Quantifying Leaf Trait Covariations and Their Relationships with Plant Adaptation Strategies along an Aridity Gradient
Abstract
:Simple Summary
Abstract
1. Introduction
- (1)
- Traits do not vary independently but show covariation and tradeoff relationships [17]. However, from the perspective of plant functional traits, plant strategies adopted to simultaneously balance conservation and resource acquisition remain unclear.
- (2)
- (3)
- Vegetation succession along an aridity gradient resulted in the replacement of larger leaf plants by plants with small and high-efficiency leaves [21]. The contribution of vegetation distribution (family) to trait covariations is still unknown along aridity gradients.
2. Materials and Methods
2.1. Study Area and Sampling Strategy
2.2. Data Descriptions of Traits, Climate and Soil
2.3. Multivariate Statistical Analysis
3. Results
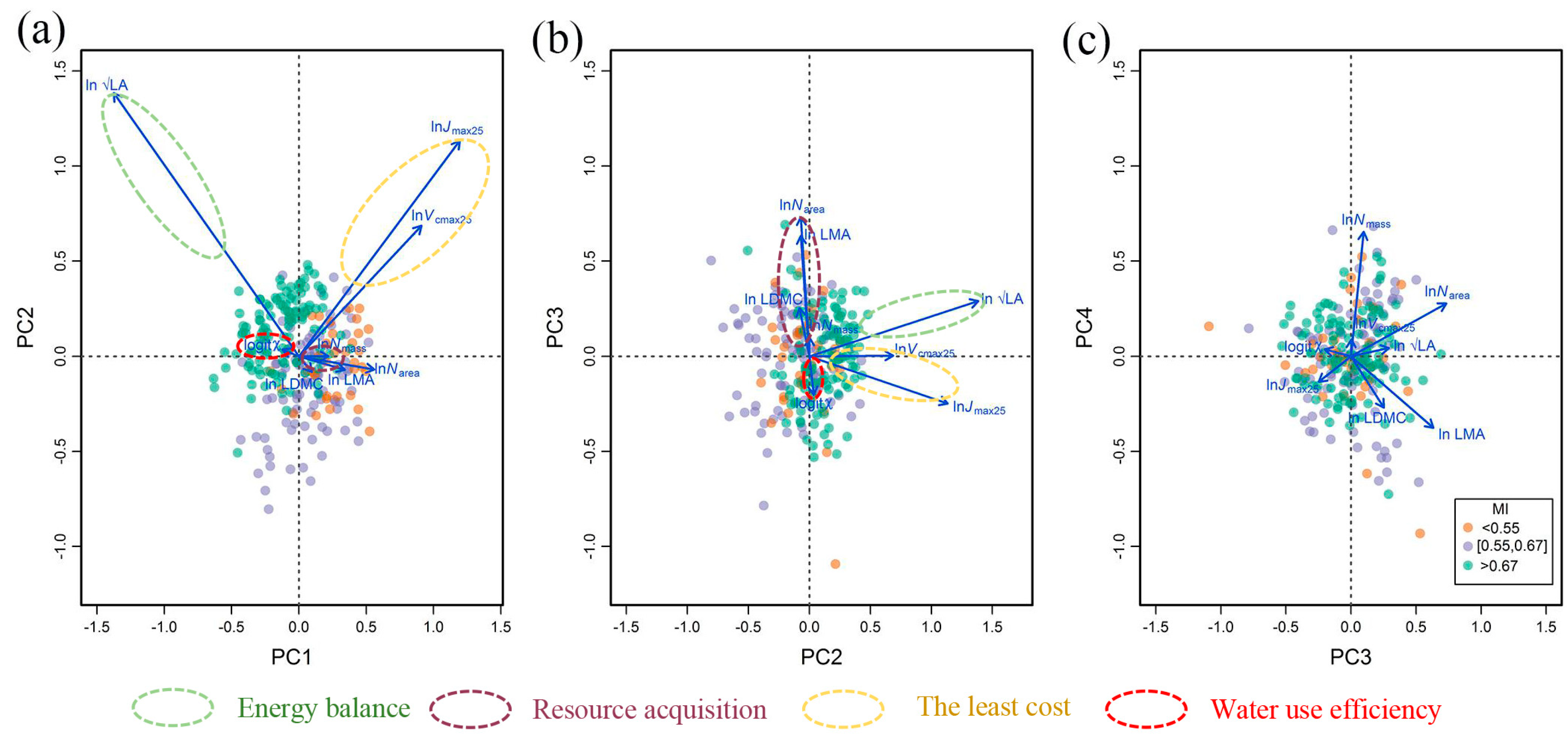
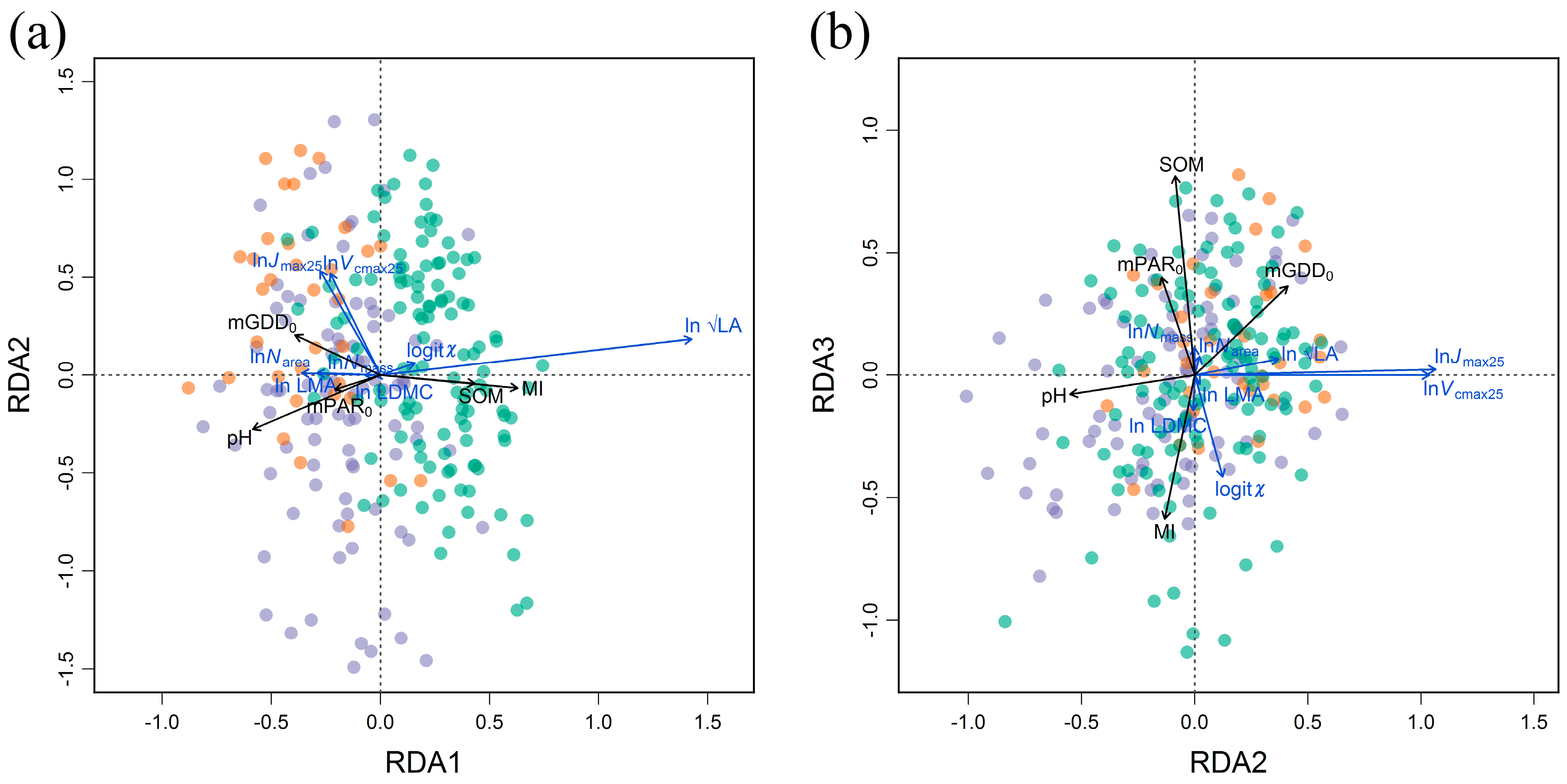
3.1. Trait Covariation and Corresponding Adaptation Strategies
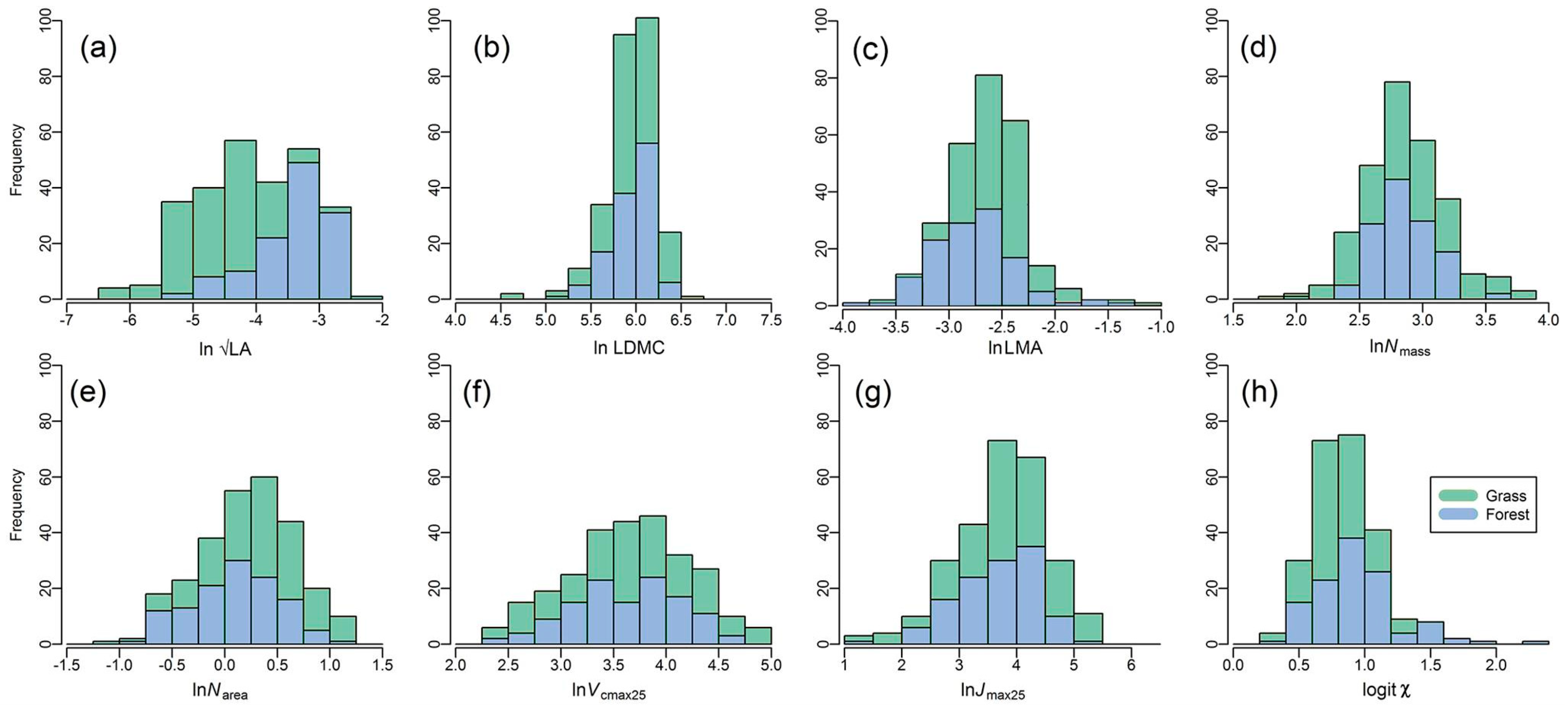
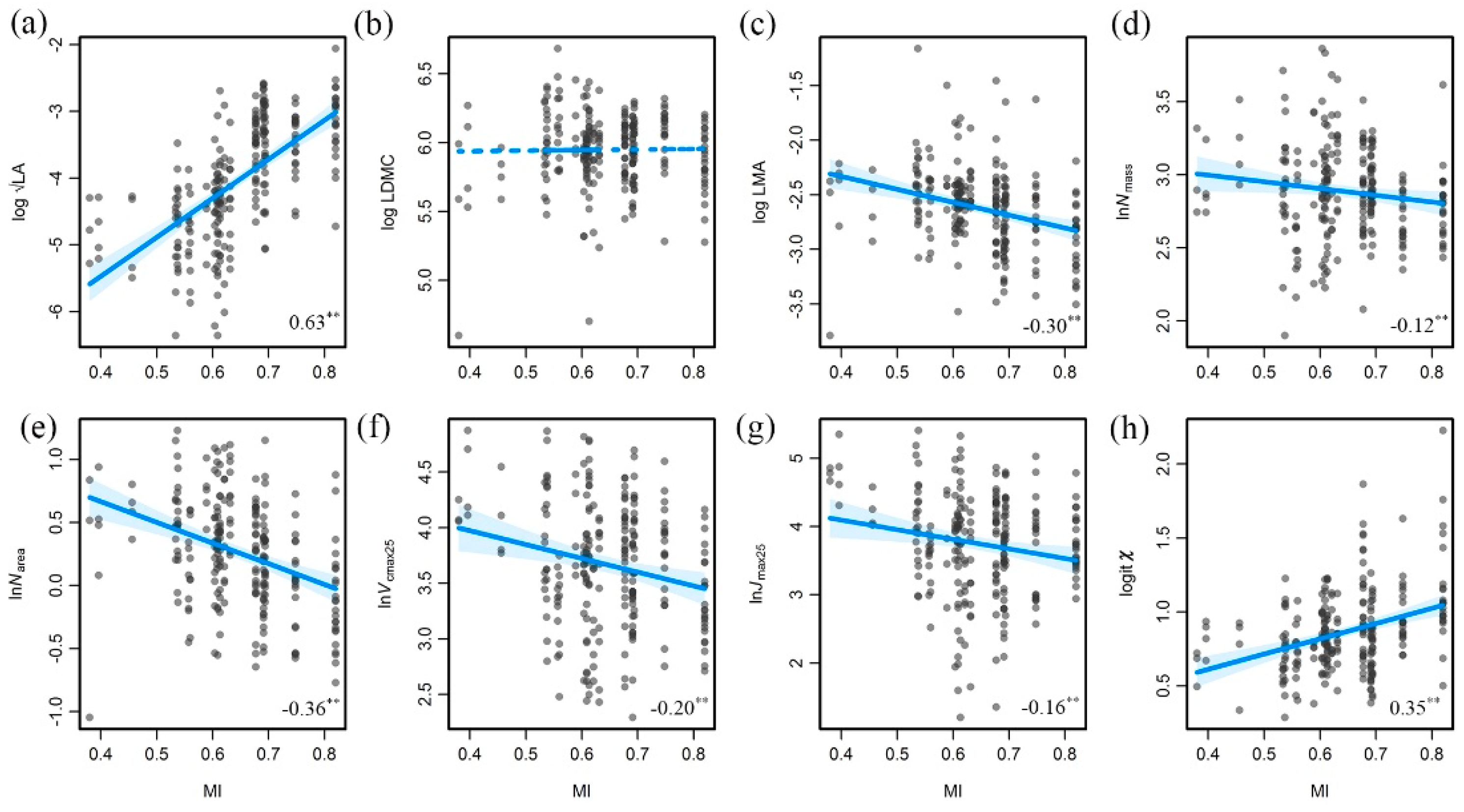
3.2. Trait Variations along the Aridity Gradient (MI Decreased)
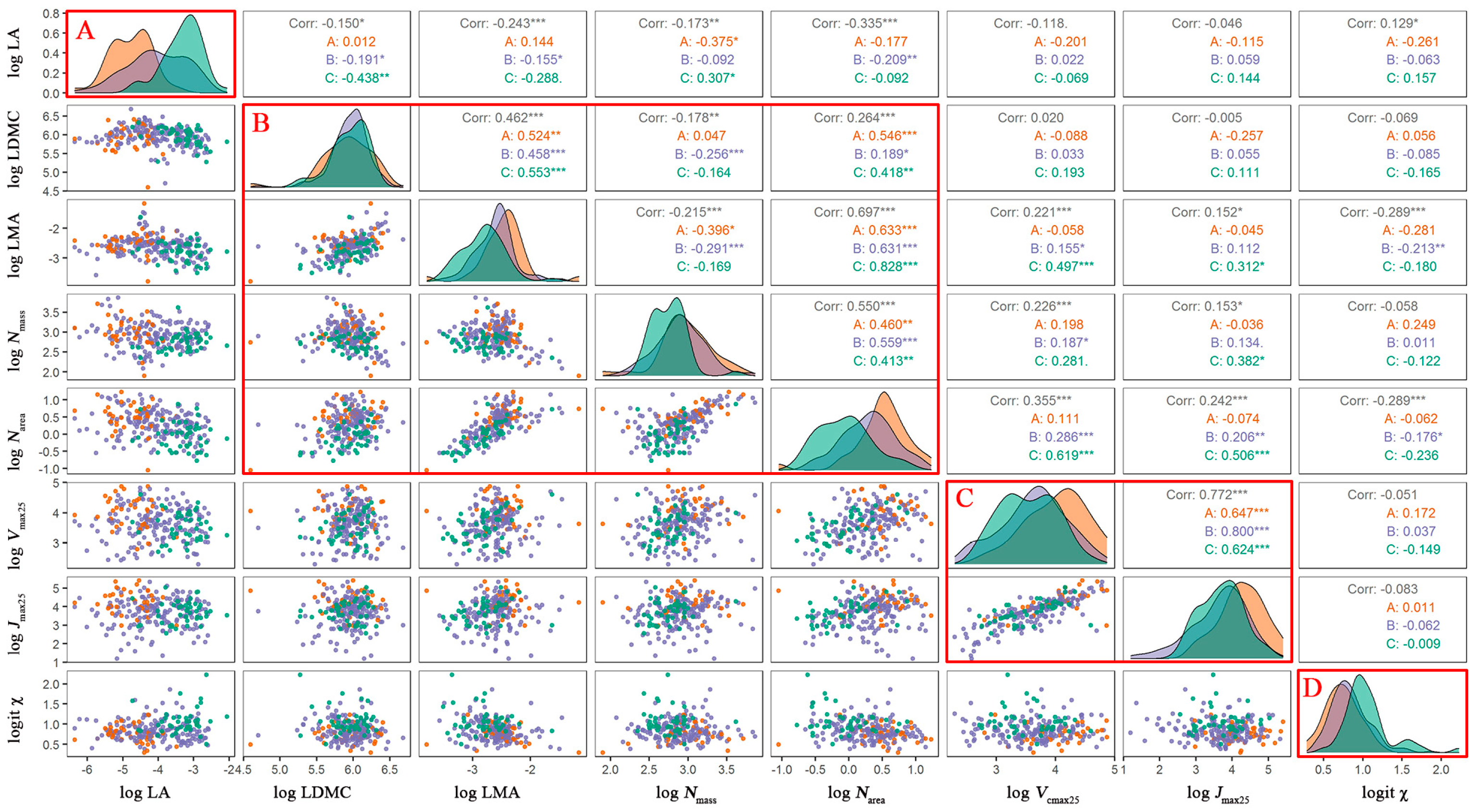
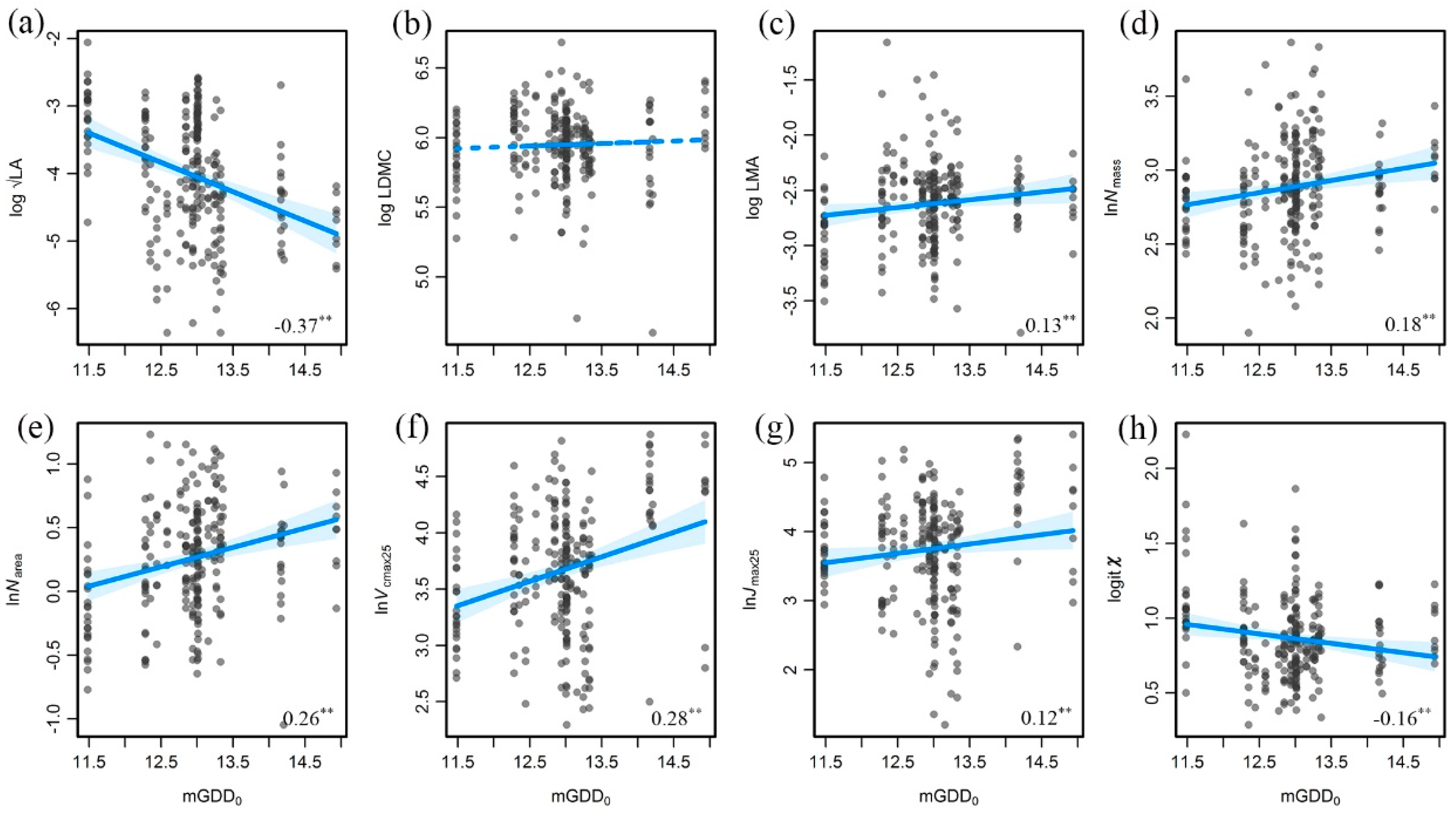
3.3. Trait Covariations and Adaptation Strategies Related to Climate and Soil Variables
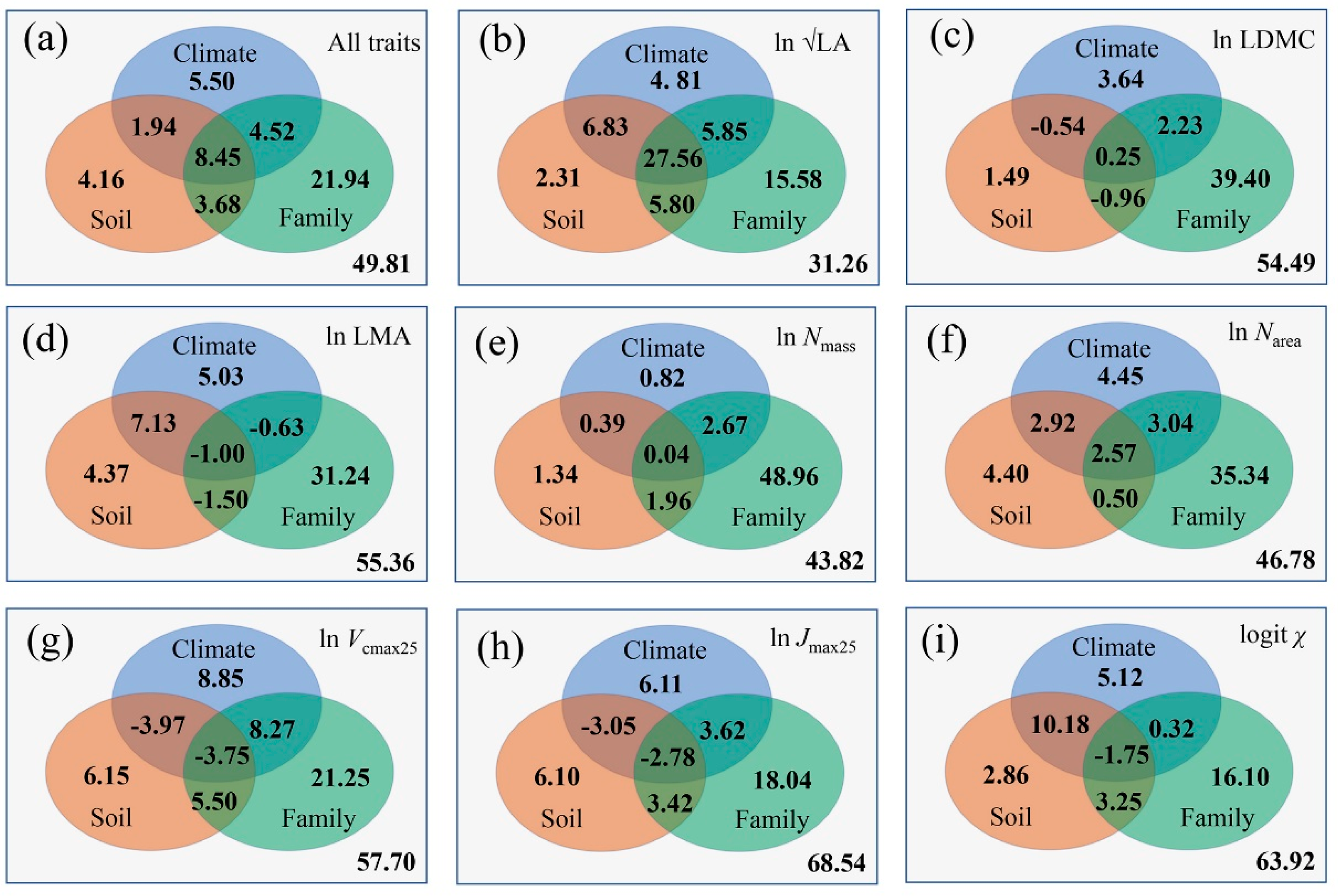
3.4. Controls of Trait Covariations along the Aridity Gradient
4. Discussion
4.1. Importance and Significance of Studying Trait Covariations in Arid Areas
4.2. Mechanism of Trait Covariations along the Aridity Gradient
4.3. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Trait | RDA1 | RDA2 | RDA3 | RDA4 | RDA5 | PC1 |
|---|---|---|---|---|---|---|
| log√LA | 2.04 | 0.26 | 0.05 | 0.00 | 0.00 | −0.07 |
| log Nmass | −0.10 | 0.00 | 0.08 | −0.17 | 0.05 | −0.25 |
| log Narea | −0.51 | 0.02 | 0.06 | −0.08 | 0.04 | −0.50 |
| log LMA | −0.41 | 0.02 | −0.03 | 0.08 | −0.01 | −0.25 |
| log LDMC | −0.04 | −0.01 | −0.10 | −0.06 | −0.18 | −0.03 |
| Log Vcmax25 | −0.33 | 0.74 | 0.00 | −0.17 | −0.03 | −1.42 |
| Log Jmax25 | −0.40 | 0.76 | 0.02 | 0.17 | 0.02 | −2.19 |
| logit χ | 0.23 | 0.09 | −0.30 | −0.04 | 0.08 | 0.09 |
| Eigenvalue | 0.47 | 0.11 | 0.01 | 0.01 | 0.00 | 0.68 |
| Proportion explained (%) | 21.30 | 5.22 | 0.48 | 0.44 | 0.19 | 31.09 |
| Cumulative proportion (%) | 21.30 | 26.52 | 27.01 | 27.45 | 27.64 | 58.74 |
| Family | Species |
|---|---|
| Aceraceae | Artemisia gmelinii, Artemisia capillaries, Artemisia mongolica, Lespedeza davurica, Artemisia giraldii, Heteropappus altaicus, Cirsium setosum, Echinops sphaerocephalus, Artemisia desertorum, Artemisia frigida, Artemisia sieversiana, Scorzonera austriaca, Artemisia argyi, Lespedeza cuneata, Carpesium cernuum, Aster tataricus |
| Gramineae | Phragmites australis, Bothriochloa ischaemum, Stipa grandis, Stipa bungeana, Cleistogenes caespitosa, Setaria viridis, Cleistogenes hancei, Cleistogenes chinensis, Leymus secalinus, Bromus inermis, Elymus dahuricus, Triarrhena sacchariflora |
| Rosaceae | Potentilla tanacetifolia, Fragaria vesca, Crataegus cuneata, Armeniaca vulgaris, Pyrus betulaefolia, Prunus salicina, Rosa xanthina, Spiraea Salicifolia, Cotoneaster multiflorus, Amygdalus davidiana, Rubus parvifolius, Pyrus betulaefolia, Amygdalus triloba |
| Leguminosae | Sophora davidii, Astragalus melilotoides, Thermopsis lanceolata, Glycyrrhiza uralensis, Sophora davidii, Oxytropis racemosa, Astragalus adsurgens, Caragana Korshinskii, Astragalus membranaceus, Lespedeza bicolor, Indigofera bungeana, |
| Caprifoliaceae | Lonicera japonica, Viburnum schensianum, Lonicera maackii |
| Elaeagnaceae | Hippophae rhamnoides, Elaeagnus umbellata, Elaeagnus pungens |
References
- Nunes, A.; Köbel, M.; Pinho, P.; Matos, P.; de Bello, F.; Correia, O.; Branquinho, C. Which plant traits respond to aridity? A critical step to assess functional diversity in Mediterranean drylands. Agric. For. Meteorol. 2017, 239, 176–184. [Google Scholar] [CrossRef]
- Maracahipes, L.; Carlucci, M.B.; Lenza, E.; Marimon, B.S.; Marimon, B.H., Jr.; Guimaraes, F.A.; Cianciaruso, M.V. Systematics, How to live in contrasting habitats? Acquisitive and conservative strategies emerge at inter-and intraspecific levels in savanna and forest woody plants. Perspect. Plant Ecol. Evol. 2018, 34, 17–25. [Google Scholar] [CrossRef]
- Enquist, B.J. Universal scaling in tree and vascular plant allometry: Toward a general quantitative theory linking plant form and function from cells to ecosystems. Tree Physiol. 2002, 22, 1045–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghalambor, C.; McKay, J.; Carroll, S.; Reznick, D. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007, 21, 394–407. [Google Scholar] [CrossRef]
- Bjorkman, A.; Myers-Smith, I.; Elmendorf, S.; Normand, S.; Rüger, N.; Beck, P.S.A.; Blach-Overgaard, A.; Blok, D.; Cornelissen, J.H.C.; Forbes, B.C.; et al. Plant functional trait change across a warming tundra biome. Nature 2018, 562, 57–62. [Google Scholar] [CrossRef]
- Boeddinghaus, R.; Marhan, S.; Berner, D.; Boch, S.; Fischer, M.; Hoelzel, N.; Kattge, J.; Klaus, V.; Kleinebecker, T.; Oelmann, Y.; et al. Dataset used in Boeddinghaus et al. Plant functional trait shifts explain concurrent changes in the structure and function of grassland soil microbial communities. J. Ecol. 2019, 107, 2197–2210. [Google Scholar] [CrossRef] [Green Version]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.; Westoby, M.; Ackerly, D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Welsh, M.E.; Cronin, J.P.; Mitchell, C.E. The role of habitat filtering in the leaf economics spectrum and plant susceptibility to pathogen infection. J. Ecol. 2016, 104, 1768–1777. [Google Scholar] [CrossRef]
- Munguía-Rosas, M.A.; Angulo, D.F.; Arceo-Gómez, G.; Parra-Tabla, V. Variation in leaf traits across a precipitation gradient in coastal sand dunes in Yucatan Peninsula. J. Arid. Environ. 2018, 162, 10–17. [Google Scholar] [CrossRef]
- Forrestel, E.J.; Donoghue, M.J.; Edwards, E.J.; Jetz, W.; du Toit, J.C.O.; Smith, M.D. Different clades and traits yield similar grassland functional responses. Proc. Natl. Acad. Sci. USA 2017, 114, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Zuo, X.; Yu, Q.; Xu, C.; Lv, P.; Zhang, J.; Knapp, A.K.; Smith, M.D. Response of plant functional traits of Leymus chinensis to extreme drought in Inner Mongolia grasslands. Plant Ecol. 2019, 220, 141–149. [Google Scholar] [CrossRef]
- Balachowski, J.A.; Volaire, F.A. Implications of plant functional traits and drought survival strategies for ecological restoration. J. Appl. Ecol. 2018, 55, 631–640. [Google Scholar] [CrossRef]
- Prentice, I.C.; Meng, T.; Wang, H.; Harrison, S.; Ni, J.; Wang, G. Evidence of a universal scaling relationship for leaf CO 2 drawdown along an aridity gradient. New Phytol. 2011, 190, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Maire, V.; Martre, P.; Kattge, J.; Gastal, F.; Esser, G.; Fontaine, S.; Soussana, J.-F. The Coordination of Leaf Photosynthesis Links C and N Fluxes in C3 Plant Species. PLoS ONE 2012, 7, e38345. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; Loy, X.; Markham, I.P.; Elmer, C.M.; Hovenden, M.J.; HilleRisLambers, J.; Mayfield, M.M. Aridity drives coordinated trait shifts but not decreased trait variance across the geographic range of eight Australian trees. New Phytol. 2021, 229, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.; Piper, F. Intraspecific trait variation and covariation in a widespread tree species (Nothofagus pumilio) in southern Chile. New Phytol. 2011, 189, 259–271. [Google Scholar] [CrossRef]
- Zang, U.; Goisser, M.; Meyer, N.; Häberle, K.-H.; Borken, W. Chemical and morphological response of beech saplings (Fagus sylvatica L.) to an experimental soil drought gradient. For. Ecol. Manag. 2021, 498, 119569. [Google Scholar] [CrossRef]
- Zarzosa, P.S.; Herraiz, A.D.; Olmo, M.; Ruiz-Benito, P.; Barrón, V.; Bastias, C.C.; de la Riva, E.G.; Villar, R. Linking functional traits with tree growth and forest productivity in Quercus ilex forests along a climatic gradient. Sci. Total. Environ. 2021, 786, 147468. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gou, R.; Li, W.; Kassout, J.; Wu, J.; Wang, L.; Peng, C.; Lin, G. Leaf Trait Covariation and Its Controls: A Quantitative Data Analysis Along a Subtropical Elevation Gradient. J. Geophys. Res. Biogeosci. 2021, 126, 7. [Google Scholar] [CrossRef]
- Gong, Y.; Ling, H.; Lv, G.; Chen, Y.; Guo, Z.; Cao, J. Disentangling the influence of aridity and salinity on community functional and phylogenetic diversity in local dryland vegetation. Sci. Total. Environ. 2019, 653, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Song, X.; Mu, X.; Gao, P.; Wang, F.; Zhao, G. Spatiotemporal vegetation cover variations associated with climate change and ecological restoration in the Loess Plateau. Agric. For. Meteorol. 2015, 209-210, 87–99. [Google Scholar] [CrossRef]
- Li, S.; Liang, W.; Fu, B.; Lü, Y.; Fu, S.; Wang, S.; Su, H. Vegetation changes in recent large-scale ecological restoration projects and subsequent impact on water resources in China’s Loess Plateau. Sci. Total Environ. 2016, 569–570, 1032–1039. [Google Scholar] [CrossRef]
- Fu, B.; Wang, S.; Liu, Y.; Liu, J.; Liang, W.; Miao, C. Hydrogeomorphic Ecosystem Responses to Natural and Anthropogenic Changes in the Loess Plateau of China. Annu. Rev. Earth Planet. Sci. 2017, 45, 223–243. [Google Scholar] [CrossRef]
- Shi, H.; Shao, M. Soil and water loss from the Loess Plateau in China. J. Arid. Environ. 2000, 45, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.; ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Mediavilla, S.; Martínez-Ortega, M.; Andrés, S.; Bobo, J.; Escudero, A. Premature losses of leaf area in response to drought and insect herbivory through a leaf lifespan gradient. J. For. Res. 2021, 1–12. [Google Scholar] [CrossRef]
- Reich, P.; Walters, M. Photosynthesis-nitrogen relations in Amazonian tree species. II. Variation in nitrogen vis-a-vis specific leaf area influences mass-and area-based expressions. Oecologia 1994, 91, 73–81. [Google Scholar] [CrossRef]
- Cui, E.; Weng, E.; Yan, E.; Xia, J. Robust leaf trait relationships across species under global environmental changes. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zuo, X.; Yue, P.; Zhao, S.; Guo, X.; Li, X.; Medina-Roldán, E. Increased Precipitation Shapes Relationship between Biochemical and Functional Traits of Stipa glareosa in Grass-Dominated Rather than Shrub-Dominated Community in a Desert Steppe. Plants 2020, 9, 1463. [Google Scholar] [CrossRef] [PubMed]
- De Kauwe, M.G.; Lin, Y.S.; Wright, I.J.; Medlyn, B.E.; Crous, K.Y.; Ellsworth, D.S.; Maire, V.; Prentice, I.C.; Atkin, O.K.; Rogers, A.; et al. A test of the ‘one-point method’for estimating maximum carboxylation capacity from field-measured, light-saturated photosynthesis. New Phytol. 2016, 210, 1130–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niinemets, Ü.; Keenan, T.; Hallik, L. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taud, H.; Mas, J. Multilayer perceptron (MLP). In Geomatic Approaches for Modeling Land Change Scenarios; Springer: Dordrecht, The Netherlands, 2018; pp. 451–455. [Google Scholar]
- Cornwell, W.K.; Wright, I.; Turner, J.; Maire, V.; Barbour, M.M.; Cernusak, L.; Dawson, T.; Ellsworth, D.; Farquhar, G.D.; Griffiths, H.; et al. Climate and soils together regulate photosynthetic carbon isotope discrimination within C3plants worldwide. Glob. Ecol. Biogeogr. 2018, 27, 1056–1067. [Google Scholar] [CrossRef] [Green Version]
- Maire, V.; Wright, I.J.; Prentice, I.C.; Batjes, N.H.; Bhaskar, R.; van Bodegom, P.M.; Cornwell, W.K.; Ellsworth, D.; Niinemets, U.; Ordonez, A.; et al. Global effects of soil and climate on leaf photosynthetic traits and rates. Glob. Ecol. Biogeogr. 2015, 24, 706–717. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Harrison, S.P.; Prentice, I.C.; Wright, I.J.; Peng, C.; Lin, G. Quantifying leaf-trait covariation and its controls across climates and biomes. New Phytol. 2019, 221, 155–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; O’hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Multivariate Analysis of Ecological Communities. Version 1. 2020, pp. 1–4. Available online: http://cran.rproject.org/package=vegan (accessed on 20 July 2021).
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Dong, N.; Prentice, I.C.; Wright, I.J.; Evans, B.J.; Togashi, H.F.; Caddy-Retalic, S.; McInerney, F.A.; Sparrow, B.; Leitch, E.; Lowe, A.J. Components of leaf-trait variation along environmental gradients. New Phytol. 2020, 228, 82–94. [Google Scholar] [CrossRef]
- De la Riva, E.G.; Violle, C.; Perez-Ramos, I.M.; Maranon, T.; Navarro-Fernandez, C.M.; Olmo, M.; Villar, R. A Multidimensional Functional Trait Approach Reveals the Imprint of Environmental Stress in Mediterranean Woody Communities. Ecosystems 2018, 21, 248–262. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.J.; Yuan, T.F.; Li, Z.; Li, N.; Liu, H.Y.; Bai, S.H.; Xia, J.Y.; Lu, M.; Zhou, X.H. Plant evolutionary history mainly explains the variance in biomass responses to climate warming at a global scale. New Phytol. 2019, 222, 1338–1351. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef]
- Willmore, K.E.; Young, N.M.; Richtsmeier, J.T. Phenotypic Variability: Its Components, Measurement and Underlying Developmental Processes. Evol. Biol. 2007, 34, 99–120. [Google Scholar] [CrossRef]
- Karagatzides, J.D.; Ellison, A.M. Construction Costs, Payback Times, and the Leaf Economics of Carnivorous Plants. Am. J. Bot. 2009, 96, 1612–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poorter, H.; Pepin, S.; Rijkers, T.; De Jong, Y.; Evans, J.; Körner, C. Construction costs, chemical composition and payback time of high- and low-irradiance leaves. J. Exp. Bot. 2005, 57, 355–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-L.; Reynolds, J.F.; Harley, P.C.; Tenhunen, J.D. Coordination theory of leaf nitrogen distribution in a canopy. Oecologia 1993, 93, 63–69. [Google Scholar] [CrossRef]
- Farquhar, G.; Von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Prentice, I.C.; Keenan, T.F.; Davis, T.W.; Wright, I.J.; Cornwell, W.K.; Evans, B.J.; Peng, C. Towards a universal model for carbon dioxide uptake by plants. Nat. Plants 2017, 3, 734–741. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Shane, M.W.; Cramer, M.; Pearse, S.J.; Veneklaas, E.J. Root Structure and Functioning for Efficient Acquisition of Phosphorus: Matching Morphological and Physiological Traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, S.; Leuschner, C.; Link, R.M.; Schuldt, B. Hydraulic variability of three temperate broadleaf tree species along a water availability gradient in central Europe. New Phytol. 2021, 231, 1387–1400. [Google Scholar] [CrossRef]
- Scheiter, S.; Langan, L.; Higgins, S.I. Next-generation dynamic global vegetation models: Learning from community ecology. New Phytol. 2013, 198, 957–969. [Google Scholar] [CrossRef]
- Harrison, S.P.; Cramer, W.; Franklin, O.; Prentice, I.C.; Wang, H.; Brännström, Å.; de Boer, H.; Dieckmann, U.; Joshi, J.; Keenan, T.F.; et al. Eco-evolutionary optimality as a means to improve vegetation and land-surface models. New Phytol. 2021, 231, 2125–2141. [Google Scholar] [CrossRef]
- Sakschewski, B.; Von Bloh, W.; Boit, A.; Poorter, L.; Peña-Claros, M.; Heinke, J.; Thonicke, K. Resilience of Amazon forests emerges from plant trait diversity. Nat. Clim. Chang. 2016, 6, 1032–1036. [Google Scholar] [CrossRef]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, M.; Bonin, C.; Bruelheide, H.; De Luca, E.; et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 2015, 526, 574–577. [Google Scholar] [CrossRef] [PubMed]
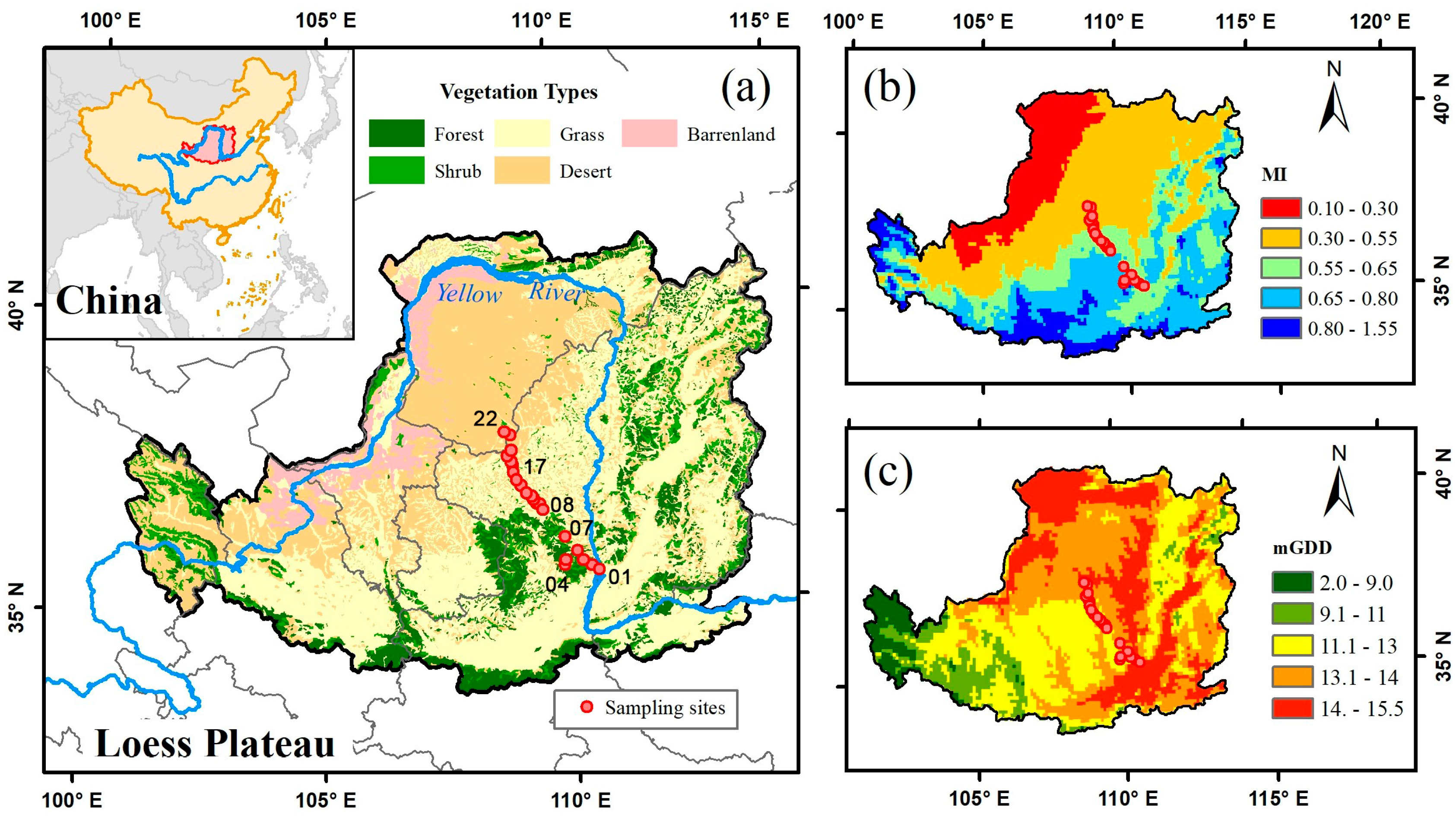
| Scheme | Site Name | Latitude (Degree) | Longitude (Degree) | Elevation (m) | Vegetation Types | No. of Species Samples | Moisture Index (MI) |
|---|---|---|---|---|---|---|---|
| 01 | Jiumen | 35.64 | 110.58 | 440.3 | grassland | 10 | 0.54 |
| 02 | Wangfeng | 35.72 | 110.44 | 721 | grassland | 12 | 0.61 |
| 03 | Qiaozigou | 35.81 | 110.27 | 1219.9 | forestland | 24 | 0.69 |
| 04 | Caijiachuan1 | 35.75 | 109.89 | 1603.2 | forestland | 26 | 0.82 |
| 05 | Caijiachuan2 | 35.82 | 109.91 | 1244.92 | forestland | 20 | 0.75 |
| 06 | Laohugou | 35.97 | 110.16 | 1061.32 | forestland | 21 | 0.69 |
| 07 | Zhonglousi | 36.21 | 109.92 | 1087.42 | forestland | 32 | 0.68 |
| 08 | Lushanmiao | 36.67 | 109.48 | 1220 | grassland | 14 | 0.63 |
| 09 | Houjiacun | 36.77 | 109.42 | 1200 | grassland | 11 | 0.62 |
| 10 | Jiugou1 | 36.8 | 109.36 | 1071 | grassland | 10 | 0.61 |
| 11 | Jiugou2 | 36.8 | 109.36 | 1067 | grassland | 8 | 0.61 |
| 12 | Caozhuang | 36.86 | 109.3 | 1154 | grassland | 10 | 0.61 |
| 13 | Liuping | 36.91 | 109.27 | 1204 | grassland | 7 | 0.61 |
| 14 | Caohe | 36.97 | 109.16 | 1311 | grassland | 15 | 0.6 |
| 15 | Fengcigeda | 37.09 | 109.05 | 1434 | grassland | 7 | 0.59 |
| 16 | Liandaowan | 37.19 | 108.97 | 1476 | grassland | 10 | 0.56 |
| 17 | Tianciwan | 37.32 | 108.91 | 1592 | grassland | 8 | 0.56 |
| 18 | Lugouqu | 37.44 | 108.91 | 1560 | grassland | 8 | 0.53 |
| 19 | Xiasandun | 37.5 | 108.87 | 1584 | grassland | 7 | 0.54 |
| 20 | Shuanghaize | 37.69 | 108.87 | 1347 | desert | 4 | 0.46 |
| 21 | Guojiazhuang | 37.94 | 108.88 | 1153 | desert | 4 | 0.4 |
| 22 | Batuwan | 37.99 | 108.74 | 1155 | desert | 3 | 0.38 |
| Traits | PC1 | PC2 | PC3 |
|---|---|---|---|
| log√LA | −1.97 a | 1.98 | 0.42 |
| log Nmass | 0.31 | 0.00 | 0.14 |
| log Narea | 0.80 | −0.10 | 1.05 |
| log LMA | 0.49 | −0.10 | 0.90 |
| log LDMC | 0.14 | −0.12 | 0.36 |
| log Vcmax25 | 1.30 | 0.98 | −0.00 |
| log Jmax25 | 1.71 | 1.62 | −0.35 |
| logit χ | −0.17 | 0.05 | −0.29 |
| Eigenvalue | 0.90 | 0.71 | 0.23 |
| Proportion explained (%) | 41.13 | 32.61 | 10.58 |
| Cumulative proportion (%) | 41.13 | 73.73 | 84.32 |
| Traits | Climate (%) | Soil (%) | Family (%) |
|---|---|---|---|
| All traits | 20.41 | 18.23 | 38.57 |
| log√LA | 45.05 | 42.50 | 54.79 |
| log LDMC | 5.58 | 0.24 | 40.92 |
| log LMA | 10.53 | 8.99 | 28.10 |
| log Nmass | 3.92 | 3.73 | 53.63 |
| log Narea | 12.98 | 10.38 | 41.44 |
| log Vcmax25 | 9.40 | 3.93 | 31.27 |
| log Jmax25 | 3.90 | 3.69 | 22.30 |
| logit χ | 13.87 | 14.55 | 17.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Kang, L.; Zhao, J.; Qi, N.; Li, R.; Wen, Z.; Kassout, J.; Peng, C.; Lin, G.; Zheng, H. Quantifying Leaf Trait Covariations and Their Relationships with Plant Adaptation Strategies along an Aridity Gradient. Biology 2021, 10, 1066. https://doi.org/10.3390/biology10101066
Yang Y, Kang L, Zhao J, Qi N, Li R, Wen Z, Kassout J, Peng C, Lin G, Zheng H. Quantifying Leaf Trait Covariations and Their Relationships with Plant Adaptation Strategies along an Aridity Gradient. Biology. 2021; 10(10):1066. https://doi.org/10.3390/biology10101066
Chicago/Turabian StyleYang, Yanzheng, Le Kang, Jun Zhao, Ning Qi, Ruonan Li, Zhongming Wen, Jalal Kassout, Changhui Peng, Guanghui Lin, and Hua Zheng. 2021. "Quantifying Leaf Trait Covariations and Their Relationships with Plant Adaptation Strategies along an Aridity Gradient" Biology 10, no. 10: 1066. https://doi.org/10.3390/biology10101066








