Haematological, Biochemical and Hormonal Biomarkers of Heat Intolerance in Military Personnel
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.3. Heat Tolerance Test
2.4. Venous Blood Collection
2.5. Statistical Analyses
2.6. Ethics Approval
3. Results
3.1. Differences in Haematological and Biochemical Variables between Heat-Tolerant and Intolerant Participants
3.2. Differences in Haematological and Biochemical Measures between Participants with a History of EHS and Participants without a History of EHS
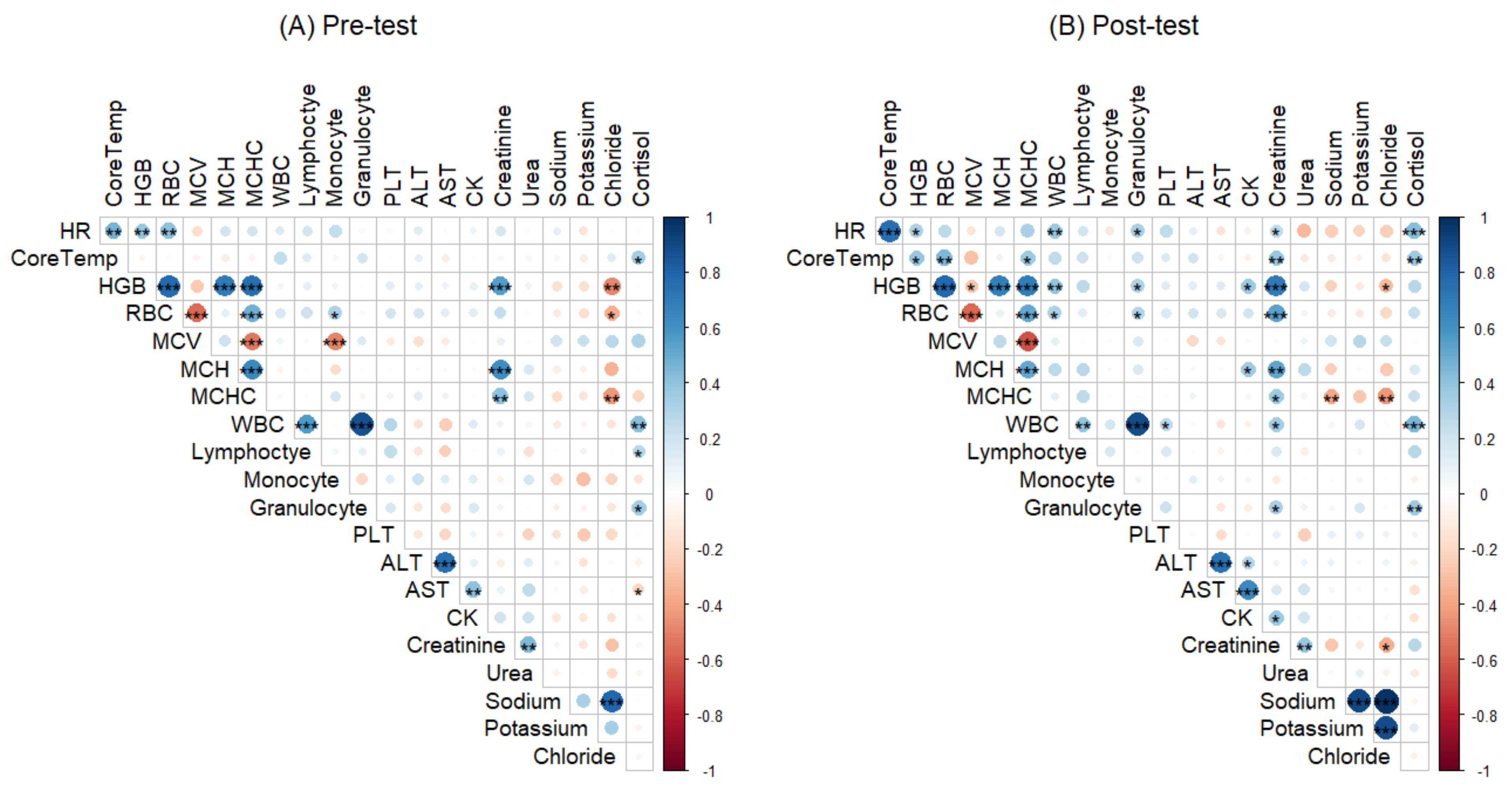
3.3. Correlations between Haematological, Biochemical Measures, Core Temperature and Heart Rate
4. Discussion
4.1. Limitations
4.2. Implications for Policy and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Cowan, T.; Purich, A.; Perkins, S.; Pezza, A.; Boschat, G.; Sadler, K. More Frequent, Longer, and Hotter Heat Waves for Australia in the Twenty-First Century. J. Clim. 2014, 27, 5851–5871. [Google Scholar] [CrossRef]
- Hughes, L.; Hanna, E.; Fenwick, J. The Silent Killer: Climate Change and the Health Impacts of Extreme Heat; 0994492642; Climate Council of Australia Ltd.: New South Wales, Australia, 2016; pp. 1–4. [Google Scholar]
- Australian Government Department of Defence. Defence at a Glance. Available online: https://www1.defence.gov.au/about/at-a-glance (accessed on 19 April 2021).
- Paterakis, T. Management of heat emergencies in the military setting. Int. Paramed. Pr. 2011, 1, 4–8. [Google Scholar] [CrossRef]
- Binkley, H.M.; Beckett, J.; Casa, D.J.; Kleiner, D.M.; Plummer, P.E. National Athletic Trainers’ Association Position Statement: Exertional Heat Illnesses. J. Athl. Train. 2002, 37, 329–343. [Google Scholar] [PubMed]
- O’Connor, F.G.; Heled, Y.; Deuster, P.A. Exertional heat stroke, the return to play decision, and the role of heat tolerance testing: A clinician’s dilemma. Curr. Sports Med. Rep. 2018, 17, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Druyan, A.; Ketko, I.; Yanovich, R.; Epstein, Y.; Heled, Y. Refining the distinction between heat tolerant and intolerant individuals during a heat tolerance test. J. Therm. Biol. 2013, 38, 539–542. [Google Scholar] [CrossRef]
- Shapiro, Y.; Magazanik, A.; Udassin, R.; Ben-Baruch, G.; Shvartz, E.; Shoenfeld, Y. Heat intolerance in former heatstroke patients. Ann. Intern. Med. 1979, 90, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Australian Defence Force. HD 286: Health Management for the Prevention and Treatment of Heat Casualties; Australian Defence Force, Ed.; ADF: Campbell, Australia, 2008.
- Alele, F.O.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O.; Crowe, M.J. Individual Anthropometric, Aerobic Capacity and Demographic Characteristics as Predictors of Heat Intolerance in Military Populations. Medicina 2021, 57, 173. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.M.; Cheuvront, S.N.; King, M.A.; Mayer, T.A.; Leon, L.R.; Kenefick, R.W. Use of the heat tolerance test to assess recovery from exertional heat stroke. Temperature 2019, 6, 106–119. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Shek, P.; Shephard, R.J. Immune dysfunction as a factor in heat illness. Crit. Rev. Immunol 1999, 19, 18–302. [Google Scholar] [CrossRef]
- Lim, C.L.; Mackinnon, L.T. The Roles of Exercise-Induced Immune System Disturbances in the Pathology of Heat Stroke. Sports Med. 2006, 36, 39–64. [Google Scholar] [CrossRef]
- Peake, J.; Peiffer, J.J.; Abbiss, C.R.; Nosaka, K.; Okutsu, M.; Laursen, P.B.; Suzuki, K. Body temperature and its effect on leukocyte mobilisation, cytokines and markers of neutrophil activation during and after exercise. Eur. J. Appl. Physiol. 2008, 102, 391–401. [Google Scholar] [CrossRef]
- Peake, J. Heat, Athletes, and Immunity. Am. J. Lifestyle Med. 2010, 4, 320–326. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Dugas, J.P.; McFarlin, B.K.; Nelson, M.J. Effect of exercise, heat stress, and hydration on immune cell number and function. Med. Sci. Sports Exerc. 2002, 34, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- DuBose, D.A.; Wenger, C.B.; Flinn, S.D.; Judy, T.A.; Dubovtsev, A.I.; Morehouse, D.H. Distribution and mitogen response of peripheral blood lymphocytes after exertional heat injury. J. Appl. Physiol. 2003, 95, 2381–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhind, S.G.; Gannon, G.A.; Shek, P.N.; Brenner, I.K.M.; Severs, Y.; Zamecnik, J.; Buguet, A.; Natale, V.M.; Shephard, R.J.; Radomski, M.W. Contribution of exertional hyperthermia to sympathoadrenal-mediated lymphocyte subset redistribution. J. Appl. Physiol. 1999, 87, 1178–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, I.; Shek, P.N.; Zamecnik, J.; Shephard, R.J. Stress hormones and the immunological responses to heat and exercise. Int. J. Sports Med. 1998, 19, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Stacey, M.; House, C.; Woods, D.; Brett, S.; Allsopp, A.; de Sa, D.R. A role for salivary cortisol measurement in assessing heat tolerance during exercise. In Proceedings of the Society for Endocrinology BES 2019, Brighton, UK, 11–13 November 2019. [Google Scholar]
- Choi, J.W.; Pai, S.H. Changes in hematologic parameters induced by thermal treatment of human blood. Ann. Clin. Lab. Sci. 2002, 32, 393–398. [Google Scholar]
- Saleh, M.M.; Mohammed, A.M.; Lateff, N.I.; Lattoofi, N.F.; Saleh, E.N. The Effect of High Temperature on the Hematological Parameters of Bakery Workers. Syst. Rev. Pharm. 2020, 11, 100–103. [Google Scholar]
- Shirreffs, S.M.; Sawka, M.N. Fluid and electrolyte needs for training, competition, and recovery. J. Sports Sci. 2011, 29 (Suppl. S1), S39–S46. [Google Scholar] [CrossRef]
- Sawka, M.N.; Montain, S.J. Fluid and electrolyte supplementation for exercise heat stress. Am. J. Clin. Nutr. 2000, 72, 564S–572S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenberger, G.; Candas, V.; Follenius, M.; Libert, J.P.; Kahn, J.M. Vascular fluid shifts and endocrine responses to exercise in the heat. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 123–129. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Lippi, G. Physical activity—An important preanalytical variable. Biochem. Med. 2014, 24, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Hashim, I.A. Clinical biochemistry of hyperthermia. Ann. Clin. Biochem. 2010, 47, 516–523. [Google Scholar] [CrossRef]
- Alele, F.O.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O.; Crowe, M.J. Epidemiology of Exertional Heat Illness in the Military: A Systematic Review of Observational Studies. Int. J. Environ. Res. Public Health 2020, 17, 7037. [Google Scholar] [CrossRef]
- Kazman, J.B.; Purvis, D.L.; Heled, Y.; Lisman, P.; Atias, D.; Van Arsdale, S.; Deuster, P.A. Women and exertional heat illness: Identification of gender specific risk factors. U.S. Army Med. Dep. J. 2015, 58–66. [Google Scholar]
- Lisman, P.; Kazman, J.B.; O’Connor, F.G.; Heled, Y.; Deuster, P.A. Heat tolerance testing: Association between heat intolerance and anthropometric and fitness measurements. Mil. Med. 2014, 179, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Shephard, R.J. Immune changes induced by exercise in an adverse environment. Can. J. Physiol. Pharmacol. 1998, 76, 539–546. [Google Scholar] [CrossRef]
- Schermann, H.; Hazut-Krauthammer, S.; Weksler, Y.; Spitzer, S.; Epstein, Y.; Kalmanovich, G.; Yanovich, R. When Should a Heat-Tolerance Test Be Scheduled After Clinical Recovery from an Exertional Heat Illness? J. Athl. Train. 2020, 55, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Rhind, S.G.; Gannon, G.A.; Shephard, R.J.; Buguet, A.; Shek, P.N.; Radomski, M.W. Cytokine induction during exertional hyperthermia is abolished by core temperature clamping: Neuroendocrine regulatory mechanisms. Int. J. Hyperth. 2004, 20, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.E.; McLellan, T.M.; Stapleton, J.M.; Hardcastle, S.G.; Kenny, G.P. Cortisol and interleukin-6 responses during intermittent exercise in two different hot environments with equivalent WBGT. J. Occup. Environ. Hyg. 2012, 9, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A.C. Exercise and circulating Cortisol levels: The intensity threshold effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Petruzzello, S.; Chludzinski, M.; Reed, J.; Woods, J. Selected hormonal and immunological responses to strenuous live-fire firefighting drills. Ergonomics 2005, 48, 55–65. [Google Scholar] [CrossRef]
- Caetano, P.C., Jr.; Castilho, M.L.; Raniero, L. Salivary Cortisol Responses and Session Ratings of Perceived Exertion to a Rugby Match and Fatigue Test. Percept. Mot. Ski. 2017, 124, 649–661. [Google Scholar] [CrossRef]
- Castro-Sepulveda, M.; Ramirez-Campillo, R.; Abad-Colil, F.; Monje, C.; Peñailillo, L.; Cancino, J.; Zbinden-Foncea, H. Basal Mild Dehydration Increase Salivary Cortisol After a Friendly Match in Young Elite Soccer Players. Front. Physiol. 2018, 9, 1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, K.A.; Carter, J. Overtraining, exercise, and adrenal insufficiency. J. Nov. Physiother. 2013, 3, 125. [Google Scholar]
- Costello, J.T.; Rendell, R.A.; Furber, M.; Massey, H.C.; Tipton, M.J.; Young, J.S.; Corbett, J. Effects of acute or chronic heat exposure, exercise and dehydration on plasma cortisol, IL-6 and CRP levels in trained males. Cytokine 2018, 110, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Cramer, M.N.; Jay, O. Biophysical aspects of human thermoregulation during heat stress. Auton. Neurosci. 2016, 196, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Van den Bos, R.; Harteveld, M.; Stoop, H. Stress and decision-making in humans: Performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology 2009, 34, 1449–1458. [Google Scholar] [CrossRef]
- Nowakowska, A.; Kostrzewa-Nowak, D.; Buryta, R.; Nowak, R. Blood Biomarkers of Recovery Efficiency in Soccer Players. Int. J. Environ. Res. Public Health 2019, 16, 3279. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.-A.; Park, K.D.; Ahn, J.; Park, Y.; Kim, Y.-J. Comparison of Changes in Biochemical Markers for Skeletal Muscles, Hepatic Metabolism, and Renal Function after Three Types of Long-distance Running: Observational Study. Medicine 2016, 95, e3657. [Google Scholar] [CrossRef]
- Das, A. Heat stress-induced hepatotoxicity and its prevention by resveratrol in rats. Toxicol. Mech. Methods 2011, 21, 393–399. [Google Scholar] [CrossRef]
- Ojanen, T.; Jalanko, P.; Kyröläinen, H. Physical fitness, hormonal, and immunological responses during prolonged military field training. Physiol. Rep. 2018, 6, e13850. [Google Scholar] [CrossRef] [PubMed]
- House, C.M.; Tipton, M.J.; Hopkins, P.M.; Roiz de Sa, D. Thermoregulation and markers of muscle breakdown in malignant hyperthermia susceptible volunteers during an acute heat tolerance test. J. Sci. Med. Sport 2019, 22, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, H.; Vincent, K. The effect of training status on the serum creatine kinase response, soreness and muscle function following resistance exercise. Int. J. Sports Med. 1997, 28, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Pereira, R.; Machado, M. The creatine kinase response to resistance exercise. J. Musculoskelet. Neuronal Interact. 2014, 14, 68–77. [Google Scholar]
- Carvalho, A.S.; Rodeia, S.C.; Silvestre, J.; Póvoa, P. Exertional heat stroke and acute liver failure: A late dysfunction. BMJ Case Rep. 2016, 2016, bcr2016214434. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.D.; King, M.A.; Gabrial, C.; Kenefick, R.W.; Leon, L.R. Biochemical recovery from exertional heat stroke follows a 16-day time course. PLoS ONE 2020, 15, e0229616. [Google Scholar] [CrossRef] [Green Version]
- Pryor, R.R.; Pryor, J.L.; Vandermark, L.W.; Adams, E.L.; Brodeur, R.M.; Schlader, Z.J.; Armstrong, L.E.; Lee, E.C.; Maresh, C.M.; Casa, D.J. Acute Kidney Injury Biomarker Responses to Short-Term Heat Acclimation. Int. J. Environ. Res. Public Health 2020, 17, 1325. [Google Scholar] [CrossRef] [Green Version]
- Junglee, N.A.; Felice, U.D.; Dolci, A.; Fortes, M.B.; Jibani, M.M.; Lemmey, A.B.; Walsh, N.P.; Macdonald, J.H. Exercising in a hot environment with muscle damage: Effects on acute kidney injury biomarkers and kidney function. Am. J. Physiol. Ren. Physiol. 2013, 305, F813–F820. [Google Scholar] [CrossRef] [PubMed]
- Anđelković, M.; Baralić, I.; Đorđević, B.; Stevuljević, J.K.; Radivojević, N.; Dikić, N.; Škodrić, S.R.; Stojković, M. Hematological and Biochemical Parameters in Elite Soccer Players During A Competitive Half Season. J. Med. Biochem. 2015, 34, 460–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-J.; Webb, H.E.; Garten, R.S.; Kamimori, G.H.; Evans, R.K.; Acevedo, E.O. Stress hormones and immunological responses to a dual challenge in professional firefighters. Int. J. Psychophysiol. 2010, 75, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Reichel, T.; Zeilinger, C. Role of heat shock proteins 70/90 in exercise physiology and exercise immunology and their diagnostic potential in sports. J. Appl. Physiol. 2019, 126, 916–927. [Google Scholar] [CrossRef]
- Heck, T.G.; Schöler, C.M.; de Bittencourt, P.I.H. HSP70 expression: Does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem. Funct. 2011, 29, 215–226. [Google Scholar] [CrossRef] [PubMed]
| Variables | RV ‡ | Group | Pre-Test | Post-Test | ∆ from Pre-Test to Post-Test † |
|---|---|---|---|---|---|
| Haemoglobin (g/L) * | 110–180 | Heat tolerant | 134.10 (12.82) | 134.82 (14.80) | 0.50 (3.50) |
| Heat intolerant | 140.00 (13.13) | 142.17 (14.47) | 2.00 (11.00) | ||
| RBC × 1012/L * | 4.0–6.0 | Heat tolerant | 4.59 (0.35) | 4.67 (0.38) | 0.065 (0.12) |
| Heat intolerant | 4.73 (0.28) | 4.88 (0.33) | 0.14 (0.38) | ||
| MCV (fL) * | 80.0–99.9 | Heat tolerant | 92.59 (5.46) | 92.06 (5.89) | −0.35 (1.92) |
| Heat intolerant | 92.57 (5.03) | 91.47 (5.69) | −0.60 (1.35) | ||
| MCH (pg) * | 27.0–31.0 | Heat tolerant | 29. 27 (1.64) | 28.92 (1.89) | −0.20 (0.88) |
| Heat intolerant | 29.58 (1.77) | 29.12 (1.95) | −0.20 (0.72) | ||
| MCHC (g/L) * | 330.0–370.0 | Heat tolerant | 316.69 (22.47) | 313.46 (23.95) | −1.00 (10.25) |
| Heat intolerant | 317.72 (28.88) | 320.43 (25.92) | 1.00 (12.25) | ||
| WBC × 109/L* | 4.5–10.5 | Heat tolerant | 5.40 (1.26) | 6.74 (1.70) | 0.95 (1.74) |
| Heat intolerant | 5.96 (1.15) | 7.71 (1.58) | 1.88 (1.84) | ||
| Lymphocytes × 109/L † | 1.2–3.4 | Heat tolerant | 1.70 (0.62) | 1.84 (0.50) | 0.25 (0.44) |
| Heat intolerant | 1.77 (0.83) | 1.77 (0.92) | 0.20 (0.54) | ||
| Monocytes × 109/L † | 0.1–0.6 | Heat tolerant | 0.39 (0.18) | 0.45 (0.19) | 0.03 (0.23) |
| Heat intolerant | 0.37 (0.18) | 0.44 (0.23) | 0.10 (0.24) | ||
| Granulocytes × 109/L* | 1.4–6.5 | Heat tolerant | 3.25 (1.00) | 4.37 (1.42) | 0.95 (1.46) |
| Heat intolerant | 3.65 (1.07) | 5.30 (1.69) | 1.29 (2.09) | ||
| Platelets × 109/L* | 150–450 | Heat tolerant | 232.45 (69.03) | 256.36 (78.92) | 19.50 (30.75) |
| Heat intolerant | 235.36 (74.53) | 262.55 (86.46) | 24.00 (33.00) |
| Variables | RV ‡ | Group | Pre-Test | Post-Test | ∆ from Pre-Test to Post-Test † |
|---|---|---|---|---|---|
| ALT (U/L) † | 7–52 | Heat tolerant | 18.80 (10.10) a | 20.25 (11.60) | 0.80 (1.55) |
| Heat intolerant | 24.60 (11.00) | 26.70 (12.90) | 1.90 (1.95) b | ||
| AST (U/L) † | 13–39 | Heat tolerant | 25.10 (13.10) | 24.70 (13.40) | 2.00 (3.13) |
| Heat intolerant | 26.80 (9.30) | 28.00 (11.30) | 3.25 (2.75) | ||
| CK (U/L) † | 30–223 | Heat tolerant | 140.35 (109.80) | 142.00 (121.20) | 12.75 (27.82) |
| Heat intolerant | 145.50 (141.00) | 164.85 (122.50) | 27.95 (24.50) b | ||
| Sodium (mEq/L) † | 136–145 | Heat tolerant | 138.72 (2.70) | 138.40 (3.00) | 0.45 (3.02) |
| Heat intolerant | 137.60 (2.10) | 137.70 (4.20) | −0.40 (5.50) | ||
| Potassium (mEq/L) † | 3.5–5.1 | Heat tolerant | 4.82 (2.00) | 4.31 (0.28) | −0.10 (0.33) |
| Heat intolerant | 4.33 (0.29) | 4.36 (0.54) | 0.07 (0.56) | ||
| Chloride (mEq/L) † | 98–107 | Heat tolerant | 103.15 (3.50) | 102.50 (3.00) | −0.45 (2.47) |
| Heat intolerant | 101.90 (3.30) | 99.60 (7.40) | −2.05 (6.42) | ||
| Cortisol (nmol/L) * | 193–690 | Heat tolerant | 341.37 (132.04) | 225.69(104.11) | −108.44 (186.29) b |
| Heat intolerant | 384.63(178.96) | 391.07 (182.51) | 5.07 (333.19) | ||
| Creatinine (µmol/L) * | 53–110 | Heat tolerant | 74.11 (11.93) | 78.28 (11.59) | 5.00 (5.50) |
| Heat intolerant | 75.41 (13.65) | 91.00 (13.85) | 13.00 (11.75) b | ||
| Urea (mmol/L) * | 2.50–8.93 | Heat tolerant | 5.65 (1.30) | 5.61 (1.21) | −0.07 (0.42) |
| Heat intolerant | 5.62 (1.57) | 5.65 (1.48) | 0.05 (0.68) |
| Variables | RV ‡ | Group | Pre-Test | Post-Test | ∆ from Pre-Test to Post-Test † |
|---|---|---|---|---|---|
| Haemoglobin (g/L) * | 110–180 | History of EHS | 142.93 (14.23) a | 145.81 (15.55) | 2.00 (9.00) |
| No history of EHS | 134.03 (11.93) | 134.63 (13.50) | 1.00 (3.00) | ||
| RBC × 1012/L * | 4.0–6.0 | History of EHS | 4.75 (0.29) | 4..91 (0.34) | 0.09 (0.69) |
| No history of EHS | 4.61 (0.33) | 4.70 (0.37) | 0.06 (0.16) | ||
| MCV (fL) * | 80.0–99.9 | History of EHS | 92.71 (5.54) | 91.74 (5.92) | −0.80 (1.85) |
| No history of EHS | 92.53 (5.18) | 91.82 (5.76) | −0.40 (1.45) | ||
| MCH (pg) * | 27.0–31.0 | History of EHS | 30.00 (2.00) | 30.00 (2.20) | −2.00 (0.90) |
| No history of EHS | 29.00 (1.50) | 29.00 (1.70) | −2.00 (0.60) | ||
| MCHC (g/L) * | 330–370 | History of EHS | 325.33 (24.15) | 321.38 (23.48) | 2.00 (18.50) |
| No history of EHS | 313.72 (25.11) | 314.42 (25.49) | −1.00 (9.00) | ||
| WBC × 109/L * | 4.5–10.5 | History of EHS | 6.00 (1.07) | 8.10 (1.59) | 1.90 (1.19) |
| No history of EHS | 5.49 (1.28) | 6.75 (1.60) | 0.75 (2.56) | ||
| Lymphocytes × 109/L † | 1.2–3.4 | History of EHS | 1.57 (0.90) | 1.82 (0.95) | 0.25 (0.40) |
| No history of EHS | 1.64 (0.45) | 1.84 (0.73) | 0.17 (0.57) | ||
| Monocytes × 109/L * | 0.1–0.6 | History of EHS | 0.33 (0.21) | 0.50 (0.44) | 0.10 (0.29) |
| No history of EHS | 0.40 (0.17) | 0.44 (0.15) | 0.03 (0.22) | ||
| Granulocytes × 109/L * | 1.4–6.5 | History of EHS | 3.89 (0.99) a | 5.62 (1.54) | 1.47 (1.54) |
| No history of EHS | 3.23 (1.02) | 4.41 (1.50) | 0.87 (1.85) | ||
| Platelets × 10^/L * | 150–450 | History of EHS | 267.93 (96.55) | 300.93 (105.73) | 27.00 (38.50) |
| No history of EHS | 219.44 (52.04) | 241.14 (62.21) | 19.00 (23.50) |
| Variables | RV ‡ | Group | Pre-Test | Post-Test | ∆ from Pre-Test to Post-Test † |
|---|---|---|---|---|---|
| ALT (U/L) † | 7–52 | History of EHS | 24.45 (16.00) | 26.55 (16.70) | 1.90 (2.45) |
| No history of EHS | 22.20 (12.80) | 23.50 (14.00) | 0.80 (1.90) | ||
| AST (U/L) † | 13–39 | History of EHS | 28.85 (12.30) | 31.90 (15.20) | 3.20 (4.40) |
| No history of EHS | 23.45 (9.30) | 24.80 (13.40) | 2.20 (3.20) | ||
| CK (U/L) † | 30–223 | History of EHS | 163.55 (172.80) | 176.35 (219.30) | 15.10 (26.85) |
| No history of EHS | 140.35 (96.70) | 153.50 (106.80) | 20.40 (27.10) | ||
| Sodium (mEq/L) * | 136–145 | History of EHS | 137.71 (1.62) | 137.99 (2.46) | −0.40 (2.80) |
| No history of EHS | 138.13 (3.82) | 133.99 (21.84) | 0.40 (3.95) | ||
| Potassium (mEq/L) * | 3.5–5.1 | History of EHS | 4.43 (0.34) | 4.46 (0.31) | 0.14 (0.40) |
| No history of EHS | 4.68 (1.78) | 4.20 (0.72) | −0.10 (0.33) | ||
| Chloride (mEq/L) * | 98–107 | History of EHS | 102.44 (3.39) | 101.36 (3.91) | −1.00 (2.60) |
| No history of EHS | 103.38 (3.30) | 99.10 (16.33) | −0.70 (3.80) | ||
| Cortisol (nmol/L) * | 193–690 | History of EHS | 385.06 (172.44) | 340.30 (214.10) | −25.73 (364.65) |
| No history of EHS | 351.08 (148.57) | 280.42 (137.30) | −122.84 (195.47) | ||
| Creatinine (µmol/L) * | 53–110 | History of EHS | 76.64 (13.00) | 90.50 (15.05) | 13.00 (17.50) b |
| No history of EHS | 73.92 (12.54) | 80.28 (11.966) | 6.00 (4.50) | ||
| Urea (mmol/L) * | 2.50–8.93 | History of EHS | 5.38 (1.14) | 5.59 (1.10) | 0.19 (0.68) b |
| No history of EHS | 5.73 (1.51) | 5.64 (1.42) | −0.14 (0.49) |
| Variables | B | S.E. | Wald | df | p Value | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| ALT | 0.026 | 0.018 | 2.238 | 1 | 0.135 | 1.037 | 0.992 | 1.063 |
| Creatinine | 0.149 | 0.065 | 5.152 | 1 | 0.023 | 1.160 | 1.020 | 1.319 |
| Sodium | 0.084 | 0.086 | 0.954 | 1 | 0.329 | 1.088 | 0.919 | 1.287 |
| Potassium | −2.872 | 2.253 | 1.625 | 1 | 0.202 | 0.057 | 0.001 | 4.681 |
| Cortisol | 0.015 | 0.006 | 7.185 | 1 | 0.007 | 1.015 | 1.004 | 1.026 |
| Gender (Ref: Female) | −2.192 | 1.573 | 1.942 | 1 | 0.163 | 0.112 | 0.005 | 2.436 |
| Age | −0.027 | 0.052 | 0.265 | 1 | 0.607 | 0.974 | 0.880 | 1.078 |
| EHS (Ref: No history of EHS) | 0.684 | 1.184 | 0.0334 | 1 | 0.563 | 1.983 | 0.195 | 20.175 |
| %BM Loss | −0.064 | 0.472 | 0.019 | 1 | 0.892 | 0.938 | 0.372 | 2.365 |
| RPE | 0.236 | 0.201 | 1.378 | 1 | 0.240 | 1.266 | 0.854 | 1.877 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alele, F.O.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O.; Crowe, M.J. Haematological, Biochemical and Hormonal Biomarkers of Heat Intolerance in Military Personnel. Biology 2021, 10, 1068. https://doi.org/10.3390/biology10101068
Alele FO, Malau-Aduli BS, Malau-Aduli AEO, Crowe MJ. Haematological, Biochemical and Hormonal Biomarkers of Heat Intolerance in Military Personnel. Biology. 2021; 10(10):1068. https://doi.org/10.3390/biology10101068
Chicago/Turabian StyleAlele, Faith O., Bunmi S. Malau-Aduli, Aduli E. O. Malau-Aduli, and Melissa J. Crowe. 2021. "Haematological, Biochemical and Hormonal Biomarkers of Heat Intolerance in Military Personnel" Biology 10, no. 10: 1068. https://doi.org/10.3390/biology10101068
APA StyleAlele, F. O., Malau-Aduli, B. S., Malau-Aduli, A. E. O., & Crowe, M. J. (2021). Haematological, Biochemical and Hormonal Biomarkers of Heat Intolerance in Military Personnel. Biology, 10(10), 1068. https://doi.org/10.3390/biology10101068






