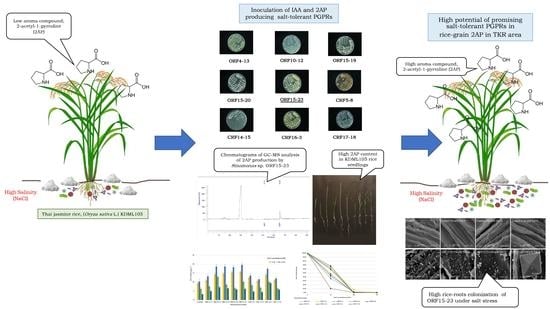
Enhancement of the Aroma Compound 2-Acetyl-1-pyrroline in Thai Jasmine Rice (Oryza sativa) by Rhizobacteria under Salt Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice Rhizobacterial Isolates
2.2. Determination of 2AP Production by Rhizobacterial Isolates in Liquid Culture
2.3. Effect of Rhizobacterial Inoculation on 2AP Level in Rice Seedlings Grown under Salt Stress
2.3.1. Experimental Design
2.3.2. Preparation of Rhizobacterial Isolates
2.3.3. Rice Seedlings
2.4. Quantification of 2-Acetyl-1-pyrroline in KDML105 Rice Leaves
2.5. Survival of Rhizobacterial Isolates
2.6. Microscopic Analysis of Rhizobacterial Isolates
2.7. Statistical Analysis
3. Results
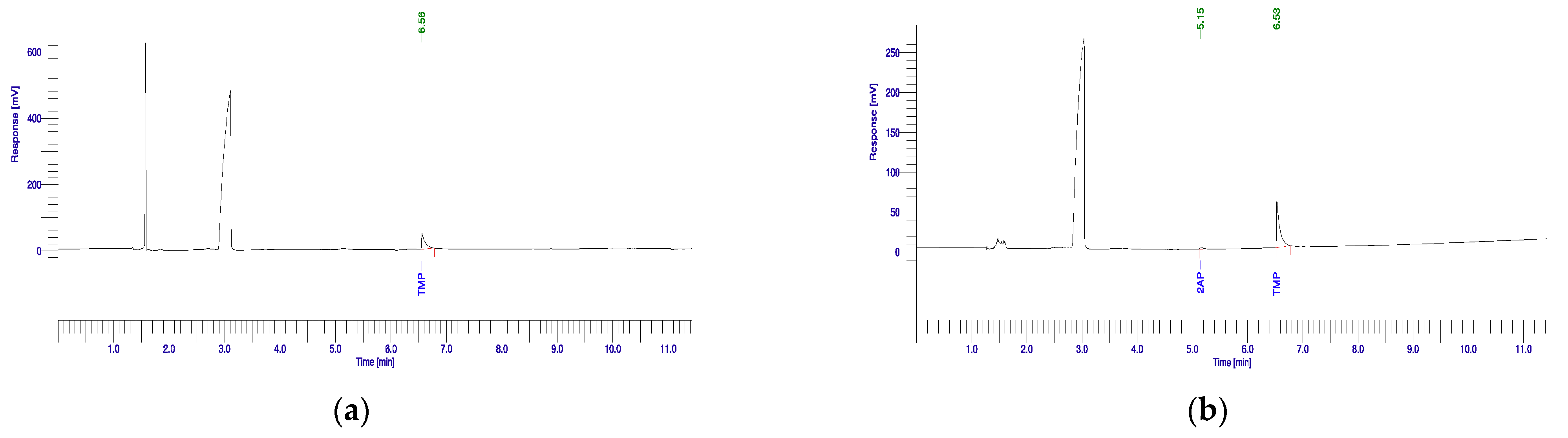
3.1. 2-Acetyl-1-pyrroline Production Potential of Rhizobacterial Isolates
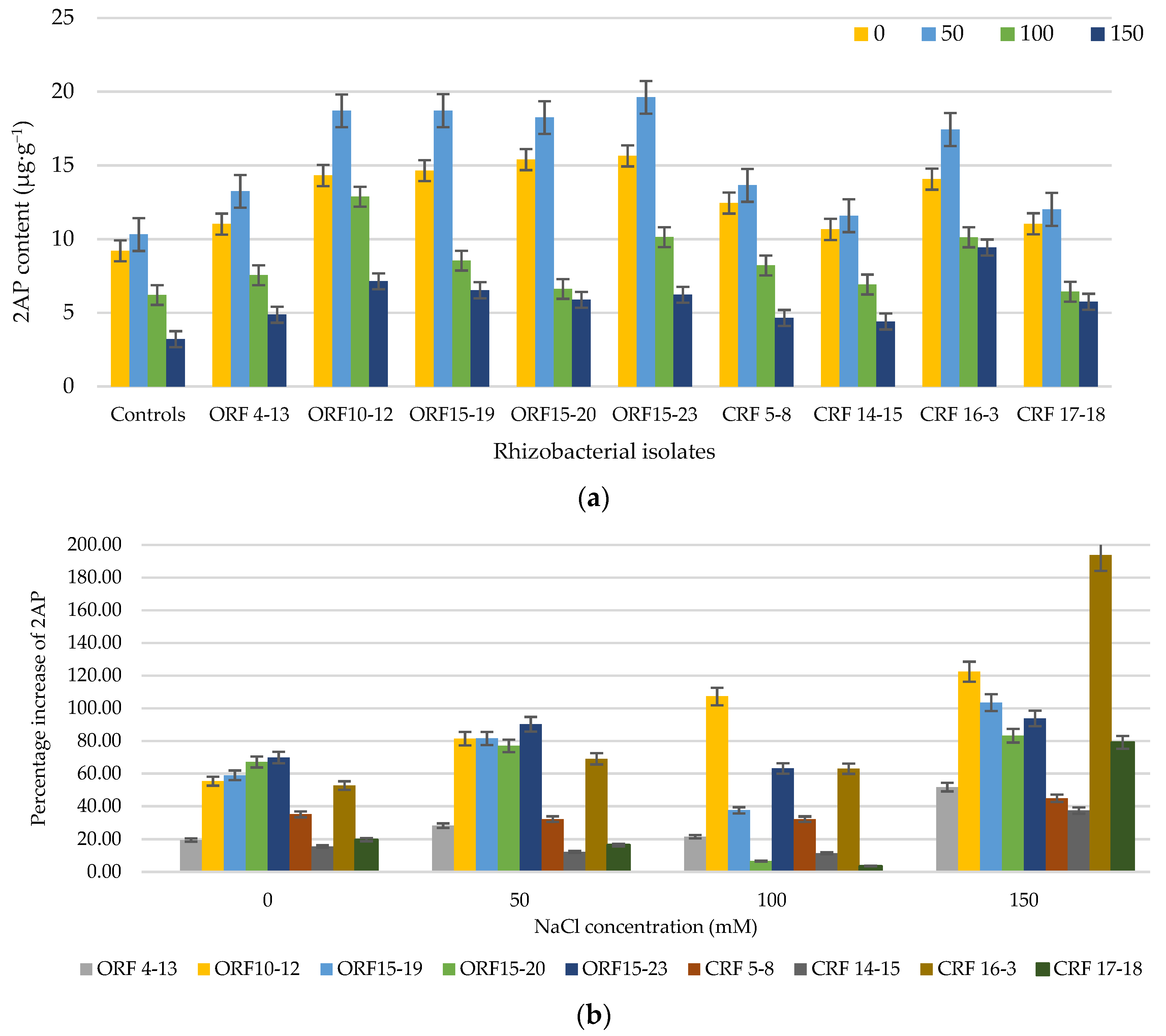
3.2. Effect of Rhizobacterial Isolate Inoculation on the 2AP Level of KDML105 Rice Seedlings
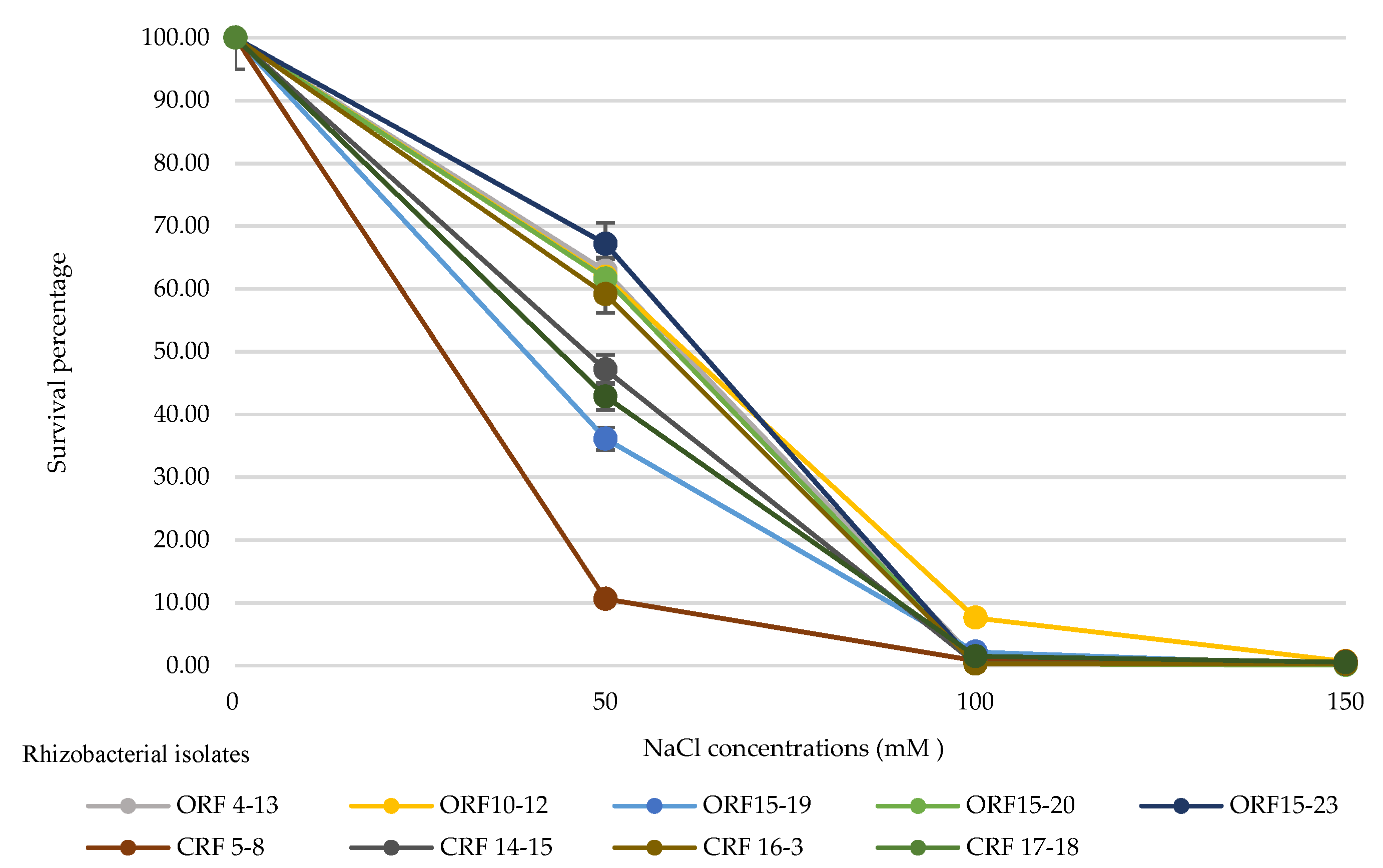
3.3. Effect of Different NaCl Concentrations on the Survival of Rhizobacterial Isolates at 30 Days after Inoculation
3.4. Microscopic Observations of Root Colonization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kongpun, A.; Jaisiri, P.; Rerkasem, B.; Prom-u-thai, C. Impact of soil salinity on grain yield and aromatic compound in Thai Hom Mali rice cv. Khao Dawk Mali 105. Agric. Nat. Resour. 2020, 54, 74–78. [Google Scholar]
- Arunin, S.; Pongwichian, P. Salt-affected soils and management in Thailand. Bull. Soc. Sea Water Sci. Jpn. 2015, 69, 319–325. [Google Scholar]
- Suebpongsang, P.; Ekasingh, B.; Cramb, R. Commercialisation of Rice Farming in Northeast Thailand. In White Gold: The Commercialisation of Rice Farming in the Lower Mekong Basin; Cramb, R., Ed.; Palgrave Macmillan: Singapore, 2020; pp. 39–68. [Google Scholar] [CrossRef] [Green Version]
- Yoshihashi, T.; Nguyen, T.T.H.; Kabaki, N. Area dependency of 2-acetyl-1-pyrroline content in an aromatic rice variety, Khao Dawk Mali 105. Jpn. Agric. Res. Q. 2004, 38, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Dhondge, H.V.; Pable, A.A.; Barvkar, V.T.; Dastager, S.G.; Nadaf, A.B. Rhizobacterial consortium mediated aroma and yield enhancement in basmati and non-basmati rice (Oryza sativa L.). J. Biotechnol. 2021, 328, 47–58. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, J.C.; Bland, W.L. Genotypic variation in crop plant root systems. Adv. Agron. 1987, 41, 91–145. [Google Scholar]
- Lasudee, K.; Tokuyama, S.; Lumyong, S.; Pathom-aree, W. Actinobacteria associated with arbuscular mycorrhizal Funneliformis mosseae spores, taxonomic characterization and their beneficial traits to plants: Evidence obtained from mung bean (Vigna radiata) and Thai jasmine rice (Oryza sativa). Front. Microbiol. 2018, 9, 1247. [Google Scholar] [CrossRef]
- Vanavichit, A.; Kamolsukyeunyong, W.; Siangliw, M.; Siangliw, J.L.; Traprab, S.; Ruengphayak, S.; Chaichoompu, E.; Saensuk, C.; Phuvanartnarubal, E.; Toojinda, T.; et al. Thai Hom Mali Rice: Origin and breeding for subsistence rainfed lowland rice system. Rice 2018, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Gay, F.; Maraval, I.; Roques, S.; Gunata, Z.; Boulanger, R.; Audebert, A.; Mestres, C. Effect of salinity on yield and 2-acetyl-1-pyrroline content in the grains of three fragrant rice cultivars (Oryza sativa L.) in Camargue (France). Field Crop. Res. 2010, 117, 154–160. [Google Scholar] [CrossRef]
- Hakim, M.; Juraimi, A.S.; Hanafi, M.; Ismail, M.R.; Selamat, A.; Rafii, M.; Latif, M. Biochemical and anatomical changes and yield reduction in rice (Oryza sativa L.) under varied salinity regimes. BioMed Res. Int. 2014, 2014, 11. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Zhang, J.-H.; Zhong, C.; Zhu, L.-F.; Cao, X.-C.; Yu, S.-M.; Allen, B.J.; Hu, J.-J.; Jin, Q.-Y. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, P.; Samaddar, S.; Niinemets, U.; Sa, T.-M. Brevibacterium linens RS16 confers salt tolerance to Oryza sativa genotypes by regulating antioxidant defense and H+ ATPase activity. Microbiol. Res. 2018, 215, 89–101. [Google Scholar] [CrossRef]
- Fahad, S.; Noor, M.; Adnan, M.; Khan, M.A.; Rahman, I.U.; Alam, M.; Khan, I.A.; Ullah, H.; Mian, I.A.; Hassan, S.; et al. Abiotic Stress and Rice Grain Quality. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J., Eds.; Elsevier: Cambridge, UK, 2019; pp. 571–583. [Google Scholar]
- Razzaz, A.; Ali, A.; Safdar, L.B.; Zafar, M.M.; Rui, Y.; Shakeel, A.; Shaukat, A.; Ashraf, M.; Gong, W.; Yuan, Y. Salt stress induces physiochemical alterations in rice grain composition and quality. J. Food Sci. 2020, 85, 14–20. [Google Scholar] [CrossRef] [PubMed]
- US Salinity Laboratory Staff. Diagnosis and Improvement of Saline and Alkali Soils; US Department of Agriculture, Agricultural Handbook, US Government Printer: Washington, DC, USA, 1954; Volume 60.
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613. [Google Scholar] [CrossRef]
- Suwanarit, A.; Somchai, K.; Warunee, V.; Patcharee, T.; Sirichai, S.; Romyen, P.; Wattanapryapkul, S.; Naklangs, K.; Rotjanakusol, S.; Pornurisnits, P.; et al. Effects of the Fertilizer Elements, Sulphur, Sodium and Salinity on Yield and Quality of Rice; Kasetsart University Annual Report: Bangkok, Thailand, 1991. [Google Scholar]
- Wanichananan, P.; Kirdmaneea, C.; Vutiyano, C. Effect of salinity on biochemical and physiological characteristics in correlation to selection of salt-tolerance in aromatic rice (Oryza sativa L.). ScienceAsia 2003, 29, 333–339. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Mahal, S.S.; Kaur, A. Physicochemical, cooking quality and productivity of rice as influenced by planting methods, planting density and nitrogen management. Int. J. Food Agric. Vet. Sci. 2015, 5, 33–40. [Google Scholar]
- Ayuso-Calles, M.; Flores-Felix, J.D.; Rivas, R. Overview of the role of rhizobacteria in plant salt stress tolerance. Agronomy 2021, 11, 1759. [Google Scholar] [CrossRef]
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, M.; Mubeen, F.; Naqqash, T. Halotolerant PGPR: A hope for cultivation of saline soils. J. King Saud Univ.-Sci. 2019, 31, 1195–1201. [Google Scholar] [CrossRef]
- Singh, R.; Singh, U.; Khush, G.; Rohilla, R.; Singh, J.; Singh, G.; Shekhar, K. Small and Medium Grained Aromatic Rices of India. In Aromatic Rices; Oxford and IBH: New Delhi, India, 2000; pp. 155–177. [Google Scholar]
- Singh, R.K.; Singh, U.S. Treatise on the Scented Rices of India; Kalyani Publishers: New Delhi, India, 2003. [Google Scholar]
- Mathure, S.V.; Jawali, N.; Thengane, R.J.; Nadaf, A.B. Comparative quantitative analysis of headspace volatiles and their association with BADH2 marker in non-basmati scented, basmati and non-scented rice (Oryza sativa L.) cultivars of India. Food Chem. 2014, 142, 383–391. [Google Scholar] [CrossRef]
- Saetung, W.; Trelo-ges, V. Monitoring in soil fertility change in Tung Kula Rong Hai using geographic information systems. Int. J. Eng. Res. 2017, 2, 189–193. [Google Scholar]
- Yoshihashi, T.; Huong, N.T.T.; Inatomi, H. Precursors of 2-acetyl-1 pyrroline, a potent flavor compound of an aromatic rice variety. J. Agric. Food Chem. 2002, 50, 2001–2004. [Google Scholar] [CrossRef]
- Adams, A.; Kimpe, D.N. Formation of pyrazines and 2-acetyl-1-pyrroline by Bacillus cereus. Food Chem. 2007, 101, 1230–1238. [Google Scholar] [CrossRef]
- Wakte, K.; Zanan, R.; Hinge, V.; Khandagale, K.; Nadaf, A.; Henry, R. Thirtythree years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): A status review. J. Sci. Food Agri. 2016, 97, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, Y.; Khare, P.; Patra, D. Rhizobacteria elevate principal basmati aroma compound accumulation in rice variety. Rhizosphere 2016, 1, 53–57. [Google Scholar] [CrossRef]
- Chinachanta, K.; Herrmann, L.; Lesueur, D.; Jongkaewwattana, S.; Santasup, C.; Shutsrirung, A. Influences of farming practices on soil properties and the 2-acetyl-1-pyrroline content of Khao Dawk Mali 105 rice grains. Appl. Environ. Soil Sci. 2020, 2020, 8818922. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant plant growth-promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Gamalero, E.; Elisa Bona, E.; Valeria Todeschini, V.; Guido Lingua, G. Saline and arid soils: Impact on bacteria, plants, and their interaction. Biology 2020, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [Green Version]
- Talaat, N.B.; Shawky, B.T. Modulation of nutrient acquisition and polyamine pool in salt-stressed wheat (Triticum aestivum L.) plants inoculated with arbuscular mycorrhizal fungi. Acta Physiol. Plant. 2013, 35, 2601–2610. [Google Scholar] [CrossRef]
- Shabani, L.; Sabzalian, M.R. Arbuscular mycorrhiza affects nickel translocation and expression of ABC transporter and metallothionein genes in Festuca arundinacea. Mycorrhiza 2016, 26, 67–76. [Google Scholar] [CrossRef]
- Rangseekaew, P.; Barros-Rodriguez, A.; Pathom-aree, W.; Manzanera, M. Deep-sea actinobacteria mitigate salinity stress in tomato seedlings and their biosafety testing. Plants 2021, 10, 1687. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Lugtenberg, B. Use of plant growth promoting rhizobacteria to alleviate salinity stress in plants. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer: New York, NY, USA, 2014; pp. 73–96. [Google Scholar]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Fan, C.; Wang, Y.; Xia, Y.; Wei Xiao, W.; Cui, X. Salt-tolerant and plant-growth-promoting bacteria isolated from high-yield paddy soil. Can. J. Microbiol. 2018, 64, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Suksaard, P.; Pathom-aree, W.; Duangmal, K. Diversity and plant growth promoting activities of actinomycetes from mangroves. Chiang Mai J. Sci. 2017, 44, 1210–1223. [Google Scholar]
- Pathom-aree, W.; Kreawsa, S.; Kamjam, M.; Tokuyama, S.; Yoosathaporn, S.; Lumyong, S. Potential of selected mangrove streptomyces as plant growth promoter and rice bakanae disease control agent. Chiang Mai J. Sci. 2019, 46, 261–276. [Google Scholar]
- Prittesh, P.; Avnika, P.; Kinjai, P.; Jinal, H.N.; Sakthivel, K.; Amaresan, N. Amelioration effect of salt-tolerant plant growth-promoting bacteria on growth and physiological properties of rice (Oryza sativa) under salt-stressed conditions. Arch. Microbiol. 2020, 202, 2419–2428. [Google Scholar] [CrossRef]
- Chinachanta, K.; Shutsrirung, A. Screening for P- and K- Solubilizing, and Siderophore Producing Capacity of Rhizobacteria from Khao Dawk Mali 105 Aromatic Rice. IOP Conf. Ser. Earth and Environ. Sci. 2021, 858, 012004. [Google Scholar] [CrossRef]
- Rungsardthong, V.; Noomhoom, A. Production of 2-acetyl-1-pyrroline by microbial cultures. Flavour Fragr. J. 2005, 20, 710–714. [Google Scholar] [CrossRef]
- Premsuriya, J.; Panyoo, W.; Patarapuwadol, S.; Kositratana, W. Development of rapid method for screening of plant growth promoting rhizobacteria in rice. Agric. Sci. J. 2017, 8, 11–22. (In Thai) [Google Scholar]
- Nawara, W.; Bennett, C.; Norkaew, O.; Sookwong, P.; Moolkam, S.; Naruebal, S.; Mahatheeranont, S. Variation of the impact aroma compound, 2-acetyl-1-pyrroline, content in Thai fragrant rice plants and its enhanced accumulation by soil nutritional elements. J. Agric. Sci. 2020, 12, 36–45. [Google Scholar]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; Wiley: New York, NY, USA, 1972. [Google Scholar]
- Wongpornchai, S.; Sriseadka, T.; Choonvisase, S. Identification and quantitation of the rice aroma compound, 2-acetyl-1-pyrroline, in bread flowers (Vallaris glabra Ktze). J. Agric. Food Chem. 2003, 51, 457–462. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and procedures of statistics. In A Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- Widjaja, R.; Craske, J.D.; Wootton, M. Comparative studies on volatile components of non-fragrant and fragrant rices. J. Sci. Food Agric. 1996, 71, 218–224. [Google Scholar] [CrossRef]
- Jezussek, M.; Juliano, B.O.; Schieberle, P. Comparison of key aroma compounds in cooked brown rice varieties based on aroma extract dilution analyses. J. Agric. Food Chem. 2002, 50, 1101–1105. [Google Scholar] [CrossRef]
- Mo, Z.; Li, W.; Pan, S.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.; Wang, Y.; Duan, M.; Tian, H.; Tang, X. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maraval, I.; Sen, K.; Agrebi, A.; Menut, C.; Morere, A.; Boulanger, R.; Gay, F.; Mestres, C.; Gunata, Z. Quantification of 2-acetyl-1-pyrroline in rice by stable isotope dilution assay through headspace solid-phase microextraction coupled to gas chromatography–tandem mass spectrometry. Anal. Chim. Acta 2010, 675, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Boontakham, P.; Sookwong, P.; Jongkaewwattana, S.; Wangtueai, S.; Mahatheeranont, S. Comparison of grain yield and 2-acetyl-1-pyrroline (2AP) content in leaves and grain of two Thai fragrant rice cultivars cultivated at greenhouse and open-air conditions. Aust. J. Crop Sci. 2019, 13, 159–169. [Google Scholar] [CrossRef]
- Sriseadka, T.; Wongpornchai, S.; Kitsawatpaiboon, P. Rapid method for quantitative analysis of the aroma impact compound, 2-acetyl-1-pyrroline, in fragrant rice using automated headspace gas chromatography. J. Agric. Food Chem. 2006, 54, 8183–8189. [Google Scholar] [CrossRef] [PubMed]
- Poonlaphdecha, J.; Maraval, I.; Roques, S.; Audebert, A.; Boulanger, R.; Bry, X.; Gunata, Z. Effect of timing and duration of salt treatment during growth of a fragrant rice variety on yield and 2-Acetyl-1-pyrroline, proline, and GABA levels. J. Agric. Food Chem. 2012, 60, 3824–3830. [Google Scholar] [CrossRef] [PubMed]
- Hinge, V.R.; Patil, H.B.; Nadaf, A.B. Aroma volatile analyses and 2AP characterization at various developmental stages in Basmati and Non-Basmati scented rice (Oryza sativa L.) cultivars. Rice 2016, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Yong Zhang, Y.; Wei, C.; Zhang, X. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef] [Green Version]
- Hairmansis, A.; Nafisah, N.; Jamil, A. Towards developing salinity tolerant rice adaptable for coastal regions in Indonesia. KnE Life Sci. 2017, 2, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Subiramani, S.; Ramalingam, S.; Muthu, T.; Nile, S.H.; Venkidasamy, B. Development of abiotic stress tolerance in crops by plant growth- promoting rhizobacteria (PGPR). In Phyto-Microbiome in Stress Regulation; Kumar, M., Etesami, H., Kumar, V., Eds.; Springer: Cham, Switzerland, 2020; pp. 125–145. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, W.; Wang, X.; Lai, R. Proposal of Sinomonas flava gen. nov., sp. nov., and description of Sinomonas atrocyanea comb. nov. to accommodate Arthrobacter atrocyaneus. Int. J. Syst. Evol. Microbiol. 2009, 59, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Planchamp, C.; Glauser, G.; Mauch-Mani, B. Root inoculation with Pseudomonas putida KT2440 induces transcriptional and metabolic changes and systemic resistance in maize plants. Front. Plant Sci. 2015, 5, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonito, G.; Reynolds, H.; Robeson, M.S.; Nelson, J.; Hodkinson, B.P.; Tuskan, G.; Schadt, C.W.; Vilgalys, R. Plant host and soil origin influence fungal and bacterial assemblages in the roots of woody plants. Mol. Ecol. 2014, 23, 3356–3370. [Google Scholar] [CrossRef] [PubMed]
- Begonia, M.; Kremer, R.; Stanley, L.; Jamshedi, A. Association of bacteria with velvetleaf roots. Trans. Missouri Acad. Sci. 1990, 24, 17–26. [Google Scholar]
- Jofre, E.; Fischer, S.; Rivarola, V.; Balegno, H.; Mori, G. Saline stress affects the attachment of Azospirillum brasilense Cd to maize and wheat roots. Can. J. Microbiol. 1998, 44, 416–422. [Google Scholar] [CrossRef]

| Farming Practice | Rhizobacterial Isolate | Genus | Phosphate (Ca-P) Solubilization | Potassium (K-Feldspar) Solubilization | Siderophore Production |
|---|---|---|---|---|---|
| Soluble P | Soluble K | Hydroxamate-Type | |||
| (mg·L−1) | (mg·L−1) | (µmol·L−1) | |||
| Organic farming | ORF4-13 | Sinomonas sp. | 35.6 | 37.3 | 110.8 |
| ORF10-12 | Enterobacter sp. | 31.6 | 39.4 | 162.5 | |
| ORF15-19 | Micrococcus sp. | 11.6 | 34.6 | 64.2 | |
| ORF15-20 | Micrococcus sp. | 25.0 | 30.7 | 83.3 | |
| ORF15-23 | Sinomonas sp. | 20.9 | 49.5 | 22.5 | |
| Conventional farming | CRF5-8 | unidentified | 31.6 | 44.8 | 2.5 |
| CRF14-15 | Sinomonas sp. | 36.6 | 38.6 | 1.7 | |
| CRF16-3 | Burkholderia sp. | 17.5 | 43.0 | 618.3 | |
| CRF17-18 | Bacillus sp. | 35.0 | 28.7 | 0.5 |
| Farming Practice | Rhizobacterial Isolate | 2AP Production (μg·kg−1) |
|---|---|---|
| Uninoculated control | ND 1 | |
| Organic farming | ORF4-13 | 30.91 ± 0.43 e |
| ORF10-12 | 330.24 ± 1.70 b | |
| ORF15-19 | 254.04 ± 3.89 c | |
| ORF15-20 | 96.94 ± 1.49 d | |
| ORF15-23 | 372.76 ± 7.99 a | |
| Average 216.98 | ||
| Farming Practice | Rhizobacterial Isolate | 2AP Production (μg·kg−1) |
| Conventional farming | CRF5-8 | ND |
| CRF14-15 | 12.00 ± 1.53 f | |
| CRF16-3 | 254.95 ± 2.48 c | |
| CRF17-18 | ND | |
| Average 133.48 | ||
| F-test | ** | |
| LSD(0.05) | 6.37 | |
| CV% | 1.88 |
| Source of Variance | The 2AP Content |
|---|---|
| PGPR isolates (A) | ** |
| NaCl concentration (B) | * |
| A × B | ** |
| Turkey’s HSD (0.01) for (A × B) | 1.0995 |
| CV% | 3.6756 |
| Rhizobacteria Isolates | Number of Viable Cells (CFU·mL−1) | |||
|---|---|---|---|---|
| NaCl Concentrations (mM) | ||||
| 0 | 50 | 100 | 150 | |
| Organic rice farming | ||||
| ORF4-13 | 5.8 × 108 | 3.7 × 108 | 6.0 × 106 | 2.0 × 106 |
| ORF10-12 | 3.5 × 108 | 2.2 × 108 | 2.7 × 107 | 2.3 × 106 |
| ORF15-19 | 2.0 × 109 | 7.2 × 108 | 4.3 × 107 | 2.3 × 106 |
| ORF15-20 | 1.4 × 109 | 8.8 × 108 | 4.3 × 106 | 1.0 × 106 |
| ORF15-23 | 2.2 × 109 | 1.5 × 109 | 6.1 × 106 | 4.8 × 106 |
| Conventional rice farming | ||||
| CRF5-8 | 8.0 × 108 | 8.5 × 107 | 6.1 × 106 | 4.8 × 106 |
| CRF14-15 | 1.2 × 109 | 5.5 × 108 | 4.5 × 106 | 3.1 × 106 |
| CRF16-3 | 1.2 × 109 | 7.0 × 108 | 3.3 × 106 | 3.0 × 106 |
| CRF17-18 | 4.7 × 108 | 2.0 × 108 | 7.0 × 106 | 2.5 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinachanta, K.; Shutsrirung, A.; Herrmann, L.; Lesueur, D.; Pathom-aree, W. Enhancement of the Aroma Compound 2-Acetyl-1-pyrroline in Thai Jasmine Rice (Oryza sativa) by Rhizobacteria under Salt Stress. Biology 2021, 10, 1065. https://doi.org/10.3390/biology10101065
Chinachanta K, Shutsrirung A, Herrmann L, Lesueur D, Pathom-aree W. Enhancement of the Aroma Compound 2-Acetyl-1-pyrroline in Thai Jasmine Rice (Oryza sativa) by Rhizobacteria under Salt Stress. Biology. 2021; 10(10):1065. https://doi.org/10.3390/biology10101065
Chicago/Turabian StyleChinachanta, Kawiporn, Arawan Shutsrirung, Laetitia Herrmann, Didier Lesueur, and Wasu Pathom-aree. 2021. "Enhancement of the Aroma Compound 2-Acetyl-1-pyrroline in Thai Jasmine Rice (Oryza sativa) by Rhizobacteria under Salt Stress" Biology 10, no. 10: 1065. https://doi.org/10.3390/biology10101065
APA StyleChinachanta, K., Shutsrirung, A., Herrmann, L., Lesueur, D., & Pathom-aree, W. (2021). Enhancement of the Aroma Compound 2-Acetyl-1-pyrroline in Thai Jasmine Rice (Oryza sativa) by Rhizobacteria under Salt Stress. Biology, 10(10), 1065. https://doi.org/10.3390/biology10101065