to Cerium Oxide Nanoparticles on Experimental Acute Kidney Injury. Cell Physiol. BioChem. 2019, 52, 439–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, M.; Stinson, K.; George, R. Reflectance structured illumination imaging of internalized cerium oxide nanoparticles modulating dose-dependent reactive oxygen species in breast cancer cells. BioChem. Biophys. Rep. 2020, 22, 100745. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Chen, M.H.; Li, C.Y.; Tung, F.I.; Chen, S.Y.; Liu, T.Y. Using Gold-Nanorod-Filled Mesoporous Silica Nanobeads for Enhanced Radiotherapy of Oral Squamous Carcinoma. Nanomaterials 2021, 11, 2235. [Google Scholar] [CrossRef] [PubMed]
- Bromma, K.; Cicon, L.; Beckham, W.; Chithrani, D.B. Gold nanoparticle mediated radiation response among key cell components of the tumour microenvironment for the advancement of cancer nanotechnology. Sci. Rep. 2020, 10, 12096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Luo, Z.; Chen, J.; Shen, X.; Song, S.; Sun, Y.; Fan, S.; Fan, F.; Leong, D.T.; Xie, J. Ultrasmall Au(10-12)(SG)(10-12) nanomolecules for high tumor specificity and cancer radiotherapy. Adv. Mater. 2014, 26, 4565–4568. [Google Scholar] [CrossRef] [Green Version]
- Ghita, M.; McMahon, S.J.; Taggart, L.E.; Butterworth, K.T.; Schettino, G.; Prise, K.M. A mechanistic study of gold nanoparticle radiosensitisation using targeted microbeam irradiation. Sci. Rep. 2017, 7, 44752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Wei, L.Y.; Lu, J.R.; Xu, H.Z.; Patel, A.; Chen, Z.S.; Chen, G.F. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wason, M.S.; Lu, H.; Yu, L.; Lahiri, S.K.; Mukherjee, D.; Shen, C.; Das, S.; Seal, S.; Zhao, J. Cerium Oxide Nanoparticles Sensitize Pancreatic Cancer to Radiation Therapy through Oxidative Activation of the JNK Apoptotic Pathway. Cancers 2018, 10, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinnon, S.; Guatelli, S.; Incerti, S.; Ivanchenko, V.; Konstantinov, K.; Corde, S.; Lerch, M.; Tehei, M.; Rosenfeld, A. Local dose enhancement of proton therapy by ceramic oxide nanoparticles investigated with Geant4 simulations. Phys. Med. 2016, 32, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.; Corde, S.; Oktaria, S.; Brown, R.; Rosenfeld, A.; Lerch, M.; Konstantinov, K.; Tehei, M. Cerium oxide nanoparticles: Influence of the high-Z component revealed on radioresistant 9L cell survival under X-ray irradiation. Nanomedicine 2013, 9, 1098–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Zhang, X.H.; Hu, X.D.; Zhang, W.; Lou, Z.C.; Xie, L.H.; Liu, P.D.; Zhang, H.Q. Enhancement of radiotherapy by ceria nanoparticles modified with neogambogic acid in breast cancer cells. Int. J. Nanomed. 2015, 10, 4957–4969. [Google Scholar] [CrossRef] [Green Version]
- Popov, A.L.; Zaichkina, S.I.; Popova, N.R.; Rozanova, O.M.; Romanchenko, S.P.; Ivanova, O.S.; Smirnov, A.A.; Mironova, E.V.; Selezneva, I.I.; Ivanov, V.K. Radioprotective effects of ultra-small citrate-stabilized cerium oxide nanoparticles in vitro and in vivo. RSC Adv. 2016, 6, 106141–106149. [Google Scholar] [CrossRef]
- Colon, J.; Hsieh, N.; Ferguson, A.; Kupelian, P.; Seal, S.; Jenkins, D.W.; Baker, C.H. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine 2010, 6, 698–705. [Google Scholar] [CrossRef]
- Colon, J.; Herrera, L.; Smith, J.; Patil, S.; Komanski, C.; Kupelian, P.; Seal, S.; Jenkins, D.W.; Baker, C.H. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine 2009, 5, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Edoff, K.; Caputo, F.; Kallman, T.; Blom, H.; Karlsson, H.L.; Ghibelli, L.; Traversa, E.; Ceccatelli, S.; Fadeel, B. Cerium oxide nanoparticles inhibit differentiation of neural stem cells. Sci. Rep. 2017, 7, 9284. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Li, J.M.; Wang, S.M.; Yong, X.; Tang, B.; Jie, M.M.; Dong, H.; Yang, X.C.; Yang, S.M. Cerium oxide nanoparticles inhibit the migration and proliferation of gastric cancer by increasing DHX15 expression. Int. J. Nanomed. 2016, 11, 3023–3034. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kumar, A.; Karakoti, A.; Seal, S.; Self, W.T. Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol. Biosyst. 2010, 6, 1813–1820. [Google Scholar] [CrossRef] [Green Version]
- Yuan, K.; Mei, J.; Shao, D.; Zhou, F.; Qiao, H.; Liang, Y.; Li, K.; Tang, T. Cerium Oxide Nanoparticles Regulate Osteoclast Differentiation Bidirectionally by Modulating the Cellular Production of Reactive Oxygen Species. Int. J. Nanomed. 2020, 15, 6355–6372. [Google Scholar] [CrossRef]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijink, A.M.; Everts, M.; Honeywell, M.E.; Richards, R.; Kok, Y.P.; de Vries, E.G.E.; Lee, M.J.; van Vugt, M. Modeling of Cisplatin-Induced Signaling Dynamics in Triple-Negative Breast Cancer Cells Reveals Mediators of Sensitivity. Cell Rep. 2019, 28, 2345–2357.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xie, T. Ghrelin inhibits cisplatin-induced MDA-MB-231 breast cancer cell apoptosis via PI3K/Akt/mTOR signaling. Exp. Ther. Med. 2020, 19, 1633–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhakaran, P.; Hassiotou, F.; Blancafort, P.; Filgueira, L. Cisplatin induces differentiation of breast cancer cells. Front. Oncol. 2013, 3, 134. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Qu, X.G. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdi Goushbolagh, N.; Abedi Firouzjah, R.; Ebrahimnejad Gorji, K.; Khosravanipour, M.; Moradi, S.; Banaei, A.; Astani, A.; Najafi, M.; Zare, M.H.; Farhood, B. Estimation of radiation dose-reduction factor for cerium oxide nanoparticles in MRC-5 human lung fibroblastic cells and MCF-7 breast-cancer cells. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S1215–S1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goushbolagh, N.A.; Farhood, B.; Astani, A.; Nikfarjam, A.; Kalantari, M.; Zare, M.H. Quantitative Cytotoxicity, Cellular Uptake and Radioprotection Effect of Cerium Oxide Nanoparticles in MRC-5 Normal Cells and MCF-7 Cancerous Cells. Bionanoscience 2018, 8, 769–777. [Google Scholar] [CrossRef]
- Colon, J.; Seal, S.; Konduri, S.; Limaye, A.; Abdelrahim, M.; Baker, C. Selective radioprotection of normal tissues by new cerium oxide nanoparticles. Cancer Res. 2007, 67 (Suppl. 9), 754. [Google Scholar]
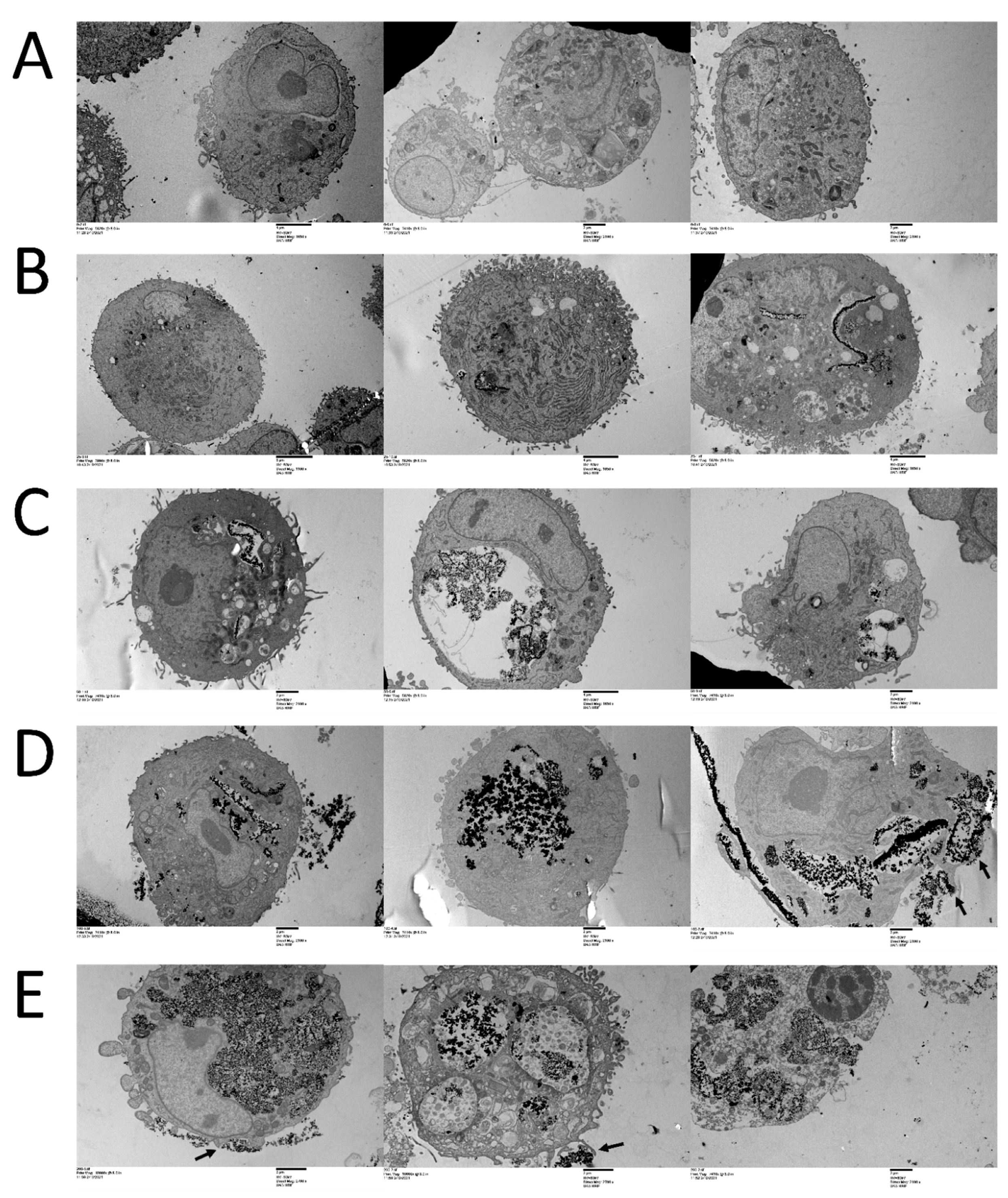
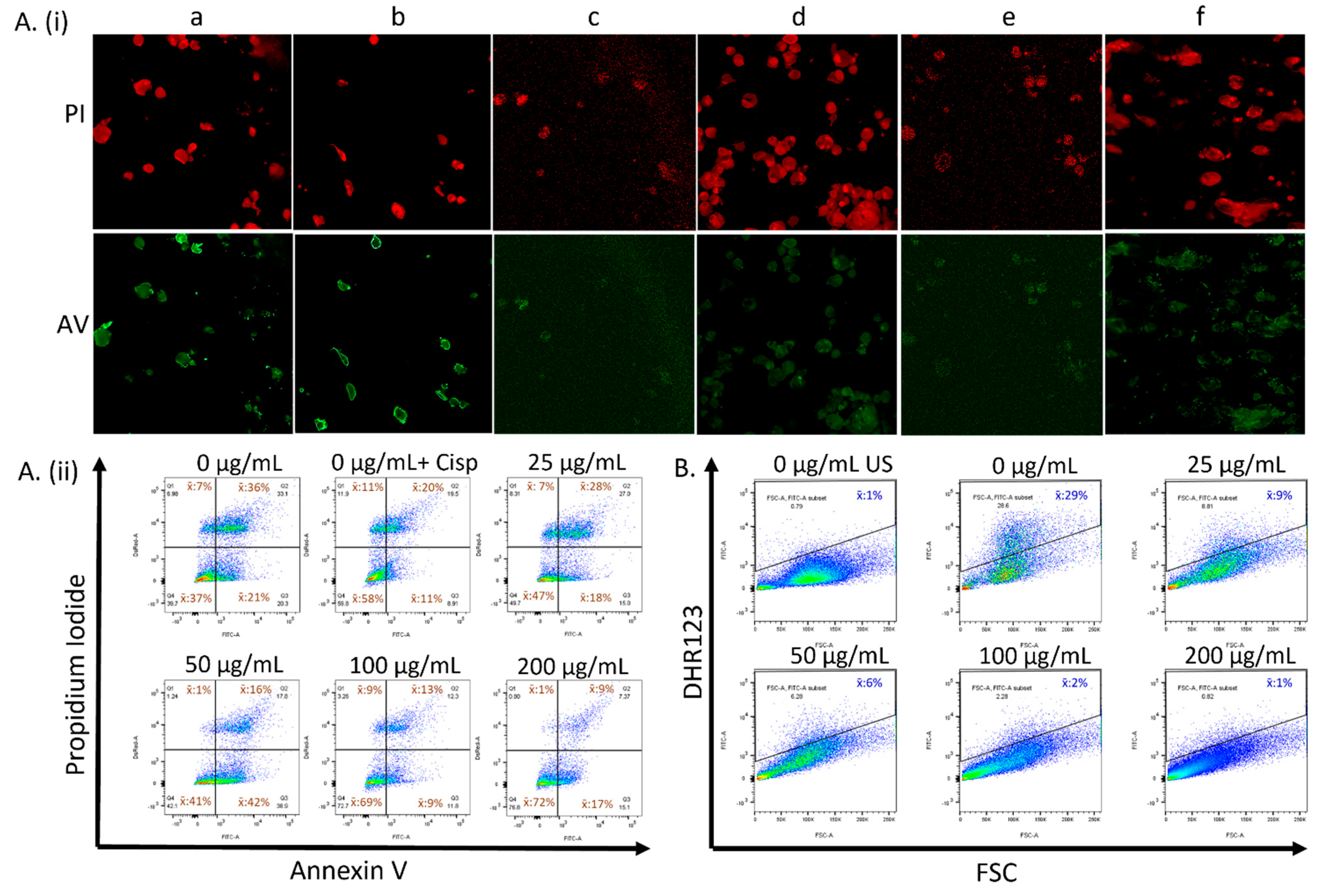
| Treatment | AV−/PI− | AV+/PI− | AV+/PI+ | AV−/PI+ |
|---|---|---|---|---|
| 0 µg mL−1 CeO2 | 37% | 21% | 36% | 7% |
| 25 µg mL−1 CeO2 | 47% | 18% | 28% | 7% |
| 50 µg mL−1 CeO2 | 41% | 42% | 16% | 1% |
| 100 µg mL−1 CeO2 | 69% | 9% | 13% | 9% |
| 200 µg mL−1 CeO2 | 72% | 17% | 9% | 1% |
| Cisplatin | 58% | 11% | 20% | 11% |
| Treatment | AV−/PI− | AV+/PI− | AV+/PI+ | AV−/PI+ |
|---|---|---|---|---|
| 0 µg mL−1 CeO2 | 57% | 10% | 23% | 10% |
| 0 µg mL−1 + Cisplatin | 43% | 13% | 25% | 19% |
| 25 µg mL−1 CeO2 | 66% | 5% | 17% | 12% |
| 50 µg mL−1 CeO2 | 52% | 13% | 26% | 9% |
| 100 µg mL−1 CeO2 | 85% | 12% | 18% | 6% |
| 200 µg mL−1 CeO2 | 84% | 12% | 4% | 1% |
| Treatment | AV−/PI− | AV+/PI− | AV+/PI+ | AV−/PI+ |
|---|---|---|---|---|
| 0 µg mL−1 CeO2 | 68% | 6% | 18% | 7% |
| 0 µg mL−1 + Cisplatin | 54% | 8% | 28% | 9% |
| 25 µg mL−1 CeO2 | 56% | 8% | 28% | 7% |
| 50 µg mL−1 CeO2 | 70% | 11% | 14% | 5% |
| 100 µg mL−1 CeO2 | 70% | 10% | 15% | 5% |
| 200 µg mL−1 CeO2 | 73% | 16% | 10% | 1% |
| Treatment | AV−/PI− | AV+/PI− | AV+/PI+ | AV−/PI+ |
|---|---|---|---|---|
| 0 µg mL−1 CeO2 | 65% | 6% | 22% | 7% |
| 0 µg mL−1 + Cisplatin | 56% | 10% | 26% | 8% |
| 25 µg mL−1 CeO2 | 59% | 6% | 23% | 12% |
| 50 µg mL−1 CeO2 | 64% | 10% | 18% | 8% |
| 100 µg mL−1 CeO2 | 64% | 11% | 21% | 4% |
| 200 µg mL−1 CeO2 | 73% | 14% | 11% | 2% |
| Treatment | 0.1 Gy | 1 Gy | 10 Gy |
|---|---|---|---|
| 0 µg mL−1 CeO2 (unlabeled) | 0% | 0% | 0% |
| 0 µg mL−1 CeO2 | 33% | 36% | 36% |
| 25 µg mL−1 CeO2 | 12% | 12% | 20% |
| 50 µg mL−1 CeO2 | 6% | 7% | 14% |
| 100 µg mL−1 CeO2 | 2% | 3% | 5% |
| 200 µg mL−1 CeO2 | 1% | 1% | 1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibb, E.; Alajlan, N.; Alsuwailem, S.; Mitchell, B.; Brady, A.; Maqbool, M.; George, R. Internalized Nanoceria Modify the Radiation-Sensitivity Profile of MDA MB231 Breast Carcinoma Cells. Biology 2021, 10, 1148. https://doi.org/10.3390/biology10111148
Bibb E, Alajlan N, Alsuwailem S, Mitchell B, Brady A, Maqbool M, George R. Internalized Nanoceria Modify the Radiation-Sensitivity Profile of MDA MB231 Breast Carcinoma Cells. Biology. 2021; 10(11):1148. https://doi.org/10.3390/biology10111148
Chicago/Turabian StyleBibb, Emory, Noura Alajlan, Saad Alsuwailem, Benjamin Mitchell, Amy Brady, Muhammad Maqbool, and Remo George. 2021. "Internalized Nanoceria Modify the Radiation-Sensitivity Profile of MDA MB231 Breast Carcinoma Cells" Biology 10, no. 11: 1148. https://doi.org/10.3390/biology10111148
APA StyleBibb, E., Alajlan, N., Alsuwailem, S., Mitchell, B., Brady, A., Maqbool, M., & George, R. (2021). Internalized Nanoceria Modify the Radiation-Sensitivity Profile of MDA MB231 Breast Carcinoma Cells. Biology, 10(11), 1148. https://doi.org/10.3390/biology10111148







