Serum Level of Vitamin D Is Associated with Severity of Coronary Atherosclerosis in Postmenopausal Women
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort and Clinical Data
2.2. Measurements
2.3. Examinations
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Gańczak, M.; Miazgowski, T.; Kożybska, M.; Kotwas, A.; Korzeń, M.; Rudnicki, B.; Nogal, T.; Andrei, C.L.; Ausloos, M.; Banach, M.; et al. Changes in disease burden in Poland between 1990-2017 in comparison with other Central European countries: A systematic analysis for the Global Burden of Disease Study 2017. PLoS ONE 2020, 15, e0226766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greendale, G.A.; Lee, N.P.; Arriola, E.R. The menopause. Lancet 1999, 353, 571–580. [Google Scholar] [CrossRef]
- Mosca, L.; Collins, P.; Herrington, D.M.; Mendelsohn, M.E.; Pasternak, R.C.; Robertson, R.M.; Schenck-Gustafsson, K.; Smith, S.C., Jr.; Taubert, K.A.; Wenger, N.K. Hormone replacement therapy and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 104, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Gordon, T.; Kannel, W.B.; Hjortland, M.C.; McNamara, P.M. Menopause and coronary heart disease. The Framingham Study. Ann. Intern. Med. 1978, 89, 157–161. [Google Scholar] [CrossRef]
- Anand, S.S.; Islam, S.; Rosengren, A.; Franzosi, M.G.; Steyn, K.; Yusufali, A.H.; Keltai, M.; Diaz, R.; Rangarajan, S.; Yusuf, S. Risk factors for myocardial infarction in women and men: Insights from the INTERHEART study. Eur. Heart J. 2008, 29, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Barrett-Connor, E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation 1997, 95, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998, 280, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Khoudary, S.R. Age at menopause onset and risk of cardiovascular disease around the world. Maturitas 2020, 141, 33–38. [Google Scholar] [CrossRef]
- Mondul, A.M.; Rodriguez, C.; Jacobs, E.J.; Calle, E.E. Age at natural menopause and cause-specific mortality. Am. J. Epidemiol. 2005, 162, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Cooper, G.S.; Baird, D.D.; Weinberg, C.R.; Ephross, S.A.; Sandler, D.P. Age at menopause and childbearing patterns in relation to mortality. Am. J. Epidemiol. 2000, 151, 620–623. [Google Scholar] [CrossRef] [Green Version]
- Jansen, S.C.; Temme, E.H.; Schouten, E.G. Lifetime estrogen exposure versus age at menopause as mortality predictor. Maturitas 2002, 43, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.K.; Heuch, I.; Kvåle, G. Age at natural menopause and all-cause mortality: A 37-year follow-up of 19,731 Norwegian women. Am. J. Epidemiol. 2003, 157, 923–929. [Google Scholar] [CrossRef] [Green Version]
- Merz, C.N.; Kelsey, S.F.; Pepine, C.J.; Reichek, N.; Reis, S.E.; Rogers, W.J.; Sharaf, B.L.; Sopko, G. The Women’s Ischemia Syndrome Evaluation (WISE) study: Protocol design, methodology and feasibility report. J. Am. Coll. Cardiol. 1999, 33, 1453–1461. [Google Scholar] [CrossRef] [Green Version]
- Coventry, L.L.; Finn, J.; Bremner, A.P. Sex differences in symptom presentation in acute myocardial infarction: A systematic review and meta-analysis. Heart Lung 2011, 40, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Mehilli, J.; Presbitero, P. Coronary artery disease and acute coronary syndrome in women. Heart 2020, 106, 487–492. [Google Scholar] [CrossRef]
- Johnston, N.; Schenck-Gustafsson, K.; Lagerqvist, B. Are we using cardiovascular medications and coronary angiography appropriately in men and women with chest pain? Eur. Heart J. 2011, 32, 1331–1336. [Google Scholar] [CrossRef] [Green Version]
- Steg, P.G.; Greenlaw, N.; Tardif, J.C.; Tendera, M.; Ford, I.; Kääb, S.; Abergel, H.; Fox, K.M.; Ferrari, R. Women and men with stable coronary artery disease have similar clinical outcomes: Insights from the international prospective CLARIFY registry. Eur. Heart J. 2012, 33, 2831–2840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansky, A.J.; Ng, V.G.; Maehara, A.; Weisz, G.; Lerman, A.; Mintz, G.S.; De Bruyne, B.; Farhat, N.; Niess, G.; Jankovic, I.; et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc. Imaging 2012, 5, S62–S72. [Google Scholar] [CrossRef] [Green Version]
- Mehta, L.S.; Beckie, T.M.; DeVon, H.A.; Grines, C.L.; Krumholz, H.M.; Johnson, M.N.; Lindley, K.J.; Vaccarino, V.; Wang, T.Y.; Watson, K.E.; et al. Acute Myocardial Infarction in Women: A Scientific Statement from the American Heart Association. Circulation 2016, 133, 916–947. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.; Garcia, M.A.; Ardissino, D.; Buszman, P.; Camici, P.G.; Crea, F.; Daly, C.; De Backer, G.; Hjemdahl, P.; Lopez-Sendon, J.; et al. Guidelines on the management of stable angina pectoris: Executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur. Heart J. 2006, 27, 1341–1381. [Google Scholar] [CrossRef]
- Hamm, C.W.; Bassand, J.P.; Agewall, S.; Bax, J.; Boersma, E.; Bueno, H.; Caso, P.; Dudek, D.; Gielen, S.; Huber, K.; et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 2999–3054. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.C., Jr.; Benjamin, E.J.; Bonow, R.O.; Braun, L.T.; Creager, M.A.; Franklin, B.A.; Gibbons, R.J.; Grundy, S.M.; Hiratzka, L.F.; Jones, D.W.; et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: A guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011, 124, 2458–2473. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.A.; Mager, N.A. Women’s involvement in clinical trials: Historical perspective and future implications. Pharm. Pract. (Granada) 2016, 14, 708. [Google Scholar] [CrossRef] [Green Version]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef] [PubMed]
- Legarth, C.; Grimm, D.; Krüger, M.; Infanger, M.; Wehland, M. Potential Beneficial Effects of Vitamin D in Coronary Artery Disease. Nutrients 2019, 12, 99. [Google Scholar] [CrossRef] [Green Version]
- Jorde, R.; Grimnes, G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog. Lipid Res. 2011, 50, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, A.; Dave, P.; Lokshin, V.; Bahtiyar, G. Type 2 Diabetes Mellitus, Insulin Resistance, and Vitamin D. Curr. Diab. Rep. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Talmor, Y.; Golan, E.; Benchetrit, S.; Bernheim, J.; Klein, O.; Green, J.; Rashid, G. Calcitriol blunts the deleterious impact of advanced glycation end products on endothelial cells. Am. J. Physiol. Renal. Physiol. 2008, 294, 1059–1064. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Wong, S.L.; Lau, C.W.; Lee, H.K.; Ng, C.F.; Zhang, L.; Yao, X.; Chen, Z.Y.; Vanhoutte, P.M.; Huang, Y. Calcitriol protects renovascular function in hypertension by down-regulating angiotensin II type 1 receptors and reducing oxidative stress. Eur. Heart J. 2012, 33, 2980–2990. [Google Scholar] [CrossRef] [Green Version]
- Boisvert, W.A.; Curtiss, L.K.; Terkeltaub, R.A. Interleukin-8 and its receptor CXCR2 in atherosclerosis. Immunol. Res. 2000, 21, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Weng, S.; Felton, S.K.; Bhandare, S.; Riek, A.; Butler, B.; Proctor, B.M.; Petty, M.; Chen, Z.; Schechtman, K.B.; et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 2009, 120, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Osborne, J.E.; Hutchinson, P.E. Vitamin D and systemic cancer: Is this relevant to malignant melanoma? Br. J. Dermatol. 2002, 147, 197–213. [Google Scholar] [CrossRef]
- Ohsawa, M.; Koyama, T.; Yamamoto, K.; Hirosawa, S.; Kamei, S.; Kamiyama, R. 1alpha,25-dihydroxyvitamin D(3) and its potent synthetic analogs downregulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation 2000, 102, 2867–2872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Decsi, T.; Dobrzańska, A.; Franek, E.; et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—Recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef]
- Liu, B.; Taioli, E. Seasonal Variations of Complete Blood Count and Inflammatory Biomarkers in the US Population—Analysis of NHANES Data. PLoS ONE 2015, 10, e0142382. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Ringqvist, I.; Fisher, L.D.; Mock, M.; Davis, K.B.; Wedel, H.; Chaitman, B.R.; Passamani, E.; Russell, R.O., Jr.; Alderman, E.L.; Kouchoukas, N.T.; et al. Prognostic value of angiographic indices of coronary artery disease from the Coronary Artery Surgery Study (CASS). J. Clin. Investig. 1983, 71, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 2298. [Google Scholar] [CrossRef]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Di Giovine, G.; Marino, P.; Suryapranata, H.; De Luca, G. Impact of gender difference on vitamin D status and its relationship with the extent of coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 464–470. [Google Scholar] [CrossRef]
- Morgan, C.; Kyvernitakis, A.; Cho, R.; Pappas, O.; Ranganathan, K.; Fischer, M.R.; Srinivasan, V. Vitamin D deficiency and degree of coronary artery luminal stenosis in women undergoing coronary angiography: A prospective observational study. Am. J. Cardiovasc. Dis. 2018, 8, 14–18. [Google Scholar] [PubMed]
- Milazzo, V.; De Metrio, M.; Cosentino, N.; Marenzi, G.; Tremoli, E. Vitamin D and acute myocardial infarction. World J. Cardiol. 2017, 9, 14–20. [Google Scholar] [CrossRef]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pludowski, P.; Grant, W.B.; Bhattoa, H.P.; Bayer, M.; Povoroznyuk, V.; Rudenka, E.; Ramanau, H.; Varbiro, S.; Rudenka, A.; Karczmarewicz, E.; et al. Vitamin d status in central europe. Int. J. Endocrinol. 2014, 2014, 589587. [Google Scholar] [CrossRef]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilkkinen, A.; Knekt, P.; Aro, A.; Rissanen, H.; Marniemi, J.; Heliövaara, M.; Impivaara, O.; Reunanen, A. Vitamin D status and the risk of cardiovascular disease death. Am. J. Epidemiol. 2009, 170, 1032–1039. [Google Scholar] [CrossRef]
- Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; März, W.; Pilz, S. Vitamin D and Cardiovascular Disease: An Updated Narrative Review. Int. J. Mol. Sci. 2021, 22, 2896. [Google Scholar] [CrossRef]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Hollis, B.W.; Rimm, E.B. 25-hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch. Intern. Med. 2008, 168, 1174–1180. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.L.; Sandhu, J.K.; Squire, I.B.; Davies, J.E.; Jones, D.J. Vitamin D and prognosis in acute myocardial infarction. Int. J. Cardiol. 2013, 168, 2341–2346. [Google Scholar] [CrossRef]
- Chen, W.R.; Qian, Y.A.; Chen, Y.D.; Shi, Y.; Yin, D.W.; Wang, H.; Zhu, P.; Liu, H.W.; Sha, Y. The effects of low vitamin D on coronary artery disease. Heart Lung Circ. 2014, 23, 314–319. [Google Scholar] [CrossRef]
- Nardin, M.; Verdoia, M.; Schaffer, A.; Barbieri, L.; Marino, P.; De Luca, G. Vitamin D status, diabetes mellitus and coronary artery disease in patients undergoing coronary angiography. Atherosclerosis 2016, 250, 114–121. [Google Scholar] [CrossRef]
- Sogomonian, R.; Alkhawam, H.; Jolly, J.; Vyas, N.; Ahmad, S.; Moradoghli Haftevani, E.; Al-Khazraji, A.; Finkielstein, D.; Vittorio, T.J. Serum vitamin D levels correlate to coronary artery disease severity: A retrospective chart analysis. Expert. Rev. Cardiovasc. Ther. 2016, 14, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.H.; Allai, M.S.; Shah, Z.A.; Changal, K.H.; Raina, M.A.; Bhat, F.A. Association of Low Levels of Vitamin D with Chronic Stable Angina: A Prospective Case-Control Study. N. Am. J. Med. Sci. 2016, 8, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Wang, T.; Zhu, S.; Li, L. Effects of vitamin D supplementation as an adjuvant therapy in coronary artery disease patients. Scand. Cardiovasc. J. 2016, 50, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83 000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019, 4, 765–776. [Google Scholar] [CrossRef]
- Gold, E.B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. N. Am. 2011, 38, 425–440. [Google Scholar] [CrossRef] [Green Version]
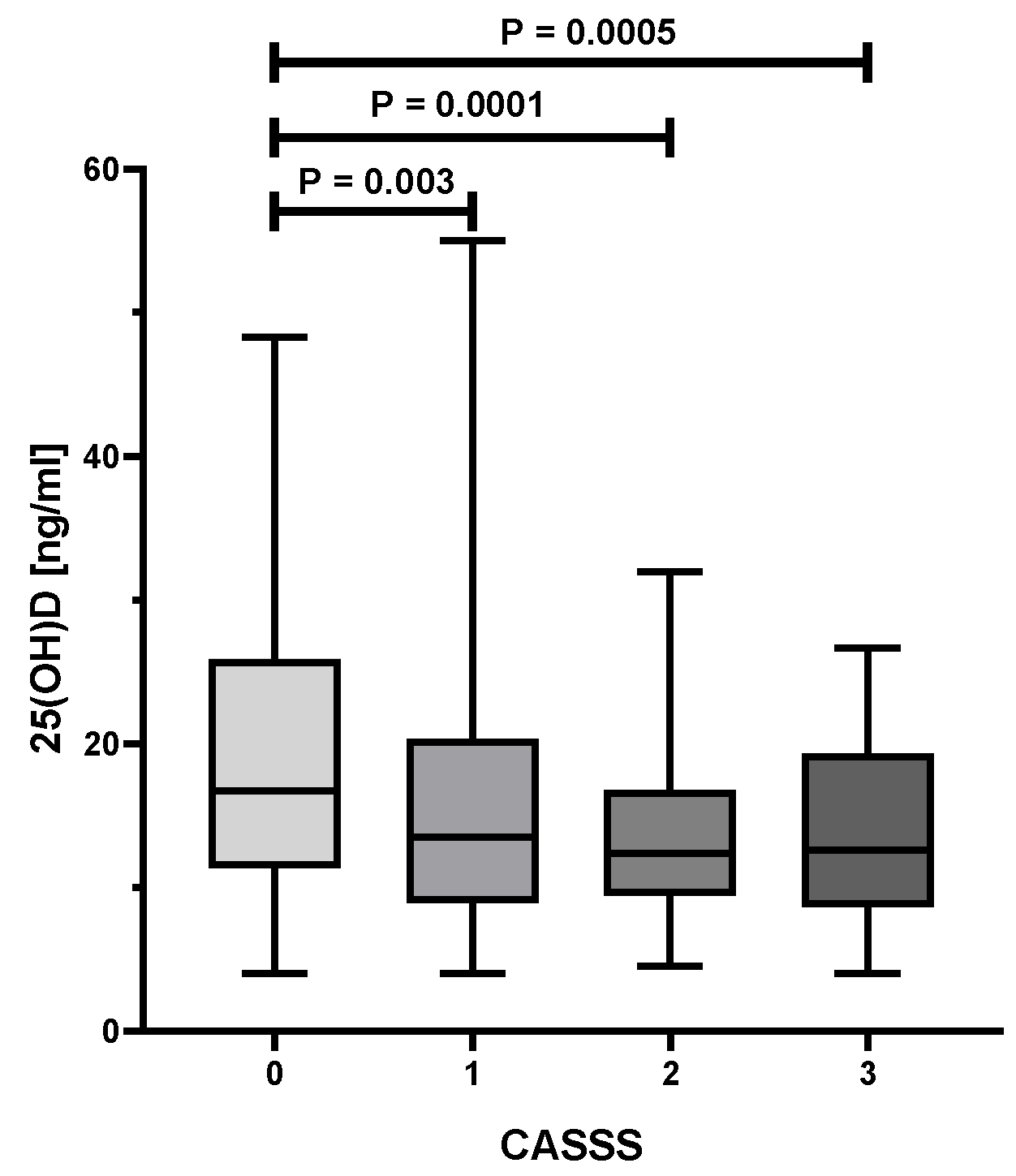
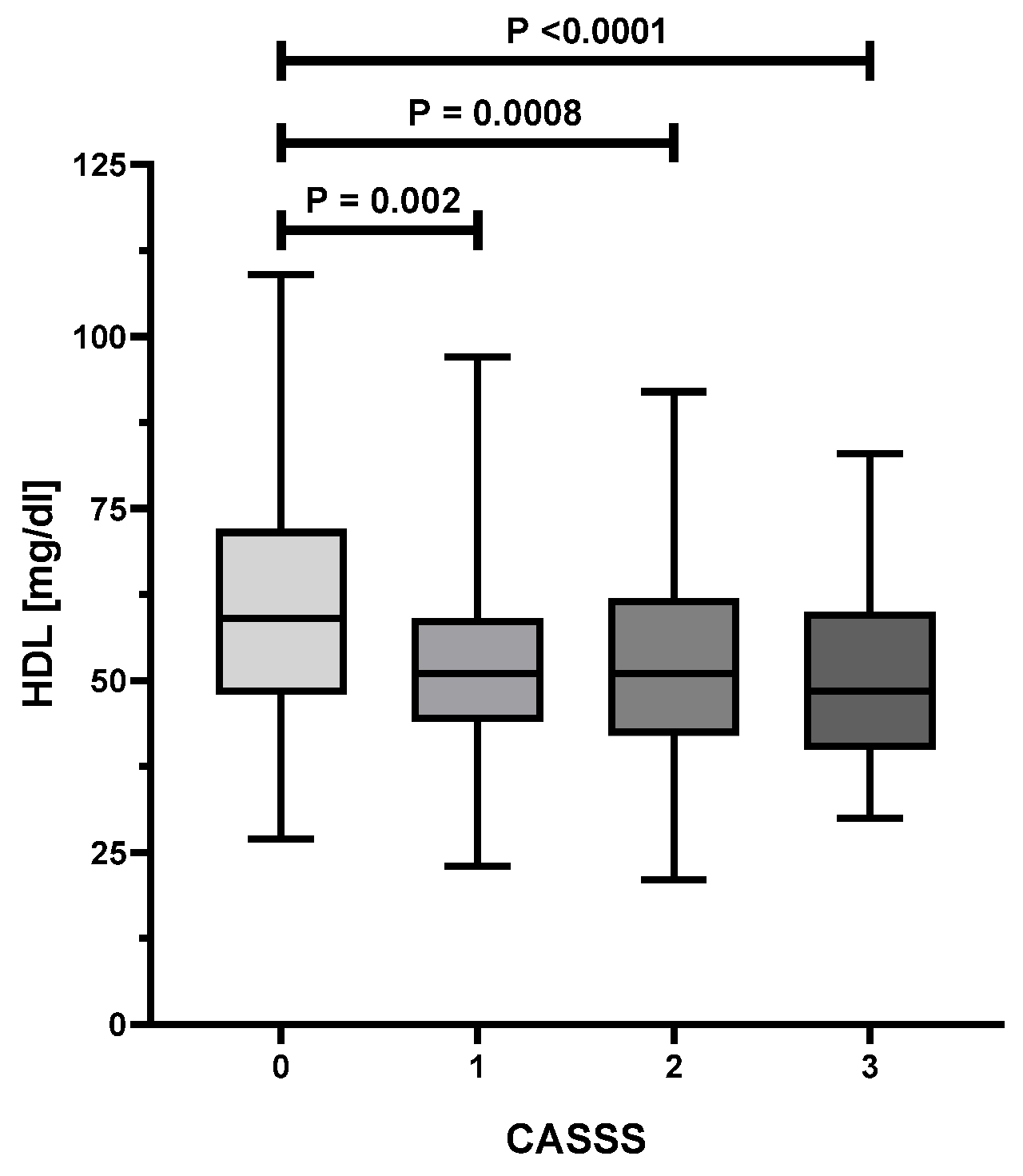
| CASS Score | ||||||
|---|---|---|---|---|---|---|
| Entire Cohort | 0 | 1 | 2 | 3 | p | |
| Total participants, n | 351 | 132 (37.6%) | 89 (25.4%) | 73 (20.8%) | 57 (16.2%) | |
| Age (years) | 71 (50–92) | 69 (52–91) | 71 (51–92) | 73 (52–87) | 75 (52–91) | 0.002 |
| Smoking, n | 66 (18.8%) | 20 (15.1%) | 24 (27.0%) | 15 (20.5%) | 7 (12.3%) | 0.18 |
| BMI [kg/m2] | 28.1 (16.9–47.8) | 28.2 (19.2–44.8) | 26.8 (16.9–47.8) | 28.5 (17.4–38.4) | 28.4 (18.3–43.3) | 0.46 |
| Hyperlipidemia, n | 205 (58.4%) | 69 (52.3%) | 52 (58.4%) | 45 (61.6%) | 39 (68.4%) | 0.19 |
| Dyslipidemia, n | 296 (90.5%) | 112 (84.9%) | 71 (79.8%) | 61 (83.6%) | 52 (91.2%) | 0.42 |
| Total cholesterol [mg/dL] | 181.8 (70.9–367.0) | 182.2 (85.4–366.3) | 179.8 (110.0–351.6) | 188.7 (70.9–338.3) | 178.0 (84.8–310.8) | 0.66 |
| Triglycerides [mg/dL] | 15.6 (31.3–386.3) | 115.5 (31.3–281.0) | 110.8 (48.7–357.6) | 18.7 (54.0–386.3) | 112.2 (46.9–306.4) | 0.50 |
| HDL [mg/dL] | 52.3 (21.3–54.8) | 58.8 (26.8–109.0) | 50.6 (23.5–97.4) | 51.0 (21.3–92.1) | 48.6 (29.7–82.6) | <0.0001 |
| LDL [mg/dL] | 101.6 (20.5–289.2) | 94.5 (20.5–253.0) | 105.2 (39.7–289.2) | 105.7 (24.4–235.0) | 101.9 (27.3–228.3) | 0.83 |
| Serum 25(OH)D [ng/mL] | 14.0 (4.0–55.0) | 16.7 (4.0–48.3) | 13.5 (4.0–55.0) | 12.4 (4.5–32.0) | 12.6 (4.0–26.7) | 0.0001 |
| Diabetes mellitus, n | 112 (31.9%) | 33 (25.0%) | 29 (32.6%) | 25 (34.2%) | 25 (43.9%) | 0.07 |
| Hypertension, n | 303 (86.3%) | 108 (81.8%) | 74 (83.1%) | 65 (89.0%) | 56 (98.2%) | 0.02 |
| MI in history, n | 111 (31.6%) | 9 (6.8%) | 33 (37.0%) | 36 (49.3%) | 33 (57.9%) | <0.0001 |
| Acute coronary syndrome, n | 131 (37.3%) | 16 (12.1%) | 51 (57.3%) | 33 (45.2%) | 31 (54.4%) | <0.0001 |
| β | 95% CI | OR (95% CI) | p | |
|---|---|---|---|---|
| Age | 0.02 | 0.01–0.03 | 1.02 (1.01–1.03) | 0.003 |
| BMI | −0.01 | −0.04–0.01 | 0.99 (0.97–1.01) | 0.25 |
| 25(OH)D | −0.02 | −0.03–−0.01 | 0.98 (0.97–1.00) | 0.016 |
| Total cholesterol | 0.03 | −0.07–0.13 | 1.03 (0.93–1.14) | 0.58 |
| LDL | −0.03 | −0.13–0.07 | 0.97 (0.88–1.07) | 0.58 |
| HDL | −0.04 | −0.14–0.06 | 0.96 (0.87–1.06) | 0.40 |
| Triglycerides | −0.01 | −0.03–0.02 | 1.00 (0.98–1.02) | 0.60 |
| Diabetes mellitus | 0.13 | −0.11–0.37 | 1.14 (0.90–1.44) | 0.28 |
| Hypertension | 0.44 | 0.06–0.82 | 1.55 (1.06–2.28) | 0.025 |
| Hyperlipidemia | 0.20 | −0.12–0.51 | 1.22 (0.89–1.67) | 0.21 |
| Dyslipidemia | 0.30 | −0.09–0.69 | 1.35 (0.92–2.00) | 0.13 |
| MI in history | 0.63 | 0.41–0.85 | 1.81 (1.51–2.35) | <0.0001 |
| Smoking status | 0.05 | −0.24–0.34 | 1.05 (0.78–1.41) | 0.76 |
| Season during the examination | 0.01 | −0.25–0.27 | 1.01 (0.78–1.31) | 0.94 |
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | VIF | p | β | VIF | p | |
| Age | −0.07 | 0.21 | −0.13 | 1.17 | 0.03 | −0.13 | 1.16 | 0.03 |
| BMI | −0.03 | 0.63 | 0.002 | 1.18 | 0.97 | 0.00 | 1.17 | 0.99 |
| Total cholesterol | −0.13 | 0.02 | −0.43 | 398.17 | 0.69 | NA | NA | NA |
| LDL | −0.15 | <0.01 | 0.30 | 312.99 | 0.76 | NA | NA | NA |
| HDL | 0.13 | 0.02 | 0.21 | 40.63 | 0.54 | 0.07 | 1.32 | 0.24 |
| Triglycerides | −0.15 | <0.01 | 0.01 | 21.14 | 0.99 | −0.09 | 1.53 | 0.21 |
| Diabetes mellitus | −0.03 | 0.64 | 0.05 | 1.21 | 0.40 | 0.05 | 1.20 | 0.46 |
| Hypertension | −0.01 | 0.07 | −0.08 | 1.12 | 0.19 | −0.08 | 1.10 | 0.18 |
| Hyperlipidemia | −0.19 | <0.01 | −0.10 | 2.15 | 0.20 | −0.16 | 1.26 | 0.01 |
| Dyslipidemia | −0.08 | 0.16 | 0.03 | 1.36 | 0.77 | 0.004 | 1.30 | 0.95 |
| MI in history | −0.14 | 0.01 | −0.13 | 1.11 | 0.02 | −0.13 | 1.13 | 0.02 |
| Smoking status | −0.04 | 0.49 | −0.04 | 1.18 | 0.54 | −0.04 | 1.17 | 0.54 |
| Season during the examination | −0.06 | 0.25 | −0.09 | 1.04 | 0.12 | −0.09 | 1.03 | 0.13 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Factor | OR (95% CI) | p | OR (95% CI) | p |
| AGE | 1.03 (1.01–1.06) | 0.02 | 1.04 (1.02–1.07) | 0.003 |
| BMI | 1.00 (0.96–1.05) | 0.96 | - | - |
| 25(OH)D | 0.97 (0.95–1.00) | 0.03 | 0.99 (0.96–1.02) | 0.38 |
| TOTAL CHOLESTEROL | 1.00 (0.99–1.00) | 0.36 | - | - |
| LDL | 1.00 (0.99–1.01) | 0.54 | - | - |
| HDL | 0.97 (0.95–0.98) | <0.0001 | 0.97 (0.95–0.99) | <0.0001 |
| TRIGLYCERIDES | 1.00 (0.99–1.00) | 0.43 | - | - |
| DIABETES MELLITUS | 1.37 (0.87–2.17) | 0.18 | - | - |
| HYPERTENSION | 1.19 (0.62–2.26) | 0.61 | - | - |
| HYPERLIPIDEMIA | 1.26 (0.79–2.01) | 0.33 | - | - |
| DYSLIPIDEMIA | 1.30 (0.59–2.85) | 0.52 | - | |
| SMOKING STATUS | 1.77 (1.04–3.02) | 0.04 | 2.31 (1.26–4.24) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedzic, E.A.; Smyk, W.; Sowińska, I.; Dąbrowski, M.; Jankowski, P. Serum Level of Vitamin D Is Associated with Severity of Coronary Atherosclerosis in Postmenopausal Women. Biology 2021, 10, 1139. https://doi.org/10.3390/biology10111139
Dziedzic EA, Smyk W, Sowińska I, Dąbrowski M, Jankowski P. Serum Level of Vitamin D Is Associated with Severity of Coronary Atherosclerosis in Postmenopausal Women. Biology. 2021; 10(11):1139. https://doi.org/10.3390/biology10111139
Chicago/Turabian StyleDziedzic, Ewelina Anna, Wiktor Smyk, Izabela Sowińska, Marek Dąbrowski, and Piotr Jankowski. 2021. "Serum Level of Vitamin D Is Associated with Severity of Coronary Atherosclerosis in Postmenopausal Women" Biology 10, no. 11: 1139. https://doi.org/10.3390/biology10111139
APA StyleDziedzic, E. A., Smyk, W., Sowińska, I., Dąbrowski, M., & Jankowski, P. (2021). Serum Level of Vitamin D Is Associated with Severity of Coronary Atherosclerosis in Postmenopausal Women. Biology, 10(11), 1139. https://doi.org/10.3390/biology10111139






