Simple Summary
Following Borreliae Part 1, this second review describes Borreliae of the relapsing fever group (RFG) and unclassified Borreliae. The RFG is further divided according to vector transmission and geographical distribution in other subgroups, namely the soft-tick-borne relapsing fever (STBRF) group, the hard-tick-borne relapsing fever (HTBRF) one, the louse-borne relapsing fever (LBRF) group, and the Avian relapsing fever group. Where possible, according to the literature description, each sub-group of the RFG Borreliae is organized here in sections explaining the geographical distribution, the vectors, the hosts, the epidemiology, and the microbiology. In case of human infectiveness, clinical aspects are also discussed. Isolation and sequencing of Borrelia species is ongoing, and in addition to the groups mentioned in these reviews, there are Borreliae that at present cannot be cultivated, but according to sequencing data they share some characteristics with one specific group. Nevertheless, they still cannot be classified in one of them. This is the case of Borreliae with not-yet-identified pathogenicity for humans or animals, which are here named “unclassified Borreliae” and described separately, recalling the similarities with Borreliae already classified. In the future, we expect that those Borreliae are going to be characterized, including them in one of the previous groups.
Abstract
Borreliae of the relapsing fever group (RFG) are heterogenous and can be divided mainly into three groups according to vectors, namely the soft-tick-borne relapsing fever (STBRF) Borreliae, the hard-tick-borne relapsing fever (HTBRF) Borreliae, the louse-borne relapsing fever (LBRF) Borreliae, and the avian relapsing fever ones. With respect to the geographical distribution, the STBRF Borreliae are further subdivided into Old World and New World strains. Except for the Avian relapsing fever group Borreliae, which cause avian spirochetosis, all the others share infectivity in humans. They are indeed the etiological agent of both endemic and epidemic forms of relapsing fever, causing high spirochaetemia and fever. Vectors are primarily soft ticks of Ornithodoros spp. in the STBRF group; hard ticks, notably Ixodes sp., Amblyomma sp., Dermacentor sp., and Rhipicephalus sp., in the HTBRF group; and the louse pediculus humanus humanus in the TBRF one. A recent hypothesis was supported for a common ancestor of RFG Borreliae, transmitted at the beginning by hard-body ticks. Accordingly, STBRF Borreliae switched to use soft-bodied ticks as a vector, which was followed by the use of lice by Borrelia recurrentis. There are also new candidate species of Borreliae, at present unclassified, which are also described in this review.
1. Introduction
Borrelia species are part of the Spirochaetaceae family; therefore, they are characterized by a spiral shape. Spirochaetes cause many important diseases in humans, including relapsing fever (RF), which is a widespread bacterial disease caused by microaerophilic spirochetes of the genus Borrelia. Most Borrelia spp. are transmitted by ticks (endemic forms), while the epidemic forms of relapsing fever are caused by body lice, through scratching that induces the rupture of the louse and micro skin wounds. Borrelia can be transmitted by soft ticks or by hard ticks. Depending on the geographic area and vectors, many Borrelia spp. can infect humans. Those types of Borreliosis are not clinically easy to distinguish from other febrile diseases, such as malaria in Africa [1].
The first description of “relapsing fever” was made by Hippocrates in 430 B.C. on the island of Thasos in the Northern Aegean Sea: “The vast majority (of sufferers) had a seizure on the sixth day, with an interval of six days followed by a seizure on the fifth day after relapse” [2]. Other typical features of the louse-borne relapsing fever (LBRF) were severe rigors, jaundice, profuse epistaxis, and a tendency to precipitate abortion [3]. Several episodes of “epidemic fever” in the following centuries were due to relapsing fever, based on the symptoms of the disease. The “yellow plague” that swept Europe in 550 (Justinian plague), of which the distinguishing feature was jaundice, was also probably predominantly RF transmitted by lice [4]. In Dublin in 1770, Rutty described a “fever lasting six or seven days, with multiple relapses” [5]. An epidemic of RF in 1812 struck one third of Napoleon’s Grande Armée in Vilnius, on the route from Moscow to Warsaw [6]. In Edinburgh, during the epidemic, which involved Ireland, Craigie in 1843 distinguished this infection transmitted by lice (Pediculus humanus corporis) from typhus and coined the name of “relapsing fever” [7]. This infection spread throughout the British Isles [8]. David Livingstone described fatal tick-borne RF in Angola in 1857, an infection transmitted by soft ticks (endemic tick-borne relapsing fever). Obermeier saw spirochetes, now recognized as B. recurrentis, in the blood of febrile patients in Berlin in 1866 [9]. In Eurasia, RF transmitted by ticks was first described by Dschunkowsky [10]; later, the microorganism was named Borrelia persica. Dramatic epidemics of louse-borne relapsing fever (LBRF) responsible for several millions of cases and a high fatality rate occurred throughout Africa after World Wars I and II, when French and British colonial soldiers infected in Europe or North Africa returned to their countries [11].
In addition to febrile episodes, symptoms include tachycardia, headache, conjunctivitis, hepatomegaly, splenomegaly, discoloration of the urine, asthenia, vomiting, myalgia, and arthralgia. Diagnosis is based on patient history, physical examination, and May–Grunwald–Giemsa staining of blood smears for microscopic confirmation of spirochetes. Morphology alone does not allow distinguishing spirochetes, which can be identified by molecular methods such as PCR and sequencing [12]. The mortality of tick-borne relapsing fever is generally low and can be linked to the Jarisch–Herxheimer reaction, which occurs in less than half of cases, but can also manifest itself with very serious symptoms [13].
RF borreliosis is well known and common in the African continent, but unfortunately, many African laboratories are not able to perform biomolecular tests. Therefore, the exact species distribution and potential animal reservoirs are often unknown. Within central, Southern, and Eastern Africa, Borrelia duttonii has mainly been described, while in northernmost Africa, B. crocidurae and B. hispanica can also be found as human pathogens [14]. According to vectors, Borreliae of the relapsing fever group can be divided into four subgroups, as described in Table 1.

Table 1.
Borreliae of relapsing fever group subgroupings according to symptoms, reservoirs, and vectors.
2. Endemic Relapsing Fever Borreliosis (STBRF)
Soft-tick-borne relapsing fever (SBRF) was first recognized in Eastern and central-southern Africa in 1904 [15], in North Africa in 1928 [16], and in West Africa in 1932 [17].
Vectors of the STBRF are argasid ticks of the genus Ornithodorus sp., which lack the dorsal shield.
Hosts and reservoirs for these Borreliae are animals such as porcupines, foxes, rodents, and monkeys, while man is an occasional host [18], except for Borrelia duttonii in Africa, for which no animal has been identified as reservoir. It is also possible that the Ornithodoros moubata tick itself acts as both vector and reservoir [19].
STBRF spirochetes adapt and colonize the salivary glands of Ornithodoros sp. in the long term and are maintained trans-stadially and transovarially. Transmission of Borrelia after tick bite occurs within a few seconds. A notable feature of their vector biology is the specificity of a given species of STBRF for a specific species of Ornithodoros. This peculiarity allows one to know the distribution of the Borrelia spp. based on its vector.
STBRFs are well known and common in the African continent [20]. Typical habitats for soft ticks are caves and burrows of rodents and small mammals in forested mountains at altitudes over 900 m. In Northern countries, O. tholozani also lives in houses and stables where the floor is soil. The infection is transmitted to humans and animals through the bite of the soft tick, and the spirochete is inoculated into the new organism at the end of the meal through the regurgitation of saliva. A blood meal lasts from 15 to 90 min and is usually carried out at night.
While several tick saliva proteins have been characterized in hard ticks and have been shown to be essential in the transmission of pathogens, very few have been identified in Ornithodoros sp. Similarly, the process of transmission and persistence of RF bacteria in the vertebrate host is not clear, although antigenic variations and erythrocyte rosetting have been described as potential virulence factors. The first study made on Ornithodoros’ saliva showed its anti-hemostatic activity [21]. Subsequently, with advances in proteomics and transcriptomic techniques, some further investigations on the saliva of O. moubata and O. erraticus were published [22]. The saliva of Ornithodoros sp. supports the feeding process by providing a cocktail of anti-hemostatic, anti-inflammatory, and immunomodulatory molecules [23]. The salivary transcriptome of the Ornithodoros parkeri soft tick includes genes of the lipocalin family, as well as certain genes with Kunitz domains indicative of serine protease inhibitors. Ornithodoros sonrai, O. erraticus, or O. normandi ticks have been collected in burrows in Morocco, Algeria, Tunisia, Mauritania, Senegal, Gambia, Mali, and Spain [24]. In all countries where Ornithodoros ticks have been found, the distribution is generally contiguous, and the burrows are located near the floodplain of the Niger River [24].
2.1. Epidemiology
The STBRF Borreliae are conventionally divided geographically into the “Old World” strains, including Borrelia crocidurae, Borrelia duttonii, and Borrelia hispanica; and the “New World” strains, with Borrelia hermsii, Borrelia parkeri, and Borrelia turicatae.
2.1.1. Old World Strains
Details on Old World strains are reported in Table 2. The four main species responsible for STBRF in Europe are: B. hispanica, B. persica, B. caucasica, and B. crocidurae [25]. In Eurasia, relapsing fever is sporadic, mainly affecting those who involuntarily enter tick-infested caves, ruins, or animal shelters. Most infections are associated with Borrelia persica transmitted by the bite of the Ornithodoros tholozani tick [26]. About 30–60% of O. tholozani ticks from Israel were reported to carry this spirochaete. As for other Ornithodoros ticks, this species has great longevity, surviving between 5 and 10 years of fasting [27]. In Eurasia, other species that cause recurrent fever coexist with Borrelia caucasica and Borrelia latyschewii. The taxonomic position of these species and other relapsing fever spirochetes is not known. In Western European countries such as Spain and Portugal, RF is often caused by Borrelia hispanica [28]. Its vector is Ornithodorus erraticus, a soft tick that is usually found in old places with pigs’ herds. In Portugal, the first human case of tick-borne relapsing fever (TBRF) was reported in 1942, but up to the early 1960s, the disease had rarely been described [29,30].
The infection is sporadic, usually following an opportunistic infection in individuals exposed to ticks. Furthermore, the infection has been seen as an imported disease in those traveling from endemic regions [31]. For this reason, in patients with recurrent episodes of fever, it is important to consider this diagnosis if patients have recently traveled to Africa, America, or the Middle East [32]. New species have also been described, but their clinical relevance remains to be clarified [33].

Table 2.
Old World soft-tick-borne relapsing fever Borreliae with geographical areas and vectors.
Table 2.
Old World soft-tick-borne relapsing fever Borreliae with geographical areas and vectors.
| Borrelia | Ornithodoros Tick | Geographical Areas | Disease (Reservoirs) | References |
|---|---|---|---|---|
| B. armenica | O. verrucosus | Ukraine | Mouse infection (mouse, guinea pigs) | [34] |
| B. babylonensis | O. verrucosus | Russia, Ukraine | Mouse infection | [35] |
| B. baltazardii | O. tholozani | Iran | STBRF | [36,37] |
| B. caucasica | O. verrucosus | Ukraine, Caucasus, southeast Europe | STBRF | [34,38] |
| B. crocidurae | O. marocanus | Western and Northern Africa | STBRF mild symptoms | [39] |
| B duttonii | O. moubata | Central, Eastern, and Southern Africa | STBRF neurological signs, neonatal infection | [40,41] |
| B. graingeri | O.graingeri | Kenya | Flu-like syndrome | [42] |
| B. harveyi | unknown | Kenya, East Africa | Bacteremia of monkeys (Cercopithecus aethiops) | [43] |
| B. hispanica | O. erraticus | Iberian Peninsula and Northern Africa | STBRF Ocular and neurological symptoms | [32] |
| B. kalaharica | O. savignyi | Kalahari desert (Botswana and Namibia) | STBRF | [44] |
| B. latyschewii | O. tartakovsky | Iran, Middle East | STBRF | [45] |
| B. mazzottii | O. talaje | Mexico, Central America, and western USA | STBRF | [46] |
| B. merionesi | O. costalis and O. merionesi | Morocco and Atlantic coastal areas of the Sahara desert | Non-infectious to humans (rodent, monkeys) | [24,47] |
| B. microtti | O. erraticus | Iran, Afghanistan, Eastern Africa | STBRF | [48] |
| B. persica | O. tholozani | Central Asia, Middle East, Egypt, and India | STBRF, infectious for dogs and cats | [49,50] |
| B. tillae | O. zumpti | Southern Africa | Rodents | [51] |
STBRF—soft-tick-borne relapsing fever.
Cases of STBRF after arthropod bite have been reported in the Kalahari desert. The causative agent is a new RF Borrelia, referred to as “Candidatus Borrelia kalaharica”, which shares more homologies with New World recurrent fever Borreliae, such as B. parkeri and B. hermsii, than with Old World species, such as B. duttonii or B. crocidurae [52].
Borrelia baltazardii was isolated from O. tholozani in a region of Iran where B. persica had already been known, from which it differs regarding its pathogenicity in animals. The name of Borrelia baltazardii has been proposed for this new Borrelia sp. nov. [36,37]. B. merionesi, first isolated in Meriones shawi in 1937, was recognized as a new species by Blanc and Maurice due to differences in pathogenicity for laboratory animals and humans in comparison to B. hispanica, B. crocidurae, and B. duttoni [47].
Geographical Distribution
In Central Asia and Middle Eastern countries, STBRF is mainly caused by Borrelia persica, and to a lesser extent by B. microtti, B. latyschewii, B. baltazardi, and B. caucasica [33]. In Iran, several species have been associated with human STBRF, including B. microtti, B. persica, and another genotype close to B. duttonii and B. recurrentis [34].
Tick-borne relapsing fever was first recognized in East Africa in 1904 [35], in North Africa in 1928 [36], and in West Africa in 1932 [26]. Studies made between 1905 and 1960 have progressively established the classic picture of STBRF in Africa, with three different vector/pathogen complexes involving soft ticks (Argasidae) of the genus Ornithodoros sp. In the eastern Savannah areas (Tanzania) and Southern Africa (Eritrea, South Africa), STBRF is caused by Borrelia duttonii, which has O. moubata and O. porcinus as vectors [37]. In nature, these two ticks live in large burrows of ants, warthogs, and porcupines, and they have subsequently adapted to human dwellings and pet shelters, where they live in the crevices of walls and floors. There are no known mammalian reservoirs, and O. moubata enables Borrelia spp. to survive for very long periods through vertical transmission. It seems that O. moubata can act as both vector and reservoir of B. duttonii [53]. The annual incidence of the disease is very high in children younger than 5 years, especially in Tanzania. B. duttonii causes several clinical manifestations, often with neurological involvement [38]. In North Africa, from Morocco to Algeria and Tunisia, STBRF is classically caused by Borrelia hispanica, with O. erraticus as vector and small mammals as reservoir hosts [39]. O. erraticus is found in both large and small burrows and under stones, and has adapted to pet shelters. Most human infections occur during summertime in people sleeping in fields or farm buildings [40]. In West Africa, in Senegal and other countries, STBRF is caused by Borrelia crocidurae, with O. sonrai as vector and rodents and insectivores as reservoir hosts [25]. B. crocidurae infection is indeed the most common bacterial infection in this region [41]. O. sonrai inhabits rodent burrows and, as with other Ornithodoros sp., feeds quickly. Blood meals last only a few minutes. People are usually infected when sleeping, with burrows opening in their bedrooms [42]. In Africa there is a high incidence of STBRF, which contrasts with the low number of reports. This infection is maybe not often recognized, or it may be confused with malaria fever, which is an important differential diagnosis [25].
2.1.2. New World Strains
Details on New World strains are reported in Table 3. In the United States, the following specificities are observed: Borrelia hermsii with Ornithodoros hermsi, Borrelia parkeri with Ornithodoros parkeri, and Borrelia turicatae with Ornithodoros turicata. B. hermsii, B. parkeri, and B. turicatae are predominant and have been identified and studied in the western part of the US [43].
Borrelia braziliensis was first reported by Davis in 1952 in Brazil. It is transmitted by an Argasid sp., which lives underground in the basements of houses and farms, where the floor is the natural ground and hygienic conditions are poor. In addition of transmitting STBRF, it introduces a poison by its bite, which can cause a toxic reaction associated with severe symptoms [54].
B. johnsonii, a new tick-borne relapsing fever Borrelia, was found in the bat tick Carios kelleyi. It is more closely related to B. turicatae and B. parkeri than to B. hermsii, but is clearly distinct from them [55]. The human health implications of this new relapsing fever group spirochaete are not yet known [55]; however, recently B. johnsonii was detected in a patient reporting tick-borne illness symptoms [56]. The name B. johnsonii was given in honor of Dr. Russell C. Johnson [57].

Table 3.
New World soft-tick-borne relapsing fever Borreliae with geographical areas and vectors.
Table 3.
New World soft-tick-borne relapsing fever Borreliae with geographical areas and vectors.
| Borrelia | Ornithodoros Tick | Geographical Areas | Hosts and Disease | Ref. |
|---|---|---|---|---|
| B. braziliensis | O. braziliensis | Plateau of southern Brazil | STBRF (dogs, armadillos) | [54] |
| B. coriaceae | O.coriaceus | Western North America, northwest California | Deer bacteremia, dogs, bovine epizootic abortion | [58] |
| B. dugesii | O. dugesii | Mexico | (Rodents, Neotoma micropus) | [59] |
| B. hermsii | O. hermsi | Western North, White Mountains (Arizona, US), British Columbia (Canada) | STBRF (rodents, squirrels) | [60] |
| B. mazzottii | O. talaje | Mexico, Central America, and western US | STBRF | [46] |
| B. parkeri | O. parkeri | Western US | STBRF | [61,62] |
| B. queenslandica | O. gurneyi | Australia | Bacteremia with relapse (Rodents, Rattus villosissimus, Kangaroo) | [63] |
| B turicatae | O. turicata | British Columbia (Canada), southwestern and south-central US and Mexico | STBRF | [64,65] |
| B. venezualensis | O. rudis | Central America and northern South America, Venezuela, Brazil, Colombia, Panama | STBRF | [66] |
| B. johnsonii | Carios kelleyi | Canada, US, China, Mexico, Cuba, Costa Rica, Chile | Bats, tick-borne illness to be defined | [56,67,68] |
Geographical Distribution
STBRF is endemic in the western United States, predominately in mountain regions, in 12 US states, namely Arizona, California, Colorado, Idaho, Montana, Nevada, New Mexico, North Dakota, Oregon, Texas, Utah, and Washington. Most RF cases in the US are caused by Borrelia hermsii and transmitted by Ornithodoros hermsi soft ticks, which typically live in nests of rodents such as ground squirrels, tree squirrels, and chipmunks in coniferous forests at altitudes between a 500 and 2500 m. Soft ticks can acquire RF Borrelia by feeding on infected rodents, the reservoir hosts. Once infected, soft ticks are infectious for life [44].
In California, sciurid rodents, including chipmunks (Tamias spp.) were found positive for serum antibodies to Borrelia hermsii [69]. Borrelia turicatae is the primary known causative species of STBRF at low-altitude, arid regions, in the southern US and Texas, inducing neurologic symptoms. O. turicata ticks are considered an arthropod reservoir of B. turicatae because they have a 10-year life span and might endure years of starvation while retaining the ability to transmit B. turicatae [46]. Borrelia parkeri is present in the western US, Colorado, and California, and is transmitted to humans by Ornithodoros parkeri ticks [47].
The first case of TBRF in Mexico was reported by Brumpt as an infestation from B. turicatae, which has as vector O. turicata [70]. Since RF is considered a forgotten and neglected tropical disease, and given the small number of cases reported by the Mexican Ministry of Health, it is conceivable that many patients who have a fever of unknown origin are suffering from RF [70]. In Mexico, the following specificities of Borrelia tick association are observed: Ornithodoros turicata with B. turicatae, Ornithodoros duguesi with B. duguesii, and Ornithodoros talaje with B. mazzottii [71]. In Brazil, Venezuela, Colombia, and Panama STBRF is caused by Borrelia venezualensis, which is transmitted by Ornithodoros rudis [66]. In central Chile, Borrelia johnsonii has been identified in Ornithodoros atacamensis ticks, infesting small mammals in a national reserve in the Andes Mountains [72]. Imported cases of STBRF have also been described in the literature. Infections have been acquired in West Africa (Senegal, Mali, Mauretania, Gambia), East Africa (Ethiopia), North Africa (Morocco), or Central Asia (Uzbekistan, Tajikistan). In most cases, the causative species was Borrelia crocidurae (acquired mainly in Senegal, Gambia, Mauretania, Mali) [73]. Cases of imported Borrelia hispanica (acquired in Morocco) [31] and Borrelia persica (acquired in Uzbekistan, Tajikistan) have also been described [49].
Carios kelleyi, the tick bat which is the vector of B. johnsonii, requires prolonged feeding. Therefore, bats could cause significant spreading of this tick and its associated spirochaete over a large geographic area. Carios kelleyi ticks have been documented in the US, Canada, China, Mexico, and Costa Rica [64,65,71]. In addition to bats, these ticks can also feed on humans and host Rickettsia spp. [67], with possible implications for tick-transmitted human diseases.
2.2. Microbiology
RF borrelia are motile, chemo-organotrophic, microaerophilic and host-associated bacteria [74]. The spiral body tends to be shorter than in the Lyme group Borreliae, and it is lacking cytoplasmic tubules. Several endoflagella (15–20) wind around the protoplasmic cylinder and overlap in the middle. Sections of cells reveal a triple-layered outer membrane. Actually, most RF Borreliae have been adapted to BSKII medium cultivation; however, not-yet-cultivable Spirochaetes can be maintained through serial passages in vivo. Notably, 10-week-old Swiss mice can be inoculated intraperitoneally with 0.4 mL infected blood preserved in liquid nitrogen [75]. The maximal yield of spirochaetes is around 106–107 spirochete/mL of blood, and it is usually achieved 3 days after inoculation of infected blood. The in vivo inoculation represents an important step for the isolation of RF Borreliae from hosts. Strains cultivated in vitro don’t lose their infectivity in mice.
STBRF spirochetes adapt and colonize in the salivary glands of Ornithodoros sp. and are maintained trans-stadially and transovarially. This means that the transmission of Borrelia in mammals after a bite occurs in a few seconds, developing high spirochaetemia. These Borreliae spp. usually grow at temperatures between 33 and 35 °C, corresponding to the mammalian host temperature, but can also multiply at 22 °C (tick temperature) as demonstrated in vitro for B. turicatae [76]. During a blood meal, STBRF spirochetes enter the middle intestine, and in the following weeks they, in part, migrate and colonize the salivary glands, which is important for rapid transmission [77]. Spirochetes populating salivary gland are therefore predisposed to establish early mammalian infection [78].
The infectious cycle of argasid-transmitted RF spirochetes requires adaptation to three environments, namely the midgut and salivary glands of the tick and the vertebrate host.
Pathogens regulate genes involved in antigenic variation to facilitate escape from the host antibody response. The dynamics between host antibody response and antigenic variation can continue for several months, providing multiple opportunities for the acquisition of spirochetes from uninfected ticks [79].
2.2.1. Phylogenetic Analysis
The typing of RF-group Borrelia is challenging because of their segmented genomes, with essential genes on large linear plasmids [80]. This is further complicated by the antigenic variation. Intergenic spacer heterogeneity (IGS) between 16S–23S genes is resolutive to discriminate between Lyme group and some relapsing fever Borreliae [80], but not for clustering RF Borreliae. The phylogenetic tree derived from nucleotide sequences of the flaB gene clearly separates New World from Old World RF Borreliae [81]. Nevertheless, for a more precise classification of the different RF Borreliae, non-coding intragenic spacer regions are more useful for their variability, because they were not under selective pressure and thus probably reflect changes accumulated over time, without functional constraints [82]. Differences obtained from gene-sequence-based comparisons more likely reflect the accumulation of adaptive changes due to different vectors for transmission, therefore allowing the subgrouping of RF Borreliae in common species groups [82]. Phylogenetic trees have been obtained among a limited number of species, being the registered data of sequences not completely available. An example of a phylogenetic tree is shown in Figure 1, where three clusters of RF Borrelia have been identified on the basis of the analysis of 13 species representative of the main RF Borreliae.
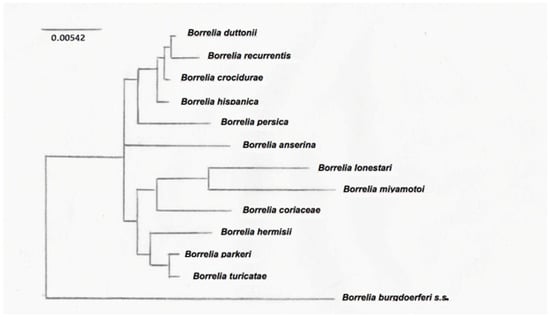
Figure 1.
Phylogenetic tree based on the 16SrRNA sequences of the main RF Borrelia species. The 50% majority rule consensus tree based on 12 trees was obtained by using a maximum parsimony method [75].
2.2.2. Genome
RF Borrelia species have segmented genomes and maintain essential genes on large linear plasmids [80]. The genome size of RF Borreliae (1–1.5 Mb) is smaller than the other pathogenic bacteria with a more versatile lifestyle (e.g., P. aeruginosa, 6.3 Mb). This 160 kb linear mega-plasmid has fairly conserved syntony between B. duttonii (lp165), B. hermsii (lp174), and B. turicatae (lp150), which are not found in the Borreliae Lyme group [83].
Genes encoding enzymes for the synthesis of most amino acids, fatty acids, enzyme cofactors, and nucleotides are absent in RF Borreliae genomes, as shown in Lyme group Borreliae [84]. RF Borrelia genomes have a limited repertoire that reflects very well their adapted lifestyle, including only a few genes associated with virulence.
The Argasidae and Ixodidae cuticle, which contains chitin derived from the polymerization of N-Acetyl Glucosamine (NAG), could be an important source of nutrients for Borreliae during the arthropod-associated phase [85]. Evolution by genome reduction is well correlated with the degenerate lifestyle of several well-adapted pathogens [86], which probably include RF Borreliae, which have a narrow niche in the vertebrate vector and host. B. turicatae’s mega-plasmid undergoes a shift in its transcriptional profile between in vitro tick-like growth conditions and infected murine blood, identifying a cluster of encoding putative surface lipoproteins likely involved in vector colonization and host–vector interactions [76].
2.2.3. Species Identification of the STBRF
Borrelia species were initially distinguished on the basis of geography and vectors. This classification was based on a co-specialization hypothesis, which postulated that only one species of relapsing fever Borrelia could be found in a particular host and vector in a given geographic area. However, recent evidence showed the coexistence of B. duttonii and B. crocidurae in Togo and of B. crocidurae and B. hispanica in North Africa; therefore, previous geographic distribution studies were not exhaustive [47].
Molecular typing of species can be carried out by restriction fragment length polymorphism through the amplification (PCR) of 16S-rRNA genes and/or species-specific PCR of the glycerophosphodiester phosphodiesterase (GlpQ) gene [87], which seems to be more variable than 16S-rRNA [88].
At least 10 different species of Borrelia with STBRF have been documented in Africa, including four species that infect humans, namely B. hispanica, B. crocidurae, B. duttonii, and B. recurrentis. Other species have been found in non-human hosts. The loci used for the initial species determination were 16S rRNA and flaB. Although they are highly conserved (especially 16S) and may have low discriminatory power in RF species, they are very useful for a first approximation of species assignment, because they have been used for many STBRF species and strains, therefore many sequences are available [89].
2.2.4. Antigens
The 40 KDa Borrelia A repeat protein (BrpA) is characterized by the repetition of a particular amino acid motif. Deletion of the BrpA gene neither avoided infection of inoculated mice, nor inhibited further colonization of the O. turicata salivary glands and subsequent transmission [90].
Many virulence factors described for the Borreliae Lyme group are also present in RF Borreliae. The initial phase of the passage of the RF Borrelia from the vector to the mammal and to man (i.e., from the initial deposition of the spirochaetes on the skin to the subsequent colonization in various tissues) exposes the spirochaetes to host defense mechanisms, which Borrelia tries to neutralize by developing adaptation mechanisms. RF and Lyme group Borreliae share many characteristics [91], such as the general organization of the genome with a well-preserved tuning of the chromosome. Notwithstanding, there is an evident difference in linear and circular plasmids. The RF group has a more variable pathological spectrum, including RF transmitted by ticks and lice as well as the different and specific types of avian and bovine Borreliae. There is also a difference in the disease that separates these two types of spirochaetes: TBRF is characterized by high spirochetaemia, while Lyme borreliosis is characterized by tissue tropism (except for Borrelia mayonii), in particular the skin, joints, and nervous system [92]. They also differ in vectors, usually Ixodes sp. in Lyme, and several vector types in RF (soft ticks, hard ticks, louses) with transovarial transmission of Borrelia, especially in ticks. The tick bite is often painless, as the tick’s saliva contains pain-relieving substances as well as anticoagulant factors and anti-inflammatory substances. Additionally, tick saliva inhibits polymorphonuclear leukocytes (PMN) activity, which further reduces killing of the spirochaete [93]. Hence, RF Borrelia must penetrate both the extracellular matrix (ECM) and the endothelial lining of blood vessels to maintain and spread the infection to adjacent tissues. Furthermore, the use of host proteases supports the diffusion of the RF spirochaete [94]. The activation of matrix metalloproteinases (MMPs) also follows the binding and activation of plasminogen on the surface of the spirochaetes [95].
In the early stages, STBRF is a blood-borne infection with high spirochaetemia. Several species, such as B. duttonii, B. crocidurae, and B. hispanica, often interact with erythrocytes, causing red blood cells to clump together. This interaction allows the spirochaete to access nutrients, nestling and hiding them from the immune response [96]. RF group Borreliae have specific genes that allow using purines from serum as metabolites for the synthesis of different macromolecules [97]. These genes are lacking in the Borrelia Lyme group, which does not reach high spirochaetemia (except for Borrelia mayonii). RF Borrelia interactions with circulating cells could be a virulence strategy to increase and lengthen the time this pathogen can be retained in the host. The most important virulence factor of RF Borrelia is the mechanism of antigenic variation of the membrane proteins, located on the surface (variable major protein—Vmp), which allows escaping the antibody response, with a mechanism similar to the VlsE in the Borrelia Lyme group [98]. After the feverish peak there is an antibody response, and subsequently, Borreliae with this modified antigen are generated, allowing for a further spirochaetemia with a new feverish peak [99]. This process of antigenic variation can be repeated several times: each relapse corresponds to the increase of a new immunogenic variant of RF Borreliae that hosts a modified “main variable protein” (Vmp) on its surface. There are two different families of Vmps: large variable proteins (Vlps) of about 40 KDa and small variable proteins (Vsps) of about 20 KDa. Both Vlps and Vsps families are encoded on linear plasmids [100].
The progression of RF involves tissue invasion and colonization associated with further escape from the immune defense [18]. Several mechanisms of gene conversion, DNA rearrangements, mutations, and change in the transcription locus seem to be involved in the replacement of the active vsp or vlp gene [101]. The repertoire of genes encoding Vmp differs highly among genomes of B. recurrentis, B. hermsii [102], and B. duttonii [103].
The mechanism of antigenic variation is likely to be a common feature to all the different RF Borreliae. Up to now, these multiphase changes have been demonstrated for B. hermsii and B. turicatae [104]. Vsps and Vlps are phylogenetically related to the surface proteins of the Borrelia Lyme group OspC and VlsE, respectively [105].
2.2.5. Clinical Aspects
Incubation lasts approximately 1 week; between the tick bite and the first STBRF symptoms, a length of about 3–10 days is observed [106]. An initial febrile episode with high fever with temperature ranging from 38.7 to 40/41 °C is followed by a series of relapses (3–5 in LBRF and 9–13 in STBRF) interspersed with remissions of a few days [107]. Clinical manifestations vary in severity among patients and in some cases, if left untreated, the disease can be fatal. It is usually more severe in young children [8]. The first period of fever is usually the longest and lasts about 4–7 days. The following relapses correspond to spirochaetemia peaks spaced out by a few days of remission [107]. In addition to high fever, typical symptoms include malaise, headache, neurological symptoms, myoarticular pains, nausea, vomiting, and diarrhea. Patients may have petechial-type skin rashes, different from erythema migrans, which is characteristic of Lyme group Borreliae [91,92]. During the spirochaete peak, more or less severe bleeding can be observed, and in some cases hemorrhages of the retina and brain. Internal organs, such as the liver, with jaundice, and the spleen can also be affected, causing enlargement and rupture. Respiratory symptoms with cough and myocarditis may also be observed [60].
Neurological symptoms are common during RF, but are more severe for B. duttonii and B. turicatae infection due to their greater neurotropism [108]. The most common symptoms are indeed meningitis and facial palsy. Ocular complications have also been reported in some cases. Anemia and thrombocytopenia are also observed, and in untreated patients a progressive worsening of general conditions can be observed, with asthenia and weight loss [108]. RF spirochaetes are susceptible to broad-spectrum antibiotics. However, upon treatment, 54% of patients develop Jarisch–Herxheimer reaction [13].
According to international organizations reports, around 10–15% of neonatal deaths worldwide are caused by serious infections [109], including tick-borne RF in endemic regions. Spirochaetes can cross the placental barrier, causing congenital infections [110]. The consequences and complications of these cases of borreliosis in pregnancy can be mild, with a slight decrease in birth weight and preterm delivery, but also severe, resulting in miscarriage or death of the newborn and pregnant woman [111]. In Rwanda, the death rate among pregnant women suffering from the disease is 16% [112]. In STBRF caused by B. duttonii, intrauterine growth retardation, placental damage and inflammation, impaired fetal circulation, and maternal anemia have been described.
In the United States, TBRF in dogs is caused by Borrelia turicatae and Borrelia hermsii, which are transmitted by Ornithodoros spp. ticks. The distinctive diagnostic feature of this infection is the visualization of several spirochaetes in standard blood smear examination. Although the course of the spirochaetemia has not been fully documented in dogs, it likely evolves with episodes of intermittent spirochaetemia and fever as in human infection [113].
In addition to infection in dogs, cases of TBRF have been reported in horses, in which it can cause abortion [114].
3. Hard-Tick-Borne Relapsing Fever (HTBRF)
HTBRF Borreliae are transmitted by several genera of hard ticks, notably Ixodes sp., Amblyomma sp., Dermacentor sp., and Rhipicephalus sp. Human infection has surely been documented for Borrelia miyamotoi, which was not the first Borrelia of the RF group transmitted by a hard tick and not by a soft tick (Ornithodoros sp.) to be described. More than a century ago, Arnold Theiler reported that Rhipicephalus sp. transmitted a spirochaete (Borrelia theileri) to cattle [115]. Recently, B. lonestari has been discovered in Amblyomma americanum [116]. Nucleotide sequences of B. miyamotoi isolates in Japan and North America confirmed that B. miyamotoi and the other aforementioned species are phylogenetically linked to HTBRF [117,118]. HTBRF Borreliae strains correlate with those of vector species rather than geographical distance [119]. Therefore, the genetic diversification of HTBRF Borreliae can be due to the speciation of vector ticks, and this relationship might be required for efficient transmission of HTBRF Borreliae within its vector [119]. Recently, the hypothesis of a common ancestor of Borreliae transmitted by hard-body ticks was formulated. Accordingly, STBRF Borreliae switched to use soft-bodied ticks as a vector, which was followed by the emergence of Borrelia recurrentis, the louse-borne RF Borrelia [119]. HTBRF Borreliae are summarized in Table 4 with their vectors.

Table 4.
Hard-tick-borne relapsing fever Borreliae.
Borrelia miyamotoi is a spirochaete of the RF group, transmitted by the same hard ticks (Ixodes sp.) of the Borreliae Lyme group [128]. It was identified in Japan in 1994 from Ixodes persulcatus ticks collected from the small Japanese country mouse Apodemus argenteus [129]. The name of this Borrelia was given in honor of Kenji Miyamoto, who first reported this spirochaete in Hokkaido, Japan. In 2000 in the northeastern US, a Borrelia closely related to B. miyamotoi was identified in Ixodes scapularis ticks. As early as 1985, in the United States spirochaetes had been observed in ticks, probably related to B. miyamotoi but mistakenly identified as B. burgdorferi due to the cross-reactivity of the antibodies used in serological tests. These spirochaetes were present in the ovarian tissue of adult ticks, eggs, and larvae of Ixodes scapularis and Ixodes pacificus and led to the false conclusion that B. burgdorferi could be transmitted transovarially by ticks [130]. Recent evidence has confirmed the transovarial (vertical) transmission of B. miyamotoi (similarly to the other RFG Borreliae), but not of B. burgdorferi in I. scapularis [131]. B. miyamotoi was later identified in other Ixodes sp. ticks, which are vectors of Lyme disease in Asia, North America, and Europe [132]. The discovery of B. miyamotoi expands the geographic range of RF group Borreliae, which are classically transmitted by soft. ticks (Argasidae) and lice, have different ecological niches, and are only occasionally found in the same geographic areas of the vectors of Lyme disease [133].
3.1. Epidemiology
Although the novelty and wide geographic distribution of B. miyamotoi have been recognized for several years, this spirochaete has received relatively little attention until human cases of relapsing febrile-like illness from B. miyamotoi infection were reported in 2011 in Russia, and later in the United States and Europe [134]. This spirochaete has a global distribution and circulates together (co-infection) with the Lyme borreliosis agent, B. burgdorferi s.l., which shares the same ticks (Ixodes spp.) as vectors [123].
Borrelia miyamotoi infection in ticks and hosts has been reported in Japan and Russia (Ixodes persulcatus), in the eastern and upper Midwest United States (Ixodes scapularis), in the western US (Ixodes pacificus in California), and in Europe (Ixodes ricinus). A few cases were reported in humans in China, but due to the increment of patients, this bacterium is considered an emerging pathogen [135]. In Italy the first detection of B. miyamotoi in Ixodes ricinus ticks was dated 2018 and referred to ticks collected in two independent studies in 2016 in the north-eastern and north-western Alps [136]. These reports highlight the importance of B. miyamotoi for public health [137]. Antigenic cross-reactivity in immunoassays between Borrelia species in North America can complicate the diagnosis of both Lyme disease and relapsing fever [138].
3.2. Hosts and Reservoirs
In Table 4, the main information about HTBRF Borreliae is summarized. In 1996, phylogenetic analysis of Borrelia DNA sequences from Amblyomma americanum led to the identification of Borrelia lonestari sp. nov., related to the RF group and not to the Lyme group [116]. Sequences of B. lonestari, found mainly in the southeastern US, have also been detected in ticks in the northern US, due to the fact that Amblyomma americanum is a parasite of migratory water birds and non-migratory wild turkeys (Meleagris gallopavo silvestris) [139]. The host of Borrelia lonestari is likely the white-tailed deer (Odocoileus virginianus), which is a reservoir host for several pathogens associated with A. americanum [120]. This deer can develop bacteremia after B. lonestari inoculation [121]; however, other studies have not confirmed this hypothesis [122].
Little is known about B. miyamotoi reservoirs, but it can be hypothesized that common characteristics are shared with bacteria belonging to the B. burgdorferi sl complex: small rodents such as Apodemus argenteus in Japan [129], Peromyscus leucopus in North America [140], Apodemus flavicollis in Europe [141], Myodes glareolus in Northern Europe [142], and also passerine birds [143] are responsible for the large distribution of this Borrelia. Sequence analysis of B. miyamotoi from North America [117] and Japan a confirmed B. miyamotoi as an RF Borrelia [118]. B. miyamotoi can be clustered in Japanese/Russian genotypes [144], which differ from European and North American ones [145]. It has been shown that in Estonia, the Asian genotype of B. miyamotoi could be associated with both I. ricinus and I. persulcatus ticks [146]. B. miyamotoi is infectious and pathogenic for humans and evades human antibodies through the mechanism of antigenic variation, which is a common feature to all Borreliae (Lyme and RF group) [147].
3.3. Clinical Aspects
The clinical manifestations in humans are characterized by fever, chills, nausea, headache, myalgia, skin rash, and lymphadenopathy. In immunocompromised patients, meningoencephalitis also can be observed. Unlike STBRF, a small number of patients experienced more febrile episodes. B. miyamotoi and B. burgdorferi (and other pathogens transmitted by Ixodes spp.) can simultaneously infect ticks, reservoirs, and humans. Spirochaetemia is also lower than in patients infected by STBRF Borreliae. Laboratory tests for B. miyamotoi infections show leukopenia, thrombocytopenia, and elevated liver enzymes. Spirochaetes can be observed in the acute phase in blood smears stained with May–Grünwald–Giemsa. Two-step serology for B. miyamotoi includes ELISA and Western blot. Glycerophosphodiester phosphodiesterase (GlpQ) was chosen as target because of its absence in the Borrelia Lyme group. GlpQ is found in other relapsing fever Borrelia species. However, this could represent a specificity problem in areas where other relapsing febrile spirochaetes are enzootic. In the acute phase, serum samples should be collected within 7 days of symptoms onset, while in the convalescent phase, they should be collected approximately 3 weeks after symptoms onset. Currently, blood PCR is the most specific test for B. miyamotoi, because of the high spirochaetemia in the acute phase. In vitro cultivation of B. miyamotoi was reported in modified and serum-supplemented Kelly–Pettenkorfer medium [148].
Borrelia lonestari was found in both A. americanum removed from a patient with the so called southern tick-associated rash illness (STARI), initially referred as Lyme-like illness, and in a biopsy of the rash [149], leading to the assumption that it was the causative agent of STARI, which manifests itself after A. americanum bites [150]. However, the association between Borrelia lonestari and STARI, and therefore human infection, has not yet been confirmed. STARI is nowadays clearly linked to A. americanum tick bites, but further evidence is needed to link B. lonestari to STARI [151].
4. Louse-Borne Relapsing Fever (LBRF)
Louse- borne RF (LBRF) is an epidemic disease linked to war, famine, refugees, poverty, and poor sanitation. In previous centuries, it caused severe epidemics around the world. More recently, certain epidemics occurred in Sudan. LBRF still persists in the mountains of Ethiopia, where it is endemic. Nowadays, it is a forgotten disease in Western countries, but refugees from Africa to Europe could recall it [3]. The epidemic form is caused by B. recurrentis, for which the vector is the human body louse (Pediculus humanus humanus or Pediculus humanus corporis). Human body lice transmission was demonstrated by Mackie in 1907 [152]. It occurs from human to human through lice, which ingest infected blood and transmit the spirochaetes to a new host through the skin or mucous membranes when the body of the louse itself is crushed by scratching [1].
4.1. Epidemiology
In Ethiopian highlands and in Somalia, there are still annual epidemics of thousands of cases coinciding with the rainy period. In Sudan, in 1999–2000, 20,000 cases with around 2000 deaths were reported [3]. In 1985, in the Andes of Peru, at altitudes above 3800 m, 60 cases were reported among the inhabitants of villages infested with lice [3]. In the second half of 2015, more than 40 cases of LBRF were imported into central Europe by migrants [153].
Since July 2015, LBRF has been diagnosed in about one hundred refugees arriving in Europe, mainly from Ethiopia, Eritrea, Somalia, and other African countries, representing the most frequently reported infection in Eritrean immigrants [3]. Two patients diagnosed in Turin, Italy, had been residing in Italy for years and shared accommodation with recently arrived immigrants, suggesting the possibility of native infection [154].
4.2. Transmission
The transmission of B. recurrentis is limited to one vector, the human body louse Pediculus humanus corporis. Fluid from a crushed louse, or louse feces infected with B. recurrentis, can be inoculated through broken skin or mucous membranes through scratching. Lice, differently from ticks, cannot infect their progeny; therefore, they do not act as reservoirs. No animal reservoir is known, therefore the persistence of infection between outbreaks can occur only through mild or asymptomatic human infections. B. recurrentis has adapted to lice transmission. Phylogenetics and sequencing data suggest that B. recurrentis evolved from Borrelia duttonii [103]. The louse vector transmission seems to be less robust than that of the ticks. Transovarial transmission has not been demonstrated. In addition to the human body louse, the Pediculus humanus capitis was supposed as a possible vector, since B. recurrentis and B. theileri were detected in African pygmies’ head lice [155].
4.3. Microbiology
LBRF is caused by B. recurrentis, a large, mobile, loosely coiled spirochaete with tapered ends, 12–22 μm long and 0.2–0.6 μm thick, with an average wavelength of 1.8 μm, and 8–10 periplasmic flagella [156]. B. recurrentis can be cultured in vitro in serum-supplemented Barbour–Stoenner–Kelly (BSK) medium [157] and Kelly–Pettenkofer modified medium (MKP) [158], with higher isolation rate, morphology, and motility in MKP-based cultures [159].
B. recurrentis has the simplest genome among Borreliae, composed of one linear chromosome of 1 Mb, 7 linear plasmids, and 990 protein-coding genes. Furthermore, it also has low genetic variability [158]. The B. recurrentis genome is similar to the B. duttonii one, but it differs in genome reduction, which allowed adaptation to Pediculus humanus humanus. The B. recurrentis genome does not include RecA and RadA proteins, which are responsible for DNA repair [103]. Recently, the genome of B. recurrentis was recovered from the skeleton of a young woman retrieved during excavations in a cemetery in Oslo. Radiocarbon dating suggested she lived among 1430–1465. The resulting European lineage of B. recurrentis was discrete, showing ancestral oppA-1 gene and gene loss at sites of antigenic variation [160].
4.4. Clinical Aspects
The incubation period is 4–18 (average 7) days. The attack begins abruptly, with fever rising to almost 40 °C in a few days, accompanied by stiffness. Early symptoms include headaches, dizziness, nightmares, generalized aches and joint pains, anorexia, nausea, vomiting, and diarrhea. Upper abdominal pain, cough and hemorrhages of the conjunctiva, epistaxis, and jaundice develop later. A petechial or ecchymotic rash, involving particularly the trunk, is often observed. There is an important spirochaetemia, which is mainly localized around the lumen of blood vessels of various organs, causing miliary abscesses and infarcts of the spleen with possible rupture, liver failure, and involvement of the central nervous system, up to cerebral hemorrhage. Perivascular histiocytic interstitial myocarditis is responsible for arrhythmias, up to myocardial failure [161]. Lungs and bowel are studded with petechial and sometimes massive hemorrhages [162].
Untreated seizures resolve in 4–10 days (average 5), followed by 5–9 days of afebrile remission, followed by relapses of decreasing severity (up to 5), where nosebleeds but no purpuric eruptions may occur. Pregnant women are particularly susceptible to severe illness and premature birth, which is frequent.
B. recurrentis Vmps, which is expressed in the host, is the main factor inducing TNF (tumor necrosis factor), which is likely involved in the Jarisch–Herxheimer reaction, which can be extremely severe with the beginning of the antibiotic treatment [163].
5. Avian Relapsing Fever Borreliae
Borreliae can infect birds, which can also act as reservoirs (see Table 5). The first report was made by Sakharoff in 1891 [164], who described the Spirochaeta anserina later named Borrelia anserina [165]. The micro-organism was identified as a member of the genus Borrelia by analyzing the 16S rRNA gene sequence, showing characteristics of the RFG Borreliae [166]. B. anserina was also isolated in BSK culture from a domestic chicken in California [167].

Table 5.
Avian relapsing fever Borreliae.
Borrelia anserina is globally distributed, and it is the infectious agent of the avian spirochetosis, a tick-borne disease of poultry [168], with important economic concerns. This Borrelia makes use of Argas sp. as vectors (Table 5). The tick Carios capensis infests the nests of brown pelicans, Pelecanus occidentalis, and other ground-nesting birds along the coast of South Carolina. These ticks harbored Borreliae related to “Borrelia lonestari” [169]. Borreliae (6.8%) were recently detected in Carios (Ornithodoros) sawaii ticks collected from seabird nests. Sequencing analysis of 16S rRNA gene showed that they were phylogenetically strictly related to Borrelia turicatae [170].
B. anserina differs phenotypically from the other species of the RFG Borreliae: it has a smaller genome, made by a linear chromosome of ~900 kb, and a mega-plasmid, like other members of the genus, but in total, fewer plasmids [171,172]. In comparison to other Borreliae, hosts are limited to birds. The main advantage for spirochaete in infecting avian hosts is the opportunity for spreading in wide geographic regions. The impact of avian borreliosis on its host is not fully known; however, it could be associated with mortality. Acute septicemic spirochaetosis has been diagnosed in an adult male spotted owl (Strix occidentalis caurina) found dead in Washington, USA. Silver-stained biopsy specimens of the liver, spleen, brain, and blood vessels showed several spiral-shaped bacteria [166].
6. Unclassified Borreliae
A possible list of unclassified Borreliae is reported in Table 6.

Table 6.
Unclassified Borreliae.
Borrelia texasensis was isolated in Texas (TXW-1) from the Dermacentor variabilis, also known as the American dog tick. The TXW-1 strain has a small 38 KDa endoflagellar protein, and it lacks the OspC gene. TXW-1 isolate seems to represent an undescribed species in RFG Borreliae. These Borreliae are microaerophilous and can be grown in BSK-H medium at 34 °C. They are Gram-negative and Giemsa-positive. Their pathogenicity and infectiveness in animals and humans are unknown [173].
Borreliae in hard ticks were found in a low percentage (1%) in the Ivory Coast, by amplifying a fragment of the flaB gene and the 16S rRNA gene sequence, which were related to undescribed species similar to Borrelia duttonii. These new species had not previously been isolated by culture, therefore the names “Candidatus Borrelia africana” for the TCI22 genotype and “Candidatus Borrelia ivorensis” for the TCI140 and TCI351 genotypes were proposed. Based on a phylogenetic tree analysis of the flaB gene, the sequences of Candidatus Borrelia africana and Candidatus Borrelia ivorensis are located in the genus Borrelia close to that identified in Amblyomma cohaerens in Ethiopia (GenBank JX089967), and are closer to the RFG than to the Lyme group [174]. These potential new Borreliae form a new clade between Borrelia Lyme group clades and the RF group [175].
In Oromia (Ethiopia), 3.8% of ticks, which parasitize domestic animals, tested positive for Borrelia DNA. The prevalence of Borrelia DNA was significantly higher in ticks of the genus Amblyomma spp. (6.7%) compared to ticks of the genus Rhipicephalus spp. (2.1%). Sequencing data of the flaB and 16S rRNA genes of Borrelia spp. from Amblyomma ticks showed the presence of a new intermediate species between the RFG and Lyme group Borreliae. The pathogenicity for humans of Borrelia sp. found in the Amblyomma ticks of Ethiopia has not yet been studied, while Borrelia sp. detected in Boophilus/Rhipicephalus ticks is the causative agent of bovine borreliosis [176].
In the UK, in a bat parasitized by the Argas vespertilionis tick, commonly known as the short-legged bat tick, several spirochaetes were detected. The bat showed at autopsy muscle damage, liver and splenomegaly, enlarged lymph nodes, and adrenal hemorrhage. Specific PCR analysis of fragment of the genes encoding 16S rRNA, glpQ, and flaB highlighted differences from B. johnsonii [177], which has been found in bats’ ticks and also linked to human disease [55,56]. It remains to be clarified whether bats are an extension of the spirochaetic reservoir of RF or represent an enzootic cycle with only negligible significance to humanity.
In the Andean valley in Chile, Borrelia sp. Cachapoal was found in rodent Ornitodoros ticks, closely related to Ornitodoros atacamensis. Even if the role of this Borrelia as etiologic agent of RF in human is unknown, its phylogenetic position is very close to Borrelia johnsonii [72].
Author Contributions
Conceptualization, G.T. and S.B.; methodology, S.B. and M.R.; writing—original draft preparation, G.T., S.B. and M.C.; writing—review and editing, S.T., N.d.M. and P.F.; visualization, M.C., G.T.; supervision, G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, but the APC to publish this review was funded by the “Associazione Lyme Italia e Coinfezioni”.
Institutional Review Board Statement
Ethical review and approval were waived for this study because this review is based on the analyses of publicly accessible documents.
Informed Consent Statement
Informed consent is not applicable to this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank the Associazione Lyme Italia e Conifezioni for supporting Lyme Borreliosis studies and dissemination.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cutler, S.J.; Abdissa, A.; Trape, J.F. New concepts for the old challenge of African relapsing fever borreliosis. Clin. Microbiol. Infect. 2009, 15, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.E.R.; Chadwick, J.; Mann, W.N. Hippocratic Writings; Harmondsworth: New York, NY, USA, 1983. [Google Scholar]
- Warrell, D.A. Louse-borne relapsing fever (Borrelia recurrentis infection). Epidemiol. Infect. 2019, 147, e106. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, W. Historical notes on some epidemic diseases associated with jaundice. Br. Med. Bull. 1957, 13, 146–149. [Google Scholar]
- Rutty, J. A Chronological History of the Weather and Seasons, and of the Prevailing Diseases in Dublin; Robinson and Roberts: London, UK, 1770. [Google Scholar]
- Raoult, D.; Dutour, O.; Houhamdi, L.; Jankauskas, R.; Fournier, P.E.; Ardagna, Y.; Drancourt, M.; Signoli, M.; La, V.D.; Macia, Y.; et al. Evidence for louse-transmitted diseases in soldiers of Napoleon’s Grand Army in Vilnius. J. Infect. Dis. 2006, 193, 112–120. [Google Scholar] [CrossRef]
- Craige, D. Notice of a febrile disorder which has prevailed at Edinburgh during the Summer of 1843. Edinb. Med. Surg. J. 1843, 60, 410–418. [Google Scholar]
- Southern, P.M.; Sanford, J.P. Relapsing fever: A clinical and microbiological review. Medicine 1969, 48, 129–149. [Google Scholar] [CrossRef]
- Wright, D.J.; Maria, B. Ich bin ein Berliner*. Clin. Microbiol. Infect. 2011, 17, 484–486. [Google Scholar] [CrossRef]
- Dschunkowsky, D. Das Rückfallfieber in Persien. Dtsch. Med. Wochenschr. 1913, 39, 419–420. [Google Scholar] [CrossRef][Green Version]
- Weldon, E.D. World Distribution of Spirochetal Diseases. 2, Relapsing Fevers, Louse-Borne and Tick-Borne; American Geographical Society of New York: New York, NY, USA, 1955. [Google Scholar]
- Elbir, H.; Gimenez, G.; Sokhna, C.; Bilcha, K.D.; Ali, J.; Barker, S.C.; Cutler, S.J.; Raoult, D.; Drancourt, M. Multispacer sequence typing relapsing fever Borreliae in Africa. PLoS Negl. Trop. Dis. 2012, 6, e1652. [Google Scholar] [CrossRef]
- Dworkin, M.S.; Anderson, D.E., Jr.; Schwan, T.G.; Shoemaker, P.C.; Banerjee, S.N.; Kassen, B.O.; Burgdorfer, W. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin. Infect. Dis. 1998, 26, 122–131. [Google Scholar] [CrossRef]
- Elbir, H.; Raoult, D.; Drancourt, M. Relapsing fever borreliae in Africa. Am. J. Trop. Med. Hyg. 2013, 89, 288–292. [Google Scholar] [CrossRef]
- Margos, G.; Vollmer, S.A.; Ogden, N.H.; Fish, D. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect. Genet. Evol. 2011, 11, 1545–1563. [Google Scholar] [CrossRef]
- Schotthoefer, A.M.; Frost, H.M. Ecology and Epidemiology of Lyme Borreliosis. Clin. Lab. Med. 2015, 35, 723–743. [Google Scholar] [CrossRef]
- Pritt, B.S.; Respicio-Kingry, L.B.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; Bjork, J.; Liu, G.; Kingry, L.C.; Mead, P.S.; Neitzel, D.F.; et al. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int. J. Syst. Evol. Microbiol. 2016, 66, 4878–4880. [Google Scholar] [CrossRef]
- Talagrand-Reboul, E.; Boyer, P.H.; Bergstrom, S.; Vial, L.; Boulanger, N. Relapsing Fevers: Neglected Tick-Borne Diseases. Front. Cell Infect. Microbiol. 2018, 8, 98. [Google Scholar] [CrossRef]
- Lopez, J.E.; Krishnavahjala, A.; Garcia, M.N.; Bermudez, S. Tick-Borne relapsing fever spirochetes in the Americas. Vet. Sci. 2016, 3, 16. [Google Scholar] [CrossRef]
- Felsenfeld, O. Chapter IV Borrelia. In Methods in Microbiology; Norris, J.R., Ribbons, D.W., Eds.; Academic Press: Cambridge, MA, USA, 1973; Volume 8, pp. 75–94. [Google Scholar]
- Ribeiro, J.C.; Endris, T.M.; Endris, R. Saliva of the soft tick, ornithodoros moubata, contains anti-platelet and apyrase activities. Comp. Biochem. Physiol.—Part A Physiol. 1991, 100, 109–112. [Google Scholar] [CrossRef]
- Oleaga, A.; Escudero-Poblacion, A.; Camafeita, E.; Perez-Sanchez, R. A proteomic approach to the identification of salivary proteins from the argasid ticks Ornithodoros moubata and Ornithodoros erraticus. Insect. Biochem. Mol. Biol. 2007, 37, 1149–1159. [Google Scholar] [CrossRef]
- Francischetti, I.M.; Sa-Nunes, A.; Mans, B.J.; Santos, I.M.; Ribeiro, J.M. The role of saliva in tick feeding. Front. Biosci. (Landmark Ed. India) 2009, 14, 2051–2088. [Google Scholar] [CrossRef]
- Trape, J.F.; Diatta, G.; Arnathau, C.; Bitam, I.; Sarih, M.; Belghyti, D.; Bouattour, A.; Elguero, E.; Vial, L.; Mane, Y.; et al. The epidemiology and geographic distribution of relapsing fever borreliosis in West and North Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida). PLoS ONE 2013, 8, e78473. [Google Scholar] [CrossRef]
- Rebaudet, S.; Parola, P. Epidemiology of relapsing fever borreliosis in Europe. FEMS Immunol. Med. Microbiol. 2006, 48, 11–15. [Google Scholar] [CrossRef]
- Assous, M.V.; Wilamowski, A. Relapsing fever borreliosis in Eurasia--forgotten, but certainly not gone! Clin. Microbiol. Infect. 2009, 15, 407–414. [Google Scholar] [CrossRef]
- Safdie, G.; Farrah, I.Y.; Yahia, R.; Marva, E.; Wilamowski, A.; Sawalha, S.S.; Wald, N.; Schmiedel, J.; Moter, A.; Gobel, U.B.; et al. Molecular characterization of Borrelia persica, the agent of tick borne relapsing fever in Israel and the Palestinian Authority. PLoS ONE 2010, 5, e14105. [Google Scholar] [CrossRef]
- Palma, M.; Lopes de Carvalho, I.; Osorio, H.; Ze-Ze, L.; Cutler, S.J.; Nuncio, M.S. Portuguese hosts for Ornithodoros erraticus ticks. Vector Borne Zoonotic Dis. 2013, 13, 775–777. [Google Scholar] [CrossRef]
- Palma, M.; Lopes de Carvalho, I.; Figueiredo, M.; Amaro, F.; Boinas, F.; Cutler, S.J.; Nuncio, M.S. Borrelia hispanica in Ornithodoros erraticus, Portugal. Clin. Mictobiol. Infect. 2012, 18, 696–701. [Google Scholar] [CrossRef]
- Pineda Cantero, A.; Perez de Pedro, I.; Martin Tellez, S.; Costo Muriel, C.; Caballero Martinez, L.F.; Gomez Huelgas, R. Borrelia hispanica as a cause of recurrent fever. Med. Clin. (Barc.) 2020, 154, 380. [Google Scholar] [CrossRef]
- Wyplosz, B.; Mihaila-Amrouche, L.; Baixench, M.T.; Bigel, M.L.; Berardi-Grassias, L.; Fontaine, C.; Hornstein, M.; Izri, A.; Baranton, G.; Postic, D. Imported tickborne relapsing fever, France. Emerg. Infect. Dis. 2005, 11, 1801–1803. [Google Scholar] [CrossRef]
- Heida, J.; Van Arkel, A.; Verweij, J.J.; Tijssen, C.C. Meningitis due to infection with Borrelia hispanica. Ned. Tijdschr. Voor Geneeskd. 2019, 163, D3859. [Google Scholar]
- Anda, P.; Sanchez-Yebra, W.; del Mar Vitutia, M.; Perez Pastrana, E.; Rodriguez, I.; Miller, N.S.; Backenson, P.B.; Benach, J.L. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet 1996, 348, 162–165. [Google Scholar] [CrossRef]
- Filatov, S.; Krishnavajhala, A.; Armstrong, B.A.; Kneubehl, A.R.; Nieto, N.C.; Perez De Leon, A.A.; Lopez, J.E. Isolation and Molecular Characterization of Tick-Borne Relapsing Fever Borrelia Infecting Ornithodoros (Pavlovskyella) verrucosus Ticks Collected in Ukraine. J. Infect. Dis. 2020, 221, 804–811. [Google Scholar] [CrossRef]
- Goubau, P.F. Relapsing fevers. A review. Ann. Soc. Belg. Med. Trop. 1984, 64, 335–364. [Google Scholar] [PubMed]
- Moradi-Asl, E.; Jafari, S. The habitat suitability model for the potential distribution of Ornithodoros tholozani (Laboulbene et Megnin, 1882) and Ornithodoros lahorensis (Neumann, 1908) (Acari: Argasidae): The main vectors of tick-borne relapsing fever in Iran. Ann. Parasitol. 2020, 66, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Karimi, Y.; Hovind-Hougen, K.; Birch-Andersen, A.; Asmar, M. Borrelia persica and B. baltazardi sp. nov.: Experimental pathogenicity for some animals and comparison of the ultrastructure. Annales de Microbiologie 1979, 130 B, 157-168. Ann. Microbiol. 1979, 130 B, 157–168. [Google Scholar]
- Kandelaki, S.P. On the Relapsing fever transmitted by ticks in Transcaucasia. Med. Parasitol. 1935, 4, 65–66. [Google Scholar]
- Souidi, Y.; Boudebouch, N.; Ezikouri, S.; Belghyti, D.; Trape, J.F.; Sarih, M. Borrelia crocidurae in Ornithodoros ticks from northwestern Morocco: A range extension in relation to climatic change? J. Vector. Ecol. 2014, 39, 316–320. [Google Scholar] [CrossRef]
- van Holten, J.; Tiems, J.; Jongen, V.H. Neonatal Borrelia duttoni infection: A report of three cases. Trop. Doct. 1997, 27, 115–116. [Google Scholar] [CrossRef]
- Obolo-Mvoulouga, P.; Oleaga, A.; Manzano-Roman, R.; Perez-Sanchez, R. Evaluation of the protective efficacy of Ornithodoros moubata midgut membrane antigens selected using omics and in silico prediction algorithms. Ticks Tick Borne Dis. 2018, 9, 1158–1172. [Google Scholar] [CrossRef]
- Heisch, R.B. On a spirochaete isolated from ornithodoros graingeri. Parasitology 1953, 43, 133–135. [Google Scholar] [CrossRef]
- Garnham, P.C. A new blood spirochaete in the grivet monkey; Ceropithecus aethiops. East Afr. Med. J. 1947, 24, 47–51. [Google Scholar]
- Cutler, S.J.; Idris, J.M.; Ahmed, A.O.; Elelu, N. Ornithodoros savignyi, the Tick Vector of “Candidatus Borrelia kalaharica” in Nigeria. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Baltazard, M.; Pournaki, R.; Bahmanyar, M.; Chamsa, M. Ornithodorus tartakovskyi Olenev 1931 and Borrelia (Spirochaeta) latychevii Sofiev 1941; supplementary note. Ann. Parasitol. Hum. Comp. 1955, 30, 225–242. [Google Scholar] [CrossRef]
- Davis, G.E. A relapsing fever spirochete, Borrelia mazzottii (sp. nov.) from Ornithodoros talaje from Mexico. Am. J. Hyg. 1956, 63, 13–17. [Google Scholar] [CrossRef]
- Diatta, G.; Souidi, Y.; Granjon, L.; Arnathau, C.; Durand, P.; Chauvancy, G.; Mane, Y.; Sarih, M.; Belghyti, D.; Renaud, F.; et al. Epidemiology of tick-borne borreliosis in Morocco. PLoS Negl. Trop. Dis. 2012, 6, e1810. [Google Scholar] [CrossRef]
- Assmar, M.; Soleimani, M.; Oreiz, F.; Piazak, N.; Hossini, S.M.; Saghiri, R.; Zamani, Z. Purification of periplasmic flagellar antigen from Borrelia microtti. Scand. J. Infect. Dis. 2002, 34, 267–272. [Google Scholar] [CrossRef]
- Colin de Verdiere, N.; Hamane, S.; Assous, M.V.; Sertour, N.; Ferquel, E.; Cornet, M. Tickborne relapsing fever caused by Borrelia persica, Uzbekistan and Tajikistan. Emerg. Infect. Dis. 2011, 17, 1325–1327. [Google Scholar] [CrossRef]
- Baneth, G.; Nachum-Biala, Y.; Halperin, T.; Hershko, Y.; Kleinerman, G.; Anug, Y.; Abdeen, Z.; Lavy, E.; Aroch, I.; Straubinger, R.K. Borrelia persica infection in dogs and cats: Clinical manifestations, clinicopathological findings and genetic characterization. Parasites Vectors 2016, 9, 244. [Google Scholar] [CrossRef]
- Geigy, R.; Aeschlimann, A. Comparative Study of the Biology of Borrelia duttoni and Borrelia tillae. Rev. Suisse Zool. 1965, 72, 87–98. [Google Scholar] [CrossRef]
- Fingerle, V.; Pritsch, M.; Wachtler, M.; Margos, G.; Ruske, S.; Jung, J.; Loscher, T.; Wendtner, C.; Wieser, A. “Candidatus Borrelia kalaharica” Detected from a Febrile Traveller Returning to Germany from Vacation in Southern Africa. PLoS Negl. Trop. Dis. 2016, 10, e0004559. [Google Scholar] [CrossRef]
- Morel, P.-C. Les Tiques d’Afrique et du Bassin Méditerranéen; CIRAD-EMVT: Montpellier, France, 2003. [Google Scholar]
- Reck, J.; Marks, F.S.; Guimaraes, J.A.; Termignoni, C.; Martins, J.R. Epidemiology of Ornithodoros brasiliensis (mouro tick) in the southern Brazilian highlands and the description of human and animal retrospective cases of tick parasitism. Ticks Tick Borne Dis. 2013, 4, 101–109. [Google Scholar] [CrossRef]
- Gill, J.S.; Ullmann, A.J.; Loftis, A.D.; Schwan, T.G.; Raffel, S.J.; Schrumpf, M.E.; Piesman, J. Novel relapsing fever spirochete in bat tick. Emerg. Infect. Dis. 2008, 14, 522–523. [Google Scholar] [CrossRef]
- Kingry, L.C.; Anacker, M.; Pritt, B.; Bjork, J.; Respicio-Kingry, L.; Liu, G.; Sheldon, S.; Boxrud, D.; Strain, A.; Oatman, S.; et al. Surveillance for and Discovery of Borrelia Species in US Patients Suspected of Tickborne Illness. Clin. Infect. Dis. 2018, 66, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Schwan, T.G.; Raffel, S.J.; Schrumpf, M.E.; Gill, J.S.; Piesman, J. Characterization of a novel relapsing fever spirochete in the midgut, coxal fluid, and salivary glands of the bat tick Carios kelleyi. Vector Borne Zoonotic Dis. 2009, 9, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.C.; Burgdorfer, W.; Lane, R.S. Borrelia coriaceae sp. nov.: Putative agent of epizootic bovine abortion. Int. J. Syst. Bacteriol. 1987, 37, 72–74. [Google Scholar] [CrossRef]
- Guzman-Cornejo, C.; Herrera-Mares, A.; Robbins, R.G.; Rebollo-Hernandez, A. The soft ticks (Parasitiformes: Ixodida: Argasidae) of Mexico: Species, hosts, and geographical distribution. Zootaxa 2019, 4623, 485–525. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.; Artsob, H.; Margos, G.; Tsao, J.I. Other Tick-borne Bacterial Diseases (including Lyme disease, relapsing feverand Tularemia). In Biology of Ticks; Sonenshine, D., Roe, M.R., Eds.; Oxford University Press: New York, NY, USA, 2014; Volume 2, pp. 278–312. [Google Scholar]
- Davis, G.E. Species unity or plurality of the relapsing fever spirochetes. In A Symposium on Relapsing Fever in Americas; Moulton, F.R., Ed.; American Association for the Advancement of Science: Washington, DC, USA, 1942; pp. 41–47. [Google Scholar]
- Gage, K.L.; Eggleston, M.E.; Gilmore, R.D., Jr.; Dolan, M.C.; Montenieri, J.A.; Tanda, D.T.; Piesman, J. Isolation and characterization of Borrelia parkeri in Ornithodoros parkeri (Ixodida: Argasidae) collected in Colorado. J. Med. Entomol. 2001, 38, 665–674. [Google Scholar] [CrossRef]
- Hussain-Yusuf, H.; Stenos, J.; Vincent, G.; Shima, A.; Abell, S.; Preece, N.D.; Tadepalli, M.; Hii, S.F.; Bowie, N.; Mitram, K.; et al. Screening for Rickettsia, Coxiella and Borrelia Species in Ticks from Queensland, Australia. Pathogens 2020, 9, 1016. [Google Scholar] [CrossRef]
- Donaldson, T.G.; Perez de Leon, A.A.; Li, A.Y.; Castro-Arellano, I.; Wozniak, E.; Boyle, W.K.; Hargrove, R.; Wilder, H.K.; Kim, H.J.; Teel, P.D.; et al. Assessment of the Geographic Distribution of Ornithodoros turicata (Argasidae): Climate Variation and Host Diversity. PLoS Negl. Trop. Dis. 2016, 10, e0004383. [Google Scholar] [CrossRef]
- Krishnavajhala, A.; Armstrong, B.A.; Lopez, J.E. Vector Competence of Geographical Populations of Ornithodoros turicata for the Tick-Borne Relapsing Fever Spirochete Borrelia turicatae. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Munoz-Leal, S.; Faccini-Martinez, A.A.; Costa, F.B.; Marcili, A.; Mesquita, E.; Marques, E.P., Jr.; Labruna, M.B. Isolation and molecular characterization of a relapsing fever Borrelia recovered from Ornithodoros rudis in Brazil. Ticks Tick Borne Dis 2018, 9, 864–871. [Google Scholar] [CrossRef]
- Nadolny, R.M.; Kennedy, A.C.; Rodgers, J.M.; Vincent, Z.T.; Cornman, H.; Haynes, S.A.; Casal, C.; Robbins, R.G.; Richards, A.L.; Jiang, J.; et al. Carios kelleyi (Acari: Ixodida: Argasidae) Infected With Rickettsial Agents Documented Infesting Housing in Kansas, United States. J. Med. Entomol. 2021, tjab069. [Google Scholar] [CrossRef]
- Li, Z.M.; Xiao, X.; Zhou, C.M.; Liu, J.X.; Gu, X.L.; Fang, L.Z.; Liu, B.Y.; Wang, L.R.; Yu, X.J.; Han, H.J. Human-pathogenic relapsing fever Borrelia found in bats from Central China phylogenetically clustered together with relapsing fever borreliae reported in the New World. PLoS Negl. Trop. Dis. 2021, 15, e0009113. [Google Scholar] [CrossRef]
- Fritz, C.L.; Payne, J.R.; Schwan, T.G. Serologic evidence for Borrelia hermsii infection in rodents on federally owned recreational areas in California. Vector Borne Zoonotic Dis. 2013, 13, 376–381. [Google Scholar] [CrossRef]
- Brumpt, E.; Mazzotti, L.; Brumpt, L.C. Étude épidémiologique de la fièvre récurrente endémique des hauts plateaux mexicains. Ann. Parasitol. Hum. Comp. 1939, 17, 275–286. [Google Scholar] [CrossRef]
- Colunga-Salas, P.; Sanchez-Montes, S.; Volkow, P.; Ruiz-Remigio, A.; Becker, I. Lyme disease and relapsing fever in Mexico: An overview of human and wildlife infections. PLoS ONE 2020, 15, e0238496. [Google Scholar] [CrossRef]
- Munoz-Leal, S.; Marcili, A.; Fuentes-Castillo, D.; Ayala, M.; Labruna, M.B. A relapsing fever Borrelia and spotted fever Rickettsia in ticks from an Andean valley, central Chile. Exp. Appl. Acarol. 2019, 78, 403–420. [Google Scholar] [CrossRef]
- van Dam, A.P.; van Gool, T.; Wetsteyn, J.C.; Dankert, J. Tick-borne relapsing fever imported from West Africa: Diagnosis by quantitative buffy coat analysis and in vitro culture of Borrelia crocidurae. J. Clin. Microbiol. 1999, 37, 2027–2030. [Google Scholar] [CrossRef]
- Adeolu, M.; Gupta, R.S. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: The emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 2014, 105, 1049–1072. [Google Scholar] [CrossRef]
- Ras, N.M.; Lascola, B.; Postic, D.; Cutler, S.J.; Rodhain, F.; Baranton, G.; Raoult, D. Phylogenesis of relapsing fever Borrelia spp. Int. J. Syst. Bacteriol. 1996, 46, 859–865. [Google Scholar] [CrossRef]
- Wilder, H.K.; Raffel, S.J.; Barbour, A.G.; Porcella, S.F.; Sturdevant, D.E.; Vaisvil, B.; Kapatral, V.; Schmitt, D.P.; Schwan, T.G.; Lopez, J.E. Transcriptional Profiling the 150 kb Linear Megaplasmid of Borrelia turicatae Suggests a Role in Vector Colonization and Initiating Mammalian Infection. PLoS ONE 2016, 11, e0147707. [Google Scholar] [CrossRef]
- Boyle, W.K.; Wilder, H.K.; Lawrence, A.M.; Lopez, J.E. Transmission dynamics of Borrelia turicatae from the arthropod vector. PLoS Negl. Trop. Dis. 2014, 8, e2767. [Google Scholar] [CrossRef]
- Krishnavajhala, A.; Armstrong, B.A.; Lopez, J.E. The impact of in vitro cultivation on the natural life cycle of the tick-borne relapsing fever spirochete Borrelia turicatae. PLoS ONE 2020, 15, e0239089. [Google Scholar] [CrossRef]
- Barbour, A.G. Antigenic variation of a relapsing fever Borrelia species. Ann. Rev. Microbiol. 1990, 44, 155–171. [Google Scholar] [CrossRef]
- Scott, J.C. Typing African relapsing fever spirochetes. Emerg. Infect. Dis. 2005, 11, 1722–1729. [Google Scholar] [CrossRef]
- Mitani, H.; Talbert, A.; Fukunaga, M. New World relapsing fever Borrelia found in Ornithodoros porcinus ticks in central Tanzania. Microbiol. Immunol. 2004, 48, 501–505. [Google Scholar] [CrossRef]
- Cutler, S.J.; Scott, J.C.; Wright, D.J.M. Phylogenetic origins of Borrelia recurrentis. Int. J. Med. Microbiol. 2008, 298, 193–202. [Google Scholar] [CrossRef]
- Miller, S.C.; Porcella, S.F.; Raffel, S.J.; Schwan, T.G.; Barbour, A.G. Large linear plasmids of Borrelia species that cause relapsing fever. J. Bacteriol. 2013, 195, 3629–3639. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.M.; Casjens, S.; Huang, W.M.; Sutton, G.G.; Clayton, R.; Lathigra, R.; White, O.; Ketchum, K.A.; Dodson, R.; Hickey, E.K.; et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 1997, 390, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Tilly, K.; Elias, A.F.; Errett, J.; Fischer, E.; Iyer, R.; Schwartz, I.; Bono, J.L.; Rosa, P. Genetics and regulation of chitobiose utilization in Borrelia burgdorferi. J. Bacteriol. 2001, 183, 5544–5553. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dobrindt, U.; Hacker, J. Whole genome plasticity in pathogenic bacteria. Curr. Opin. Microbiol. 2001, 4, 550–557. [Google Scholar] [CrossRef]
- Halperin, T.; Orr, N.; Cohen, R.; Hasin, T.; Davidovitch, N.; Klement, E.; Kayouf, R.; Baneth, G.; Cohen, D.; Yavzori, M. Detection of relapsing fever in human blood samples from Israel using PCR targeting the glycerophosphodiester phosphodiesterase (GlpQ) gene. Acta Trop. 2006, 98, 189–195. [Google Scholar] [CrossRef]
- Oshaghi, M.A.; Rafinejad, J.; Choubdar, N.; Piazak, N.; Vatandoost, H.; Telmadarraiy, Z.; Mohtarami, F.; Ravasan, N.M. Discrimination of relapsing fever Borrelia persica and Borrelia microtti by diagnostic species-specific primers and polymerase chain reaction-restriction fragment length polymorphism. Vector Borne Zoonotic Dis. 2011, 11, 201–207. [Google Scholar] [CrossRef]
- Toledo, A.; Anda, P.; Escudero, R.; Larsson, C.; Bergstrom, S.; Benach, J.L. Phylogenetic analysis of a virulent Borrelia species isolated from patients with relapsing fever. J. Clin. Microbiol. 2010, 48, 2484–2489. [Google Scholar] [CrossRef]
- Lopez, J.E.; Wilder, H.K.; Hargrove, R.; Brooks, C.P.; Peterson, K.E.; Beare, P.A.; Sturdevant, D.E.; Nagarajan, V.; Raffel, S.J.; Schwan, T.G. Development of genetic system to inactivate a Borrelia turicatae surface protein selectively produced within the salivary glands of the arthropod vector. PLoS Negl. Trop. Dis. 2013, 7, e2514. [Google Scholar] [CrossRef]
- Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Chersi, K.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology 2021, 10, 1036. [Google Scholar] [CrossRef]
- Trevisan, G.; Bonin, S.; Ruscio, M. A Practical Approach to the Diagnosis of Lyme Borreliosis: From Clinical Heterogeneity to Laboratory Methods. Front. Med. (Lausanne) 2020, 7, 265. [Google Scholar] [CrossRef]
- Montgomery, R.R.; Lusitani, D.; De Boisfleury Chevance, A.; Malawista, S.E. Tick saliva reduces adherence and area of human neutrophils. Infect. Immun. 2004, 72, 2989–2994. [Google Scholar] [CrossRef]
- Nordstrand, A.; Shamaei-Tousi, A.; Ny, A.; Bergstrom, S. Delayed invasion of the kidney and brain by Borrelia crocidurae in plasminogen-deficient mice. Infect. Immun. 2001, 69, 5832–5839. [Google Scholar] [CrossRef]
- Gebbia, J.A.; Coleman, J.L.; Benach, J.L. Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect. Immun. 2001, 69, 456–462. [Google Scholar] [CrossRef]
- Burman, N.; Shamaei-Tousi, A.; Bergstrom, S. The spirochete Borrelia crocidurae causes erythrocyte rosetting during relapsing fever. Infect. Immun. 1998, 66, 815–819. [Google Scholar] [CrossRef]
- Pettersson, J.; Schrumpf, M.E.; Raffel, S.J.; Porcella, S.F.; Guyard, C.; Lawrence, K.; Gherardini, F.C.; Schwan, T.G. Purine salvage pathways among Borrelia species. Infect. Immun. 2007, 75, 3877–3884. [Google Scholar] [CrossRef]
- Barbour, A.G.; Tessier, S.L.; Stoenner, H.G. Variable major proteins of Borrellia hermsii. J. Exp. Med. 1982, 156, 1312–1324. [Google Scholar] [CrossRef]
- Alugupalli, K.R.; Gerstein, R.M.; Chen, J.; Szomolanyi-Tsuda, E.; Woodland, R.T.; Leong, J.M. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 2003, 170, 3819–3827. [Google Scholar] [CrossRef]
- Hinnebusch, B.J.; Barbour, A.G.; Restrepo, B.I.; Schwan, T.G. Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect. Immun. 1998, 66, 432–440. [Google Scholar] [CrossRef]
- Raffel, S.J.; Battisti, J.M.; Fischer, R.J.; Schwan, T.G. Inactivation of genes for antigenic variation in the relapsing fever spirochete Borrelia hermsii reduces infectivity in mice and transmission by ticks. PLoS Pathog. 2014, 10, e1004056. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Restrepo, B.I.; Porcella, S.F.; Raffel, S.J.; Schwan, T.G.; Barbour, A.G. Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Mol. Microbiol. 2006, 60, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Audic, S.; Robert, C.; Nguyen, T.T.; Blanc, G.; Cutler, S.J.; Wincker, P.; Couloux, A.; Claverie, J.M.; Raoult, D.; et al. The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PLoS Genet. 2008, 4, e1000185. [Google Scholar] [CrossRef] [PubMed]
- Ras, N.M.; Postic, D.; Ave, P.; Huerre, M.; Baranton, G. Antigenic variation of Borrelia turicatae Vsp surface lipoproteins occurs in vitro and generates novel serotypes. Res. Microbiol. 2000, 151, 5–12. [Google Scholar] [CrossRef]
- Zuckert, W.R.; Kerentseva, T.A.; Lawson, C.L.; Barbour, A.G. Structural conservation of neurotropism-associated VspA within the variable Borrelia Vsp-OspC lipoprotein family. J. Biol. Chem. 2001, 276, 457–463. [Google Scholar] [CrossRef]
- Crowder, C.D.; Ghalyanchi Langeroudi, A.; Shojaee Estabragh, A.; Lewis, E.R.G.; Marcsisin, R.A.; Barbour, A.G. Pathogen and Host Response Dynamics in a Mouse Model of Borrelia hermsii Relapsing Fever. Vet. Sci. 2016, 3, 19. [Google Scholar] [CrossRef]
- Cutler, S.J. Relapsing Fever Borreliae: A Global Review. Clin. Lab. Med. 2015, 35, 847–865. [Google Scholar] [CrossRef]
- Cadavid, D.; Barbour, A.G. Neuroborreliosis during relapsing fever: Review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clin. Infect. Dis. 1998, 26, 151–164. [Google Scholar] [CrossRef]
- Oza, S.; Lawn, J.E.; Hogan, D.R.; Mathers, C.; Cousens, S.N. Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: 2000-2013. Bull. World Health Organ. 2015, 93, 19–28. [Google Scholar] [CrossRef]
- Larsson, C.; Andersson, M.; Guo, B.P.; Nordstrand, A.; Hagerstrand, I.; Carlsson, S.; Bergstrom, S. Complications of pregnancy and transplacental transmission of relapsing-fever borreliosis. J. Infect. Dis. 2006, 194, 1367–1374. [Google Scholar] [CrossRef]
- UNICEF. Levels and Trends in Child Mortalit 2017; United Nations Interagency Group for Child Mortality: New York, NY, USA, 2017. [Google Scholar]
- Goubau, P.F.; Munyangeyo, C. Tick-borne relapsing fever and pregnancy. A clinical study in Rwanda. Ann. Soc. Belg. Med. Trop. 1983, 63, 347–355. [Google Scholar]
- Piccione, J.; Levine, G.J.; Duff, C.A.; Kuhlman, G.M.; Scott, K.D.; Esteve-Gassent, M.D. Tick-Borne Relapsing Fever in Dogs. J. Vet. Intern. Med. 2016, 30, 1222–1228. [Google Scholar] [CrossRef]
- Elelu, N. Tick-borne relapsing fever as a potential veterinary medical problem. Vet. Med. Sci. 2018, 4, 271–279. [Google Scholar] [CrossRef]
- Theiler, A. Spirillosis of cattle. J. Comp. Pathol. Ther. 1904, 17, 47–55. [Google Scholar] [CrossRef]
- Barbour, A.G.; Maupin, G.O.; Teltow, G.J.; Carter, C.J.; Piesman, J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: Possible agent of a Lyme disease-like illness. J. Infect. Dis. 1996, 173, 403–409. [Google Scholar] [CrossRef]
- Hue, F.; Ghalyanchi Langeroudi, A.; Barbour, A.G. Chromosome Sequence of Borrelia miyamotoi, an Uncultivable Tick-Borne Agent of Human Infection. Genome Announc. 2013, 1. [Google Scholar] [CrossRef]
- Barbour, A.G. Phylogeny of a relapsing fever Borrelia species transmitted by the hard tick Ixodes scapularis. Infect. Genet. Evol. 2014, 27, 551–558. [Google Scholar] [CrossRef]
- Nakao, R.; Kasama, K.; Boldbaatar, B.; Ogura, Y.; Kawabata, H.; Toyoda, A.; Hayashi, T.; Takano, A.; Maeda, K. The evolution of hard tick-borne relapsing fever borreliae is correlated with vector species rather than geographical distance. BMC Ecol. Evol. 2021, 21, 105. [Google Scholar] [CrossRef]
- Allan, B.F.; Goessling, L.S.; Storch, G.A.; Thach, R.E. Blood meal analysis to identify reservoir hosts for Amblyomma americanum ticks. Emerg. Infect. Dis. 2010, 16, 433–440. [Google Scholar] [CrossRef]
- Moyer, P.L.; Varela, A.S.; Luttrell, M.P.; Moore, V.A.t.; Stallknecht, D.E.; Little, S.E. White-tailed deer (Odocoileus virginianus) develop spirochetemia following experimental infection with Borrelia lonestari. Vet. Microbiol. 2006, 115, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Krishnavajhala, A.; Wilder, H.K.; Boyle, W.K.; Damania, A.; Thornton, J.A.; Perez de Leon, A.A.; Teel, P.D.; Lopez, J.E. Imaging of Borrelia turicatae Producing the Green Fluorescent Protein Reveals Persistent Colonization of the Ornithodoros turicata Midgut and Salivary Glands from Nymphal Acquisition through Transmission. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Gugliotta, J.L.; Goethert, H.K.; Berardi, V.P.; Telford, S.R., 3rd. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N. Engl. J. Med. 2013, 368, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peña, A.; Horak, I.G. The Hard Ticks of the World, 1st ed.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Laveran, A. Sur la spirillose des bovidés. CR Acad. Sci. Paris 1903, 136, 939–941. [Google Scholar]
- Theiler, A.; Bruce, D. Transmission and inoculability of Spirillum theileri (Laveran). Proc. R. Soc. Lond. 1905, 76, 504–506. [Google Scholar] [CrossRef]
- Callow, L.L. Observations on tick-transmitted spirochaetes of cattle in Australia and South Africa. Br. Vet. J. 1967, 123, 492–497. [Google Scholar] [CrossRef]
- Krause, P.J.; Fish, D.; Narasimhan, S.; Barbour, A.G. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect. 2015, 21, 631–639. [Google Scholar] [CrossRef]
- Fukunaga, M.; Takahashi, Y.; Tsuruta, Y.; Matsushita, O.; Ralph, D.; McClelland, M.; Nakao, M. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int. J. Syst. Bacteriol. 1995, 45, 804–810. [Google Scholar] [CrossRef]
- Piesman, J.; Donahue, J.G.; Mather, T.N.; Spielman, A. Transovarially acquired Lyme disease spirochetes (Borrelia burgdorferi) in field-collected larval Ixodes dammini (Acari: Ixodidae). J. Med. Entomol. 1986, 23, 219. [Google Scholar] [CrossRef]
- Rollend, L.; Fish, D.; Childs, J.E. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks Tick Borne Dis. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823. [Google Scholar] [CrossRef]
- Schwan, T.G.; Piesman, J. Vector interactions and molecular adaptations of lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 2002, 8, 115–121. [Google Scholar] [CrossRef]
- Franck, M.; Ghozzi, R.; Pajaud, J.; Lawson-Hogban, N.E.; Mas, M.; Lacout, A.; Perronne, C. Borrelia miyamotoi: 43 Cases Diagnosed in France by Real-Time PCR in Patients With Persistent Polymorphic Signs and Symptoms. Front. Med. (Lausanne) 2020, 7, 55. [Google Scholar] [CrossRef]
- Szekeres, S.; Lakos, A.; Foldvari, G. Borrelia miyamotoi: A recently identified human pathogenic tick-borne relapsing fever spirochete. Orv. Hetil. 2017, 158, 1124–1130. [Google Scholar] [CrossRef]
- Ravagnan, S.; Tomassone, L.; Montarsi, F.; Krawczyk, A.I.; Mastrorilli, E.; Sprong, H.; Milani, A.; Rossi, L.; Capelli, G. First detection of Borrelia miyamotoi in Ixodes ricinus ticks from northern Italy. Parasites Vectors 2018, 11, 130. [Google Scholar] [CrossRef]
- Cutler, S.; Vayssier-Taussat, M.; Estrada-Pena, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. A new Borrelia on the block: Borrelia miyamotoi—A human health risk? Eurosurveillance 2019, 24. [Google Scholar] [CrossRef]
- Magnarelli, L.A.; Anderson, J.F.; Johnson, R.C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J. Infect. Dis. 1987, 156, 183–188. [Google Scholar] [CrossRef]
- Jordan, B.E.; Onks, K.R.; Hamilton, S.W.; Hayslette, S.E.; Wright, S.M. Detection of Borrelia burgdorferi and Borrelia lonestari in birds in Tennessee. J. Med. Entomol. 2009, 46, 131–138. [Google Scholar] [CrossRef]
- Barbour, A.G.; Bunikis, J.; Travinsky, B.; Hoen, A.G.; Diuk-Wasser, M.A.; Fish, D.; Tsao, J.I. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg. 2009, 81, 1120–1131. [Google Scholar] [CrossRef]
- Hamsikova, Z.; Coipan, C.; Mahrikova, L.; Minichova, L.; Sprong, H.; Kazimirova, M. Borrelia miyamotoi and Co-Infection with Borrelia afzelii in Ixodes ricinus Ticks and Rodents from Slovakia. Microb. Ecol. 2017, 73, 1000–1008. [Google Scholar] [CrossRef]
- Wagemakers, A.; Jahfari, S.; de Wever, B.; Spanjaard, L.; Starink, M.V.; de Vries, H.J.C.; Sprong, H.; Hovius, J.W. Borrelia miyamotoi in vectors and hosts in The Netherlands. Ticks Tick Borne Dis. 2017, 8, 370–374. [Google Scholar] [CrossRef]
- Hamer, S.A.; Hickling, G.J.; Keith, R.; Sidge, J.L.; Walker, E.D.; Tsao, J.I. Associations of passerine birds, rabbits, and ticks with Borrelia miyamotoi and Borrelia andersonii in Michigan, U.S.A. Parasites Vectors 2012, 5, 231. [Google Scholar] [CrossRef]
- Takano, A.; Toyomane, K.; Konnai, S.; Ohashi, K.; Nakao, M.; Ito, T.; Andoh, M.; Maeda, K.; Watarai, M.; Sato, K.; et al. Tick surveillance for relapsing fever spirochete Borrelia miyamotoi in Hokkaido, Japan. PLoS ONE 2014, 9, e104532. [Google Scholar] [CrossRef]
- Crowder, C.D.; Carolan, H.E.; Rounds, M.A.; Honig, V.; Mothes, B.; Haag, H.; Nolte, O.; Luft, B.J.; Grubhoffer, L.; Ecker, D.J.; et al. Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerg. Infect. Dis. 2014, 20, 1678–1682. [Google Scholar] [CrossRef]
- Geller, J.; Nazarova, L.; Katargina, O.; Jarvekulg, L.; Fomenko, N.; Golovljova, I. Detection and genetic characterization of relapsing fever spirochete Borrelia miyamotoi in Estonian ticks. PLoS ONE 2012, 7, e51914. [Google Scholar] [CrossRef]
- Wagemakers, A.; Koetsveld, J.; Narasimhan, S.; Wickel, M.; Deponte, K.; Bleijlevens, B.; Jahfari, S.; Sprong, H.; Karan, L.S.; Sarksyan, D.S.; et al. Variable Major Proteins as Targets for Specific Antibodies against Borrelia miyamotoi. J. Immunol. 2016, 196, 4185–4195. [Google Scholar] [CrossRef]
- Koetsveld, J.; Kolyasnikova, N.M.; Wagemakers, A.; Toporkova, M.G.; Sarksyan, D.S.; Oei, A.; Platonov, A.E.; Hovius, J.W. Development and optimization of an in vitro cultivation protocol allows for isolation of Borrelia miyamotoi from patients with hard tick-borne relapsing fever. Clin. Microbiol. Infect. 2017, 23, 480–484. [Google Scholar] [CrossRef]
- James, A.M.; Liveris, D.; Wormser, G.P.; Schwartz, I.; Montecalvo, M.A.; Johnson, B.J. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J. Infect. Dis. 2001, 183, 1810–1814. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.J.; Grigery, C.N.; Masters, R.W. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect. Dis. Clin. N. Am. 2008, 22, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.; Varela-Stokes, A.S. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet. Parasitol. 2009, 160, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Mackie, F.P. The Part Played by Pediculus Corporis in the Transmission of Relapsing Fever. Br. Med. J. 1907, 2, 1706–1709. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoch, M.; Wieser, A.; Loscher, T.; Margos, G.; Purner, F.; Zuhl, J.; Seilmaier, M.; Balzer, L.; Guggemos, W.; Rack-Hoch, A.; et al. Louse-borne relapsing fever (Borrelia recurrentis) diagnosed in 15 refugees from northeast Africa: Epidemiology and preventive control measures, Bavaria, Germany, July to October 2015. Eurosurveillance 2015, 20. [Google Scholar] [CrossRef]
- Antinori, S.; Mediannikov, O.; Corbellino, M.; Raoult, D. Louse-borne relapsing fever among East African refugees in Europe. Travel Med. Infect. Dis. 2016, 14, 110–114. [Google Scholar] [CrossRef]
- Amanzougaghene, N.; Akiana, J.; Mongo Ndombe, G.; Davoust, B.; Nsana, N.S.; Parra, H.J.; Fenollar, F.; Raoult, D.; Mediannikov, O. Head Lice of Pygmies Reveal the Presence of Relapsing Fever Borreliae in the Republic of Congo. PLoS Negl. Trop. Dis. 2016, 10, e0005142. [Google Scholar] [CrossRef]
- Cutler, S.J.; Moss, J.; Fukunaga, M.; Wright, D.J.; Fekade, D.; Warrell, D. Borrelia recurrentis characterization and comparison with relapsing-fever, Lyme-associated, and other Borrelia spp. Int. J. Syst. Bacteriol. 1997, 47, 958–968. [Google Scholar] [CrossRef]
- Cutler, S.J.; Fekade, D.; Hussein, K.; Knox, K.A.; Melka, A.; Cann, K.; Emilianus, A.R.; Warrell, D.A.; Wright, D.J. Successful in-vitro cultivation of Borrelia recurrentis. Lancet 1994, 343, 242. [Google Scholar] [CrossRef]
- Marosevic, D.; Margos, G.; Wallich, R.; Wieser, A.; Sing, A.; Fingerle, V. First insights in the variability of Borrelia recurrentis genomes. PLoS Negl. Trop. Dis. 2017, 11, e0005865. [Google Scholar] [CrossRef]
- Ruzic-Sabljic, E.; Maraspin, V.; Stupica, D.; Rojko, T.; Bogovic, P.; Strle, F.; Cerar, T. Comparison of MKP and BSK-H media for the cultivation and isolation of Borrelia burgdorferi sensu lato. PLoS ONE 2017, 12, e0171622. [Google Scholar] [CrossRef]
- Guellil, M.; Kersten, O.; Namouchi, A.; Bauer, E.L.; Derrick, M.; Jensen, A.O.; Stenseth, N.C.; Bramanti, B. Genomic blueprint of a relapsing fever pathogen in 15th century Scandinavia. Proc. Natl. Acad. Sci. USA 2018, 115, 10422–10427. [Google Scholar] [CrossRef]
- Salih, S.Y.; Mustafa, D.; Abdel Wahab, S.M.; Ahmed, M.A.; Omer, A. Louse-borne relapsing fever: I. A clinical and laboratory study of 363 cases in the Sudan. Trans. R. Soc. Trop. Med. Hyg. 1977, 71, 43–48. [Google Scholar] [CrossRef]
- Bryceson, A.D.; Parry, E.H.; Perine, P.L.; Warrell, D.A.; Vukotich, D.; Leithead, C.S. Louse-borne relapsing fever. Q. J. Med. 1970, 39, 129–170. [Google Scholar]
- Vidal, V.; Scragg, I.G.; Cutler, S.J.; Rockett, K.A.; Fekade, D.; Warrell, D.A.; Wright, D.J.; Kwiatkowski, D. Variable major lipoprotein is a principal TNF-inducing factor of louse-borne relapsing fever. Nat. Med. 1998, 4, 1416–1420. [Google Scholar] [CrossRef]
- Sakharoff, M.N. Spirochaeta anserina et la septicémie des oies. Ann. Inst. Pasteur Paris 1891, 564–566. [Google Scholar]
- Hovind-Hougen, K. A morphological characterization of Borrelia anserina. Microbiology (Reading) 1995, 141 Pt 1, 79–83. [Google Scholar] [CrossRef][Green Version]
- Thomas, N.J.; Bunikis, J.; Barbour, A.G.; Wolcott, M.J. Fatal spirochetosis due to a relapsing fever-like Borrelia sp. in a northern spotted owl. J. Wildl. Dis. 2002, 38, 187–193. [Google Scholar] [CrossRef]
- DaMassa, A.J.; Adler, H.E. Avian spirochetosis: Natural transmission by Argas (Persicargas) sanchezi (Ixodoidea: Argasidae) and existence of different serologic and immunologic types of Borrelia anserina in the United States. Am. J. Vet. Res. 1979, 40, 154–157. [Google Scholar]
- McNeil, E.; Hinshaw, W.R.; Kissling, R.E. A Study of Borrelia Anserina Infection (Spirochetosis) in Turkeys. J. Bacteriol. 1949, 57, 191–206. [Google Scholar] [CrossRef]
- Reeves, W.K.; Loftis, A.D.; Sanders, F.; Spinks, M.D.; Wills, W.; Denison, A.M.; Dasch, G.A. Borrelia, Coxiella, and Rickettsia in Carios capensis (Acari: Argasidae) from a brown pelican (Pelecanus occidentalis) rookery in South Carolina, USA. Exp. Appl. Acarol. 2006, 39, 321–329. [Google Scholar] [CrossRef]
- Han, S.W.; Chae, J.B.; Jo, Y.S.; Cho, Y.K.; Kang, J.G.; Shin, N.S.; Youn, H.J.; Youn, H.Y.; Nam, H.M.; Kim, H.J.; et al. First detection of Borrelia and Rickettsia species from Ornithodoros ticks in the Republic of Korea. Ticks Tick Borne Dis. 2021, 12, 101689. [Google Scholar] [CrossRef]
- Ferdows, M.S.; Serwer, P.; Griess, G.A.; Norris, S.J.; Barbour, A.G. Conversion of a linear to a circular plasmid in the relapsing fever agent Borrelia hermsii. J. Bacteriol. 1996, 178, 793–800. [Google Scholar] [CrossRef]
- Elbir, H.; Sitlani, P.; Bergstrom, S.; Barbour, A.G. Chromosome and Megaplasmid Sequences of Borrelia anserina (Sakharoff 1891), the Agent of Avian Spirochetosis and Type Species of the Genus. Genome Announc. 2017, 5. [Google Scholar] [CrossRef]
- Lin, T.; Gao, L.; Seyfang, A.; Oliver, J.H. ‘Candidatus Borrelia texasensis’, from the American dog tick Dermacentor variabilis. Int. J. Syst. Evol. Microbiol. 2005, 55, 685–693. [Google Scholar] [CrossRef]
- Mediannikov, O.; Abdissa, A.; Socolovschi, C.; Diatta, G.; Trape, J.F.; Raoult, D. Detection of a new Borrelia species in ticks taken from cattle in Southwest Ethiopia. Vector Borne Zoonotic Dis. 2013, 13, 266–269. [Google Scholar] [CrossRef]
- Ehounoud, C.B.; Yao, K.P.; Dahmani, M.; Achi, Y.L.; Amanzougaghene, N.; Kacou N’Douba, A.; N’Guessan, J.D.; Raoult, D.; Fenollar, F.; Mediannikov, O. Multiple Pathogens Including Potential New Species in Tick Vectors in Cote d’Ivoire. PLoS Negl. Trop. Dis. 2016, 10, e0004367. [Google Scholar] [CrossRef]
- Kumsa, B.; Socolovschi, C.; Raoult, D.; Parola, P. New Borrelia species detected in ixodid ticks in Oromia, Ethiopia. Ticks Tick Borne Dis 2015, 6, 401–407. [Google Scholar] [CrossRef]
- Evans, N.J.; Bown, K.; Timofte, D.; Simpson, V.R.; Birtles, R.J. Fatal borreliosis in bat caused by relapsing fever spirochete, United Kingdom. Emerg. Infect. Dis. 2009, 15, 1331–1333. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).