The Identification of RNA Modification Gene PUS7 as a Potential Biomarker of Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Differential Expression Analysis
2.3. PUS7 Protein Level Analysis of OV Tissues in HPA and Tissue Array
2.4. PUS7 Gene Expression Analysis Using TCGA and GEO Datasets
2.5. PUS7 Gene Expression Analysis Using Oncomine Database
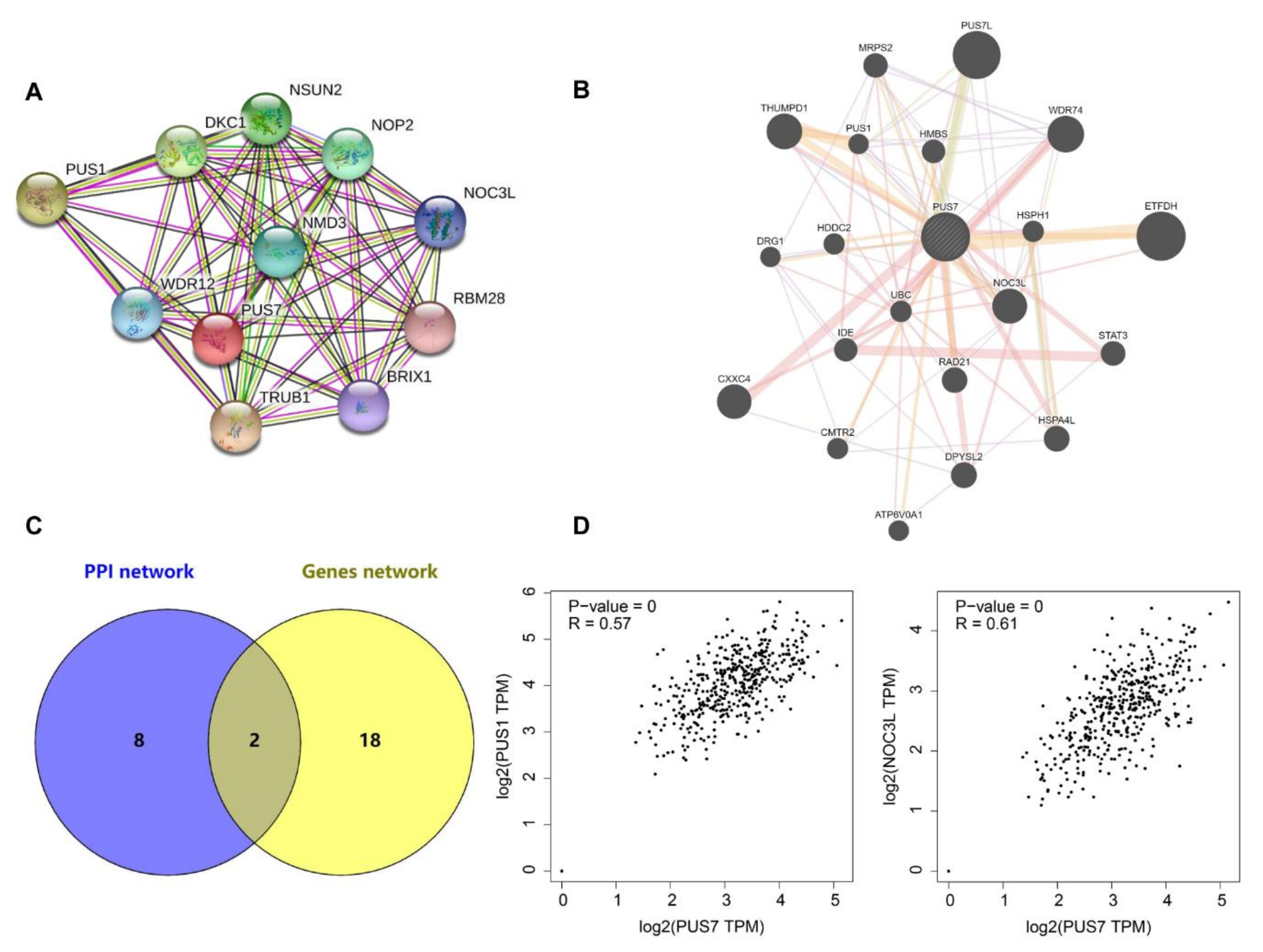
2.6. Protein–Protein Interaction (PPI) Network Analyses
2.7. The Mutation and Correlation Analyses of PUS7
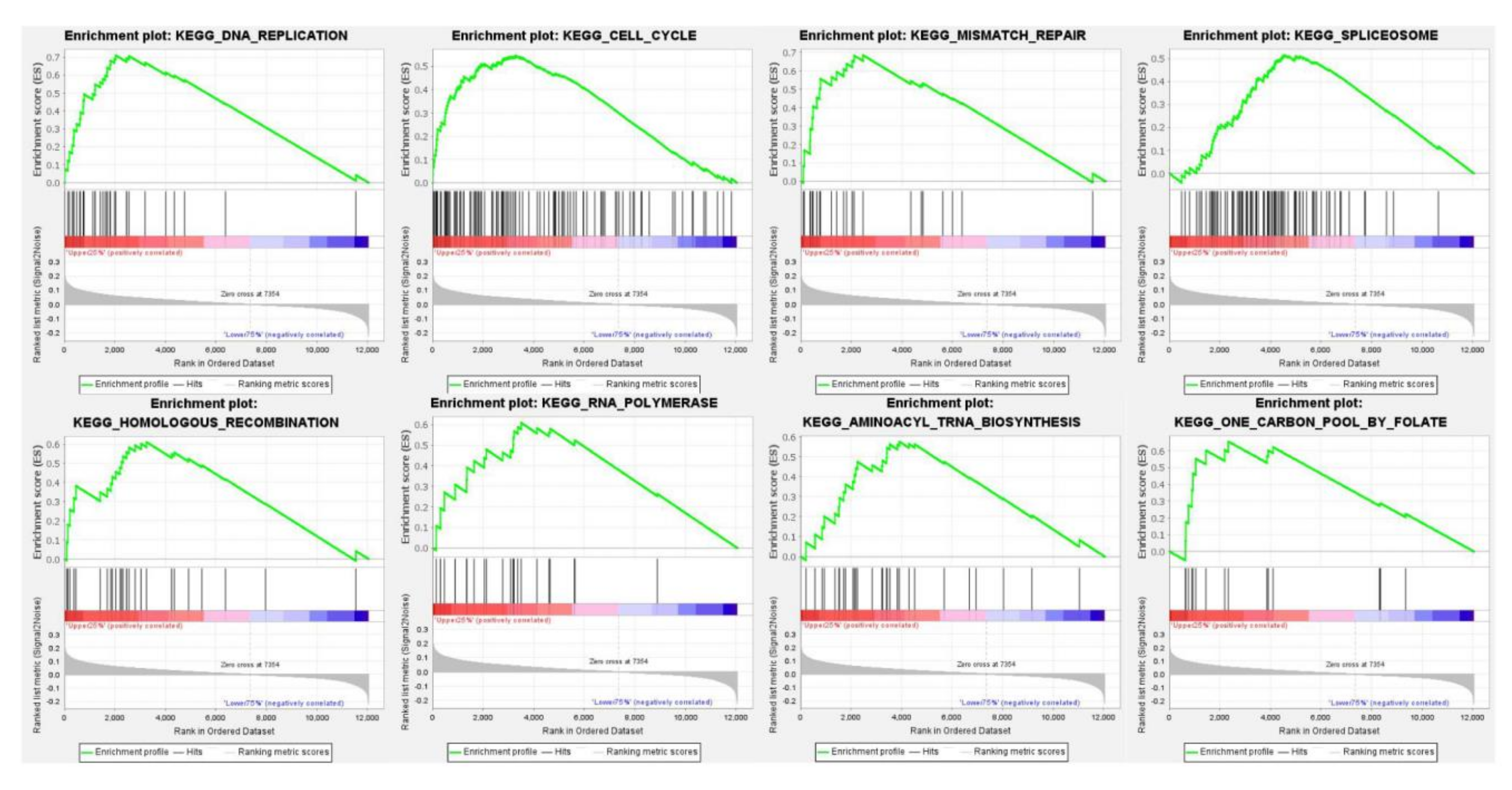
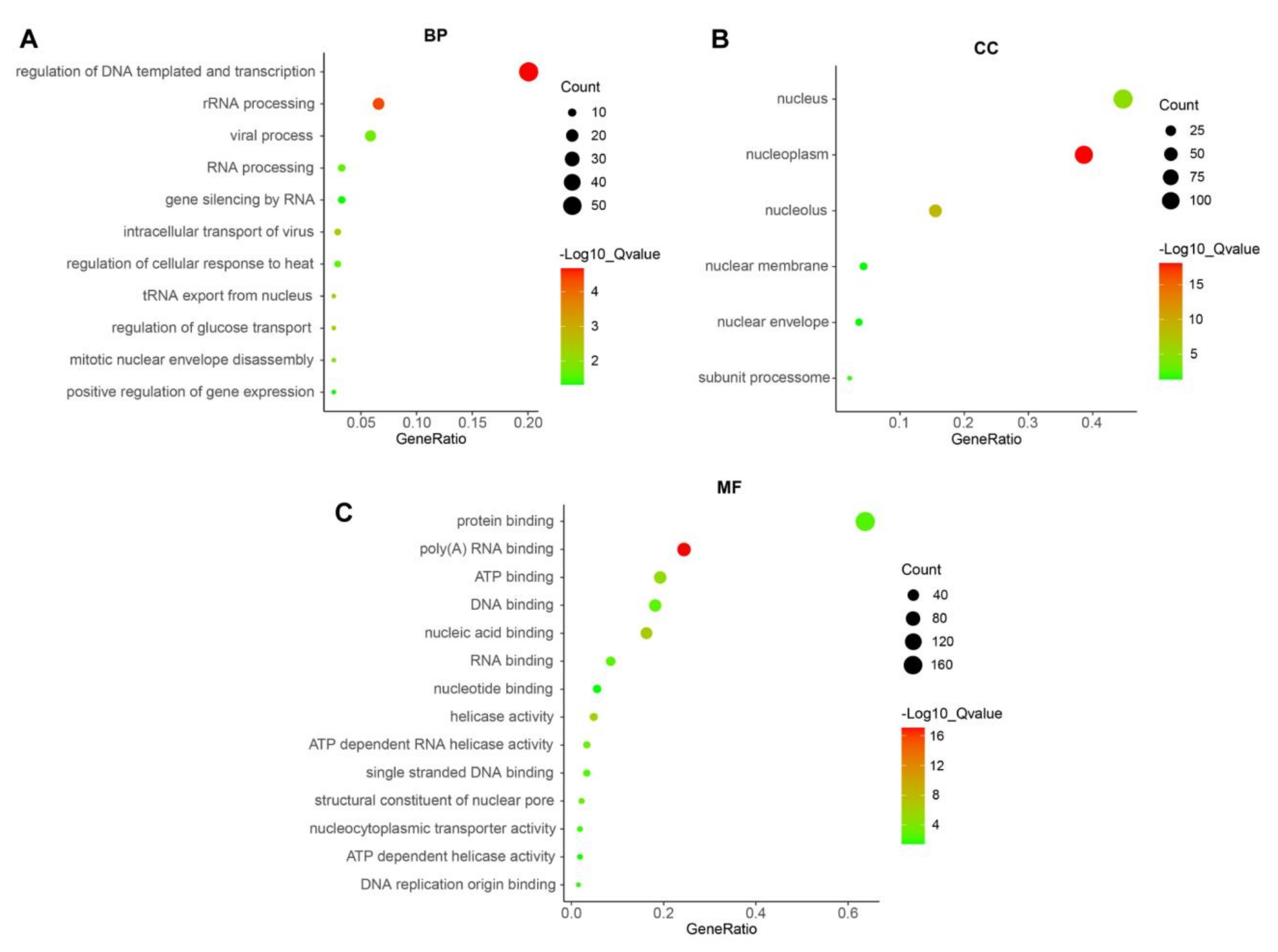
2.8. Pathways and BP, CC, and MF Analyses
2.9. Statistical Analysis
3. Results
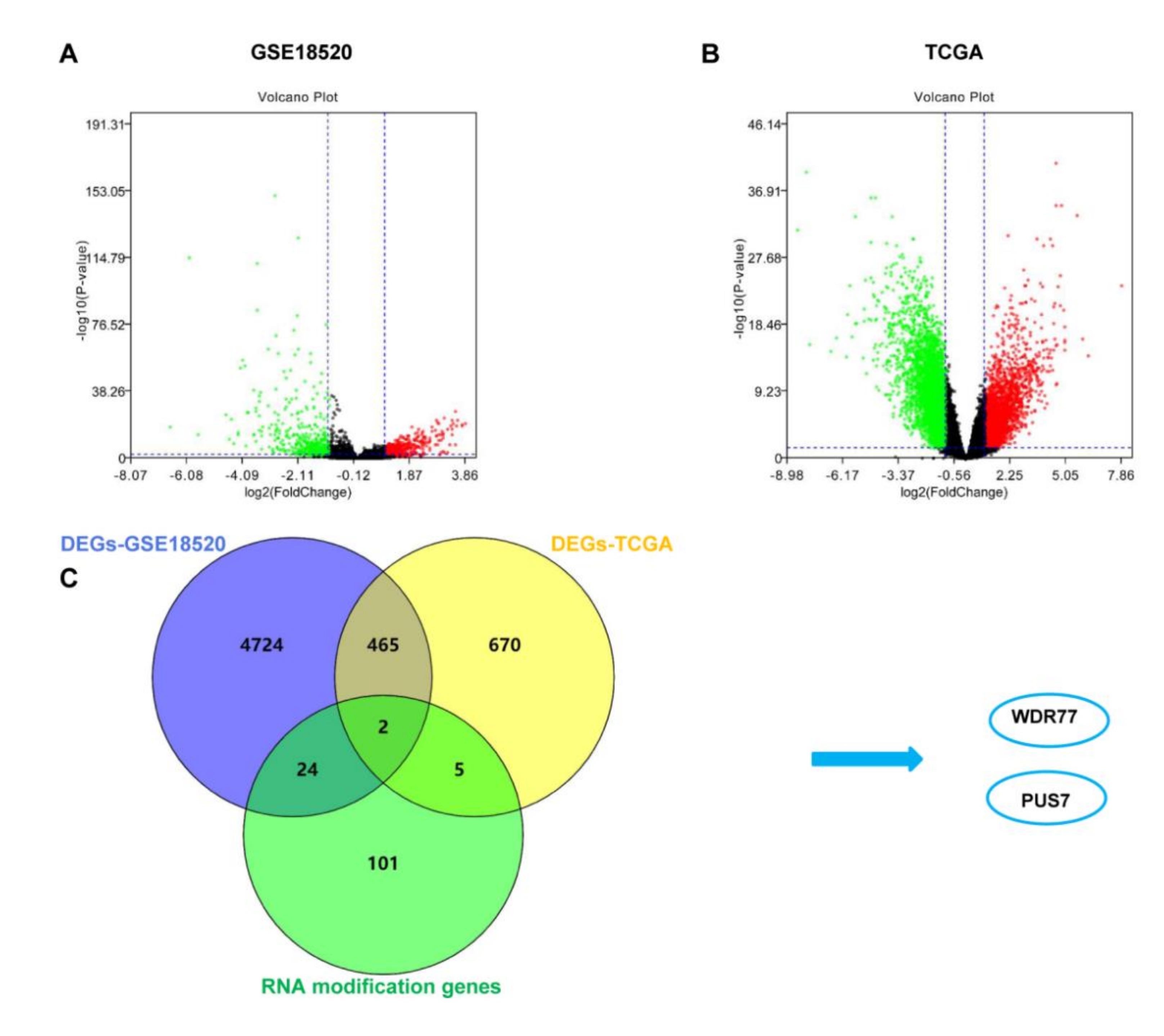
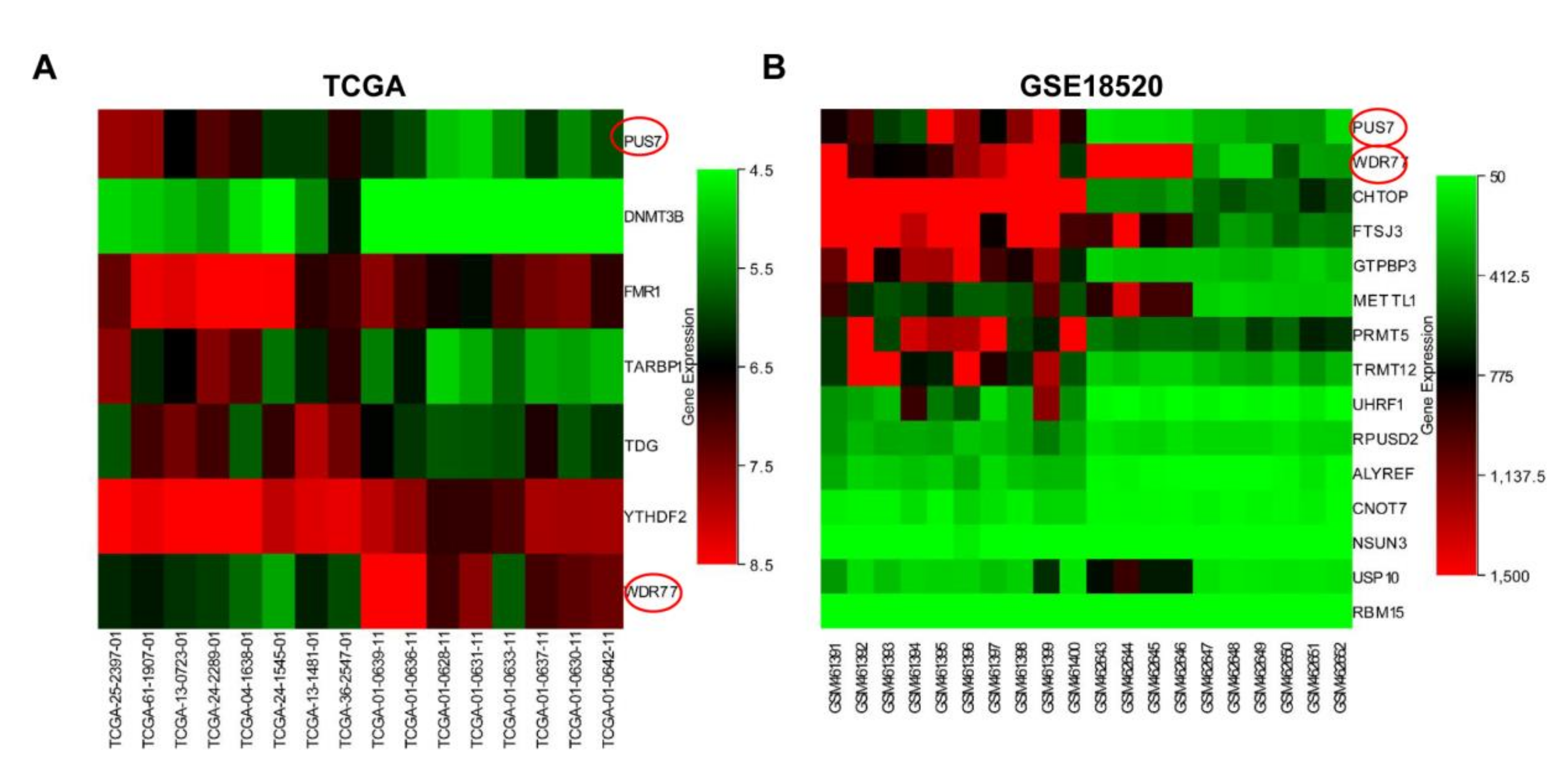
3.1. The Identification of DEGs-RMGs of OV Data in TCGA and GEO
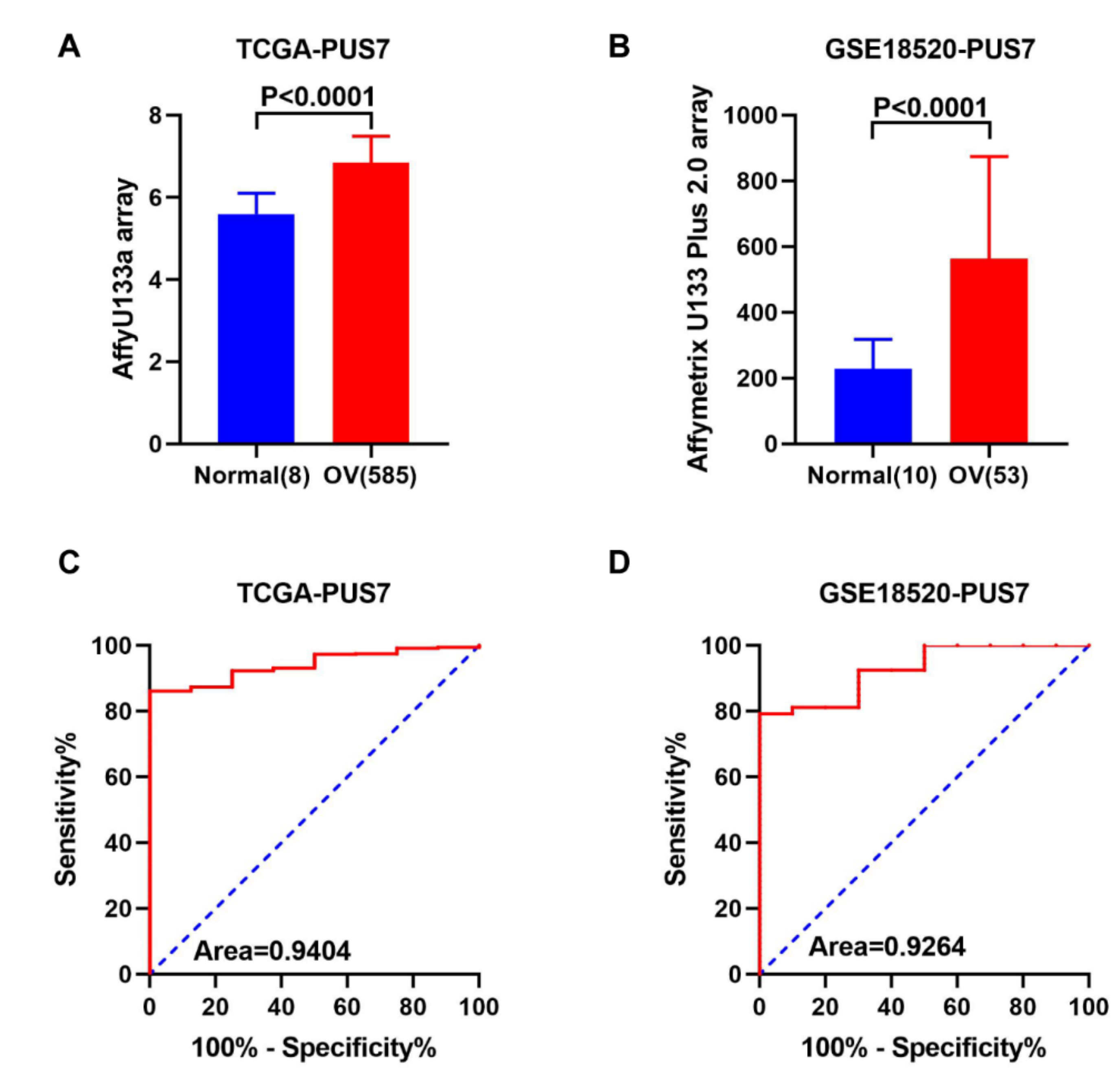
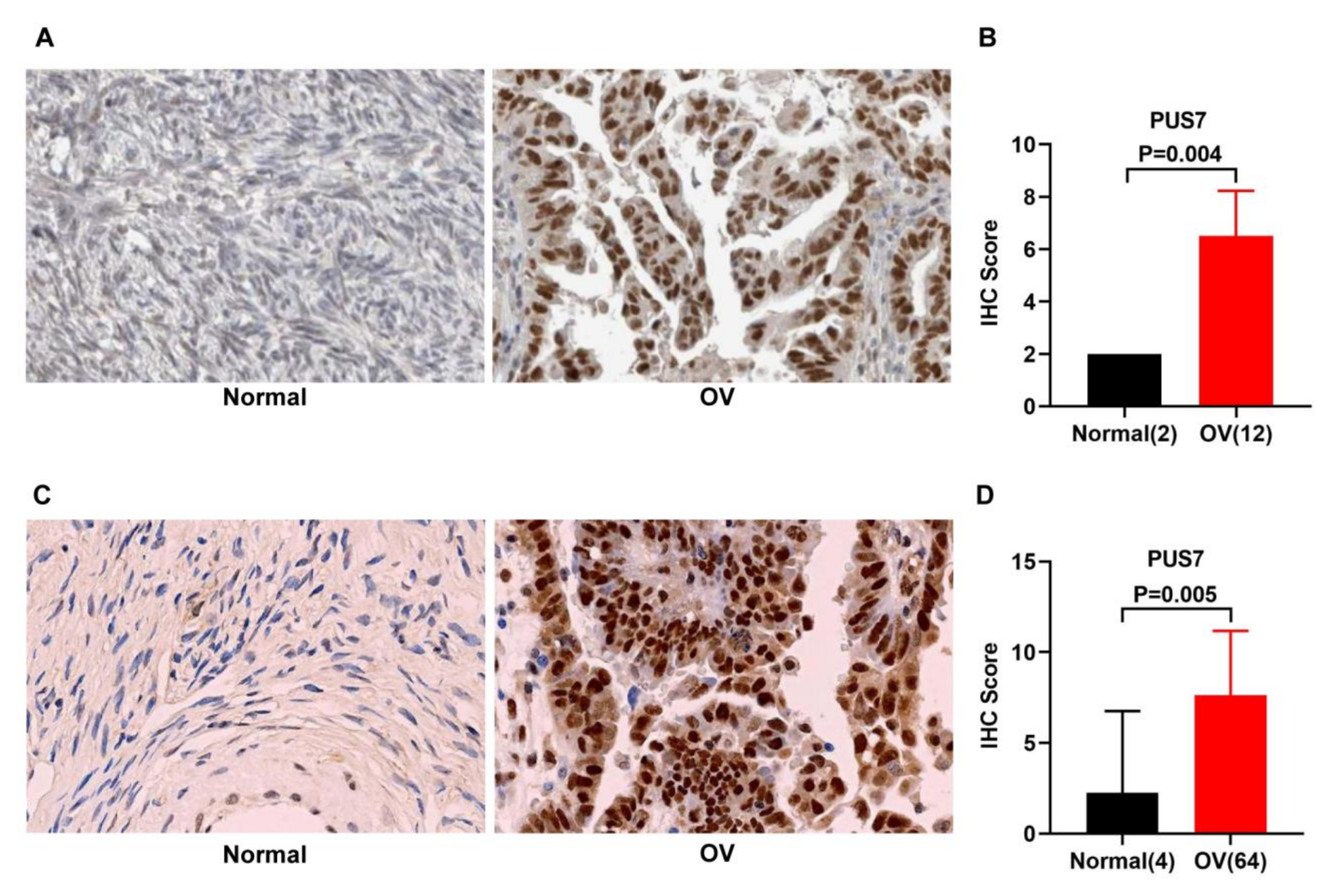
3.2. Expression Validation and Mutation Analysis for PUS7 in Ovarian Cancer
3.3. The Interaction Network of PUS7
3.4. The Pathway Enrichment Analysis of PUS7 in Ovarian Cancer
3.5. Gene Ontology (GO) Analyses of PUS7 in Ovarian Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Myriam, K.; Alexandra, L.; Jean-Yves, S.; Catherine, G. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2018, 85, 41–49. [Google Scholar]
- Giusti, I.; Bianchi, S.; Nottola, S.A.; Macchiarelli, G.; Dolo, V. Clinical electron microscopy in the study of human ovarian tissues. Euromediterranean Biomed. J. 2019, 14, 145–151. [Google Scholar]
- Brett, M.R.; Jennifer, B.P.; Thomas, A.S. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebecca, A.; Alba, M.; Tomasz, S.; Michael, J.B. Biomarkers in ovarian cancer: To be or not to be. Cancer 2019, 15, 125. [Google Scholar]
- Lindsey, A.T.; Britton, T.; Carol, E.D.; Kimberly, D.M.; Goli, S.; Carolyn, D.R.; Mia, M.G.; Ahmedin, J.; Rebecca, L.S. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef] [Green Version]
- Machnicka, M.A.; Olchowik, A.; Grosjean, H.; Bujnicki, J.M. Distribution and frequencies of post-transcriptional modifications in tRNAs. RNA Biol. 2014, 11, 1619–1629. [Google Scholar] [CrossRef] [Green Version]
- Cantara, W.A.; Crain, P.F.; Rozenski, J.; McCloskey, J.A.; Harris, K.A.; Zhang, X.; Vendeix, F.A.P.; Fabris, D.; Agris, P.F. The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Res. 2011, 39, D195–D201. [Google Scholar] [CrossRef] [Green Version]
- Carell, T.; Brandmayr, C.; Hienzsch, A.; Muller, M.; Pearson, D.; Reiter, V.; Thoma, I.; Thumbs, P.; Wagner, M. Structure and function of noncanonical nucleobases. Angew. Chem. Int. Ed. Engl. 2012, 51, 7110–7131. [Google Scholar] [CrossRef]
- Karijolich, J.; Yu, Y.T. Spliceosomal snRNA modifications and their function. RNA Biol. 2010, 7, 192–204. [Google Scholar] [CrossRef]
- Nicky, J.; Julia, T.; Martin, A.S.; Nicole, S.; John, S.M.; Eva, M.N. The RNA modification landscape in human disease. RNA 2017, 23, 1754–1769. [Google Scholar]
- Liu, J.; Ren, D.; Du, Z.; Wang, H.; Zhang, H.; Jin, Y. m6A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem. Biophys. Res. Commun. 2018, 502, 456–464. [Google Scholar] [CrossRef]
- Lin, S.; Junho, C.; Du, P.; Robinson, T.; Richard, I.G. The m6A methyltransferase METTL3 promotes translation in human Cancer cells. Mol. Cell 2016, 62, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Gao, R.; Chen, Y.; Yang, Z.; Han, P.; Zhang, H.; Dou, Y.; Liu, W.; Wang, W.; Du, G.; et al. Overexpression of NSUN2 by DNA hypomethylation is associated with metastatic progression in human breast cancer. Oncotarget 2016, 8, 20751–20765. [Google Scholar] [CrossRef] [Green Version]
- Sylvain, D.; Francesca, R.; Lars, T.; Zhou, Z.; Lukas, H.; Martin, T.; Kateryna, S.; Iva, K.; Alexandra, F.; Hadrien, D.; et al. Elp3 links tRNA modification to IRES-dependent translation of LEF1 to sustain metastasis in breast cancer. J. Exp. Med. 2016, 213, 2503–2523. [Google Scholar]
- Begley, U.; Sosa, M.S.; Avivar-Valderas, A.; Patil, A.; Endres, L.; Estrada, Y.; Chan, C.T.; Su, D.; Dedon, P.C.; Aguirre-Ghiso, J.A.; et al. A human tRNA methyltransferase 9-like protein prevents tumour growth by regulating LIN9 and HIF1-α. EMBO Mol. Med. 2013, 5, 366–383. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fu, D.; Chen, Y.; Su, J.; Wang, Y.; Li, X.; Zhai, W.; Niu, Y.; Yue, D.; Geng, H. G3BP1 promotes tumor progression and metastasis through IL-6/G3BP1/STAT3 signaling axis in renal cell carcinomas. Cell Death Dis. 2018, 9, 501. [Google Scholar] [CrossRef]
- Tomoko, H.; Adrian, R.F. Pseudouridine synthases. Chem. Biol. 2006, 13, 1125–1135. [Google Scholar]
- Rintala-Dempsey, A.C.; Kothe, U. Eukaryotic stand-alone pseudouridine synthases-RNA modifying enzymes and emerging regulators of gene expression? RNA Biol. 2017, 14, 1185–1196. [Google Scholar] [CrossRef] [Green Version]
- Guzzi, N.; Cieśla, M.; Ngoc, P.C.T.; Lang, S.; Arora, S.; Dimitriou, M.; Pimková, K.; Sommarin, M.N.E.; Munita, R.; Lubas, M.; et al. Pseudouridylation of tRNA-Derived Fragments Steers Translational Control in Stem Cells. Cell 2018, 73, 1204–1216.e25. [Google Scholar] [CrossRef] [Green Version]
- Ron, E.; Michael, D.; Alex, E.L. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar]
- Goldman, M.; Craft, B.; Swatloski, T.; Cline, M.; Morozova, O.; Diekhans, M.; David Haussler, D.; Zhu, J. The UCSC cancer genomics browser: Update 2015. Nucleic Acids Res. 2015, 43, D812–D817. [Google Scholar] [CrossRef] [Green Version]
- Pontén, F.; Jirström, K.; Uhlen, M. The Human Protein Atlas-a tool for pathology. J. Pathol. 2008, 216, 387–393. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pandey, A.; Chinnaiyan, A.M. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Snel, B.; Lehmann, G.; Bork, P.; Huynen, M.A. STRING: A web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000, 28, 3442–3444. [Google Scholar] [CrossRef] [Green Version]
- Sara, M.; Debajyoti, R.; David, W.-F.; Chris, G.; Quaid, M. GeneMANIA: A real-time multiple association network integration algorithm for predicting gene function. Genome Biol. 2008, 9, S4. [Google Scholar]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Innocenti, F.; Cooper, G.M.; Stanaway, I.B.; Gamazon, E.R.; Smith, J.D.; Mirkov, S.; Ramirez, J.; Liu, W.; Lin, Y.S.; Moloney, C.; et al. Identification, replication, and functional fine-mapping of expression quantitative trait loci in primary human liver tissue. PLoS Genet. 2011, 7, e1002078. [Google Scholar] [CrossRef]
- Denis, A.S.; Michael, M.; Eunice, S.; Richard, S.S.; Vivian, G.C. Genetic analysis of radiation-induced changes in human gene expression. Nature 2009, 459, 587–591. [Google Scholar]
- Wang, I.X.; Ramrattan, G.; Cheung, V.G. Genetic variation in insulin-induced kinase signaling. Mol. Syst. Biol. 2015, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Borgeson, B.; Phanse, S.; Tu, F.; Drew, K.; Clark, G.; Xiong, X.; Kagan, O.; Kwan, J.; Bezginov, A.; et al. Panorama of ancient metazoan macromolecular complexes. Nature 2015, 525, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, J.; Zhu, J. The role of m6A RNA methylation in human cancer. Mol. Cancer 2019, 18, 103. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Wei, Q.; Jin, J.; Luo, Q.; Liu, Y.; Yang, Y.; Cheng, C.; Li, L.; Pi, J.; Si, Y.; et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020, 48, 3816–3831. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, R.; Tasak, M.; Maddirevula, S.; Abdel-Salam, G.; Sayed, I.; Alazami, A.; Al-Sheddi, T.; Eman Alobeid, E.; Phizicky, E.; Alkuraya, F. PUS7 Mutations Impair Pseudouridylation in Humans and Cause Intellectual Disability and Microcephaly. Hum. Genet. 2019, 138, 231–239. [Google Scholar] [CrossRef]
- Hu, G.; Wang, R.; Wei, B.; Wang, L.; Yang, Q.; Kong, D.; Du, C. Prognostic Markers Identification in Glioma by Gene Expression Profile Analysis. J. Comput. Biol. 2020, 27, 81–90. [Google Scholar] [CrossRef]
- Obuchowski, N.A.; Bullen, J.A. Receiver operating characteristic (ROC) curves: Review of methods with applications in diagnostic medicine. Phys. Med. Biol. 2018, 63, 07tr01. [Google Scholar] [CrossRef]
- Zhe, H.; Jane, C.; Dawn, T. What is an ROC curve? Emerg. Med. J. 2017, 34, 357–359. [Google Scholar]
- Yan, C.; Zhu, M.; Ding, Y.; Yang, M.; Wang, M.; Li, G.; Ren, C.; Huang, T.; Yang, W.; He, B.; et al. Meta-analysis of genome-wide association studies and functional assays decipher susceptibility genes for gastric cancer in Chinese populations. Gut 2020, 69, 641–651. [Google Scholar] [CrossRef]
- Yuan, L.; Jin, T.; Yin, J.; Du, X.; Wang, Q.; Dong, R.; Wang, S.; Cui, Y.; Chen, C.; Lu, G. Polymorphisms of tumor-related genes IL-10, PSCA, MTRR and NOC3L are associated with the risk of gastric cancer in the Chinese Han population. Cancer Epidemiol. 2012, 36, e366–e372. [Google Scholar] [CrossRef]
- Tesarova, M.; Vondrackova, A.; Stufkova, H.; Veprekova, L.; Stranecky, V.; Berankova, K.; Hansikova, H.; Magner, M.; Galoova, N.; Honzik, T.; et al. Sideroblastic anemia associated with multisystem mitochondrial disorders. Pediatr. Blood Cancer 2019, 66, e27591. [Google Scholar] [CrossRef]
| Dataset | Tumor (Cases) | Normal (Cases) | Fold Change | t-Test | p-Value |
|---|---|---|---|---|---|
| Lu Ovarian | Ovarian Serous Adenocarcinoma (20) | Ovarian Surface Epithelium (5) | 1.913 | 9.134 | 2.28 × 10−9 |
| Lu Ovarian | Ovarian Endometrioid Adenocarcinoma (9) | Ovarian Surface Epithelium (5) | 1.808 | 5.846 | 0.0000904 |
| Lu Ovarian | Ovarian Mucinous Adenocarcinoma (9) | Ovarian Surface Epithelium (5) | 1.405 | 4.275 | 0.000692 |
| Lu Ovarian | Ovarian Clear Cell Adenocarcinoma (7) | Ovarian Surface Epithelium (5) | 1.457 | 2.64 | 0.017 |
| Hendrix Ovarian | Ovarian Mucinous Adenocarcinoma (13) | Ovary (4) | 1.216 | 4.26 | 0.003 |
| Hendrix Ovarian | Ovarian Clear Cell Adenocarcinoma (8) | Ovary (4) | 1.275 | 4.44 | 0.000934 |
| Hendrix Ovarian | Ovarian Endometrioid Adenocarcinoma (37) | Ovary (4) | 1.299 | 6.012 | 0.00098 |
| Hendrix Ovarian | Ovarian Serous Adenocarcinoma (37) | Ovary (4) | 1.301 | 6.304 | 0.001 |
| Yoshihara Ovarian | Ovarian Serous Adenocarcinoma (43) | Peritoneum (10) | 1.537 | 3.171 | 0.003 |
| Cancer Type | Sample ID | Fusion Partner | Copy | Mutation in Sample |
|---|---|---|---|---|
| Serious Ovarian Cancer | TCGA-24-1469-01 | Fusion, SRPK2-PUS7 | ShallowDel | 223 |
| Serious Ovarian Cancer | TCGA-31-1953-01 | Fusion, SRPK2-PUS7 | Gain | 56 |
| Serious Ovarian Cancer | TCGA-61-1740-01 | Fusion, SRPK2-PUS7 | Gain | 183 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Chen, L.; Han, Y.; Zhang, F.; Wang, Y.; Han, Y.; Wang, Y.; Wang, Q.; Guo, X. The Identification of RNA Modification Gene PUS7 as a Potential Biomarker of Ovarian Cancer. Biology 2021, 10, 1130. https://doi.org/10.3390/biology10111130
Li H, Chen L, Han Y, Zhang F, Wang Y, Han Y, Wang Y, Wang Q, Guo X. The Identification of RNA Modification Gene PUS7 as a Potential Biomarker of Ovarian Cancer. Biology. 2021; 10(11):1130. https://doi.org/10.3390/biology10111130
Chicago/Turabian StyleLi, Huimin, Lin Chen, Yunsong Han, Fangfang Zhang, Yanyan Wang, Yali Han, Yange Wang, Qiang Wang, and Xiangqian Guo. 2021. "The Identification of RNA Modification Gene PUS7 as a Potential Biomarker of Ovarian Cancer" Biology 10, no. 11: 1130. https://doi.org/10.3390/biology10111130
APA StyleLi, H., Chen, L., Han, Y., Zhang, F., Wang, Y., Han, Y., Wang, Y., Wang, Q., & Guo, X. (2021). The Identification of RNA Modification Gene PUS7 as a Potential Biomarker of Ovarian Cancer. Biology, 10(11), 1130. https://doi.org/10.3390/biology10111130







