Recent Developments in Diagnosis of Epilepsy: Scope of MicroRNA and Technological Advancements
Abstract
Simple Summary
Abstract
1. Introduction
2. Diagnostic Techniques
2.1. Electroencephalogram (EEG)
2.2. Computed Tomography (CT SCAN)
2.3. Magnetic Resonance Imaging (MRI)
2.4. Positron Emission Tomography (PET)
2.5. Single Photon Emission Tomography (SPECT)
3. Recent Developments in PET Radiotracers
3.1. Translocator Protein (TSPO)—PET
3.2. Flumazenil PET
3.3. 11C-Verapamil PET
3.4. 11C-α-Methyl-L-Tryptophan (AMT) PET
3.5. 5-HT1A Receptor Ligands and Serotonin Transporter(5-HTT)-Based PET
3.6. Dopamine-Based PET
3.7. Cannabinoid-Based PET
4. Recent Advancements in Diagnostic Techniques
4.1. High-Resolution MRI (HRMRI)
4.2. Diffusion Tensor Imaging (DTI)
4.3. Magnetic Resonance Spectroscopy (MRS)
4.4. Magnetoencephalography (MEG)
| Name of Imaging Technique | Study Population/Disease Model | Indication | Location/Tissue Examined | Effect in Epilepsy | AUC | Ref. |
|---|---|---|---|---|---|---|
| T1 and T2 weighted MRI | 4-AP-induced seizures in rats | Diagnostic biomarker of epilepsy | Brain | In the cerebral cortex, hippocampus, amygdala, and medial thalamus, T2 relaxation time changes (recovered completely after 3 days) | ------- | [3] |
| TSPO-PET | Kainic acid-induced SE in rats | Predictive biomarker of SE and SRS | Brain | Unregulated TSPO expression 14 days post-SE predicts SRS frequency and comorbidities associated with chronic SE | ------- | [52] |
| iECEEG scalp EEG | Epileptic foci vs. other brain areas in humans assessed during epilepsy surgery | Diagnostic biomarker to identify epileptic foci | Brain | Existence of HFOs Conventional HFOs with waveform similarity Spikes | -------- | [89] |
| fMRI | Patients with IGE vs. healthy controls | Diagnostic and predictive biomarker of IGE | Brain | Reduced DNM functional connectivity between anterior and posterior cortical seeds, seizure duration is positively associated with RSFC between Para hippocampal gyri and the PCC and negatively associated with connectivity between PCC and frontal lobe | --------- | [90] |
| TARC/sICAM5 ratio assisted with video-EEG monitoring | Patients with focal epilepsy | Diagnostic biomarker for drug-resistant focal epilepsy | Blood/plasma | Increased TARC/sICAM5 ratio of these two proteins serves a vital role in epileptogenesis, as one is inducer of inflammation and the other inhibits it. | 1.000 | [91] |
| PET | Kainic acid-induced SE in rats | Diagnostic biomarker of SE | Brain | Decreased GABA receptor density and affinity in the hippocampus | --------- | [92] |
| MRI | Hyperthermia-induced SE rats, with or without epilepsy | Diagnostic biomarker of epilepsy or prognostic biomarker of epilepsy development in hyperthermia-induced SE rats | Brain tissue regions– amygdala, thalamus | T2 relaxation time decreased in basolateral and medial amygdala | 0.910 (Basolateral amygdala) 0.820 (Medial amygdala) | [93] |
| FDG-PET | Pilocarpine-induced SE | Diagnostic biomarker of SE | Brain | Decreased glucose metabolism, along with decreased brain connectivity | --------- | [94] |
| PET | Pilocarpine-induced SE in rats | Diagnostic biomarker of SE | Brain | Decrease in global mGluR5 metabotropic glutamate receptor. Decreased focal in amygdala and hippocampus during chronic SE | -------- | [95] |
| MRI | PTZ-induced seizures test in seizure susceptible or non-susceptible rats after TBI induction | Diagnostic biomarker of augmented seizure susceptibility after TBI | Brain tissue–cortex, hippocampus, thalamus | Decreased T2 relaxation time in medial thalamus | 0.780 | [96] |
| Appearance of T1σ in S1 cortex, S1 HC, and Prh cortex | 0.881 (S1 cortex) 0.857 (S1 HC) 0.929 (Prh cortex) | |||||
| Appearance of T2 in the thalamus | 0.893 | |||||
| DTI-MRI | Patients with h benign vs. refractory mTLE (age and sex-harmonized) | Diagnostic biomarker for drug-resistant mTLE | Brain tissue–temporal lobe gray and white matter | Increased ipsilateral MD | 0.670 | [97] |
| Decreased ipsilateral FA | 0.770 | |||||
| Decreased ipsilateral HC volume | 0.670 | |||||
| FDG-PET | Pilocarpine-induced TLE | Prognostic biomarker of TLE | Brain | Decreased glucose metabolism in the hippocampus at the latent phase of the disease and neuronal loss | ------- | [98] |
| DTI/DWI-MRI | Right and left TLE patients vs. healthy individuals | Diagnostic biomarker for TLE | Brain tissue–anterior corpus callosum | Reduced local diffusion homogeneity | RmTLE 0.935 LmTLE 0.919 | [99] |
| MRS | Kainic acid-induced SE and amygdala kindling in rats | Biomarker of epilepsy | Brain | Sodium selenate prevents changes in mIns, NAA levels, volumetric changes, and FA | --------- | [100] |
| T1 and T2 weighted MRI | Lithium–pilocarpine-induced SE in rats | Diagnostic biomarker of SE | Brain | T2 in the amygdala after 30 days of SE induction, strongly correlated with hyperactivity in the novel open field | -------- | [101] |
| T1 and T2 weighted MRI | Kainic acid-induced MTLE in mouse | Diagnostic biomarker of MTLE | Brain | Hippocampal paroxysmal discharges (number and duration) are associated with T2 relaxation time | -------- | [102] |
| Gadolinium-MRI | Patients with epilepsy vs. without epilepsy after TBI | Diagnostic biomarker for PTE in patients with TBI | Cerebral cortex | Area of gadolinium leakage around cortical lesion after TBI | 0.850 | [103] |
| Intracortical EEG | Rats having epilepsy vs. rats deprived of epilepsy after lateral fluid-percussion-induced TBI | Diagnostic biomarker for PTE in rats after lateral fluid-percussion induced TBI | Brain | Incidence of perilesional pHFOs and rHFOSs throughout the first 2 post-TBI weeks only in rats that will grow PTE | --------- | [104] |
| MRS | Pilocarpine-induced SE -P21 rats which develop epilepsy vs. P21 rats which does not develop epilepsy | Diagnostic and prognostic biomarker for epilepsy and SE | Septal pole of the hippocampus | Augmented hippocampal mIns/tCr after 72 days of SE induction | 0.830 | [105] |
| MRS | Pilocarpine-induced SE in rats | Biomarker of SE | Brain | Increased expression of mIns post SE induction | ------- | [105] |
| Combination of EEG and fMRI | Kainic acid-induced SE in Rhesus Monkey | Diagnostic biomarker of SE | Brain | Functional brain network disruption in chronic SE | ------ | [106] |
| MRI | Rats with or deprived of epilepsy after paraoxan-induced SE | Diagnostic and prognostic biomarker for epilepsy and SE | BBB pathology in the piriform network | Amplified T2- weighted signal | 0.720 | [107] |
| HMGB1- acetylated | 65 patients with drug-resistant epilepsy and electrically induced rat SE model | Diagnostic biomarker for drug refractoriness | plasma | Acts on RAGE and TLR and increased expression in epilepsy | 1 | [108] |
| HMGB1- total | 65 patients with drug-resistant epilepsy and electrically induced rat SE model | Diagnostic biomarker for epileptogenesis | plasma | Acts on RAGE and TLR and increased expression in epilepsy | 1 | [108] |
| HMGB1- bisulfide | 65 patients with drug-resistant epilepsy and electrically induced rat SE model | Prognostic biomarker for SE | plasma | Acts on RAGE and TLR and increased expression in epilepsy | 1 | [108] |
| Diffusion MRI | Kainic acid and pilocarpine-induced SE in rats | Diagnostic biomarker of SE | Brain | Longitudinal changes in hippocampal diffusion due to astrocyte processes | ------- | [109] |
| Diffusion MRI | Spontaneous recurrent seizures in cats | Diagnostic biomarker of epilepsy | Brain | Microstructural changes and hypoperfusion in the hippocampus and parietal cortex during ictal periods in cats | ------- | [110] |
| Sleep EEG | Rats having epilepsy vs. rats deprived of epilepsy after lateral fluid-percussion-induced TBI | Diagnostic biomarker for PTE in rats after lateral fluid-percussion-induced TBI | Brain | Decrease of the extend of sleep occurrence of spindles at conversion from N3 to REM | 0.907 | [111] |
| Electrophysiology Interictal VEP-visual hyper excitability assessed using contrast response function | Patients having idiopathic generalized epilepsy vs. healthy individuals | Diagnostic biomarker for idiopathic generalized epilepsy | Brain | Relative lack of gain control at high contrasts | 0.870 | [112] |
| fMRI | 17 patients with drug-resistant TLE with good post-operative seizure control vs. healthy controls | Diagnostic biomarker of TLE | Brain | fALFF reduction in ipsilateral amygdala | ------- | [113] |
| Diffusion MRI | Spontaneous recurrent seizures in cats | Diagnostic biomarker of epilepsy | Brain | Decreased postictal hippocampal perfusion compared to ictal state | ------- | [114] |
| TSPO-PET | Electrically induced SE in rats | Prognostic biomarker of SE | Brain | Unregulated TSPO expression up to 10 weeks after SE induction | ------- | [115] |
| Theta dynamics in EEG | Rats with or without epilepsy after photo thrombotic stroke,Rats with or without epilepsy after bilateral hippocampal electrical stimulation-induced SE | Diagnostic biomarker of epilepsy or prognostic biomarker of epilepsy development in rodents having a brain injury | Brain | Absolute slope value of dynamic change in theta band | 0.910 | [116] |
| PET | Pilocarpine-induced SE in rats | Prognostic and diagnostic biomarker of SE | Brain | 48 h after SE induction, the permeability of BBB gets increased in the hippocampus, piriform cortex, thalamus, and amygdala | ------ | [117] |
| EEG | Patients having acute anterior circulation ischemic stroke, which developed into epilepsy vs. not developed into epilepsy | Diagnostic biomarker of epilepsy or prognostic biomarker of acute anterior circulation ischemic stroke developing into epilepsy | Brain | Background asymmetry, Interictal epileptiform activity | 0.810 | [118] |
| EEG | Two cohort studies with- Patients having idiopathic generalized epilepsy and generalized spike-wave discharges on EEG, who are drug-resistant vs. drug-responsive | Diagnostic biomarker of drug resistance in idiopathic generalized epilepsy | Brain | Appearance of generalized polyspikes (burst of generalized rhythmic spikes lasting less than 1 s) in EEG during sleep | ------ | [119] |
| MEG coupled with behavioral evaluation | Human and animal models TLE | Early diagnostic biomarker of TLE | Brain | Coherence and alteration of theta and gamma rhythms | -------- | [120] |
| EEG | Three different rodent models of epilepsy | Diagnostic and prognostic biomarker of epilepsy | Brain | Decrease in the non-linear dynamics dimension in EEG | ≥0.886 in different models | [121] |
| EEG | A child with type 1 RCDP | Diagnostic biomarker of impending epilepsy | Brain | Transition from normal background to the appearance of focal epileptiform abnormality | ------- | [122] |
| Interictal scalp EEG | 22 children having CSWS | Predictive biomarker of seizures and cognitive outcome in CSWS | Brain | Presence of interictal HFOs around 80–250 Hz range (ripple band) and they are negatively associated with average IQ | ------- | [123] |
| intracranial and scalp EEG | 11 patients (6 M, 5 F; age range 21–41 years) | Diagnostic biomarker of postictal generalized EEG suppression | Brain | Delta- gamma phase-amplitude coupling (gradual decrease of phase-frequency in the coupling between delta, 0.5–4 Hz and gamma, 30+ Hz, followed by an increased coupling between the phase of 0.5–1.5 Hz signal and amplitude of 30–50 Hz signal) | ------- | [124] |
| Scalp EEG | 30 patients, suspected to have infantile spasms | Objective biomarker for active epileptic spasm | Brain | Increased HFO rates and coupling were identified between HFOs and SWA. | 0.80–0.98 | [125] |
| iEEG | 11 patients having TLE (6 M, 5 F) | Diagnostic biomarker of SOZ | Brain | High amplitude of HFOs was observed in SOZs, and measuring the amplitude of HFOs serves a greater advantage over measuring the rate of HFOs, in the case of SOZ identification | 0.948–0.960 | [126] |
| MRS | 35 patients with IGE (avg. age-32) vs. 35 healthy individuals (avg. age-31) | Diagnostic biomarker of IGE | Brain | Upregulated Cr expression in left thalamus (no difference in right thalamus). Downregulated NAA expression in right and left thalamus. Downregulated NAA/Cr ration in right and left thalamus. | ------- | [127] |
| 18F-FDG-PET/rs-fMRI | Patients with mTLE-HS vs. healthy individuals | Biomarker of epilepsy surgery in patients having mTLE-HS | Bain | Positive correlation between SUVR and rs-fMRI metrics, spatial correlation between SUVR and fMRI across the gray matter, and Higher fALFF/SUVR couplings, suggested altered bioenergetic coupling across gray matter and it was also found to be associated with seizure outcome. | ------ | [128] |
| [11 C] UCB-J PET | 12 patients having TLE vs. 12 healthy controls | Diagnostic and predictive biomarker of TLE | Reduced [11 C] UCB-J binding in the seizure onset zone of patients having TLE | [129] | ||
| MRS | Kainic acid-induced MTLE in mouse (KA-MTLE model) | Biomarker of epileptic zone in MTLE | Brain | Upregulated GABA expression in the epileptic zone of mouse | ------ | [130] |
| iEEG | 27 patients with SOZ in different parts of the hemisphere | Predictive biomarker of seizures | Brain | Temporal trends in HFO rates can predict preictal state and can differentiate between preictal and interictal periods for a few patients. | 0.80 | [131] |
4.5. Quantitative Analysis of PET and MRI
4.6. EEG Analyses Methods
4.7. Other Advances in Data Analytical Techniques
5. Limitations of Neuroimaging Techniques
6. Biomarkers Associated with Diagnosis of Epileptogenesis
6.1. miRNAs as Biomarkers of Epileptogenesis
| Biomarker | Description | Role in Epilepsy | Indication | Specimen | Expression | Ref. |
|---|---|---|---|---|---|---|
| miR-34, miR-132, miR-134, miR-181a, miR-199a, miR-210 | miRNAs associated with epilepsy play a vital role in epilepsy by targeting apoptosis, neuronal microstructure, transcriptional regulation, inhibitory neurotransmission, excitatory neurotransmission and by regulating transcription. | Antagomir of them reduces SE and protects the hippocampus | Diagnostic biomarker of SE and possible therapeutic targets of SE treatment | Serum, hippocampus | Increased | [187] |
| miR-128, miR-219, miR-23b, miR-124 | miRNAs associated with epilepsy plays a vital role in epilepsy by targeting apoptosis, neuronal microstructure, transcriptional regulation, inhibitory neurotransmission, excitatory neurotransmission and by regulating transcription. | Agomir reduces SE and protects the hippocampus | Diagnostic biomarker of SE and possible therapeutic targets of SE treatment | Serum, hippocampus | Decreased | [187] |
| miR-134, miR-203 | miRNAs associated with epilepsy plays a vital role in epilepsy by targeting apoptosis, neuronal microstructure, transcriptional regulation, inhibitory neurotransmission, excitatory neurotransmission and by regulating transcription. | Antagomir reduces SRS | Diagnostic biomarker and therapeutic target for SRS | Serum, hippocampus | Increased | [187] |
| miR-22 | miRNAs associated with epilepsy plays a vital role in epilepsy by targeting apoptosis, neuronal microstructure, transcriptional regulation, inhibitory neurotransmission, excitatory neurotransmission and by regulating transcription. | Agomir reduces SRS | Diagnostic biomarker of SRS and possible therapeutic targets of SRS treatment | Serum, hippocampus | Increased | [187] |
| miR-106b-5p | A type of miRNA. MiR-106b-5p is an oncogene to attenuate the tumor suppressor CDKN1A | Inflammation, dysregulation of protein synthesis, and neurodegeneration | Diagnostic biomarker for epilepsy | Serum | Increased | [190] |
| miR-7d-5p | A type of miRNA, represses ER α expression | Inflammation, dysregulation of protein synthesis, and neuro degeneration | Diagnostic biomarker for epilepsy | Serum | Increased | [190] |
| miR-130a-3p | A type of miRNA targets HMGA1 regulation | Inflammation, dysregulation of protein synthesis and neuro degeneration | Diagnostic biomarker for epilepsy | Serum | Increased | [190] |
| miR-146a-5p | miRNA functions as a control switch between angiogenesis and cell death | Inflammation, dysregulation of protein synthesis and neuro degeneration | Diagnostic biomarker for epilepsy | Serum | Increased | [190] |
| miR-15a-5p | miRNA acts as a post-translational modifier of proto-oncogene MYB and can also target VEGF | Regulates: Fibrosis, inflammation, viability, and matrix degeneration | Diagnostic biomarker for epilepsy | Serum | Decreased | [190] |
| miR-194-5p | Tumour suppresser miRNA | Altered Nucleic and cytoplasmic functions, altered metal binding, and motif folding | Diagnostic biomarker for epilepsy | Serum | Decreased | [190] |
| miR-301a-3p | Supresses estrogen signaling, reduces the expression of ERα | Decreases the ESR1 mRNA and modulates inflammation | Diagnostic biomarker for drug-refractory epilepsy | Serum | Decreased | [191] |
| Combination of miR-194p, miR-301a-3p, miR-30b-5p, miR-4446-3p | These are various types of miRNAs affecting inflammation | Dysregulation of protein synthesis | Diagnostic biomarker for drug-refractory epilepsy | Serum | Decreased | [191] |
| miR-19b-3p | miRNA associated with epilepsy | Plays a role in epilepsy by inducing proteins that regulate apoptosis, tissue remodeling, gliosis, and neuroinflammation | Diagnostic biomarker for TLE | CSF | Decreased | [192] |
| miR-451a and miR-21-5p | miRNA associated with epilepsy | Plays a role in epilepsy by inducing proteins that regulate apoptosis, tissue remodeling, gliosis, and neuroinflammation | Diagnostic biomarker for SE | CSF | Increased | [192] |
| miR-134 | miR-134 is a family of MicroRNA precursors found in mammals, including humans | Targets Lim kinase 1, a protein involved in dendritic spine dynamics 22 and doublecortin | Diagnostic biomarker for mTLE | plasma | Decreased | [193] |
| miR-27a-3p, miR-328-3p and miR-654-3p | miRNAs associated with epilepsy | Association with growth factor and apoptosis signaling (p53 pathway) | Diagnostic biomarker of TLE | Blood plasma | Increased | [194] |
| circ-EFCAB2 | Circular RNAs, long noncoding RNAs, acts as templates and regulates transcription | Have a role in epilepsy as they regulate gene expression as microRNA sponges | Diagnostic biomarker and potential treatment target in TLE | Temporal cortices | Increased | [195] |
| circ-DROSHA | Circular RNAs, long noncoding RNAs, acts as templates and regulates transcription | Have a role in epilepsy as they regulate gene expression as microRNA sponges | Diagnostic biomarker and potential treatment target in TLE | Temporal cortices | Decreased | [195] |
| miR-145 | miRNA associated with macrophage differentiation, phagocyte migration, proliferation | Reduced expression in the hippocampus of epileptic brain | Diagnostic biomarker of mTLE-HS | Blood, hippocampus | Decreased (hippocampus), increased (blood) | [196] |
| miR-181c | miRNA targets dopaminergic, serotonergic synapses, and has a role in BBB disruption and breast cancer cell metastasis | Overexpressed in the hippocampal region of mice after epileptic seizures | Diagnostic biomarker of mTLE-HS | Blood, hippocampus | Increased in hippocampus and blood | [196] |
| miR-199a | Especially expressed in the olfactory bulb, cortex, hippocampus, hypothalamus, brain stem, dorsal root ganglia | Over-expression in epileptic brain tissues | Diagnostic biomarker of mTLE-HS | Blood, hippocampus | Increased in hippocampus and blood | [196] |
| miR-1183 | miRNA which was observed to lose its function in breast tumors, and it was observed to be downregulated in Kaposi sarcoma biopsies. Although, upregulation was observed in colorectal tumors and RHD. | It targets genes-CXCR4, EGF, and EGFR. Thus, it is considered a potential biomarker. | Diagnostic biomarker of mTLE-HS | Blood, hippocampus | Increased in hippocampus and blood | [196] |
| miR-106b miR-146a miR-301a | Epilepsy related miRNA | Regulation of TLR, IL-1, receptor associated kinases and TRAF6 | Diagnostic biomarker for epilepsy | Serum | Increased | [197] |
| miR-194-5p, Combination of miR-106b and miR-146a | Epilepsy related miRNA | Inhibits cell proliferation | Diagnostic biomarker for epilepsy | Serum | Decreased | [197] |
| miR-129-2-3p | Non-invasive miRNA biomarker for epilepsy | Repress cell growth, colony formation and targets BCL2L2 | Diagnostic biomarker for drug refractory TLE with FCD | Cortical tissue and plasma | Increased | [198] |
| miR-4521 | Promising Ovel biomarkers | Nucleic and cytoplasmic functions altered, altered metal binding and motif folding | Diagnostic biomarker for FCD with refractory TLE | Cortical brain tissue, serum | Increased | [199] |
| MMP2, MMP3 | MMP2, MMP3 | Epileptic focus formation and stimulation of seizures | Diagnostic biomarker for Epilepsy | brain | Decreased | [200,201] |
| miR-30, miR-378, miR-106b and mir-15a | miRNA associated with epilepsy | Expression is positively associated with seizure frequency, however, miR-30 expression gradual declines with the progression of the disease and negatively affects the CAMK4 expression | Diagnostic and prognostic biomarker of epilepsy | Serum | Increased | [202] |
| miR-211 | miRNA which shifts the threshold for spontaneous and pharmacologically induced seizures | Downregulation induces hyper synchronization and nonconvulsive, convulsive seizures along with alterations in cholinergic and TGFBR2 signaling pathways | Diagnostic biomarker of epilepsy | Forebrain | Decreased | [203] |
| miR-134 | miRNA associated with epilepsy serves an important role in inter-neuronal signaling by targeting dendrites | Upregulation of miR-134 was found in the rodent models of SE. | Diagnostic biomarker of epilepsy | Plasma and CSF | Increased | [204] |
| miR-323a-5p | miRNA associated with epilepsy | Associated with duration of epilepsy and seizure frequency | Diagnostic biomarker of FCD and DRE | Cerebral cortex, blood plasma | Increased | [205] |
| miR-3613-5P miR-4668-5P miR-8071, miR-197-5P | miRNA associated with epilepsy | Inflammation in brain and neuronal tissues | Diagnostic biomarker for mTLE-HS | Plasma exosomes | Decreased | [206] |
| miR-146a and miR-106b | miRNA associated with epilepsy | Important regulators of the innate immune response in the modulation of astrocyte-mediated inflammation | Diagnostic biomarker of childhood epilepsy | Plasma | Increased | [207] |
| miR-106b | Belongs to the miR-17 family and are associated with CVDs and tumors | Highly expressed in the serum of epilepsy patients | Diagnostic and prognostic biomarker of epilepsy in children | Serum | Increased | [208] |
| miR-15a-5p | miRNA associated with epilepsy and acts as a regulator in endometrial cancer | Upregulation reduced the apoptosis and increases the cell viability of hippocampal neurons, which were dysregulated by TLE | Diagnostic biomarker of TLE in children | Serum | Decreased | [209] |
| miR-135b-5p | miRNA associated with epilepsy and cancer cell proliferation, migration. | Reduces post epileptic dysregulation of cell viability and apoptosis of hippocampal neurons by targeting SIRT1. | Diagnostic biomarker of TLE in children | Plasma | Decreased | [210] |
| miR-93-5p, miR-199a-3p and miR-574-3p | miRNA associated with epilepsy | Found to be dysregulated in epilepsy | Diagnostic biomarker of TLE | Plasma | Increased | [211] |
| miR194-2-5p, miR15a-5p, miR-132-3p, and miR-145-5p | miRNA associated with epilepsy | Plays important role in the pathogenesis of FCD and refractory epilepsy by regulating mTOR, P13K-Akt, P53, TGF- β signaling pathways, and cell cycle. | Diagnostic and prognostic biomarker for refractory epilepsy | Serum | Increased | [212] |
| miR-328-3p | miRNA associated with epilepsy | Important peripheral biomarker of epilepsy | Diagnostic biomarker of mTLE-HS | Serum | Increased | [213] |
| miR-654-3p | miRNA associated with epilepsy | Important peripheral biomarker of epilepsy with statistical power to differentiate between Engel I (good surgical prognosis) and Engel III-IV (unfavorable surgical prognosis) patients | surgical prognosis biomarker of MTLE-HS | Serum | Increased | [213] |
| miR-134 and miR-146a | miRNA associated with epilepsy, and they also had a role in neuroinflammation, dendritic functionality | Their upregulated expression indicates a higher risk of developing DRE, independent of temporal lobe sclerosis, epilepsy duration, familial history, age at first seizure, age, body mass index (BMI), smoking behavior, and gender | Predictive and prognostic biomarker of DRE | Serum | Increased | [214] |
| miR-182 | miRNA associated with epilepsy, neuroinflammation, and apoptosis | Upregulation inhibited the expression of APLN, which serves a neuroprotective role in epilepsy. Moreover, it’s upregulation induces apoptosis. | Diagnostic biomarker of epilepsy | Hippocampal neurons | Increased | [215] |
| MiR-146a, miR-155 and miR-132 | miRNA associated with epilepsy | Plays important role in neuroinflammation, neuroprotection, neurodegeneration, and neuronal growth, related to epilepsy | Diagnostic and prognostic biomarker of GGE | Serum | Increased | [216] |
| miR-194-5p | miRNA associated with epilepsy | Hyperexpression increases cell viability which was reduced by TLE. Targets the IGF1R gene directly and reduces apoptosis of hippocampal neurons. | Diagnostic biomarker and treatment target of TLE in children | Plasma | Decreased | [217] |
| miR-142 | miRNA associated with epilepsy | Expression is associated with anti-inflammatory signaling in epileptogenic tubers from a tuberous sclerosis complex | Diagnostic biomarker of TLE and prognostic biomarker for drug-resistant TLE | Serum | Increased | [218] |
| miR-146a | miRNA associated with epilepsy | Diagnostic miRNA in genetically generalized epilepsy because of its role in propagating inflammation of the hippocampus | Diagnostic biomarker of TLE | Serum | Increased | [218] |
| miR-223 | miRNA associated with epilepsy | Expression is associated with anti-inflammatory signaling in epileptogenic tubers from a tuberous sclerosis complex | Diagnostic biomarker of TLE and prognostic biomarker for drug-resistant TLE | Serum | Increased | [218] |
6.2. Genetic Biomarkers
6.3. Molecular Analysis
6.4. Molecular Profiling after Surgery
7. Conclusions and Future Aspects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Singh, T.; Joshi, S.; Williamson, J.M.; Kapur, J. Neocortical injury–induced status epilepticus. Epilepsia 2020, 61, 2811–2824. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Goel, R.K. Modulatory Effect of Serotonergic System in Pentylenetetrazole-Induced Seizures and Associated Memory Deficit: Role of 5-HT1A and 5-HT2A/2C. J. Epilepsy Res. 2019, 9, 119–125. [Google Scholar] [CrossRef][Green Version]
- Sharma, P.; Wright, D.K.; Johnston, L.A.; Powell, K.L.; Wlodek, M.E.; Shultz, S.R.; O’Brien, T.J.; Gilby, K.L. Differences in white matter structure between seizure prone (FAST) and seizure resistant (SLOW) rat strains. Neurobiol. Dis. 2017, 104, 33–40. [Google Scholar] [CrossRef]
- Mishra, A.; Goel, R.K. Chronic 5-HT3 receptor antagonism ameliorates seizures and associated memory deficit in pentylenetetrazole-kindled mice. Neuroscience 2016, 339, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Shorvon, S.D. The causes of epilepsy: Changing concepts of etiology of epilepsy over the past 150 years. Epilepsia 2011, 52, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Shorvon, S.D.; Andermann, F.; Guerrini, R. (Eds.) The Causes of Epilepsy: Common and Uncommon Causes in Adults and Children; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Xu, Y.; Nguyen, D.; Mohamed, A.; Carcel, C.; Li, Q.; Kutlubaev, M.A.; Anderson, C.S.; Hackett, M.L. Frequency of a false positive diagnosis of epilepsy: A systematic review of observational studies. Seizure 2016, 41, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Defalla, B.A.; Chadwick, D.W. The misdiagnosis of epilepsy and the management of refractory epilepsy in a specialist clinic. QJM 1999, 92, 15–23. [Google Scholar] [CrossRef]
- Vilar, L.; Vilar, C.F.; Lyra, R.; Freitas, M.D.C. Pitfalls in the Diagnostic Evaluation of Hyperprolactinemia. Neuroendocrinology 2019, 109, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J. EEG Instrumentation and Safety; InEEG-fMRI 2009; Springer: Berlin, Germany, 2009; pp. 115–133. [Google Scholar]
- Priyanka, A.; Abhang, B.W.; Gawali, S.C.; Mehrotra, S.C. (Eds.) Chapter 2—Technological Basics of EEG Recording and Operation of Apparatus. In Introduction to EEG-and Speech-Based Emotion Recognition; Academic Press: London, UK, 2016; pp. 19–50. ISBN 9780128044902. [Google Scholar] [CrossRef]
- Leach, J.P.; Stephen, L.J.; Salveta, C.; Brodie, M.J. Which electroencephalography (EEG) for epilepsy? The relative usefulness of different EEG protocols in patients with possible epilepsy. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1040–1042. [Google Scholar] [CrossRef] [PubMed]
- Beniczky, S.; Aurlien, H.; Brøgger, J.C.; Hirsch, L.J.; Schomer, D.L.; Trinka, E.; Pressler, R.M.; Wennberg, R.; Visser, G.H.; Eisermann, M.; et al. Standardized computer-based organized reporting of EEG: SCORE-Second version. Clin. Neurophysiol. 2017, 128, 2334–2346. [Google Scholar] [CrossRef]
- Williams Roberson, S.; Shah, P.; Piai, V.; Gatens, H.; Krieger, A.M.; Lucas, T.H., 2nd; Litt, B. Electrocorticography reveals spatiotemporal neuronal activation patterns of verbal fluency in patients with epilepsy. Neuropsychologia 2020, 141, 107386. [Google Scholar] [CrossRef] [PubMed]
- Pacreu, S.; Vilà, E.; Moltó, L.; Bande, D.; Rueda, M.; Fernández Candil, J.L. Anaesthesia management in epilepsy surgery with intraoperative electrocorticography. Rev. Esp. Anestesiol. Reanim. 2018, 65, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Ravat, S.; Iyer, V.; Panchal, K.; Muzumdar, D.; Kulkarni, A. Surgical outcomes in patients with intraoperative Electrocorticography (EcoG) guided epilepsy surgery-experiences of a tertiary care centre in India. Int. J. Surg. 2016, 36, 420–428. [Google Scholar] [CrossRef]
- Türedi, S.; Hasanbasoglu, A.; Gunduz, A.; Yandi, M. Clinical decision instruments for CT scan in minor head trauma. J. Emerg. Med. 2008, 34, 253–259. [Google Scholar] [CrossRef]
- Kuzniecky, R.I. Neuroimaging of epilepsy: Therapeutic implications. NeuroRx 2005, 2, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Andica, C.; Hagiwara, A.; Hori, M.; Kamagata, K.; Koshino, S.; Maekawa, T.; Suzuki, M.; Fujiwara, H.; Ikeno, M.; Shimizu, T.; et al. Review of synthetic MRI in pediatric brains: Basic principle of MR quantification, its features, clinical applications, and limitations. J. Neuroradiol. 2019, 46, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Odéen, H.; Parker, D.L. Magnetic resonance thermometry and its biological applications—Physical principles and practical considerations. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 110, 34–61. [Google Scholar] [CrossRef]
- Heim, B.; Krismer, F.; De Marzi, R.; Seppi, K. Magnetic resonance imaging for the diagnosis of Parkinson’s disease. J. Neural Transm. Vienna 2017, 124, 915–964. [Google Scholar] [CrossRef]
- Risacher, S.L.; Saykin, A.J. Neuroimaging in aging and neurologic diseases. Handb. Clin. Neurol. 2019, 167, 191–227. [Google Scholar] [CrossRef]
- Sidhu, M.K.; Duncan, J.S.; Sander, J.W. Neuroimaging in epilepsy. Curr. Opin. Neurol. 2018, 31, 371–378. [Google Scholar] [CrossRef]
- Heilbrun, M.P.; Sunderland, P.M.; McDonald, P.R.; Wells, T.H., Jr.; Cosman, E.; Ganz, E. Brown-Roberts-Wells stereotactic frame modifications to accomplish magnetic resonance imaging guidance in three planes. Appl. Neurophysiol. 1987, 50, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Cendes, F.; Theodore, W.H.; Brinkmann, B.H.; Sulc, V.; Cascino, G.D. Neuroimaging of epilepsy. Handb. Clin. Neurol. 2016, 136, 985–1014. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.G.; Jakabek, D.; Ljung, H.; Velakoulis, D.; van Westen, D.; Looi, J.C.L.; Källén, K. MRI morphology of the hippocampus in drug-resistant temporal lobe epilepsy: Shape inflation of left hippocampus and correlation of right-sided hippocampal volume and shape with visuospatial function in patients with right-sided TLE. J. Clin. Neurosci. 2019, 67, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Phelps, M.E.; Hoffman, E.J.; Mullani, N.A.; Ter-Pogossian, M.M. Application of annihilation coincidence detection to transaxial reconstruction tomography. J. Nucl. Med. 1975, 16, 210–224. [Google Scholar] [PubMed]
- Herschman, H.R. Micro-PET imaging and small animal models of disease. Curr. Opin. Immunol. 2003, 15, 378–384. [Google Scholar] [CrossRef]
- Zukotynski, K.; Kuo, P.H.; Mikulis, D.; Rosa-Neto, P.; Strafella, A.P.; Subramaniam, R.M.; Black, S.E. PET/CT of Dementia. AJR. Am. J. Roentgenol. 2018, 211, 246–259. [Google Scholar] [CrossRef]
- Jupp, B.; Binns, D.; Willams, J.; Hicks, R.; O’Brien, T. Serial FDG-pet during epileptogenesis in the rat kainic acid model of tle reveals persistent cerebral hypometabolism: 3.044. Epilepsia 2005, 46, 289. [Google Scholar]
- Jupp, B.; Williams, J.; Binns, D.; Vosmanski, M.; Hicks, R.; O’Brien, T.J. MRI and FDG-PET show progressive hippocampal changes during epileptogenesis in the amygdale kindling rat model of TLE. Epilepsia 2004, 45, 19. [Google Scholar]
- Kornblum, H.I.; Araujo, D.M.; Annala, A.J.; Tatsukawa, K.J.; Phelps, M.E.; Cherry, S.R. In vivo imaging of neuronal activation and plasticity in the rat brain by high resolution positron emission tomography (microPET). Nat. Biotechnol. 2000, 18, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Liefaard, L.C.; Ploeger, B.A.; Molthoff, C.F.; de Jong, H.W.; Dijkstra, J.; van der Weerd, L.; Lammertsma, A.A.; Danhof, M.; Voskuyl, R.A. Changes in GABAA receptor properties in amygdala kindled animals: In vivo studies using [11C]flumazenil and positron emission tomography. Epilepsia 2009, 50, 88–98. [Google Scholar] [CrossRef]
- Merlet, I.; Ostrowsky, K.; Costes, N.; Ryvlin, P.; Isnard, J.; Faillenot, I.; Lavenne, F.; Dufournel, D.; Le Bars, D.; Mauguière, F. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: An [18F]MPPF-PET study. Brain 2004, 127, 900–913. [Google Scholar] [CrossRef]
- Catana, C.; Wu, Y.; Judenhofer, M.S.; Qi, J.; Pichler, B.J.; Cherry, S.R. Simultaneous acquisition of multislice PET and MR images: Initial results with a MR-compatible PET scanner. J. Nucl. Med. 2006, 47, 1968–1976. [Google Scholar] [PubMed]
- Lucas, A.J.; Hawkes, R.C.; Ansorge, R.E.; Williams, G.B.; Nutt, R.E.; Clark, J.C.; Fryer, T.D.; Carpenter, T.A. Development of a combined microPET-MR system. Technol. Cancer Res. Treat. 2006, 5, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Raylman, R.R.; Majewski, S.; Lemieux, S.K.; Velan, S.S.; Kross, B.; Popov, V.; Smith, M.F.; Weisenberger, A.G.; Zorn, C.; Marano, G.D. Simultaneous MRI and PET imaging of a rat brain. Phys. Med. Biol. 2006, 51, 6371–6379. [Google Scholar] [CrossRef]
- Myers, R.; Hume, S. Small animal PET. Eur. Neuropsychopharmacol. 2002, 12, 545–555. [Google Scholar] [CrossRef]
- Catafau, A.M. Brain SPECT in clinical practice. Part I: Perfusion. J. Nucl. Med. 2001, 42, 259–271. [Google Scholar] [PubMed]
- Lassen, N.A.; Blasberg, R.G. Technetium-99m-d,l-HM-PAO, the development of a new class of 99mTc-labeled tracers: An overview. J. Cereb. Blood Flow Metab. 1988, 8, S1–S3. [Google Scholar] [CrossRef][Green Version]
- Walovitch, R.C.; Hill, T.C.; Garrity, S.T.; Cheesman, E.H.; Burgess, B.A.; O’Leary, D.H.; Watson, A.D.; Ganey, M.V.; Morgan, R.A.; Williams, S.J. Characterization of technetium-99m-L,L-ECD for brain perfusion imaging, Part 1: Pharmacology of technetium-99m ECD in nonhuman primates. J. Nucl. Med. 1989, 30, 1892–1901. [Google Scholar] [PubMed]
- Léveillé, J.; Demonceau, G.; Walovitch, R.C. Intrasubject comparison between technetium-99m-ECD and technetium-99m-HMPAO in healthy human subjects. J. Nucl. Med. 1992, 33, 480–484. [Google Scholar]
- Moretti, J.L.; Defer, G.; Cinotti, L.; Cesaro, P.; Vigneron, N.; Pethe, C. Comparative tomoscintigraphic study of strokes using ECD Tc-99m,HMPAO Tc-99m and IMP I-123, preliminary results. Eur. J. Nucl. Med. 1988, 14, 311. [Google Scholar]
- Kim, S.; Mountz, J.M. SPECT Imaging of Epilepsy: An Overview and Comparison with F-18 FDG PET. Int. J. Mol. Imaging 2011, 2011, 813028. [Google Scholar] [CrossRef]
- McArthur, C.; Jampana, R.; Patterson, J.; Hadley, D. Applications of cerebral SPECT. Clin. Radiol. 2011, 66, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; Gallezot, J.D.; Schafbauer, T.; Lim, K.; Kloczynski, T.; Morris, E.D.; Carson, R.E.; Ding, Y.S.; Cosgrove, K.P. Endotoxin-induced systemic inflammation activates microglia: (¹¹C)PBR28 positron emission tomography in nonhuman primates. Neuroimage 2012, 63, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Ory, D.; Planas, A.; Dresselaers, T.; Gsell, W.; Postnov, A.; Celen, S.; Casteels, C.; Himmelreich, U.; Debyser, Z.; Van Laere, K.; et al. PET imaging of TSPO in a rat model of local neuroinflammation induced by intracerebral injection of lipopolysaccharide. Nucl. Med. Biol. 2015, 42, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and epilepsy: Excitability and inflammation. Trends Neurosci. 2013, 36, 174–184. [Google Scholar] [CrossRef]
- Sandiego, C.M.; Gallezot, J.D.; Pittman, B.; Nabulsi, N.; Lim, K.; Lin, S.F.; Matuskey, D.; Lee, J.Y.; O’Connor, K.C.; Huang, Y.; et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc. Natl. Acad. Sci. USA 2015, 112, 12468–12473. [Google Scholar] [CrossRef] [PubMed]
- Lavisse, S.; Guillermier, M.; Hérard, A.S.; Petit, F.; Delahaye, M.; Van Camp, N.; Ben Haim, L.; Lebon, V.; Remy, P.; Dollé, F.; et al. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J. Neurosci. 2012, 32, 10809–10818. [Google Scholar] [CrossRef]
- Liu, B.; Le, K.X.; Park, M.A.; Wang, S.; Belanger, A.P.; Dubey, S.; Frost, J.L.; Holton, P.; Reiser, V.; Jones, P.A.; et al. In Vivo Detection of Age- and Disease-Related Increases in Neuroinflammation by 18F-GE180 TSPO MicroPET Imaging in Wild-Type and Alzheimer’s Transgenic Mice. J. Neurosci. 2015, 35, 15716–15730. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, D.; Verhaeghe, J.; Santermans, E.; Amhaoul, H.; Jonckers, E.; Wyffels, L.; Van Der Linden, A.; Hens, N.; Staelens, S.; Dedeurwaerdere, S. Non-invasive PET imaging of brain inflammation at disease onset predicts spontaneous recurrent seizures and reflects comorbidities. Brain Behav. Immun. 2017, 61, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.J.; Shao, X.; Desmond, T.J.; Hockley, B.G.; Sherman, P.; Quesada, C.A.; Frey, K.A.; Koeppe, R.A.; Kilbourn, M.R.; Bohnen, N.I. Investigation of Proposed Activity of Clarithromycin at GABAA Receptors Using [(11)C]Flumazenil PET. ACS Med. Chem. Lett. 2016, 7, 746–750. [Google Scholar] [CrossRef] [PubMed][Green Version]
- la Fougère, C.; Rominger, A.; Förster, S.; Geisler, J.; Bartenstein, P. PET and SPECT in epilepsy: A critical review. Epilepsy Behav. 2009, 15, 50–55. [Google Scholar] [CrossRef]
- Vibholm, A.K.; Landau, A.M.; Møller, A.; Jacobsen, J.; Vang, K.; Munk, O.L.; Orlowski, D.; Sørensen, J.C.; Brooks, D.J. NMDA receptor ion channel activation detected in vivo with [18F]GE-179 PET after electrical stimulation of rat hippocampus. J. Cereb. Blood Flow Metab. 2021, 41, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- McGinnity, C.J.; Koepp, M.J.; Hammers, A.; Riaño Barros, D.A.; Pressler, R.M.; Luthra, S.; Jones, P.A.; Trigg, W.; Micallef, C.; Symms, M.R.; et al. NMDA receptor binding in focal epilepsies. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Koepp, M. P-glycoprotein imaging in temporal lobe epilepsy: In vivo PET experiments with the Pgp substrate [11C]-verapamil. Epilepsia 2012, 53 (Suppl. 6), 60–63. [Google Scholar] [CrossRef]
- Hartz, A.M.; Pekcec, A.; Soldner, E.L.; Zhong, Y.; Schlichtiger, J.; Bauer, B. P-gp Protein Expression and Transport Activity in Rodent Seizure Models and Human Epilepsy. Mol. Pharm. 2017, 14, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Gidal, B.E. P-glycoprotein Expression and Pharmacoresistant Epilepsy: Cause or Consequence? Epilepsy Curr. 2014, 14, 136–138. [Google Scholar] [CrossRef]
- Chugani, D.C. α-methyl-L-tryptophan: Mechanisms for tracer localization of epileptogenic brain regions. Biomark. Med. 2011, 5, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, K.; Chugani, D.C.; Asano, E.; Juhász, C.; Muzik, O.; Shah, A.; Shah, J.; Sood, S.; Kupsky, W.J.; Mangner, T.J.; et al. Epilepsy surgery outcome in children with tuberous sclerosis complex evaluated with alpha-[11C]methyl-L-tryptophan positron emission tomography (PET). J. Child Neurol. 2005, 20, 429–438. [Google Scholar] [CrossRef]
- Garibotto, V.; Picard, F. Nuclear medicine imaging in epilepsy. Epileptologie 2013, 30, 109–121. [Google Scholar]
- Hasler, G.; Bonwetsch, R.; Giovacchini, G.; Toczek, M.T.; Bagic, A.; Luckenbaugh, D.A.; Drevets, W.C.; Theodore, W.H. 5-HT1A receptor binding in temporal lobe epilepsy patients with and without major depression. Biol. Psychiatry 2007, 62, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Didelot, A.; Ryvlin, P.; Lothe, A.; Merlet, I.; Hammers, A.; Mauguière, F. PET imaging of brain 5-HT1A receptors in the preoperative evaluation of temporal lobe epilepsy. Brain 2008, 131, 2751–2764. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, I. PET studies in epilepsy. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 416–430. [Google Scholar]
- Martinez, A.; Finegersh, A.; Cannon, D.M.; Dustin, I.; Nugent, A.; Herscovitch, P.; Theodore, W.H. The 5-HT1A receptor and 5-HT transporter in temporal lobe epilepsy. Neurology 2013, 80, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Velísková, J.; Moshé, S.L. Update on the role of substantia nigra pars reticulata in the regulation of seizures. Epilepsy Curr. 2006, 6, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Bouilleret, V.; Semah, F.; Chassoux, F.; Mantzaridez, M.; Biraben, A.; Trebossen, R.; Ribeiro, M.J. Basal ganglia involvement in temporal lobe epilepsy: A functional and morphologic study. Neurology 2008, 70, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bernedo Paredes, V.E.; Buchholz, H.G.; Gartenschläger, M.; Breimhorst, M.; Schreckenberger, M.; Werhahn, K.J. Reduced D2/D3 Receptor Binding of Extrastriatal and Striatal Regions in Temporal Lobe Epilepsy. PLoS ONE 2015, 10, e0141098. [Google Scholar] [CrossRef]
- Fedi, M.; Berkovic, S.F.; Scheffer, I.E.; O’Keefe, G.; Marini, C.; Mulligan, R.; Gong, S.; Tochon-Danguy, H.; Reutens, D.C. Reduced striatal D1 receptor binding in autosomal dominant nocturnal frontal lobe epilepsy. Neurology 2008, 71, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Odano, I.; Varrone, A.; Savic, I.; Ciumas, C.; Karlsson, P.; Jucaite, A.; Halldin, C.; Farde, L. Quantitative PET analyses of regional [11C]PE2I binding to the dopamine transporter--application to juvenile myoclonic epilepsy. Neuroimage 2012, 59, 3582–3593. [Google Scholar] [CrossRef]
- Karlócai, M.R.; Tóth, K.; Watanabe, M.; Ledent, C.; Juhász, G.; Freund, T.F.; Maglóczky, Z. Redistribution of CB1 cannabinoid receptors in the acute and chronic phases of pilocarpine-induced epilepsy. PLoS ONE 2011, 6, e27196. [Google Scholar] [CrossRef]
- Goffin, K.; Van Paesschen, W.; Van Laere, K. In vivo activation of endocannabinoid system in temporal lobe epilepsy with hippocampal sclerosis. Brain 2011, 134, 1033–1040. [Google Scholar] [CrossRef]
- Riaño Barros, D.A.; McGinnity, C.J.; Rosso, L.; Heckemann, R.A.; Howes, O.D.; Brooks, D.J.; Duncan, J.S.; Turkheimer, F.E.; Koepp, M.J.; Hammers, A. Test-retest reproducibility of cannabinoid-receptor type 1 availability quantified with the PET ligand [¹¹C]MePPEP. Neuroimage 2014, 97, 151–162. [Google Scholar] [CrossRef]
- Weltzin, M.M.; George, A.A.; Lukas, R.J.; Whiteaker, P. Sleep-related hypermotor epilepsy associated mutations uncover important kinetic roles of α4β2-nicotinic acetylcholine receptor intracellular structures. PLoS ONE 2021, 16, e0247825. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Bruel, D.; Servent, D.; Saba, W.; Fruchart-Gaillard, C.; Schöllhorn-Peyronneau, M.A.; Roumenov, D.; Brodtkorb, E.; Zuberi, S.; Gambardella, A.; et al. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: A PET study. Brain 2006, 129, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, B.; Wang, Y.; Chen, Z. Cholinergic Signaling, Neural Excitability, and Epilepsy. Molecules 2021, 26, 2258. [Google Scholar] [CrossRef]
- Bodle, J.D.; Feldmann, E.; Swartz, R.H.; Rumboldt, Z.; Brown, T.; Turan, T.N. High-resolution magnetic resonance imaging: An emerging tool for evaluating intracranial arterial disease. Stroke 2013, 44, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Bammer, R.; Hope, T.A.; Aksoy, M.; Alley, M.T. Time-resolved 3D quantitative flow MRI of the major intracranial vessels: Initial experience and comparative evaluation at 1.5T and 3.0T in combination with parallel imaging. Magn. Reson. Med. 2007, 57, 127–140. [Google Scholar] [CrossRef]
- Li, M.; Le, W.J.; Tao, X.F.; Li, M.H.; Li, Y.H.; Qu, N. Advantage in Bright-blood and Black-blood Magnetic Resonance Imaging with High-resolution for Analysis of Carotid Atherosclerotic Plaques. Chin. Med. J. 2015, 128, 2478–2484. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Q.; Shi, Z.; Tian, X.; Peng, W.; Lu, J. Interstudy reproducibility of dark blood high-resolution MRI in evaluating basilar atherosclerotic plaque at 3 Tesla. Diagn. Interv. Radiol. 2018, 24, 237–242. [Google Scholar] [CrossRef]
- Yarnykh, V.L.; Terashima, M.; Hayes, C.E.; Shimakawa, A.; Takaya, N.; Nguyen, P.K.; Brittain, J.H.; McConnell, M.V.; Yuan, C. Multicontrast black-blood MRI of carotid arteries: Comparison between 1.5 and 3 tesla magnetic field strengths. J. Magn. Reson. Imaging 2006, 23, 691–698. [Google Scholar] [CrossRef]
- Anumula, S.; Song, H.K.; Wright, A.C.; Wehrli, F.W. High-resolution black-blood MRI of the carotid vessel wall using phased-array coils at 1.5 and 3 Tesla. Acad. Radiol. 2005, 12, 1521–1526. [Google Scholar] [CrossRef]
- Winston, G.P.; Yogarajah, M.; Symms, M.R.; McEvoy, A.W.; Micallef, C.; Duncan, J.S. Diffusion tensor imaging tractography to visualize the relationship of the optic radiation to epileptogenic lesions prior to neurosurgery. Epilepsia 2011, 52, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Cendes, F.; Knowlton, R.C.; Novotny, E.; Min, L.L.; Antel, S.; Sawrie, S.; Laxer, K.D.; Arnold, D. Magnetic resonance spectroscopy in epilepsy: Clinical issues. Epilepsia 2002, 43, 32–39. [Google Scholar] [CrossRef]
- Rhodes, C.J. Magnetic resonance spectroscopy. Sci. Prog. 2017, 100, 241–292. [Google Scholar] [CrossRef] [PubMed]
- Riederer, F.; Bittsanský, M.; Lehner-Baumgartner, E.; Baumgartner, C.; Mlynárik, V.; Gruber, S.; Moser, E.; Kaya, M.; Serles, W. Decrease of NAA with aging outside the seizure focus in mesial temporal lobe epilepsy—A proton-MRS study at 3 Tesla. Brain Res. 2007, 1179, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.W.; Heinrichs-Graham, E.; Proskovec, A.L.; McDermott, T.J. Neuroimaging with magnetoencephalography: A dynamic view of brain pathophysiology. Transl. Res. 2016, 175, 17–36. [Google Scholar] [CrossRef]
- Andrade-Valenca, L.P.; Dubeau, F.; Mari, F.; Zelmann, R.; Gotman, J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology 2011, 77, 524–531. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.L.; Devinsky, O.; Kelly, C.; Milham, M.; Castellanos, F.X.; Quinn, B.T.; DuBois, J.; Young, J.R.; Carlson, C.; French, J.; et al. Default mode network abnormalities in idiopathic generalized epilepsy. Epilepsy Behav. 2012, 23, 353–359. [Google Scholar] [CrossRef]
- Pollard, J.R.; Eidelman, O.; Mueller, G.P.; Dalgard, C.L.; Crino, P.B.; Anderson, C.T.; Brand, E.J.; Burakgazi, E.; Ivaturi, S.K.; Pollard, H.B. The TARC/sICAM5 Ratio in Patient Plasma is a Candidate Biomarker for Drug Resistant Epilepsy. Front. Neurol. 2013, 3, 181. [Google Scholar] [CrossRef]
- Vivash, L.; Gregoire, M.C.; Bouilleret, V.; Berard, A.; Wimberley, C.; Binns, D.; Roselt, P.; Katsifis, A.; Myers, D.E.; Hicks, R.J.; et al. In vivo measurement of hippocampal GABAA/cBZR density with [18F]-flumazenil PET for the study of disease progression in an animal model of temporal lobe epilepsy. PLoS ONE 2014, 9, e86722. [Google Scholar] [CrossRef]
- Choy, M.; Dubé, C.M.; Patterson, K.; Barnes, S.R.; Maras, P.; Blood, A.B.; Hasso, A.N.; Obenaus, A.; Baram, T.Z. A novel, noninvasive, predictive epilepsy biomarker with clinical potential. J. Neurosci. 2014, 34, 8672–8684. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, Y.K.; Kang, H.; Lee, H.; Im, H.J.; Hwang, D.W.; Kim, E.E.; Chung, J.K.; Lee, D.S. Abnormal metabolic connectivity in the pilocarpine-induced epilepsy rat model: A multiscale network analysis based on persistent homology. Neuroimage 2014, 99, 226–236. [Google Scholar] [CrossRef]
- Choi, H.; Kim, Y.K.; Oh, S.W.; Im, H.J.; Hwang, D.W.; Kang, H.; Lee, B.; Lee, Y.S.; Jeong, J.M.; Kim, E.E.; et al. In vivo imaging of mGluR5 changes during epileptogenesis using [11C]ABP688 PET in pilocarpine-induced epilepsy rat model. PLoS ONE 2014, 9, e92765. [Google Scholar] [CrossRef]
- Pitkänen, A.; Immonen, R. Epilepsy related to traumatic brain injury. Neurotherapeutics 2014, 11, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Labate, A.; Cherubini, A.; Tripepi, G.; Mumoli, L.; Ferlazzo, E.; Aguglia, U.; Quattrone, A.; Gambardella, A. White matter abnormalities differentiate severe from benign temporal lobe epilepsy. Epilepsia 2015, 56, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, Y.; Hu, H.; Wang, J.; Liu, Z.; Gao, F. FDG-PET and NeuN-GFAP immunohistochemistry of hippocampus at different phases of the pilocarpine model of temporal lobe epilepsy. Int. J. Med. Sci. 2015, 12, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Wang, J.; Chen, X.M.; Li, J.P.; Ye, W.; Zheng, J. Reduced local diffusion homogeneity as a biomarker for temporal lobe epilepsy. Medicine 2016, 95, e4032. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Zheng, P.; Wright, D.K.; Dezsi, G.; Braine, E.; Nguyen, T.; Corcoran, N.M.; Johnston, L.A.; Hovens, C.M.; Mayo, J.N.; et al. Sodium selenate retards epileptogenesis in acquired epilepsy models reversing changes in protein phosphatase 2A and hyperphosphorylated tau. Brain 2016, 139, 1919–1938. [Google Scholar] [CrossRef]
- Suleymanova, E.M.; Gulyaev, M.V.; Abbasova, K.R. Structural alterations in the rat brain and behavioral impairment after status epilepticus: An MRI study. Neuroscience 2016, 315, 79–90. [Google Scholar] [CrossRef]
- Dietrich, Y.; Eliat, P.A.; Dieuset, G.; Saint-Jalmes, H.; Pineau, C.; Wendling, F.; Martin, B. Structural and functional changes during epileptogenesis in the mouse model of medial temporal lobe epilepsy. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 2016, 4005–4008. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Löscher, W.; Vezzani, A.; Becker, A.J.; Simonato, M.; Lukasiuk, K.; Gröhn, O.; Bankstahl, J.P.; Friedman, A.; Aronica, E.; et al. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 2016, 15, 843–856. [Google Scholar] [CrossRef]
- Bragin, A.; Li, L.; Almajano, J.; Alvarado-Rojas, C.; Reid, A.Y.; Staba, R.J.; Engel, J., Jr. Pathologic electrographic changes after experimental traumatic brain injury. Epilepsia 2016, 57, 735–745. [Google Scholar] [CrossRef]
- Pascente, R.; Frigerio, F.; Rizzi, M.; Porcu, L.; Boido, M.; Davids, J.; Zaben, M.; Tolomeo, D.; Filibian, M.; Gray, W.P.; et al. Cognitive deficits and brain myo-Inositol are early biomarkers of epileptogenesis in a rat model of epilepsy. Neurobiol. Dis. 2016, 93, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Cleeren, E.; Premereur, E.; Casteels, C.; Goffin, K.; Janssen, P.; Van Paesschen, W. The effective connectivity of the seizure onset zone and ictal perfusion changes in amygdala kindled rhesus monkeys. Neuroimage Clin. 2016, 12, 252–261. [Google Scholar] [CrossRef]
- Bar-Klein, G.; Lublinsky, S.; Kamintsky, L.; Noyman, I.; Veksler, R.; Dalipaj, H.; Senatorov, V.V., Jr.; Swissa, E.; Rosenbach, D.; Elazary, N.; et al. Imaging blood-brain barrier dysfunction as a biomarker for epileptogenesis. Brain 2017, 140, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.E.; Frigerio, F.; Ravizza, T.; Ricci, E.; Tse, K.; Jenkins, R.E.; Sills, G.J.; Jorgensen, A.; Porcu, L.; Thippeswamy, T.; et al. Molecular isoforms of high-mobility group box 1 are mechanistic biomarkers for epilepsy. J. Clin. Investig. 2017, 127, 2118–2132. [Google Scholar] [CrossRef] [PubMed]
- Salo, R.A.; Miettinen, T.; Laitinen, T.; Gröhn, O.; Sierra, A. Diffusion tensor MRI shows progressive changes in the hippocampus and dentate gyrus after status epilepticus in rat—histological validation with Fourier-based analysis. Neuroimage 2017, 152, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, S.; Hasegawa, D.; Hamamoto, Y.; Yu, Y.; Kuwabara, T.; Fujiwara-Igarashi, A.; Fujita, M. Interictal diffusion and perfusion magnetic resonance imaging features of cats with familial spontaneous epilepsy. Am. J. Vet Res. 2017, 78, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Nissinen, J.; Pitkänen, A. Generalized Seizures after Experimental Traumatic Brain Injury Occur at the Transition from Slow-Wave to Rapid Eye Movement Sleep. J. Neurotrauma 2017, 34, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Won, D.; Kim, W.; Chaovalitwongse, W.A.; Tsai, J.J. Altered visual contrast gain control is sensitive for idiopathic generalized epilepsies. Clin. Neurophysiol. 2017, 128, 340–348. [Google Scholar] [CrossRef][Green Version]
- Maccotta, L.; Lopez, M.A.; Adeyemo, B.; Ances, B.M.; Day, B.K.; Eisenman, L.N.; Dowling, J.L.; Leuthardt, E.C.; Schlaggar, B.L.; Hogan, R.E. Postoperative seizure freedom does not normalize altered connectivity in temporal lobe epilepsy. Epilepsia 2017, 58, 1842–1851. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Hasegawa, D.; Mizoguchi, S.; Yu, Y.; Wada, M.; Kuwabara, T.; Fujiwara-Igarashi, A.; Fujita, M. Changes in the interictal and early postictal diffusion and perfusion magnetic resonance parameters in familial spontaneous epileptic cats. Epilepsy Res. 2017, 133, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Russmann, V.; Brendel, M.; Mille, E.; Helm-Vicidomini, A.; Beck, R.; Günther, L.; Lindner, S.; Rominger, A.; Keck, M.; Salvamoser, J.D.; et al. Identification of brain regions predicting epileptogenesis by serial [18F]GE-180 positron emission tomography imaging of neuroinflammation in a rat model of temporal lobe epilepsy. Neuroimage Clin. 2017, 15, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Milikovsky, D.Z.; Weissberg, I.; Kamintsky, L.; Lippmann, K.; Schefenbauer, O.; Frigerio, F.; Rizzi, M.; Sheintuch, L.; Zelig, D.; Ofer, J.; et al. Electrocorticographic Dynamics as a Novel Biomarker in Five Models of Epileptogenesis. J. Neurosci. 2017, 37, 4450–4461. [Google Scholar] [CrossRef]
- Breuer, H.; Meier, M.; Schneefeld, S.; Härtig, W.; Wittneben, A.; Märkel, M.; Ross, T.L.; Bengel, F.M.; Bankstahl, M.; Bankstahl, J.P. Multimodality imaging of blood-brain barrier impairment during epileptogenesis. J. Cereb. Blood Flow Metab. 2017, 37, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Bentes, C.; Martins, H.; Peralta, A.R.; Morgado, C.; Casimiro, C.; Franco, A.C.; Fonseca, A.C.; Geraldes, R.; Canhão, P.; Pinho EMelo, T.; et al. Early EEG predicts poststroke epilepsy. Epilepsia Open 2018, 3, 203–212. [Google Scholar] [CrossRef]
- Sun, Y.; Seneviratne, U.; Perucca, P.; Chen, Z.; Tan, M.K.; O’Brien, T.J.; D’Souza, W.; Kwan, P. Generalized polyspike train: An EEG biomarker of drug-resistant idiopathic generalized epilepsy. Neurology 2018, 91, e1822–e1830. [Google Scholar] [CrossRef]
- Kitchigina, V.F. Alterations of Coherent Theta and Gamma Network Oscillations as an Early Biomarker of Temporal Lobe Epilepsy and Alzheimer’s Disease. Front. Integr. Neurosci. 2018, 12, 36. [Google Scholar] [CrossRef]
- Rizzi, M.; Brandt, C.; Weissberg, I.; Milikovsky, D.Z.; Pauletti, A.; Terrone, G.; Salamone, A.; Frigerio, F.; Löscher, W.; Friedman, A.; et al. Changes of dimension of EEG/ECoG nonlinear dynamics predict epileptogenesis and therapy outcomes. Neurobiol. Dis. 2019, 124, 373–378. [Google Scholar] [CrossRef]
- Samanta, D. Rhizomelic chondrodysplasia punctata: Role of EEG as a biomarker of impending epilepsy. eNeurologicalSci 2019, 18, 100218. [Google Scholar] [CrossRef]
- Cao, D.; Chen, Y.; Liao, J.; Nariai, H.; Li, L.; Zhu, Y.; Zhao, X.; Hu, Y.; Wen, F.; Zhai, Q. Scalp EEG high frequency oscillations as a biomarker of treatment response in epileptic encephalopathy with continuous spike-and-wave during sleep (CSWS). Seizure 2019, 71, 151–157. [Google Scholar] [CrossRef]
- Grigorovsky, V.; Jacobs, D.; Breton, V.L.; Tufa, U.; Lucasius, C.; Del Campo, J.M.; Chinvarun, Y.; Carlen, P.L.; Wennberg, R.; Bardakjian, B.L. Delta-gamma phase-amplitude coupling as a biomarker of postictal generalized EEG suppression. Brain Commun. 2020, 2, fcaa182. [Google Scholar] [CrossRef]
- Nariai, H.; Hussain, S.A.; Bernardo, D.; Motoi, H.; Sonoda, M.; Kuroda, N.; Asano, E.; Nguyen, J.C.; Elashoff, D.; Sankar, R.; et al. Scalp EEG interictal high frequency oscillations as an objective biomarker of infantile spasms. Clin. Neurophysiol. 2020, 131, 2527–2536. [Google Scholar] [CrossRef]
- Charupanit, K.; Sen-Gupta, I.; Lin, J.J.; Lopour, B.A. Amplitude of high frequency oscillations as a biomarker of the seizure onset zone. Clin. Neurophysiol. 2020, 131, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Firouzjah, R.; Rostamzadeh, A.; Banaei, A.; Shafiee, M.; Moghaddam, Z.M.; Vafapour, H. Exploring Changes in Thalamus Metabolites as Diagnostic Biomarkers in Idiopathic Generalised Epilepsy Patients Using Magnetic Resonance Spectroscopy. Malay. J. Med. Sci. 2020, 27, 78–86. [Google Scholar] [CrossRef]
- Wang, J.; Shan, Y.; Dai, J.; Cui, B.; Shang, K.; Yang, H.; Chen, Z.; Shan, B.; Zhao, G.; Lu, J. Altered coupling between resting-state glucose metabolism and functional activity in epilepsy. Ann. Clin. Transl. Neurol. 2020, 7, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Finnema, S.J.; Toyonaga, T.; Detyniecki, K.; Chen, M.K.; Dias, M.; Wang, Q.; Lin, S.F.; Naganawa, M.; Gallezot, J.D.; Lu, Y.; et al. Reduced synaptic vesicle protein 2A binding in temporal lobe epilepsy: A [11 C]UCB-J positron emission tomography study. Epilepsia 2020, 61, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, S.; Stupar, V.; Mazière, L.; Guo, J.; Labriji, W.; Liu, C.; Bretagnolle, L.; Parrot, S.; Barbier, E.L.; Depaulis, A.; et al. In vivo γ-aminobutyric acid increase as a biomarker of the epileptogenic zone: An unbiased metabolomics approach. Epilepsia 2021, 62, 163–175. [Google Scholar] [CrossRef]
- Scott, J.M.; Gliske, S.V.; Kuhlmann, L.; Stacey, W.C. Viability of Preictal High-Frequency Oscillation Rates as a Biomarker for Seizure Prediction. Front. Hum. Neurosci. 2021, 14, 612899. [Google Scholar] [CrossRef]
- Niedermeyer, E.; Da Silva, F.L. Electroencephalography--Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Hari, R.; Baillet, S.; Barnes, G.; Burgess, R.; Forss, N.; Gross, J.; Hämäläinen, M.; Jensen, O.; Kakigi, R.; Mauguière, F.; et al. IFCN-endorsed practical guidelines for clinical magnetoencephalography (MEG). Clin. Neurophysiol. 2018, 129, 1720–1747. [Google Scholar] [CrossRef]
- Martínez-Cañada, P.; Ness, T.V.; Einevoll, G.T.; Fellin, T.; Panzeri, S. Computation of the electroencephalogram (EEG) from network models of point neurons. PLoS Comput. Biol. 2021, 17, e1008893. [Google Scholar] [CrossRef] [PubMed]
- Baillet, S. Magnetoencephalography for brain electrophysiology and imaging. Nat. Neurosci. 2017, 20, 327–339. [Google Scholar] [CrossRef]
- Spanaki, M.V.; Kopylev, L.; DeCarli, C.; Gaillard, W.D.; Liow, K.; Fazilat, S.; Fazilat, S.; Reeves, P.; Sato, S.; Kufta, C.; et al. Postoperative changes in cerebral metabolism in temporal lobe epilepsy. Arch. Neurol. 2000, 57, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Estrada, A.; Velasco, F.; Velasco, A.L.; Cuellar-Herrera, M.; Saucedo-Alvarado, P.E.; Marquez-Franco, R.; Rivera-Bravo, B.; Ávila-Rodríguez, M.A. Quantitative Analysis of [18F]FFMZ and [18F]FDG PET Studies in the Localization of Seizure Onset Zone in Drug-Resistant Temporal Lobe Epilepsy. Stereotact. Funct. Neurosurg. 2019, 97, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Loucks, C.M.; Park, K.; Walker, D.S.; McEwan, A.H.; Timbers, T.A.; Ardiel, E.L.; Grundy, L.J.; Li, C.; Johnson, J.L.; Kennedy, J.; et al. EFHC1, implicated in juvenile myoclonic epilepsy, functions at the cilium and synapse to modulate dopamine signaling. Elife 2019, 8, e37271. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.H.; Liu, L.N.; Zhao, B.T.; Wang, X.; Zhang, C.; Shao, X.Q.; Zhang, K.; Ma, Y.S.; Ai, L.; Li, J.J.; et al. Use of an Automated Quantitative Analysis of Hippocampal Volume, Signal, and Glucose Metabolism to Detect Hippocampal Sclerosis. Front. Neurol. 2018, 9, 820. [Google Scholar] [CrossRef]
- Peter, J.; Houshmand, S.; Werner, T.J.; Rubello, D.; Alavi, A. Applications of global quantitative 18F-FDG-PET analysis in temporal lobe epilepsy. Nucl. Med. Commun. 2016, 37, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.J.; Pan, J.; Zhou, G.; Wang, Q.; Shao, X.Q.; Zhao, X.B.; Liu, J. Semi-quantitative FDG-PET Analysis Increases the Sensitivity Compared with Visual Analysis in the Diagnosis of Autoimmune Encephalitis. Front. Neurol. 2019, 10, 576. [Google Scholar] [CrossRef]
- Tan, Y.L.; Kim, H.; Lee, S.; Tihan, T.; Ver Hoef, L.; Mueller, S.G.; Barkovich, A.J.; Xu, D.; Knowlton, R. Quantitative surface analysis of combined MRI and PET enhances detection of focal cortical dysplasias. Neuroimage 2018, 166, 10–18. [Google Scholar] [CrossRef]
- Van Paesschen, W. Qualitative and quantitative imaging of the hippocampus in mesial temporal lobe epilepsy with hippocampal sclerosis. Neuroimaging Clin. N. Am. 2004, 14, 373–400. [Google Scholar] [CrossRef]
- Traub-Weidinger, T.; Muzik, O.; Sundar, L.K.S.; Aull-Watschinger, S.; Beyer, T.; Hacker, M.; Hahn, A.; Kasprian, G.; Klebermass, E.M.; Lanzenberger, R.; et al. Utility of Absolute Quantification in Non-lesional Extratemporal Lobe Epilepsy Using FDG PET/MR Imaging. Front. Neurol. 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Sree, S.V.; Swapna, G.; Martis, R.J.; Suri, J.S. Automated EEG analysis of epilepsy: A review. Knowl.-Based Systems 2013, 45, 147–165. [Google Scholar] [CrossRef]
- Dressler, O.; Schneider, G.; Stockmanns, G.; Kochs, E.F. Awareness and the EEG power spectrum: Analysis of frequencies. Br. J. Anaesth. 2004, 93, 806–809. [Google Scholar] [CrossRef]
- Subasi, A.; Kiymik, M.K.; Alkan, A.; Koklukaya, E. Neural network classification of EEG signals by using AR with MLE preprocessing for epileptic seizure detection. Math. Comput. Appl. 2005, 10, 57–70. [Google Scholar] [CrossRef]
- Hjorth, B. EEG analysis based on time domain properties. Electroencephalogr. Clin. Neurophysiol. 1970, 29, 306–310. [Google Scholar] [CrossRef]
- Sharanreddy, M.; Kulkarni, P.K. Automated EEG signal analysis for identification of epilepsy seizures and brain tumour. J. Med. Eng. Technol. 2013, 37, 511–519. [Google Scholar] [CrossRef]
- Adeli, H.; Zhou, Z.; Dadmehr, N. Analysis of EEG records in an epileptic patient using wavelet transform. J. Neurosci. Methods 2003, 123, 69–87. [Google Scholar] [CrossRef]
- Kousarrizi, M.R.N.; Ghanbari, A.A.; Teshnehlab, M.; Aliyari, M.; Gharaviri, A. Feature extraction and classification of EEG signals using wavelet transform, SVM and artificial neural networks for brain computer interfaces. In Proceedings of the International Joint Conference on Bioinformatics, Systems Biology and Intelligent Computing (IJCBS ‘09), Shanghai, China, 3–5 August 2009; pp. 352–355. [Google Scholar]
- Cvetkovic, D.; Übeyli, E.D.; Cosic, I. Wavelet transform feature extraction from human PPG, ECG, and EEG signal responses to ELF PEMF exposures: A pilot study. Digit. Signal Process. 2008, 18, 861–874. [Google Scholar] [CrossRef]
- Pigorini, A.; Casali, A.G.; Casarotto, S.; Ferrarelli, F.; Baselli, G.; Mariotti, M.; Massimini, M.; Rosanova, M. Time-frequency spectral analysis of TMS-evoked EEG oscillations by means of Hilbert-Huang transform. J. Neurosci. Methods 2011, 198, 236–245. [Google Scholar] [CrossRef]
- Guerrero-Mosquera, C.; Vazquez, A.N. New approach in features extraction for EEG signal detection. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 13–16. [Google Scholar] [CrossRef]
- Stam, C.J. Nonlinear dynamical analysis of EEG and MEG: Review of an emerging field. Clin. Neurophysiol. 2005, 116, 2266–2301. [Google Scholar] [CrossRef]
- Subasi, A.; Erçelebi, E. Classification of EEG signals using neural network and logistic regression. Comput. Methods Progr. Biomed. 2005, 78, 87–99. [Google Scholar] [CrossRef]
- Zhang, W.; Itoh, K.; Tanida, J.; Ichioka, Y. Parallel distributed processing model with local space-invariant interconnections and its optical architecture. Appl. Opt. 1990, 29, 4790–4797. [Google Scholar] [CrossRef]
- Schirrmeister, R.; Gemein, L.; Eggensperger, K.; Hutter, F.; Ball, T. Deep learning with convolutional neural networks for decoding and visualization of EEG pathology. In Proceedings of the 2017 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 2 December 2017. [Google Scholar] [CrossRef]
- Hosseini, M.-P.; Soltanian-Zadeh, H.; Elisevich, K.; Pompili, D. Cloud-based deep learning of big EEG data for epileptic seizure prediction. In Proceedings of the 2016 IEEE Global Conference on Signal and Information Processing (GlobalSIP), Washington, WA, USA, 7–9 December 2016. [Google Scholar] [CrossRef]
- O’Brien, T.J.; O’Connor, M.K.; Mullan, B.P.; Brinkmann, B.H.; Hanson, D.; Jack, C.R.; So, E.L. Subtraction ictal SPET co-registered to MRI in partial epilepsy: Description and technical validation of the method with phantom and patient studies. Nucl. Med. Commun. 1998, 19, 31–45. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.J.; So, E.L.; Mullan, B.P.; Hauser, M.F.; Brinkmann, B.H.; Bohnen, N.I.; Hanson, D.; Cascino, G.D.; Jack, C.R., Jr.; Sharbrough, F.W. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology 1998, 50, 445–454. [Google Scholar] [CrossRef]
- O’Brien, T.J.; So, E.L.; Mullan, B.P.; Hauser, M.F.; Brinkmann, B.H.; Jack, C.R., Jr.; Cascino, G.D.; Meyer, F.B.; Sharbrough, F.W. Subtraction SPECT co-registered to MRI improves postictal SPECT localization of seizure foci. Neurology 1999, 52, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Newey, C.R.; Wong, C.; Wang, Z.I.; Chen, X.; Wu, G.; Alexopoulos, A.V. Optimizing SPECT SISCOM analysis to localize seizure-onset zone by using varying z scores. Epilepsia 2013, 54, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Dupont, P.; Van Paesschen, W.; Palmini, A.; Ambayi, R.; Van Loon, J.; Goffin, J.; Weckhuysen, S.; Sunaert, S.; Thomas, B.; Demaerel, P.; et al. Ictal perfusion patterns associated with single MRI-visible focal dysplastic lesions: Implications for the noninvasive delineation of the epileptogenic zone. Epilepsia 2006, 47, 1550–1557. [Google Scholar] [CrossRef]
- Chen, T.; Guo, L. The role of SISCOM in preoperative evaluation for patients with epilepsy surgery: A meta-analysis. Seizure 2016, 41, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, N.J.; Worrell, G.A.; Stead, S.M.; Brinkmann, B.H.; Mullan, B.P.; O’Brien, T.J.; So, E.L. Ictal SPECT statistical parametric mapping in temporal lobe epilepsy surgery. Neurology 2010, 74, 70–76. [Google Scholar] [CrossRef]
- McNally, K.A.; Paige, A.L.; Varghese, G.; Zhang, H.; Novotny, E.J., Jr.; Spencer, S.S.; Zubal, I.G.; Blumenfeld, H. Localizing value of ictal-interictal SPECT analyzed by SPM (ISAS). Epilepsia 2005, 46, 1450–1464. [Google Scholar] [CrossRef]
- Lenkov, D.N.; Volnova, A.B.; Pope, A.R.; Tsytsarev, V. Advantages and limitations of brain imaging methods in the research of absence epilepsy in humans and animal models. J. Neurosci. Methods 2013, 212, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Eugène, F.; Lévesque, J.; Mensour, B.; Leroux, J.M.; Beaudoin, G.; Bourgouin, P.; Beauregard, M. The impact of individual differences on the neural circuitry underlying sadness. Neuroimage 2003, 19, 354–364. [Google Scholar] [CrossRef]
- Peres, J.; Nasello, A.G. Psychotherapy and neuroscience: Towards closer integration. Int. J. Psychol. 2008, 43, 943–957. [Google Scholar] [CrossRef]
- Goldmann, A.; Chien, D.; Edelman, R.R. Image artifacts in fast magnetic resonance imaging. Top Magn. Reson. Imaging 1992, 4, 35–45. [Google Scholar] [CrossRef]
- Weiss, R.A.; Saint-Louis, L.A.; Haik, B.G.; McCord, C.D.; Taveras, J.L. Mascara and eyelining tattoos: MRI artifacts. Ann. Ophthalmol. 1989, 21, 129–131. [Google Scholar] [PubMed]
- Watzke, O.; Kalender, W.A. A pragmatic approach to metal artifact reduction in CT: Merging of metal artifact reduced images. Eur. Radiol. 2004, 14, 849–856. [Google Scholar] [CrossRef]
- Giglio, P.; Gilbert, M.R. Cerebral radiation necrosis. Neurologist 2003, 9, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Schussler, G. Neurobiologie und Psychotherapie [Neurobiology and psychotherapy]. Z. Psychosom. Med. Psychother. 2004, 50, 406–429. [Google Scholar]
- Engel, J., Jr.; Pitkänen, A.; Loeb, J.A.; Dudek, F.E.; Bertram, E.H., 3rd; Cole, A.J.; Moshé, S.L.; Wiebe, S.; Jensen, F.E.; Mody, I.; et al. Epilepsy biomarkers. Epilepsia 2013, 54 (Suppl. S4), 61–69. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Engel, J., Jr. Past and present definitions of epileptogenesis and its biomarkers. Neurotherapeutics 2014, 11, 231–241. [Google Scholar] [CrossRef]
- Pitkänen, A.; EkolleNdode-Ekane, X.; Lapinlampi, N.; Puhakka, N. Epilepsy biomarkers—Toward etiology and pathology specificity. Neurobiol. Dis. 2019, 123, 42–58. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.S.; Sharp, P.A. Roles for microRNAs in conferring robustness to biological processes. Cell 2012, 149, 515–524. [Google Scholar] [CrossRef]
- Tan, C.L.; Plotkin, J.L.; Venø, M.T.; von Schimmelmann, M.; Feinberg, P.; Mann, S.; Handler, A.; Kjems, J.; Surmeier, D.J.; O’Carroll, D.; et al. MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science 2013, 342, 1254–1258. [Google Scholar] [CrossRef]
- Aronica, E.; Fluiter, K.; Iyer, A.; Zurolo, E.; Vreijling, J.; van Vliet, E.A.; Baayen, J.C.; Gorter, J.A. Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur. J. Neurosci. 2010, 31, 1100–1107. [Google Scholar] [CrossRef]
- Jimenez-Mateos, E.M.; Engel, T.; Merino-Serrais, P.; McKiernan, R.C.; Tanaka, K.; Mouri, G.; Sano, T.; O’Tuathaigh, C.; Waddington, J.L.; Prenter, S.; et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat. Med. 2012, 18, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Mateos, E.M.; Engel, T.; Merino-Serrais, P.; Fernaud-Espinosa, I.; Rodriguez-Alvarez, N.; Reynolds, J.; Reschke, C.R.; Conroy, R.M.; McKiernan, R.C.; deFelipe, J.; et al. Antagomirs targeting microRNA-134 increase hippocampal pyramidal neuron spine volume in vivo and protect against pilocarpine-induced status epilepticus. Brain Struct. Funct. 2015, 220, 2387–2399. [Google Scholar] [CrossRef]
- Kan, A.A.; van Erp, S.; Derijck, A.A.; de Wit, M.; Hessel, E.V.; O’Duibhir, E.; de Jager, W.; Van Rijen, P.C.; Gosselaar, P.H.; de Graan, P.N.; et al. Genome-wide microRNA profiling of human temporal lobe epilepsy identifies modulators of the immune response. Cell Mol. Life Sci. 2012, 69, 3127–3145. [Google Scholar] [CrossRef]
- Iyer, A.; Zurolo, E.; Prabowo, A.; Fluiter, K.; Spliet, W.G.; van Rijen, P.C.; Gorter, J.A.; Aronica, E. MicroRNA-146a: A key regulator of astrocyte-mediated inflammatory response. PLoS ONE 2012, 7, e44789. [Google Scholar] [CrossRef]
- Henshall, D.C.; Hamer, H.M.; Pasterkamp, R.J.; Goldstein, D.B.; Kjems, J.; Prehn, J.H.M.; Schorge, S.; Lamottke, K.; Rosenow, F. MicroRNAs in epilepsy: Pathophysiology and clinical utility. Lancet Neurol. 2016, 15, 1368–1376. [Google Scholar] [CrossRef]
- Turchinovich, A.; Weiz, L.; Burwinkel, B. Extracellular miRNAs: The mystery of their origin and function. Trends Biochem. Sci. 2012, 37, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Spain, E.; Jimenez-Mateos, E.M.; Raoof, R.; ElNaggar, H.; Delanty, N.; Forster, R.J.; Henshall, D.C. Direct, non-amplified detection of microRNA-134 in plasma from epilepsy patients. RSC Adv. 2015, 5, 90071–90078. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.T.; Tan, L.; Tian, Y.; Ma, J.; Tan, C.C.; Wang, H.F.; Liu, Y.; Tan, M.S.; Jiang, T.; et al. Genome-wide circulating microRNA expression profiling indicates biomarkers for epilepsy. Sci. Rep. 2015, 5, 9522. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, L.; Tan, L.; Tian, Y.; Ma, J.; Tan, C.C.; Wang, H.F.; Liu, Y.; Tan, M.S.; Jiang, T.; et al. Circulating microRNAs are promising novel biomarkers for drug-resistant epilepsy. Sci. Rep. 2015, 5, 10201. [Google Scholar] [CrossRef] [PubMed]
- Raoof, R.; Jimenez-Mateos, E.M.; Bauer, S.; Tackenberg, B.; Rosenow, F.; Lang, J.; Onugoren, M.D.; Hamer, H.; Huchtemann, T.; Körtvélyessy, P.; et al. Cerebrospinal fluid microRNAs are potential biomarkers of temporal lobe epilepsy and status epilepticus. Sci. Rep. 2017, 7, 3328. [Google Scholar] [CrossRef]
- Avansini, S.H.; de Sousa Lima, B.P.; Secolin, R.; Santos, M.L.; Coan, A.C.; Vieira, A.S.; Torres, F.R.; Carvalho, B.S.; Alvim, M.K.; Morita, M.E.; et al. MicroRNA hsa-miR-134 is a circulating biomarker for mesial temporal lobe epilepsy. PLoS ONE 2017, 12, e0173060. [Google Scholar] [CrossRef]
- Raoof, R.; Bauer, S.; El Naggar, H.; Connolly, N.M.C.; Brennan, G.P.; Brindley, E.; Hill, T.; McArdle, H.; Spain, E.; Forster, R.J.; et al. Dual-center, dual-platform microRNA profiling identifies potential plasma biomarkers of adult temporal lobe epilepsy. EBioMedicine 2018, 38, 127–141. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.; Sun, Z.; Kong, G.; Yan, X.; Wang, Y.; Wang, X.; Wen, Y.; Liu, X.; Zheng, H.; et al. High-Throughput Data of Circular RNA Profiles in Human Temporal Cortex Tissue Reveals Novel Insights into Temporal Lobe Epilepsy. Cell PhysiolBiochem. 2018, 45, 677–691. [Google Scholar] [CrossRef]
- Antônio, L.G.L.; Freitas-Lima, P.; Pereira-da-Silva, G.; Assirati, J.A., Jr.; Matias, C.M.; Cirino, M.L.A.; Tirapelli, L.F.; Velasco, T.R.; Sakamoto, A.C.; Carlotti, C.G., Jr.; et al. Expression of MicroRNAs miR-145, miR-181c, miR-199a and miR-1183 in the Blood and Hippocampus of Patients with Mesial Temporal Lobe Epilepsy. J. Mol. Neurosci. 2019, 69, 580–587. [Google Scholar] [CrossRef]
- An, N.; Zhao, W.; Liu, Y.; Yang, X.; Chen, P. Elevated serum miR-106b and miR-146a in patients with focal and generalized epilepsy. Epilepsy Res. 2016, 127, 311–316. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Wang, Z.; Zhang, Y.; Che, N.; Luo, X.; Tan, Z.; Sun, X.; Li, X.; Yang, K.; et al. Expression of microRNA-129-2-3p and microRNA-935 in plasma and brain tissue of human refractory epilepsy. Epilepsy Res. 2016, 127, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Y.; Tan, Z.; Che, N.; Ji, A.; Luo, X.; Sun, X.; Li, X.; Yang, K.; Wang, G.; et al. Serum MicroRNA-4521 is a Potential Biomarker for Focal Cortical Dysplasia with Refractory Epilepsy. Neurochem. Res. 2016, 41, 905–912. [Google Scholar] [CrossRef]
- Wang, R.; Zeng, G.Q.; Liu, X.; Tong, R.Z.; Zhou, D.; Hong, Z. Evaluation of serum matrix metalloproteinase-3 as a biomarker for diagnosis of epilepsy. J. Neurol. Sci. 2016, 367, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zeng, G.Q.; Tong, R.Z.; Zhou, D.; Hong, Z. Serum matrix metalloproteinase-2: A potential biomarker for diagnosis of epilepsy. Epilepsy Res. 2016, 122, 114–119. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, W.; Liu, L.; Tao, S.; Xia, Z.; Qi, L.; Huang, M. Identification of serum miRNAs differentially expressed in human epilepsy at seizure onset and post-seizure. Mol. Med. Rep. 2016, 14, 5318–5324. [Google Scholar] [CrossRef] [PubMed]
- Bekenstein, U.; Mishra, N.; Milikovsky, D.Z.; Hanin, G.; Zelig, D.; Sheintuch, L.; Berson, A.; Greenberg, D.S.; Friedman, A.; Soreq, H. Dynamic changes in murine forebrain miR-211 expression associate with cholinergic imbalances and epileptiform activity. Proc. Natl. Acad. Sci. USA 2017, 114, E4996–E5005. [Google Scholar] [CrossRef]
- McArdle, H.; Jimenez-Mateos, E.M.; Raoof, R.; Carthy, E.; Boyle, D.; ElNaggar, H.; Delanty, N.; Hamer, H.; Dogan, M.; Huchtemann, T.; et al. “TORNADO”—Theranostic One-Step RNA Detector; microfluidic disc for the direct detection of microRNA-134 in plasma and cerebrospinal fluid. Sci. Rep. 2017, 7, 1750. [Google Scholar] [CrossRef]
- Che, N.; Zu, G.; Zhou, T.; Wang, X.; Sun, Y.; Tan, Z.; Liu, Y.; Wang, D.; Luo, X.; Zhao, Z.; et al. Aberrant Expression of miR-323a-5p in Patients with Refractory Epilepsy Caused by Focal Cortical Dysplasia. Genet. Test Mol. Biomark. 2017, 21, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, H.; Xie, W.; Meng, F.; Zhang, K.; Jiang, Y.; Zhang, X.; Zhang, J. Altered microRNA profiles in plasma exosomes from mesial temporal lobe epilepsy with hippocampal sclerosis. Oncotarget 2017, 8, 4136–4146. [Google Scholar] [CrossRef]
- Elnady, H.G.; Abdelmoneam, N.; Eissa, E.; Hamid, E.R.A.; Zeid, D.A.; Abo-Shanab, A.M.; Atta, H.; Kholoussi, N.M. MicroRNAs as Potential Biomarkers for Childhood Epilepsy. Open Access Maced. J. Med. Sci. 2019, 7, 3965–3969. [Google Scholar] [CrossRef]
- Zhao, J.; Sang, Y.; Zhang, Y.; Zhang, D.; Chen, J.; Liu, X. Efficacy of levetiracetam combined with sodium valproate on pediatric epilepsy and its effect on serum miR-106b in children. Exp. Ther. Med. 2019, 18, 4436–4442. [Google Scholar] [CrossRef]
- Li, N.; Pan, J.; Liu, W.; Li, Y.; Li, F.; Liu, M. MicroRNA-15a-5p serves as a potential biomarker and regulates the viability and apoptosis of hippocampus neuron in children with temporal lobe epilepsy. Diagn. Pathol. 2020, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, J.; Cao, S. The Clinical Significance of miR-135b-5p and Its Role in the Proliferation and Apoptosis of Hippocampus Neurons in Children with Temporal Lobe Epilepsy. Dev. Neurosci. 2020, 42, 187–194. [Google Scholar] [CrossRef]
- Brennan, G.P.; Bauer, S.; Engel, T.; Jimenez-Mateos, E.M.; Del Gallo, F.; Hill, T.D.M.; Connolly, N.M.C.; Costard, L.S.; Neubert, V.; Salvetti, B.; et al. Genome-wide microRNA profiling of plasma from three different animal models identifies biomarkers of temporal lobe epilepsy. Neurobiol. Dis. 2020, 144, 105048. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Pan, H.Y.; Huang, J.B.; Liu, X.P.; Li, J.H.; Ho, C.J.; Tsai, M.H.; Yang, J.L.; Chen, S.F.; Chen, N.C.; et al. Circulating MicroRNAs from Serum Exosomes May Serve as a Putative Biomarker in the Diagnosis and Treatment of Patients with Focal Cortical Dysplasia. Cells 2020, 10, 1867. [Google Scholar] [CrossRef] [PubMed]
- Ioriatti, E.S.; Cirino, M.L.A.; Lizarte Neto, F.S.; Velasco, T.R.; Sakamoto, A.C.; Freitas-Lima, P.; Tirapelli, D.P.C.; Carlotti, C.G., Jr. Expression of circulating microRNAs as predictors of diagnosis and surgical outcome in patients with mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res. 2020, 166, 106373. [Google Scholar] [CrossRef]
- Leontariti, M.; Avgeris, M.; Katsarou, M.S.; Drakoulis, N.; Siatouni, A.; Verentzioti, A.; Alexoudi, A.; Fytraki, A.; Patrikelis, P.; Vassilacopoulou, D.; et al. Circulating miR-146a and miR-134 in predicting drug-resistant epilepsy in patients with focal impaired awareness seizures. Epilepsia 2020, 61, 959–970. [Google Scholar] [CrossRef]
- Dong, H.; Dong, B.; Zhang, N.; Liu, S.; Zhao, H. microRNA-182 Negatively Influences the Neuroprotective Effect of Apelin Against Neuronal Injury in Epilepsy. Neuropsychiatr. Dis. Treat. 2020, 16, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Martins-Ferreira, R.; Chaves, J.; Carvalho, C.; Bettencourt, A.; Chorão, R.; Freitas, J.; Samões, R.; Boleixa, D.; Lopes, J.; Ramalheira, J.; et al. Circulating microRNAs as potential biomarkers for genetic generalized epilepsies: A three microRNA panel. Eur. J. Neurol. 2020, 27, 660–666. [Google Scholar] [CrossRef]
- Niu, X.; Zhu, H.L.; Liu, Q.; Yan, J.F.; Li, M.L. MiR-194-5p serves as a potential biomarker and regulates the proliferation and apoptosis of hippocampus neuron in children with temporal lobe epilepsy. J. Chin. Med. Assoc. 2021, 1, 510–516. [Google Scholar] [CrossRef]
- De Benedittis, S.; Fortunato, F.; Cava, C.; Gallivanone, F.; Iaccino, E.; Caligiuri, M.E.; Castiglioni, I.; Bertoli, G.; Manna, I.; Labate, A.; et al. Circulating microRNA: The Potential Novel Diagnostic Biomarkers to Predict Drug Resistance in Temporal Lobe Epilepsy, a Pilot Study. Int. J. Mol. Sci. 2021, 12, 702. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Roivainen, R.; Lukasiuk, K. Development of epilepsy after ischaemic stroke. Lancet Neurol. 2016, 15, 185–197. [Google Scholar] [CrossRef]
- Stavropoulos, I.; Pervanidou, P.; Gnardellis, C.; Loli, N.; Theodorou, V.; Mantzou, A.; Soukou, F.; Sinani, O.; Chrousos, G.P. Increased hair cortisol and antecedent somatic complaints in children with a first epileptic seizure. Epilepsy Behav. 2017, 68, 146–152. [Google Scholar] [CrossRef]
- Wester, V.L.; van Rossum, E.F. Clinical applications of cortisol measurements in hair. Eur. J. Endocrinol. 2015, 173, M1–M10. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C. Analysis of cortisol in hair--state of the art and future directions. Brain Behav. Immun. 2012, 26, 1019–1029. [Google Scholar] [CrossRef]
- Gallek, M.J.; Skoch, J.; Ansay, T.; Behbahani, M.; Mount, D.; Manziello, A.; Witte, M.; Bernas, M.; Labiner, D.M.; Weinand, M.E. Cortical gene expression: Prognostic value for seizure outcome following temporal lobectomy and amygdalohippocampectomy. Neurogenetics 2016, 17, 211–218. [Google Scholar] [CrossRef]
- Guo, D.; Zeng, L.; Brody, D.L.; Wong, M. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS ONE 2013, 8, e64078. [Google Scholar] [CrossRef]
- Miszczuk, D.; Dębski, K.J.; Tanila, H.; Lukasiuk, K.; Pitkänen, A. Traumatic Brain Injury Increases the Expression of Nos1, Aβ Clearance, and Epileptogenesis in APP/PS1 Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 7010–7027. [Google Scholar] [CrossRef]
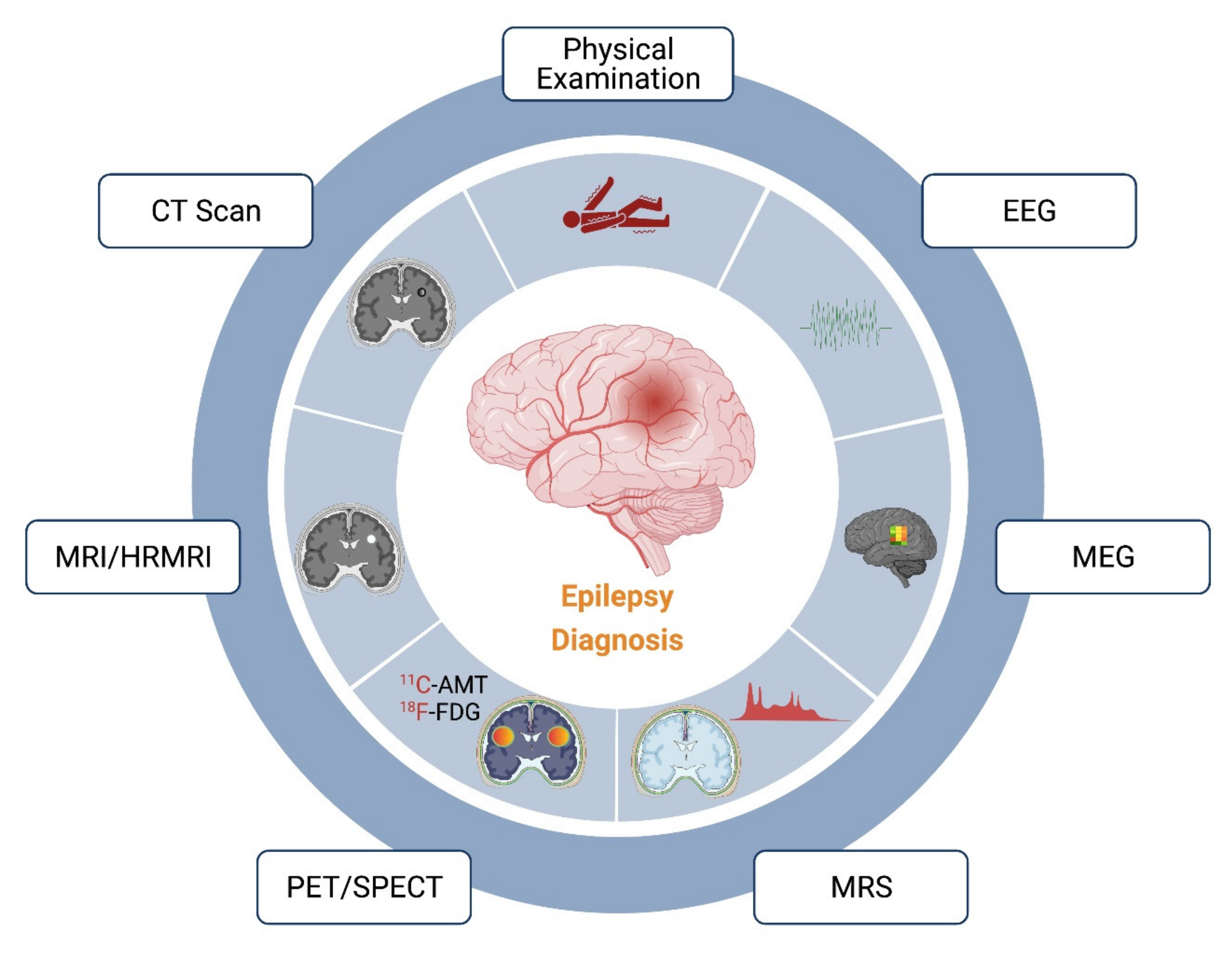
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandopadhyay, R.; Singh, T.; Ghoneim, M.M.; Alshehri, S.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C.; Ahmad, J.; Alhakamy, N.A.; Alfaleh, M.A.; et al. Recent Developments in Diagnosis of Epilepsy: Scope of MicroRNA and Technological Advancements. Biology 2021, 10, 1097. https://doi.org/10.3390/biology10111097
Bandopadhyay R, Singh T, Ghoneim MM, Alshehri S, Angelopoulou E, Paudel YN, Piperi C, Ahmad J, Alhakamy NA, Alfaleh MA, et al. Recent Developments in Diagnosis of Epilepsy: Scope of MicroRNA and Technological Advancements. Biology. 2021; 10(11):1097. https://doi.org/10.3390/biology10111097
Chicago/Turabian StyleBandopadhyay, Ritam, Tanveer Singh, Mohammed M. Ghoneim, Sultan Alshehri, Efthalia Angelopoulou, Yam Nath Paudel, Christina Piperi, Javed Ahmad, Nabil A. Alhakamy, Mohamed A. Alfaleh, and et al. 2021. "Recent Developments in Diagnosis of Epilepsy: Scope of MicroRNA and Technological Advancements" Biology 10, no. 11: 1097. https://doi.org/10.3390/biology10111097
APA StyleBandopadhyay, R., Singh, T., Ghoneim, M. M., Alshehri, S., Angelopoulou, E., Paudel, Y. N., Piperi, C., Ahmad, J., Alhakamy, N. A., Alfaleh, M. A., & Mishra, A. (2021). Recent Developments in Diagnosis of Epilepsy: Scope of MicroRNA and Technological Advancements. Biology, 10(11), 1097. https://doi.org/10.3390/biology10111097











