Humoral and Cellular Defense Mechanisms in Rebel Workers of Apis mellifera
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Hemolymph Collection
2.3. Biochemical Analysis
- −
- Phagocytic index, according to Watson et al. [1] modified by Keehnen et al. [2] method. To investigate the presence of phagocytic cells, a 10 µL suspension of heat-killed Escherichia coli (Sigma-Aldrich, Poznań, Poland; E. coli K-12 strain) in Luria–Bertani medium (A&A Biotechnology, Gdańsk, Poland) was added to 10 uL fresh hemolymph. The solution was incubated for 30 min at room temperature and gently shaking every 10 min. For each sample, a 30 µL drop of the solution was placed on a microscope slide and left to adhere for 20 min in a humid chamber at room temperature (RT). Next, the slide was fixed in 96% methanol for 5 min and 10 µL freshly prepared 0, 4% trypan blue was added. The preparation, after 1–2 min incubation and drying, was imaged under immersion under a light microscope with a magnification of 1250× and the bacterial cells absorbed by 50 phagocytes were counted. From these data, the mean number of bacteria phagocytized by one hemocyte was calculated.
- −
- JH3 titers, using Teal et al. [29] method: GC–MS analysis was carried out using a Shimadzu chromatograph coupled with a mass detector (GC MS QP-2010 Ultra EI NCI; Duisburg, Germany). The GC was equipped with both split/splitless injectors. The injection volume was 2 µL. Zebron ZB-5MSi column capillary 30 m × 0.25 mm × 0.25 was used. Temperature program from 50 °C to 260 °C in increments of 10 °C / min was used. The analysis was performed in the SIM mode (main ion 235 and additional ions 217, 189, 147, 125, 111). Isobutane was used for chemical ionization.
- −
- 135 µL of methanol and 100 µL of hexane were added to 15 µL of hemolymph. The solution was vortexed at 3200 rpm for 2 min. The emulsion was broken by centrifugation at 10,000× g for 5 min and the organic layer was removed with a 250 µL syringe. The aqueous layer was extracted an additional two times as above with 100 µL hexane. The JH3 content was determined by comparing the sample measurement result to the standard curve, which was prepared from the JH3 standard (Sigma-Aldrich, Poznań, Poland; J2000-10MG) in the concentration range 1–10 ng/mL.
- −
- Vg levels, using a Honey Bee Vitellogenin (VG) ELISA Kit (MyBioSource, Warsaw, Poland; MBS109137), according to the instructions attached to the test. This commercially available ELISA kit measures the Vg content in body fluids, tissue homogenates, secretions, or honey bee feces. The sensitivity of the test is 5 ng/mL. The measuring range is 31.2 ng/mL–1000 ng/mL (1.56–50 ng).
2.4. Fat Body Analysis
2.5. Anatomical Characteristics
2.6. Statistical Analysis
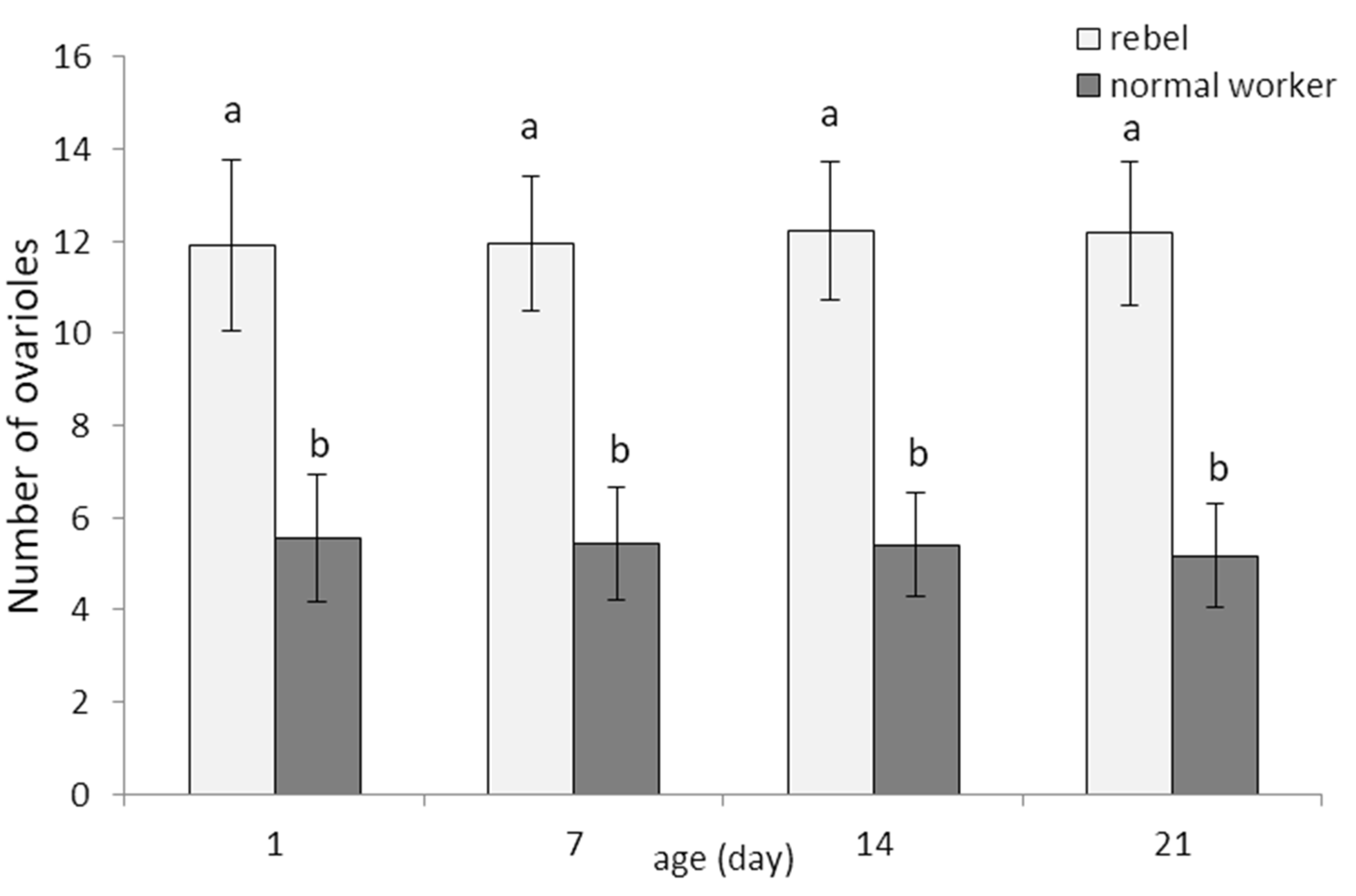
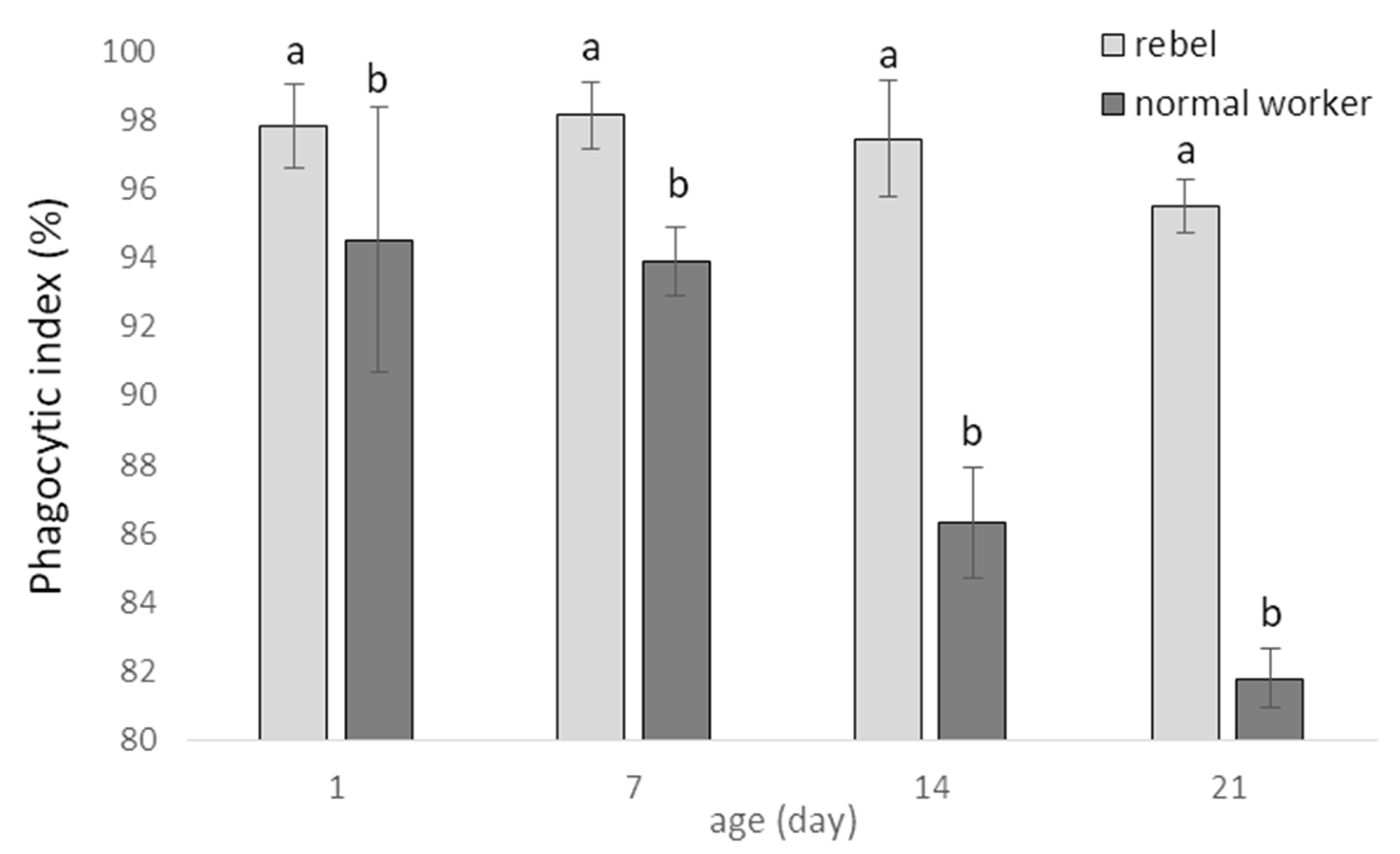
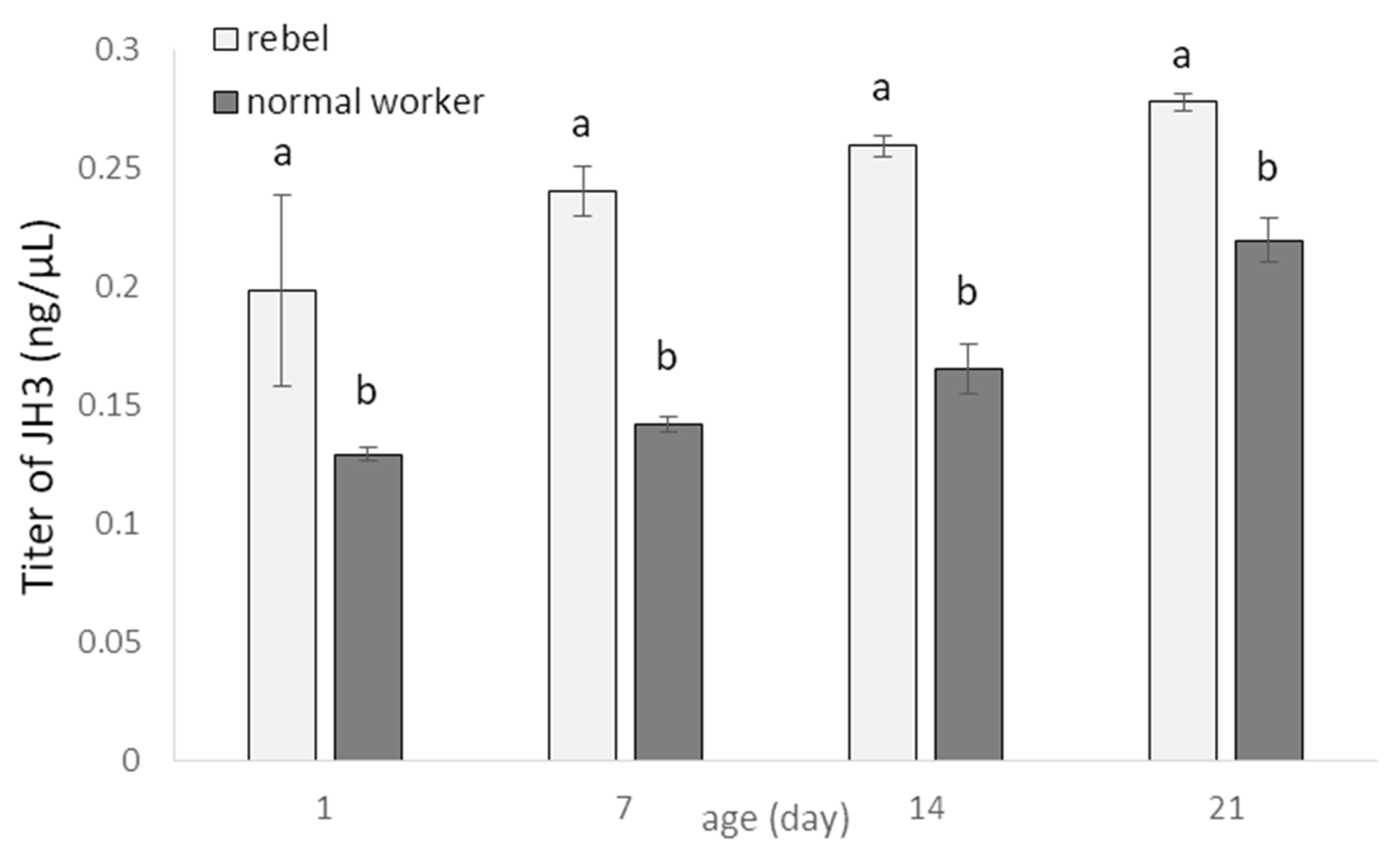
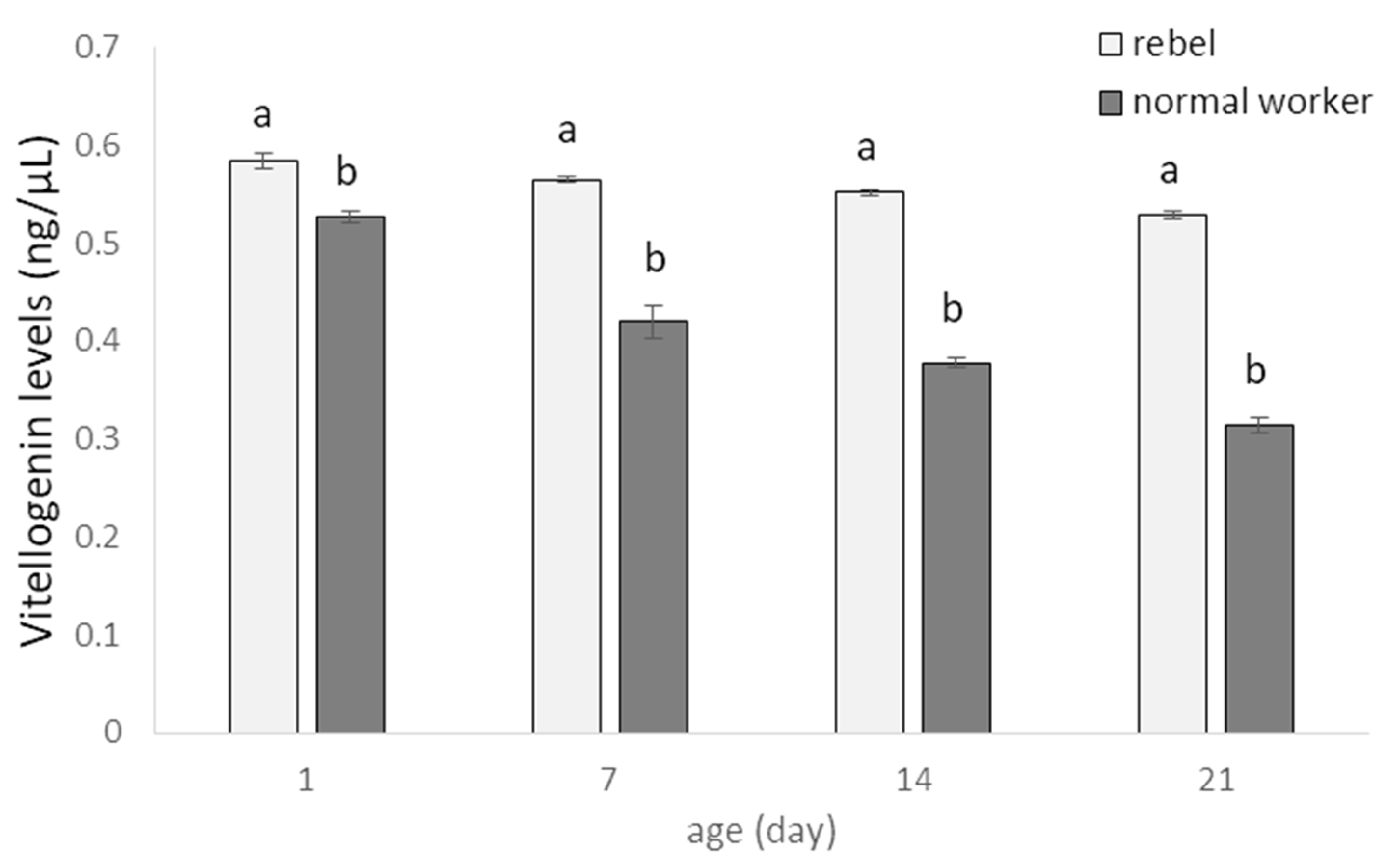
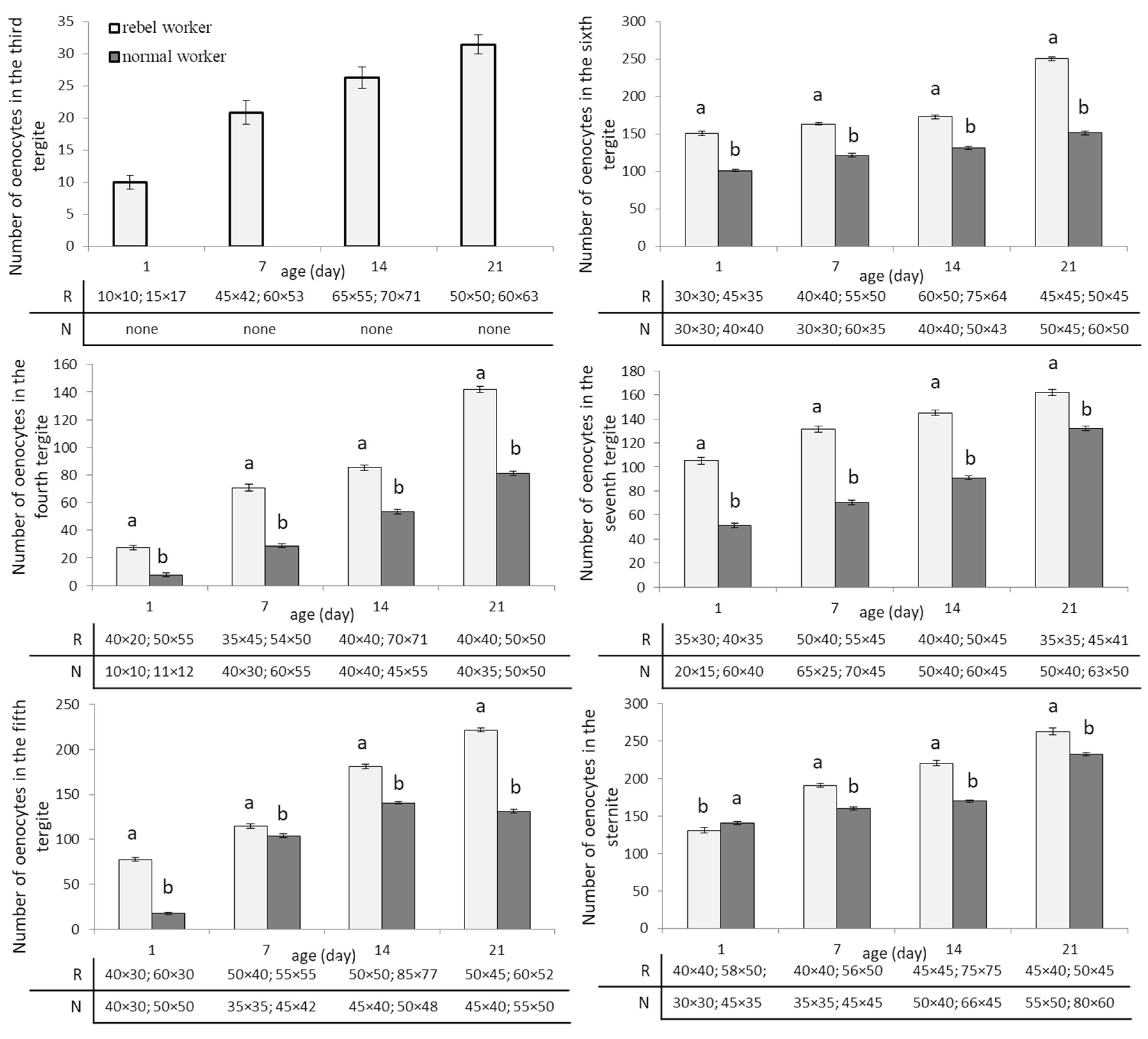
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watson, F.; Püttmann-Holgado, R.; Thomas, F.; Lamar, D.; Hughes, M.; Kondo, M.; Rebel, V.I.; Schmucker, D. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science 2005, 309, 1874–1878. [Google Scholar] [CrossRef]
- Keehnen, N.L.P.; Fors, L.; Järver, P.; Spetz, A.; Nylin, S.; Theopold, U.; Wheat, C. A Population Genomic Investigation of Immune Cell Diversity and Phagocytic Capacity in a Butterfly. Genes 2021, 12, 279. [Google Scholar] [CrossRef]
- Yan, Y.; Hillyer, J.F. The immune and circulatory systems are functionally integrated across insect evolution. Sci. Adv. 2020, 6, eabb3164. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Olszewski, K.; Kuszewska, K.; Chobotow, J.; Wójcik, Ł.; Paleolog, J.; Woyciechowski, M. Segmentation of the subcuticular fat body in Apis mellifera females with different reproductive potentials. Sci. Rep. 2021, 11, 13887. [Google Scholar] [CrossRef]
- Paes de Oliveira, V.T.; Poiani, S.B.; Antonialli, W.F.; Da Cruz-Landim, C. Morphometric changes on honeybee Apis mellifera L. workers fat body cells after juvenile hormone topic application at emergence. Micron 2008, 39, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Hartfelder, K.; Norberg, K.; Hagen, A.; Omholt, S.W. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): A factor in colony loss during overwintering? J. Econ. Entomol. 2004, 97, 741–747. [Google Scholar] [CrossRef]
- Robinson, G.E.; Strambi, C.; Strambi, A.; Feldlaufer, M.F. Comparison of juvenile hormone and ecdysteroid adult haemolymph titres in adult worker and queen honey bees (Apis mellifera). J. Insect Physiol. 1991, 37, 929–935. [Google Scholar] [CrossRef]
- Barchuk, A.R.; Bitondi, M.M.; Simões, Z.L. Effects of juvenile hormone and ecdysone on the timing of vitellogenin appearance in hemolymph of queen and worker pupae of Apis mellifera. J. Insect Sci. 2002, 2, 1. [Google Scholar] [CrossRef]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef] [PubMed]
- Paes de Oliveira, V.T.; Cruz-Landim, C. Protein contentand eletrophoretic profile of fat body and ovary extracts from workers of Melipona quadrifasciata anthidioides (Hymenoptera, Meliponini). Ineringia 2004, 94, 417–419. [Google Scholar]
- Robinson, G.E.; Page, R.E.; Strambi, C.; Strambi, A. Colony integration in honey bees: Mechanisms of behavioral reversion. Ethology 1992, 90, 336–348. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Robinson, G.E. Regulation of honey bee division of labor by colony age demography. Behav. Ecol. Sociobiol. 1996, 39, 147–158. [Google Scholar] [CrossRef]
- Winston, M. The Biology of Honeybee, 1st ed.; Harvard University Press: Cambridge, MA, USA, 1987; pp. 5–57. [Google Scholar]
- Toth, A.L.; Robinson, G.E. Worker nutrition and division of labour in honeybees. Anim. Behav. 2005, 69, 427–435. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Snyder, L.; Meador, C. Fat body lipolysis connects poor nutrition to hypopharyngeal gland degradation in Apis mellifera. J. Insect Physiol. 2019, 116, 1–9. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Deeter, M.E.; Snyder, L.; Meador, C.; Welchert, A.C.; Hoffman, A.; Obernesser, B.T. Octopamine mobilizes lipids from honey bee (Apis mellifera) hypopharyngeal glands. J. Exp. Biol. 2020, 223, jeb216135. [Google Scholar] [CrossRef] [PubMed]
- Klose, S.P.; Rolke, D.; Baumann, O. Morphogenesis of honeybee hypopharyngeal gland during pupal development. Front. Zool. 2017, 14, 22. [Google Scholar] [CrossRef]
- Hu, H.; Bezabih, G.; Feng, M.; Wei, Q.; Zhang, X.; Wu, F.; Meng, L.; Fang, Y.; Han, B.; Ma, C.; et al. In-depth Proteome of the Hypopharyngeal Glands of Honeybee Workers Reveals Highly Activated Protein and Energy Metabolism in Priming the Secretion of Royal Jelly. Mol. Cell. Prot. 2019, 18, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Makert, G.R.; Paxton, R.J.; Hartfelder, K. Ovariole number—A predictor of differential reproductive success among worker subfamilies in queenless honeybee (Apis mellifera L.) colonies. Behav. Ecol. Sociobiol. 2006, 60, 815–825. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Kuszewska, K. Swarming generates rebel workers in honeybees. Curr. Biol. 2012, 22, 707–711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Woyciechowski, M.; Kuszewska, K.; Pitorak, J.; Kierat, J. Honeybee worker larvae perceive queen pheromones in their food. Apidologie 2017, 48, 144–149. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Kuszewska, K.; Paleolog, J.; Woyciechowski, M. Reproductive potential accelerates preimaginal development of rebel workers in Apis mellifera. Apidologie 2021, in press. [Google Scholar]
- Kuszewska, K.; Miler, K.; Rojek, W.; Woyciechowski, M. Honeybee workers with higher reproductive potential live longer lives. Exp. Gerontol. 2017, 98, 8–12. [Google Scholar] [CrossRef]
- Kuszewska, K.; Miler, K.; Woyciechowski, M. Honeybee rebel workers invest less in risky foraging than normal workers. Sci. Rep. 2018, 8, 9459. [Google Scholar] [CrossRef] [PubMed]
- Kuszewska, K.; Miler, K.; Rojek, W.; Ostap-Chęć, M.; Woyciechowski, M. Rebel honeybee workers have a tendency to become intraspecific reproductive parasites. Ecol. Evol. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kuszewska, K.; Miler, K.; Woyciechowski, M. Honeybee rebel workers preferentially respond to high concentrations of sucrose. Apidologie 2019, 50, 253–261. [Google Scholar] [CrossRef]
- Kuszewska, K.; Rojek, W. Honeybee workers with higher reproductive potential have a greater learning ability. Apidologie 2021, 52, 608–619. [Google Scholar] [CrossRef]
- Łoś, A.; Strachecka, A. Fast and Cost-Effective Biochemical Spectrophotometric Analysis of Solution of Insect “Blood” and Body Surface Elution. Sensors 2018, 18, 1494. [Google Scholar] [CrossRef]
- Teal, P.E.; Proveaux, A.T.; Heath, R. Analysis and Quantitation of Insect Juvenile hormones using chemical ionization ion-trap Mass Spectrometry. Anal. Biochem. 2000, 277, 206–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carreck, N.; Andree, M.; Brent, C.; Cox-Foster, D.; Dade, H.; Ellis, J.; Hatjina, F.; Van Englesdorp, D. Standard methods for Apis mellifera anatomy and dissection. J. Apic. Res. 2013, 52, 1–40. [Google Scholar] [CrossRef]
- Amdam, G.V.; Aase, A.L.; Seehuus, S.C.; Kim, F.M.; Norberg, K.; Hartfelder, K. Social reversal of immunosenescence in honey bee workers. Exp. Gerontol. 2005, 40, 939–947. [Google Scholar] [CrossRef]
- Cini, A.; Bordoni, A.; Cappa, F.; Petrocelli, I.; Pitzalis, M.; Iovinella, I.; Dani, F.R.; Turillazzi, S.; Cervo, R. Increased immunocompetence and network centrality of allogroomer workers suggest a link between individual and social immunity in honeybees. Sci. Rep. 2020, 10, 8928. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Kozłowski, J. Division of labour by division of risk according to worker life expectancy in the honey bee (Apis mellifera). Apidologie 1998, 29, 143–157. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Moroń, D. Life expectancy and onset of foraging in the honeybee (Apis mellifera). Insectes Sociaux 2009, 56, 193–201. [Google Scholar] [CrossRef]
- Mertl, A.L.; Traniello, J.F. Behavioral evolution in the major worker subcaste of twig-nesting Pheidole (Hymenoptera: Formicidae): Does morphological specialization influence task plasticity? Behav. Ecol. Sociobiol. 2009, 63, 1411–1426. [Google Scholar] [CrossRef]
- Strachecka, A.; Chobotow, J.; Wójcik, Ł.; Kuszewska, K.; Skowronek, K.; Bryś, M.; Paleolog, J.; Woyciechowski, M. Rebel—queen or worker? Morphology of Nasonov and tergal glands in Apis mellifera rebels. Eur. Zool. J 2021, in press. [Google Scholar]
- Paleolog, J.; Kuszewsk, K.; Woyciechowski, M.; Strachecka, A. Reproductive potential impacts of life strategy parameters and global DNA methylation in honeybee workers (Apis mellifera L.). Animals 2021, in press. [Google Scholar]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuza, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect antimicrobial peptides: Potential weapons to counteract the antibiotic resistance. Cell. Mol. Life Sci. 2021, 78, 4259–4282. [Google Scholar] [CrossRef]
- Sheehan, G.; Farrell, G.; Kavanagh, K. Immune priming: The secret weapon of the insect world. Virulence 2020, 11, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Hystad, E.M.; Salmela, H.; Amdam, G.V.; Münch, D. Hemocyte-mediated phagocytosis differs between honey bee (Apis mellifera) worker castes. PLoS ONE 2017, 12, e0184108. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.T.; Ballinger, M.N.; Qian, F.; Christman, J.; Johnson, R. Morphological and functional characterization of honey bee, Apis mellifera, hemocyte cell communities. Apidologie 2018, 49, 397–410. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhang, S.C.; Zhang, J.; Liu, M.; Liu, Z.H. Vitellogenin is a cidal factor capable of killing bacteria via interaction with lipopolysaccharide and lipoteichoic acid. Mol. Immunol. 2009, 46, 3232–3239. [Google Scholar] [CrossRef]
- Amdam, G.V.; Omholt, S.W. The hive bee to forager transition in honeybee colonies: The double repressor hypothesis. J. Theor. Biol. 2003, 223, 451–464. [Google Scholar] [CrossRef]
- Guidugli, K.R.; Nascimento, A.M.; Amdam, G.V.; Barchuk, A.R.; Omholt, S.; Simões, Z.L.P.; Hartfelder, K. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. 2005, 579, 4961–4965. [Google Scholar] [CrossRef]
- Nelson, C.M.; Ihle, K.E.; Fondrk, M.K.; Page, R.E.; Amdam, G.V. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007, 5, e62. [Google Scholar] [CrossRef]
- Antonio, D.; Guidugli-Lazzarini, K.; Do Nascimento, A.M.; Simões, Z.; Hartfelder, K. RNAi-mediated silencing of vitellogenin gene function turns honeybee (Apis mellifera) workers into extremely precocious foragers. Naturwissen 2008, 95, 953–961. [Google Scholar] [CrossRef]
- Peso, M.; Even, N.; Søvik, E.; Naeger, N.L.; Robinson, G.E.; Barron, A.B. Physiology of reproductive worker honey bees (Apis mellifera): Insights for the development of the worker caste. J. Comp. Physiol. A 2016, 202, 147–158. [Google Scholar] [CrossRef]
- Lu, C.Y.; Huang, P.J.; Hsu, C.Y. The cholesterol-hydroxyecdysone-vitellogenin pathway is involved in the longevity of trophocytes and oenocytes of queen honey bees (Apis mellifera). Apidologie 2018, 49, 721–733. [Google Scholar] [CrossRef]
- Lin, H.R.; Winston, M.L.; Haunerland, N.H.; Slessor, K.N. Influence of age and population size on ovarian development, and of trophallaxis on ovarian development and vitellogenin titres of queenless worker honey bees (Apis mellifera L.). Canadian Entomologist 1999, 131, 695–706. [Google Scholar] [CrossRef]
- Nakaoka, T.; Takeuchi, H.; Kubo, T. Laying workers in queenless honeybee (Apis mellifera L.) colonies have physiological states similar to that of nurse bees but opposite that of foragers. J. Insect Physiol. 2008, 54, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Naeger, N.L.; Peso, M.; Even, N.; Barron, A.B.; Robinson, G.E. Altruistic behavior by egg-laying worker honeybees. Curr. Biol. 2013, 23, 1574–1578. [Google Scholar] [CrossRef]
- Berger, B.; Da Cruz-Landim, C. Ultrastructural analysis of the effect of mating delay on cell death in the ovaries of virgin honey bee (Apis mellifera L.) queens. J. Apic. Res. 2009, 48, 60–66. [Google Scholar] [CrossRef]
- Ronai, I.; Vergoz, V.; Oldroyd, B. The mechanistic, genetic, and evolutionary basis of worker sterility in the social hymenoptera. Adv. Stud. Behav. 2016, 48, 251–317. [Google Scholar]
- Aamidor, S.E.; Cardoso-Júnior, C.A.M.; Harianto, J.; Nowell, C.J.; Cole, L.; Oldroyd, B.P.; Ronai, I. Regulation of oogenesis in the queen honey bee (Apis mellifera). BioRxiv 2021, 434480. [Google Scholar] [CrossRef]
- Pankiw, T.; Huang, Z.; Winston, M.; Robinson, G. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. J. Insect Physiol. 1998, 44, 685–692. [Google Scholar] [CrossRef]
- Romer, F.; Emmerrich, H.; Nowock, J. Biosynthesis of ecdisteroids in isolated prothoracic glands and oenocytes of Tenebrio molitor in vitro. J. Insect Physiol. 1974, 20, 1987–1995. [Google Scholar] [CrossRef]
- Aurori, C.M.; Giurgiu, A.I.; Conlon, B.H.; Kastally, C.; Dezmirean, D.; Routtu, J.; Aurori, A. Juvenile hormone pathway in honey bee larvae: A source of possible signal molecules for the reproductive behavior of Varroa destructor. Ecol. Evol. 2021, 11, 1057–1068. [Google Scholar] [CrossRef]
- Cruz-Landim, C. Oenocytes of honey bee workers. Structural modifications during their adult life. Rev. Biol. 1985, 78, 107–122. [Google Scholar]
- Koubová, J.; Sábová, M.; Brejcha, M.; Kodrík, D.; Frydrychová, Č. Seasonality in telomerase activity in relation to cell size, DNA replication, and nutrients in the fat body of Apis mellifera. Sci. Rep. 2021, 11, 592. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strachecka, A.; Migdał, P.; Kuszewska, K.; Skowronek, P.; Grabowski, M.; Paleolog, J.; Woyciechowski, M. Humoral and Cellular Defense Mechanisms in Rebel Workers of Apis mellifera. Biology 2021, 10, 1146. https://doi.org/10.3390/biology10111146
Strachecka A, Migdał P, Kuszewska K, Skowronek P, Grabowski M, Paleolog J, Woyciechowski M. Humoral and Cellular Defense Mechanisms in Rebel Workers of Apis mellifera. Biology. 2021; 10(11):1146. https://doi.org/10.3390/biology10111146
Chicago/Turabian StyleStrachecka, Aneta, Paweł Migdał, Karolina Kuszewska, Patrycja Skowronek, Marcin Grabowski, Jerzy Paleolog, and Michał Woyciechowski. 2021. "Humoral and Cellular Defense Mechanisms in Rebel Workers of Apis mellifera" Biology 10, no. 11: 1146. https://doi.org/10.3390/biology10111146
APA StyleStrachecka, A., Migdał, P., Kuszewska, K., Skowronek, P., Grabowski, M., Paleolog, J., & Woyciechowski, M. (2021). Humoral and Cellular Defense Mechanisms in Rebel Workers of Apis mellifera. Biology, 10(11), 1146. https://doi.org/10.3390/biology10111146






