Effects of Low Stocking Densities on Zootechnical Parameters and Physiological Responses of Rainbow Trout (Oncorhynchus mykiss) Juveniles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Handling
2.2. Fish and Experimental Design
2.3. Zootechnical Variables and Analysis
2.4. Gene Expression Measurement by Real-Time Quantitative PCR
2.5. Primer Design
2.6. Cells Staining and Microscopy
2.7. Stress Biomarkers
2.7.1. Plasma Cortisol
2.7.2. Plasma Lactate
2.7.3. Plasma Lysozyme
2.8. Assay for Welfare Responses: Catecholamine and Indolamine Release
2.9. Statistical Analysis
3. Results
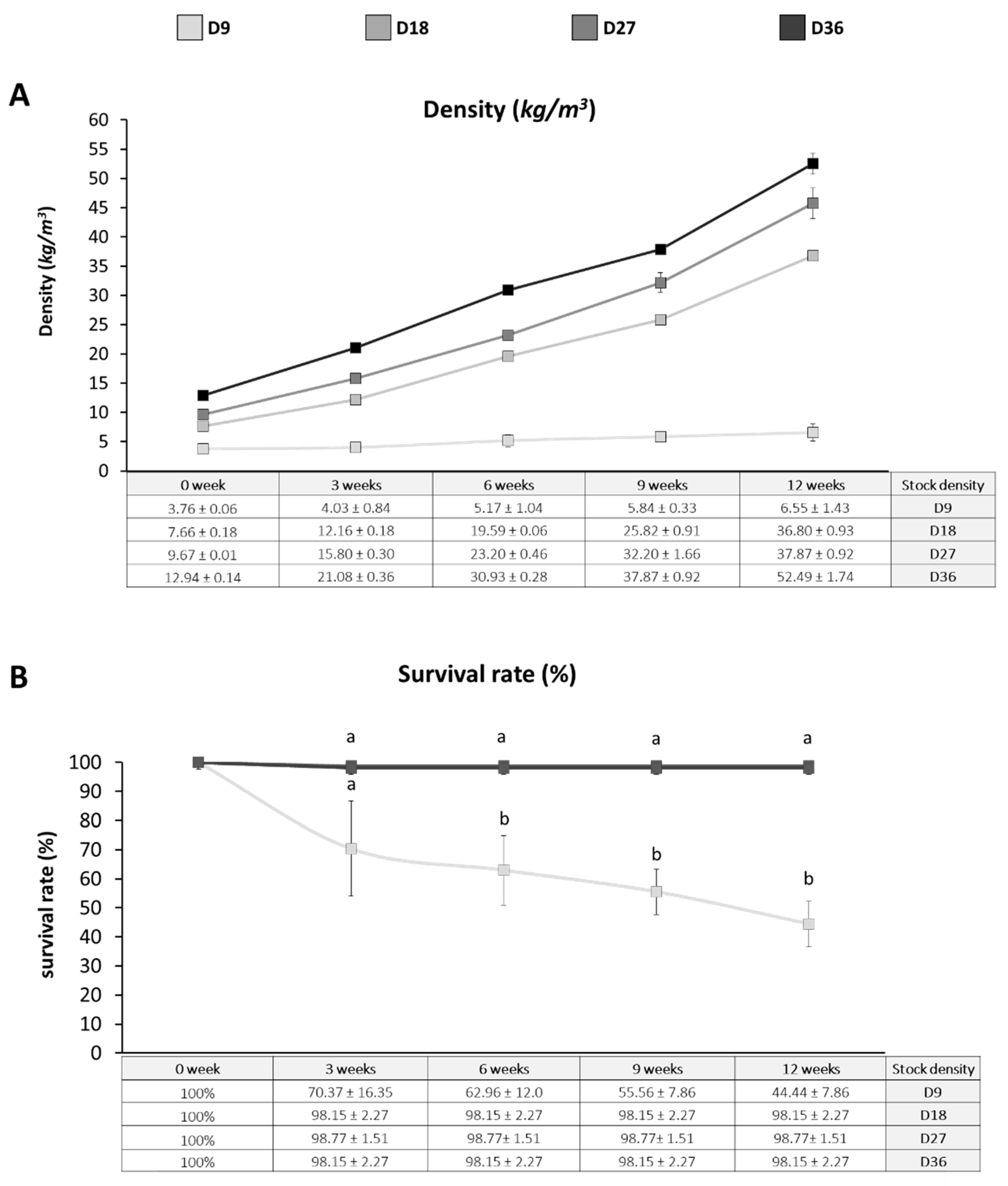
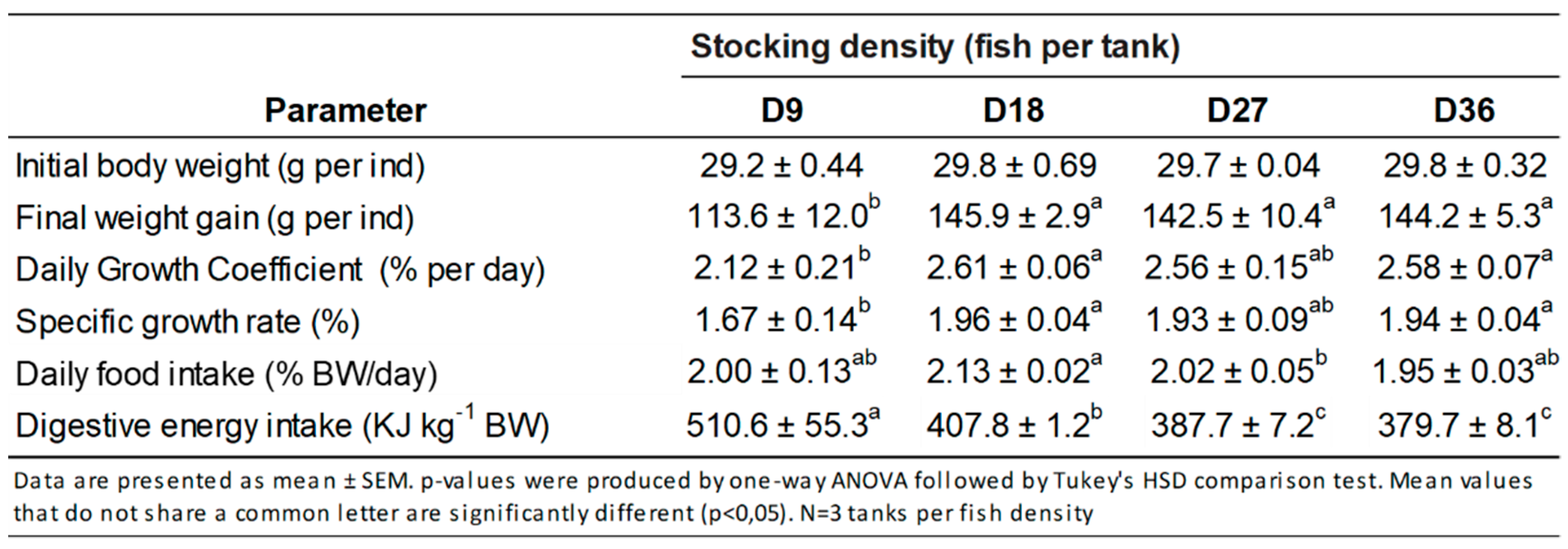
3.1. Growth Performance
3.2. Physiological Parameters
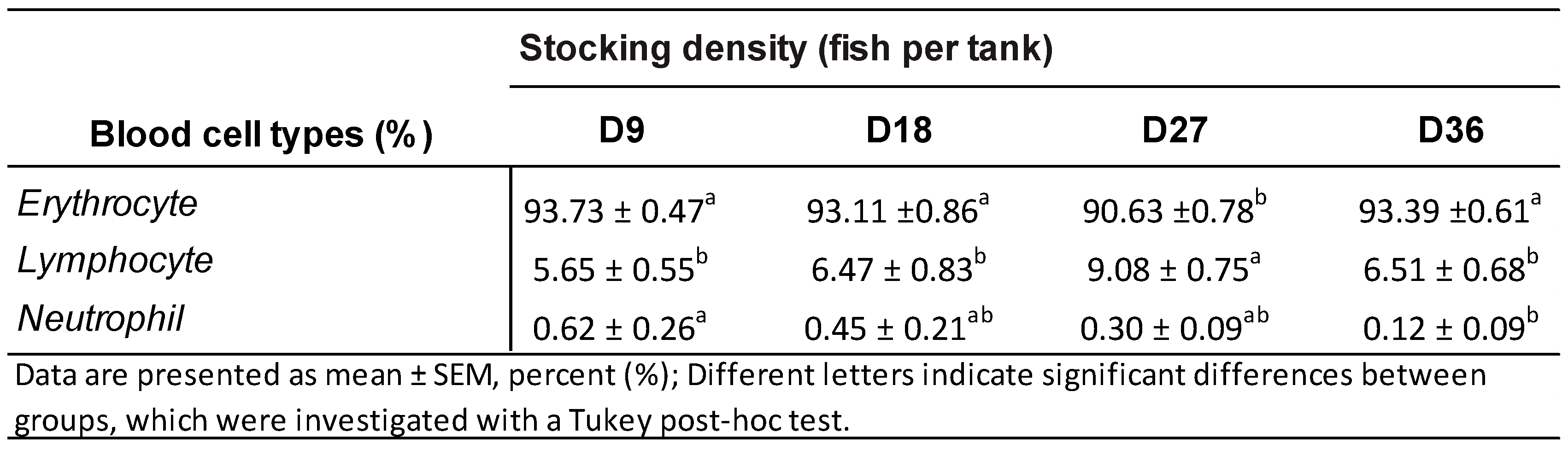
3.3. Blood Cell Type Proportions
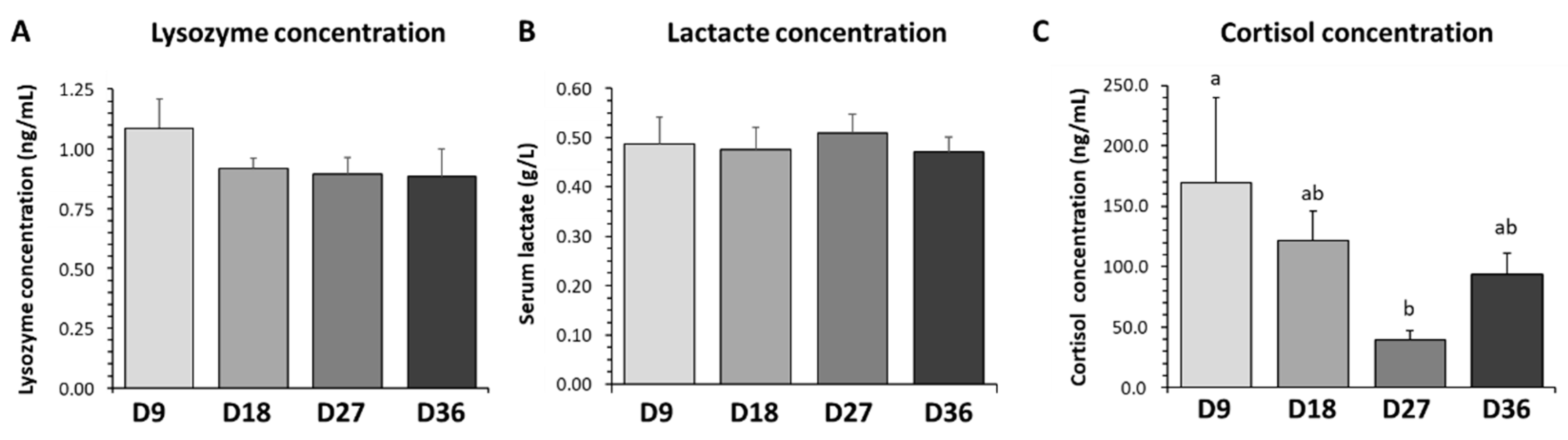
3.4. Lysozyme, Lactate and Cortisol Plasma Levels
3.5. Indolamine and Catecholamine Level Expression
3.6. Changes in mRNA Gene Transcript Expressions Related to Stress Response
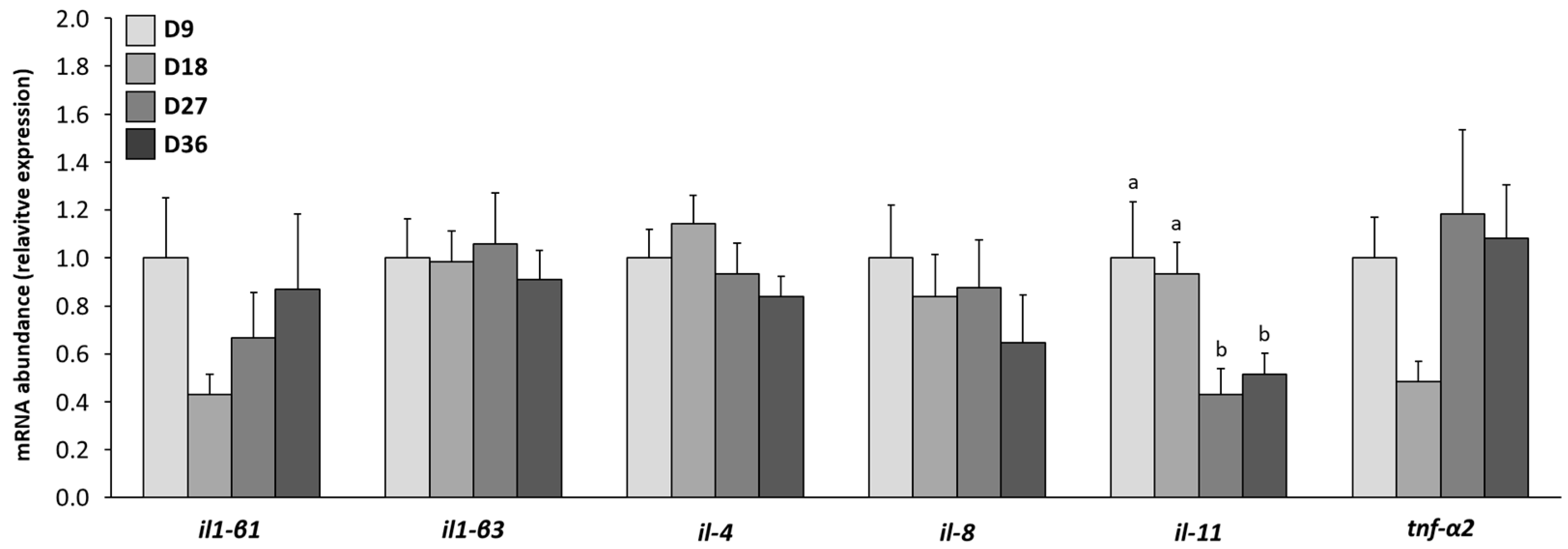
3.7. Changes in mRNA Gene Transcript Expressions Related to the Immune System
4. Discussion
4.1. Effects of Low Stocking Density on Zootechnical Parameters of Trout
4.2. Physiological Responses of Fish Were Altered by Long-Term Low Stocking Density
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montero, D.; Izquierdo, M.S.; Tort, L.; Robaina, L.; Vergara, J.M. High Stocking Density Produces Crowding Stress Altering Some Physiological and Biochemical Parameters in Gilthead seabream, Sparus aurata, Juveniles. Fish Physiol. Biochem. 1999, 20, 53–60. [Google Scholar] [CrossRef]
- Lupatsch, I.; Santos, G.A.; Schrama, J.W.; Verreth, J.A.J. Effect of Stocking Density and Feeding Level on Energy Expenditure and Stress Responsiveness in European sea bass Dicentrarchus labrax. Aquaculture 2010, 298, 245–250. [Google Scholar] [CrossRef]
- North, B.P.; Turnbull, J.F.; Ellis, T.; Porter, M.J.; Migaud, H.; Bron, J.; Bromage, N.R. The Impact of Stocking Density on the Welfare of Rainbow trout (Oncorhynchus mykiss). Aquaculture 2006, 255, 466–479. [Google Scholar] [CrossRef]
- Ellis, T.; North, B.; Scott, A.P.; Bromage, N.R.; Porter, M.; Gadd, D. The Relationships between Stocking Density and Welfare in Farmed Rainbow trout. J. Fish Biol. 2002, 61, 493–531. [Google Scholar] [CrossRef]
- Salas-Leiton, E.; Anguis, V.; Martín-Antonio, B.; Crespo, D.; Planas, J.V.; Infante, C.; Cañavate, J.P.; Manchado, M. Effects of Stocking Density and Feed Ration on Growth and Gene Expression in the senegalese sole (Solea senegalensis): Potential Effects on the Immune Response. Fish Shellfish Immunol. 2010, 28, 296–302. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Gesto, M.; López-Patiño, M.A.; Hernández, J.; Soengas, J.L.; Míguez, J.M. The Response of Brain Serotonergic and Dopaminergic Systems to an Acute Stressor in Rainbow trout: A Time Course Study. J. Exp. Biol. 2013, 216, 4435–4442. [Google Scholar] [CrossRef] [Green Version]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in Teleosts: Dynamics, Mechanisms of Action, and Metabolic Regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L. The Concept of Stress in Fish Fish Physiol. In Biology of Stress in Fish, 1st ed.; Schreck, C.B., Tort, L., Farrell, A.P., Brauner, C.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 1–34. [Google Scholar] [CrossRef]
- Yarahmadi, P.; Miandare, H.K.; Fayaz, S.; Caipang, C.M.A. Increased Stocking Density Causes Changes in Expression of Selected Stress- and Immune-Related Genes, Humoral Innate Immune Parameters and Stress Responses of Rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 48, 43–53. [Google Scholar] [CrossRef]
- Sahin, K.; Yazlak, H.; Orhan, C.; Tuzcu, M.; Akdemir, F.; Sahin, N. The Effect of Lycopene on Antioxidant Status in Rainbow trout (Oncorhynchus mykiss) Reared under High Stocking Density. Aquaculture 2014, 418–419, 132–138. [Google Scholar] [CrossRef]
- Pickering, A.D.; Pottinger, T.G. Stress Responses and Disease Resistance in Salmonid Fish: Effects of Chronic Elevation of Plasma Cortisol. Fish Physiol. Biochem. 1989, 7, 253–258. [Google Scholar] [CrossRef]
- Naderi, M.; Keyvanshokooh, S.; Salati, A.P.; Ghaedi, A. Effects of Chronic High Stocking Density on Liver Proteome of Rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2017, 43, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Noakes, D.L.G.; Leatherland, J.F. Social Dominance and Interrenal Cell Activity in Rainbow trout, Salmo gairdneri (Pisces, Salmonidae). Environ. Biol. Fish 1977, 2, 131–136. [Google Scholar] [CrossRef]
- Grobler, J.M.B.; Wood, C.M. The Physiology of Rainbow trout in Social Hierarchies: Two Ways of Looking at the Same Data. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2013, 183, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Huntingford, F.A.; Turner, A.K. Animal Conflict; Springer: Amsterdam, The Netherlands, 1987; p. 448. [Google Scholar]
- Stejskal, V.; Matoušek, J.; Prokešová, M.; Podhorec, P.; Křišťan, J.; Policar, T.; Gebauer, T. Fin Damage and Growth Parameters Relative to Stocking Density and Feeding Method in Intensively Cultured European perch (Perca fluviatilis L.). J. Fish. Dis. 2020, 43, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Sveen, L.R.; Timmerhaus, G.; Krasnov, A.; Takle, H.; Stefansson, S.O.; Handeland, S.O.; Ytteborg, E. High Fish Density Delays Wound Healing in Atlantic salmon (Salmo salar). Sci. Rep. 2018, 8, 16907. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Zanin, G.; Birolo, M.; Trocino, A.; Sambo, P.; Borin, M.; Xiccato, G. Effect of Stocking Density of Fish on Water Quality and Growth Performance of European Carp and Leafy Vegetables in a Low-Tech Aquaponic System. PLoS ONE 2019, 14, e0217561. [Google Scholar] [CrossRef] [Green Version]
- Ni, M.; Wen, H.; Li, J.; Chi, M.; Bu, Y.; Ren, Y.; Zhang, M.; Song, Z.; Ding, H. Effects of Stocking Density on Mortality, Growth and Physiology of Juvenile Amur sturgeon (Acipenser schrenckii). Aquac. Res. 2016, 47, 1596–1604. [Google Scholar] [CrossRef]
- Roy, J.; Vigor, C.; Vercauteren, J.; Reversat, G.; Zhou, B.; Surget, A.; Larroquet, L.; Lanuque, A.; Sandres, F.; Terrier, F.; et al. Characterization and Modulation of Brain Lipids Content of Rainbow trout Fed with 100% Plant Based Diet Rich in Omega-3 Long Chain Polyunsaturated Fatty Acids DHA and EPA. Biochimie 2020, 178, 137–147. [Google Scholar] [CrossRef]
- Roy, J.; Larroquet, L.; Surget, A.; Lanuque, A.; Sandres, F.; Terrier, F.; Corraze, G.; Chung-Yung Lee, J.; Skiba-Cassy, S. Impact on Cerebral Function in Rainbow trout Fed with Plant Based Omega-3 Long Chain Polyunsaturated Fatty Acids Enriched with DHA and EPA. Fish Shellfish Immunol. 2020, 103, 409–420. [Google Scholar] [CrossRef]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for General Users and for Biologist Programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [CrossRef] [Green Version]
- Miandare, H.K.; Farahmand, H.; Akbarzadeh, A.; Ramezanpour, S.; Kaiya, H.; Miyazato, M.; Rytkönen, K.T.; Nikinmaa, M. Developmental Transcription of Genes Putatively Associated with Growth in Two Sturgeon Species of Different Growth Rate. Gen. Comp. Endocrinol. 2012, 182, 41–47. [Google Scholar] [CrossRef]
- White, A.; Fletcher, T.C. Radioimmunoassay of Serum Cortisol in the Plaice (Pleuronectes platessa L.). Gen. Comp. Endocrinol. 1984, 53, 410–417. [Google Scholar] [CrossRef]
- Ellis, T.; Oidtmann, B.; St-Hilaire, S.; Turnbull, J.F.; North, B.P.; MacIntyre, C.; Knowles, T.G. Fin Erosion in Farmed Fish. In Fish Welfare; Branson, E.J., Ed.; Blackwell Science Ltd.: Oxford, UK, 2008; pp. 121–149. [Google Scholar]
- Mörsky, P. Turbidimetric Determination of Lysozyme with Micrococcus lysodeikticus Cells: Reexamination of Reaction Conditions. Anal. Biochem. 1983, 128, 77–85. [Google Scholar] [CrossRef]
- Chapman, D.W. Food and Space as Regulators of Salmonid Populations in Streams. Am. Nat. 1966, 100, 345–357. [Google Scholar] [CrossRef]
- Sloman, K.A.; Gilmour, K.M.; Metcalfe, N.B.; Taylor, A.C. Does Socially Induced Stress in Rainbow trout Cause Chloride Cell Proliferation? J. Fish Biol. 2000, 56, 725–738. [Google Scholar] [CrossRef]
- Sloman, K.A.; Armstrong, J.D. Physiological Effects of Dominance Hierarchies: Laboratory Artefacts or Natural Phenomena? J. Fish Biol. 2002, 61, 1–23. [Google Scholar] [CrossRef]
- Gurney, W.S.; Nisbet, R.M. Ecological Stability and Social Hierarchy. Theor. Popul. Biol. 1979, 16, 48–80. [Google Scholar] [CrossRef]
- Pottinger, T.G.; Pickering, A.D. The Influence of Social Interaction on the Acclimation of Rainbow trout, Oncorhynchus mykiss (Walbaum) to Chronic Stress. J. Fish Biol. 1992, 41, 435–447. [Google Scholar] [CrossRef]
- Pickering, A.D.; Pottinger, T.G.; Sumpter, J.P.; Carragher, J.F.; Le Bail, P.Y. Effects of Acute and Chronic Stress on the Levels of Circulating Growth Hormone in the Rainbow trout, Oncorhynchus mykiss. Gen. Comp. Endocrinol. 1991, 83, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Peters, G.; Delventhal, H.; Klinger, H. Physiological and Morphological Effects of Social Stress on the Eel, Anguilla anguilla L. In Fish Diseases; Springer: New York, NY, USA, 1980. [Google Scholar]
- Sloman, K.A.; Wilson, L.; Freel, J.A.; Taylor, A.C.; Metcalfe, N.B.; Gilmour, K.M. The Effects of Increased Flow Rates on Linear Dominance Hierarchies and Physiological Function in Brown trout, Salmo trutta. Can. J. Zool. 2002, 80, 1221–1227. [Google Scholar] [CrossRef]
- Sloman, K.A.; Taylor, A.C.; Metcalfe, N.B.; Gilmour, K.M. Effects of an Environmental Perturbation on the Social Behaviour and Physiological Function of Brown trout. Anim. Behav. 2001, 61, 325–333. [Google Scholar] [CrossRef]
- Gilmour, K.M.; Dibattista, J.D.; Thomas, J.B. Physiological Causes and Consequences of Social Status in Salmonid Fish. Integr. Comp. Biol. 2005, 45, 263–273. [Google Scholar] [CrossRef]
- Dalla, C.; Antoniou, K.; Kokras, N.; Drossopoulou, G.; Papathanasiou, G.; Bekris, S.; Daskas, S.; Papadopoulou-Daifoti, Z. Sex Differences in the Effects of Two Stress Paradigms on Dopaminergic Neurotransmission. Physiol. Behav. 2008, 93, 595–605. [Google Scholar] [CrossRef]
- Papoutsoglou, S.E.; Karakatsouli, N.; Pizzonia, G.; Dalla, C.; Polissidis, A.; Papadopoulou-Daifoti, Z. Effects of Rearing Density on Growth, Brain Neurotransmitters and Liver Fatty Acid Composition of Juvenile White Sea sream Diplodus sargus L. Aquac. Res. 2006, 37, 87–95. [Google Scholar] [CrossRef]
- Karakatsouli, N.; Papoutsoglou, S.E.; Pizzonia, G.; Tsatsos, G.; Tsopelakos, A.; Chadio, S.; Kalogiannis, D.; Dalla, C.; Polissidis, A.; Papadopoulou-Daifoti, Z. Effects of Light Spectrum on Growth and Physiological Status of Gilthead seabream Sparus aurata and Rainbow trout Oncorhynchus mykiss Reared under Recirculating System Conditions. Aquac. Eng. 2007, 36, 302–309. [Google Scholar] [CrossRef]
- Winberg, S.; Nilsson, G.E.; Olsén, K.H. Changes in Brain Serotonergic Activity during Hierarchic Behavior in Arctic charr (Salvelinus alpinus L.) Are Socially Induced. J. Comp. Physiol A 1992, 170, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Winberg, S.; Nilsson, G.E. Time course of changes in brain serotonergic activity and brain tryptophan levels in dominant and subordinate juvenile artic charr. J. Exp. Biol. 1993, 179, 181–195. [Google Scholar] [CrossRef]
- Höglund, E.; Kolm, N.; Winberg, S. Stress-Induced Changes in Brain Serotonergic Activity, Plasma Cortisol and Aggressive Behavior in Arctic charr (Salvelinus alpinus) Is Counteracted by L-DOPA. Physiol. Behav. 2001, 74, 381–389. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Galhardo, L.; Noble, C.; Damsgård, B.; Spedicato, M.T.; Zupa, W.; Beauchaud, M.; Kulczykowska, E.; Massabuau, J.-C.; Carter, T.; et al. Behavioural Indicators of Welfare in Farmed Fish. Fish Physiol. Biochem. 2012, 38, 17–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendelaar Bonga, S.E. The Stress Response in Fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. Enhancing versus Suppressive Effects of Stress on Immune Function: Implications for Immunoprotection and Immunopathology. Neuroimmunomodulation 2009, 16, 300–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bly, J.E.; Quiniou, S.M.; Clem, L.W. Environmental Effects on Fish Immune Mechanisms. Dev. Biol. Stand. 1997, 90, 33–43. [Google Scholar] [PubMed]
- Davidson, A.J.; Zon, L.I. The “definitive” (and ’Primitive’) Guide to Zebrafish Hematopoiesis. Oncogene 2004, 23, 7233–7246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harikrishnan, R.; Rani, M.; Balasundaram, C. Hematological and Biochemical Parameters in ommon Carp, Cyprinus carpio, Following Herbal Treatment for Aeromonas hydrophila Infection. Aquaculture 2003, 221, 41–50. [Google Scholar] [CrossRef]
- Workenhe, S.T.; Kibenge, M.J.T.; Wright, G.M.; Wadowska, D.W.; Groman, D.B.; Kibenge, F.S.B. Infectious Salmon Anaemia Virus Replication and Induction of Alpha Interferon in Atlantic salmon Erythrocytes. Virol. J. 2008, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Yanai, H.; Savitsky, D.; Taniguchi, T. The IRF Family Transcription Factors in Immunity and Oncogenesis. Annu Rev. Immunol. 2008, 26, 535–584. [Google Scholar] [CrossRef]
- Sigh, J.; Lindenstrøm, T.; Buchmann, K. Expression of Pro-Inflammatory Cytokines in Rainbow trout (Oncorhynchus mykiss) during an Infection with Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2004, 17, 75–86. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, J.; Terrier, F.; Marchand, M.; Herman, A.; Heraud, C.; Surget, A.; Lanuque, A.; Sandres, F.; Marandel, L. Effects of Low Stocking Densities on Zootechnical Parameters and Physiological Responses of Rainbow Trout (Oncorhynchus mykiss) Juveniles. Biology 2021, 10, 1040. https://doi.org/10.3390/biology10101040
Roy J, Terrier F, Marchand M, Herman A, Heraud C, Surget A, Lanuque A, Sandres F, Marandel L. Effects of Low Stocking Densities on Zootechnical Parameters and Physiological Responses of Rainbow Trout (Oncorhynchus mykiss) Juveniles. Biology. 2021; 10(10):1040. https://doi.org/10.3390/biology10101040
Chicago/Turabian StyleRoy, Jérôme, Frederic Terrier, Michael Marchand, Alexandre Herman, Cécile Heraud, Anne Surget, Anthony Lanuque, Franck Sandres, and Lucie Marandel. 2021. "Effects of Low Stocking Densities on Zootechnical Parameters and Physiological Responses of Rainbow Trout (Oncorhynchus mykiss) Juveniles" Biology 10, no. 10: 1040. https://doi.org/10.3390/biology10101040
APA StyleRoy, J., Terrier, F., Marchand, M., Herman, A., Heraud, C., Surget, A., Lanuque, A., Sandres, F., & Marandel, L. (2021). Effects of Low Stocking Densities on Zootechnical Parameters and Physiological Responses of Rainbow Trout (Oncorhynchus mykiss) Juveniles. Biology, 10(10), 1040. https://doi.org/10.3390/biology10101040






