Decreased Expression of Cytotoxic Proteins in Decidual CD8+ T Cells in Preeclampsia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Preparation and Clinical Data
2.2. Double Immunofluorescence Staining
2.3. Isolation of CD8+ T Cells from mPBL
2.4. Flow Cytometry Analysis
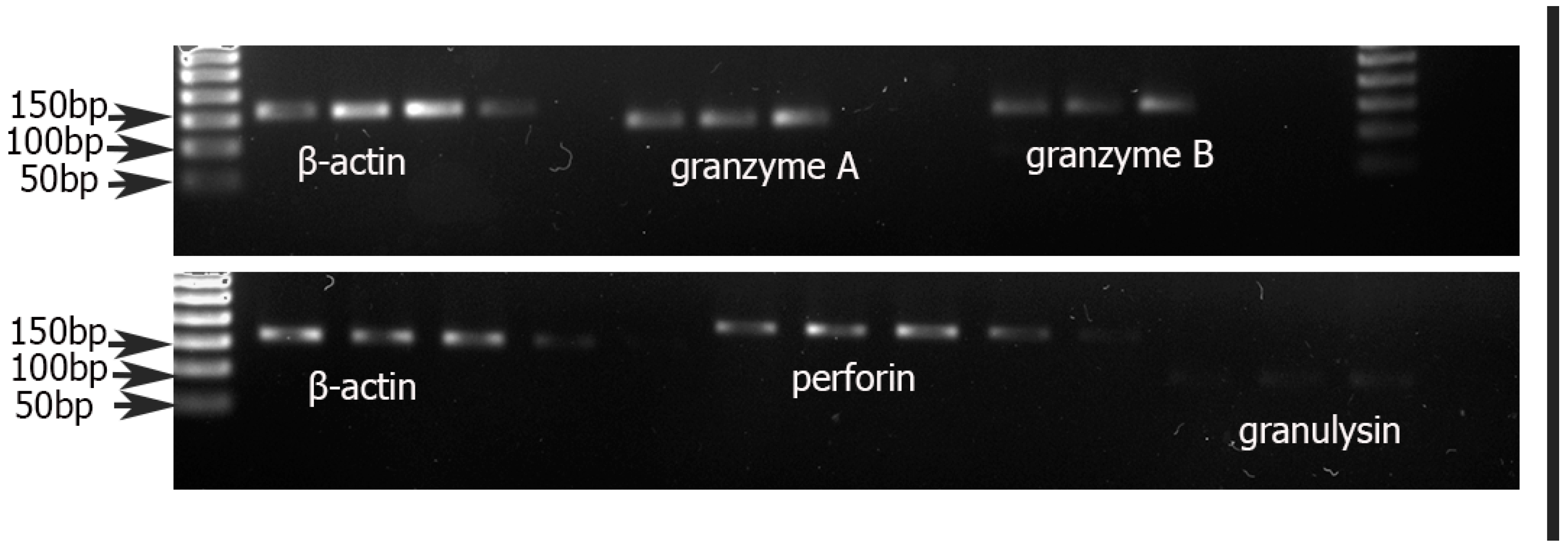
2.5. RT-PCR
2.6. qPCR
2.7. Statistical Analysis
3. Results
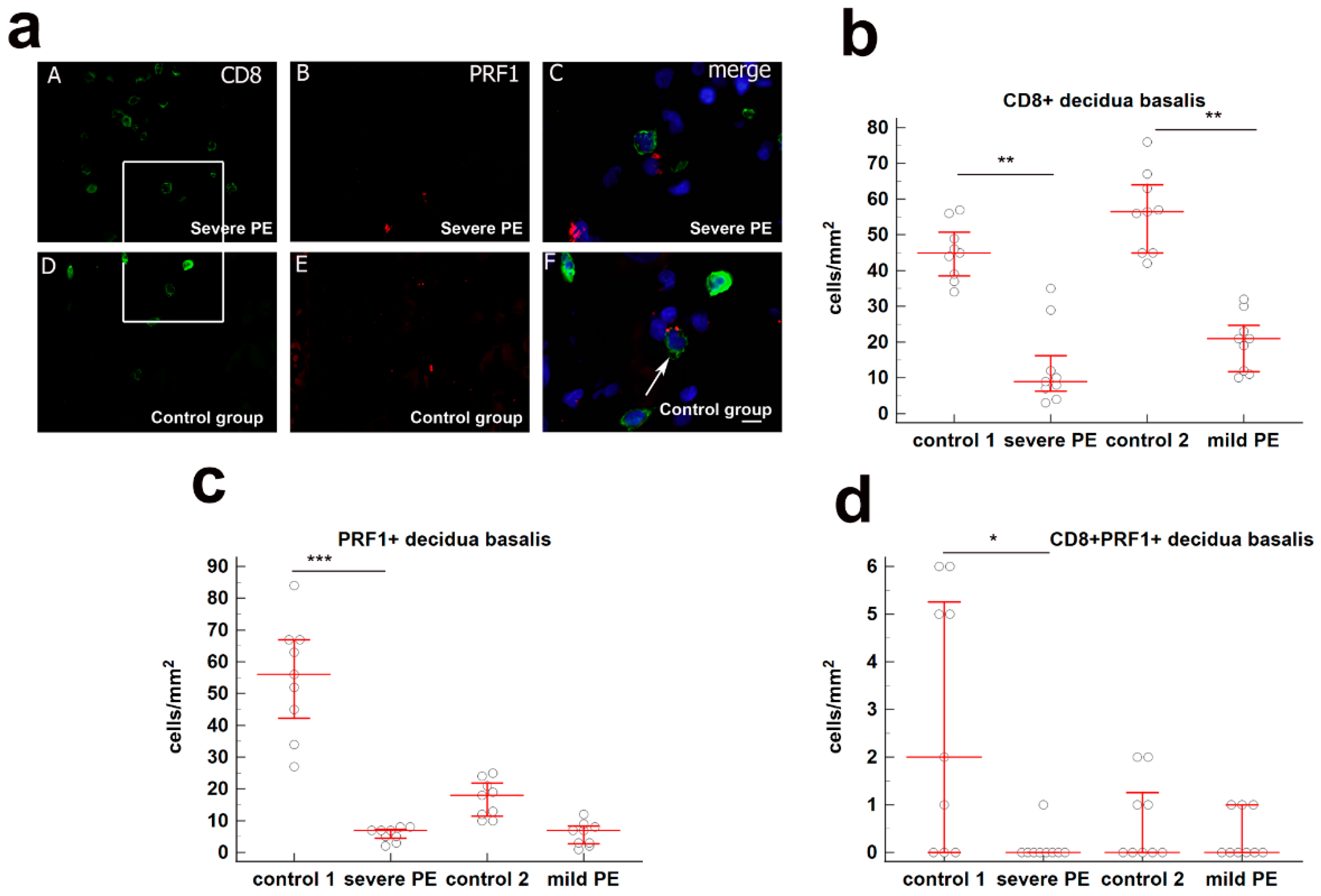
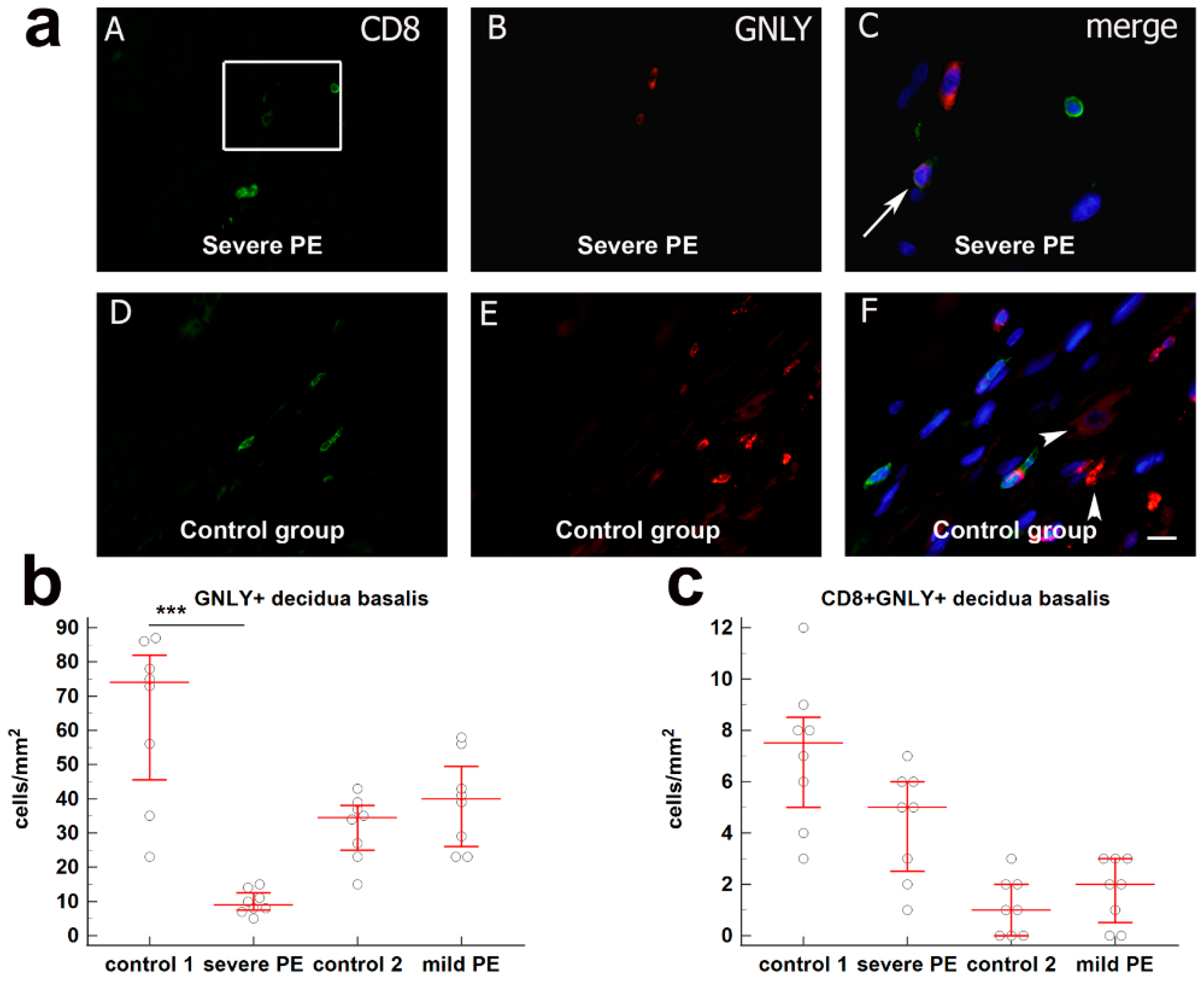
3.1. Quantification of Cytotoxic CD8+ T Cells in Decidua Basalis of Severe and Mild PE Compared to Normal Healthy Pregnancies
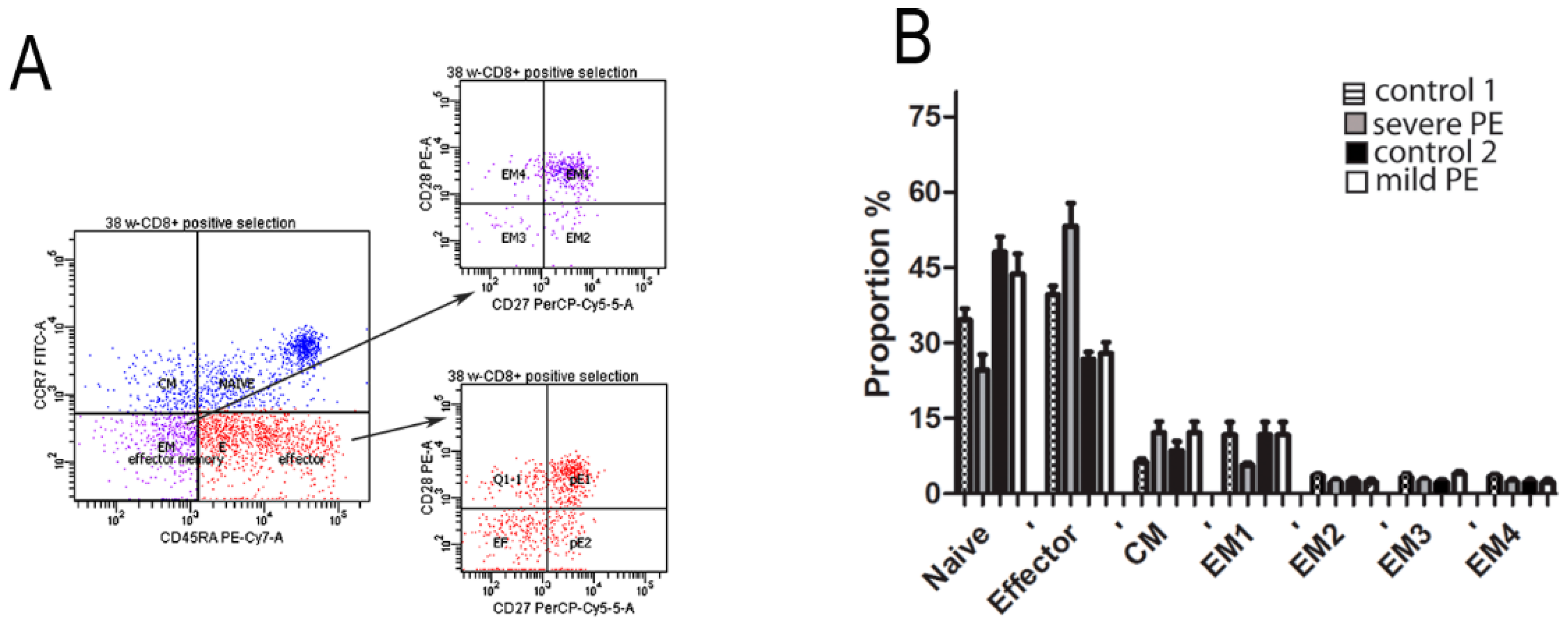
3.2. Quantification of Cytotoxic Proteins PRF1, GNLY, GZMA, GzB, and FOXP3 in CD8+ T Cells from mPBL of Severe and Mild Preeclampsia Compared to Normal Healthy Pregnancies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohan-Ghadr, H.R.; Kadam, L.; Jain, C.; Armant, D.R.; Drewlo, S. Potential role of epigenetic mechanisms in regulation of trophoblast differentiation, migration, and invasion in the human placenta. Cell Adhes. Migr. 2016, 10, 126–135. [Google Scholar] [CrossRef]
- Nelissen, E.C.; van Montfoort, A.P.; Dumoulin, J.C.; Evers, J.L. Epigenetics and the placenta. Hum. Reprod. Update 2011, 17, 397–417. [Google Scholar] [CrossRef]
- Farah, O.; Nguyen, C.; Tekkatte, C.; Parast, M.M. Trophoblast lineage-specific differentiation and associated alterations in preeclampsia and fetal growth restriction. Placenta 2020, 102, 4–9. [Google Scholar] [CrossRef]
- Cunningham, G.; Leveno, K.; Bloom, S.; Spong, C.; Dashe, J.; Hoffman, B. Williams Obstetrics; McGraw Hill Education: New York, NY, USA, 2014; Volume 24, p. 1305. [Google Scholar]
- von Dadelszen, P.; Magee, L.A.; Roberts, J.M. Subclassification of preeclampsia. Hypertens. Pregnancy 2003, 22, 143–148. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Egbor, M.; Ansari, T.; Morris, N.; Green, C.J.; Sibbons, P.D. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Redman, C.W.G. The differences between early- and late-onset pre-eclampsia. In Preeclampsia, Comprehensive Gynecology and Obstetrics; Springer: Singapore, 2018; pp. 157–172. [Google Scholar]
- Crespo, A.C.; van der Zwan, A.; Ramalho-Santos, J.; Strominger, J.L.; Tilburgs, T. Cytotoxic potential of decidual NK cells and CD8+ T cells awakened by infections. J. Reprod. Immunol. 2017, 119, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.W.; King, A.; Burrows, T.D. Decidua in human implantation. Hum. Reprod. 1995, 10, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Tanaka, S.; Okada, H. Immune Tolerance of the Human Decidua. J. Clin. Med. 2021, 10, 351. [Google Scholar] [CrossRef]
- Schust, D.J.; Bonney, E.A.; Sugimoto, J.; Ezashi, T.; Roberts, R.M.; Choi, S.; Zhou, J. The Immunology of Syncytialized Trophoblast. Int. J. Mol. Sci. 2021, 22, 1767. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Lombardelli, L.; Logiodice, F.; Kullolli, O.; Romagnani, S.; Le Bouteiller, P. T helper cell mediated-tolerance towards fetal allograft in successful pregnancy. Clin. Mol. Allergy CMA 2015, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sanguansermsri, D.; Pongcharoen, S. Pregnancy immunology: Decidual immune cells. Asian. Pac. J. Allergy. Immunol. Launched Allergy Immunol. Soc. Thail. 2008, 26, 171–181. [Google Scholar]
- PrabhuDas, M.; Bonney, E.; Caron, K.; Dey, S.; Erlebacher, A.; Fazleabas, A.; Fisher, S.; Golos, T.; Matzuk, M.; McCune, J.M.; et al. Immune mechanisms at the maternal-fetal interface: Perspectives and challenges. Nat. Immunol. 2015, 16, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Lissauer, D.; Kilby, M.D.; Moss, P. Maternal effector T cells within decidua: The adaptive immune response to pregnancy? Placenta 2017, 60, 140–144. [Google Scholar] [CrossRef] [PubMed]
- White, H.D.; Crassi, K.M.; Givan, A.L.; Stern, J.E.; Gonzalez, J.L.; Memoli, V.A.; Green, W.R.; Wira, C.R. CD3+ CD8+ CTL activity within the human female reproductive tract: Influence of stage of the menstrual cycle and menopause. J. Immunol. 1997, 158, 3017–3027. [Google Scholar]
- Klentzeris, L.D.; Bulmer, J.N.; Warren, A.; Morrison, L.; Li, T.C.; Cooke, I.D. Endometrial lymphoid tissue in the timed endometrial biopsy: Morphometric and immunohistochemical aspects. Am. J. Obstet. Gynecol. 1992, 167, 667–674. [Google Scholar] [CrossRef]
- Scaife, P.J.; Bulmer, J.N.; Robson, S.C.; Innes, B.A.; Searle, R.F. Effector activity of decidual CD8+ T lymphocytes in early human pregnancy. Biol. Reprod. 2006, 75, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, F.; Li, M.; Qian, J.; Chen, C.; Wang, M.; Zang, X.; Li, D.; Yu, M.; Du, M. The appropriate frequency and function of decidual Tim-3(+)CTLA-4(+)CD8(+) T cells are important in maintaining normal pregnancy. Cell Death Dis. 2019, 10, 407. [Google Scholar] [CrossRef]
- Liu, S.; Diao, L.; Huang, C.; Li, Y.; Zeng, Y.; Kwak-Kim, J.Y.H. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol. 2017, 124, 44–53. [Google Scholar] [CrossRef]
- Hamann, D.; Roos, M.T.; van Lier, R.A. Faces and phases of human CD8 T-cell development. Immunol. Today 1999, 20, 177–180. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Basic Immunology: Functions and Disorders of the Immune System, 4th ed.; Elsevier: Philadelphia, PA, USA, 2014. [Google Scholar]
- Nagasawa, M.; Ogawa, K.; Nagata, K.; Shimizu, N. Serum granulysin as a possible biomarker of natural killer cell neoplasms. Br. J. Haematol. 2010, 148, 812–814. [Google Scholar] [CrossRef]
- Bade, B.; Boettcher, H.E.; Lohrmann, J.; Hink-Schauer, C.; Bratke, K.; Jenne, D.E.; Virchow, J.C., Jr.; Luttmann, W. Differential expression of the granzymes A, K and M and perforin in human peripheral blood lymphocytes. Int. Immunol. 2005, 17, 1419–1428. [Google Scholar] [CrossRef]
- Bratke, K.; Kuepper, M.; Bade, B.; Virchow, J.C., Jr.; Luttmann, W. Differential expression of human granzymes A, B, and K in natural killer cells and during CD8+ T cell differentiation in peripheral blood. Eur. J. Immunol. 2005, 35, 2608–2616. [Google Scholar] [CrossRef]
- Tilburgs, T.; Schonkeren, D.; Eikmans, M.; Nagtzaam, N.M.; Datema, G.; Swings, G.M.; Prins, F.; van Lith, J.M.; van der Mast, B.J.; Roelen, D.L.; et al. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J. Immunol. 2010, 185, 4470–4477. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Tsuda, S.; Kobayashi, E.; Hamana, H.; Tsuda, K.; Shima, T.; Nakashima, A.; Ushijima, A.; Kishi, H.; Saito, S. Analysis of TCR Repertoire and PD-1 Expression in Decidual and Peripheral CD8(+) T Cells Reveals Distinct Immune Mechanisms in Miscarriage and Preeclampsia. Front. Immunol. 2020, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; Strominger, J.L. CD8+ effector T cells at the fetal-maternal interface, balancing fetal tolerance and antiviral immunity. Am. J. Reprod. Immunol. 2013, 69, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, G.M.; Storvold, G.L.; Alnaes-Katjavivi, P.H.; Roald, B.; Golic, M.; Dechend, R.; Redman, C.W.G.; Staff, A.C. Lymphocyte characterization of decidua basalis spiral arteries with acute atherosis in preeclamptic and normotensive pregnancies. J. Reprod. Immunol. 2019, 132, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Shiozaki, A.; Nakashima, A.; Sakai, M.; Sasaki, Y. The role of the immune system in preeclampsia. Mol. Asp. Med. 2007, 28, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.T.; Fortner, K.A.; Oppenheimer, K.H.; Bonney, E.A. Evidence that CD8 T-cell homeostasis and function remain intact during murine pregnancy. Immunology 2010, 131, 426–437. [Google Scholar] [CrossRef]
- van der Zwan, A.; Bi, K.; Norwitz, E.R.; Crespo, A.C.; Claas, F.H.J.; Strominger, J.L.; Tilburgs, T. Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc. Natl. Acad. Sci. USA 2018, 115, 385–390. [Google Scholar] [CrossRef]
- Qiu, C.; Saito, S.; Sakai, M.; Ogawa, K.; Nagata, K.; Williams, M.A. Plasma granulysin concentrations and preeclampsia risk. Clin Biochem. 2006, 39, 1016–1021. [Google Scholar] [CrossRef]
- Veljkovic Vujaklija, D.; Sucic, S.; Gulic, T.; Dominovic, M.; Rukavina, D. Cell death mechanisms at the maternal-fetal interface: Insights into the role of granulysin. Clin. Dev. Immunol. 2012, 2012, 180272. [Google Scholar] [CrossRef]
- Kieffer, T.E.C.; Laskewitz, A.; Vledder, A.; Scherjon, S.A.; Faas, M.M.; Prins, J.R. Decidual memory T-cell subsets and memory T-cell stimulatory cytokines in early- and late-onset preeclampsia. Am. J. Reprod. Immunol. 2020, 84, e13293. [Google Scholar] [CrossRef] [PubMed]
- Somerset, D.A.; Zheng, Y.; Kilby, M.D.; Sansom, D.M.; Drayson, M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 2004, 112, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Orlovic, M.; Tomic, V.; Vukojevic, K.; Hudic, I.; Mandic, V.; Azinovic, I.; Soldo, D.; Kajic, M.; Soljic, V. Decreased expression of MMP-9 in CD8+ cells in placenta with severe preeclampsia. Biotech. Histochem. Off. Publ. Biol. Stain. Comm. 2017, 92, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Klentzeris, L.D.; Bulmer, J.N.; Warren, M.A.; Morrison, L.; Li, T.C.; Cooke, I.D. Lymphoid tissue in the endometrium of women with unexplained infertility: Morphometric and immunohistochemical aspects. Hum. Reprod. 1994, 9, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Zhang, X.; Hamana, H.; Shima, T.; Ushijima, A.; Tsuda, K.; Muraguchi, A.; Kishi, H.; Saito, S. Clonally Expanded Decidual Effector Regulatory T Cells Increase in Late Gestation of Normal Pregnancy, but Not in Preeclampsia, in Humans. Front. Immunol. 2018, 9, 1934. [Google Scholar] [CrossRef] [PubMed]
- Toldi, G.; Svec, P.; Vasarhelyi, B.; Meszaros, G.; Rigo, J.; Tulassay, T.; Treszl, A. Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta Obstet. Gynecol. Scand. 2008, 87, 1229–1233. [Google Scholar] [CrossRef]
- Steinborn, A.; Schmitt, E.; Kisielewicz, A.; Rechenberg, S.; Seissler, N.; Mahnke, K.; Schaier, M.; Zeier, M.; Sohn, C. Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin. Exp. Immunol. 2012, 167, 84–98. [Google Scholar] [CrossRef]
- Steinborn, A.; Haensch, G.M.; Mahnke, K.; Schmitt, E.; Toermer, A.; Meuer, S.; Sohn, C. Distinct subsets of regulatory T cells during pregnancy: Is the imbalance of these subsets involved in the pathogenesis of preeclampsia? Clin. Immunol. 2008, 129, 401–412. [Google Scholar] [CrossRef]
- Sasaki, Y.; Darmochwal-Kolarz, D.; Suzuki, D.; Sakai, M.; Ito, M.; Shima, T.; Shiozaki, A.; Rolinski, J.; Saito, S. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin. Exp. Immunol. 2007, 149, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.H.; Lacoursiere, D.Y.; Cui, L.; Bui, J.; Parast, M.M. The unique pathophysiology of early-onset severe preeclampsia: Role of decidual T regulatory cells. J. Reprod. Immunol. 2011, 91, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Orlovic Vlaho, M.; Tomic, V.; Vukojevic, K.; Vasilj, A.; Pejic, R.; Lesko, J.; Soljic, V. CD25(+) FOXP3(+) and CD4(+) CD25(+) cells distribution in decidual departments of women with severe and mild pre-eclampsia: Comparison with healthy pregnancies. Am. J. Reprod. Immunol. 2020, 84, e13281. [Google Scholar] [CrossRef] [PubMed]
- Bezie, S.; Anegon, I.; Guillonneau, C. Advances on CD8+ Treg Cells and Their Potential in Transplantation. Transplantation 2018, 102, 1467–1478. [Google Scholar] [CrossRef]
- Zahran, A.M.; Nafady-Hego, H.; Mansor, S.G.; Abbas, W.A.; Abdel-Malek, M.O.; Mekky, M.A.; Hetta, H.F. Increased frequency and FOXP3 expression of human CD8(+)CD25(High+) T lymphocytes and its relation to CD4 regulatory T cells in patients with hepatocellular carcinoma. Hum. Immunol. 2019, 80, 510–516. [Google Scholar] [CrossRef]
- Jorgensen, N.; Persson, G.; Hviid, T.V.F. The Tolerogenic Function of Regulatory T Cells in Pregnancy and Cancer. Front. Immunol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Vianna, P.; Mondadori, A.G.; Bauer, M.E.; Dornfeld, D.; Chies, J.A. HLA-G and CD8+ regulatory T cells in the inflammatory environment of pre-eclampsia. Reproduction 2016, 152, 741–751. [Google Scholar] [CrossRef][Green Version]
- Tilburgs, T.; Scherjon, S.A.; Roelen, D.L.; Claas, F.H. Decidual CD8+CD28- T cells express CD103 but not perforin. Hum. Immunol. 2009, 70, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Li, Y.H.; Piao, H.L.; Hong, X.W.; Zhang, D.; Xu, Y.Y.; Tao, Y.; Wang, Y.; Yuan, M.M.; Li, D.J.; et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015, 6, e1738. [Google Scholar] [CrossRef]
- Rieger, L.; Segerer, S.; Bernar, T.; Kapp, M.; Majic, M.; Morr, A.K.; Dietl, J.; Kammerer, U. Specific subsets of immune cells in human decidua differ between normal pregnancy and preeclampsia--a prospective observational study. Reprod. Biol. Endocrinol. RBE 2009, 7, 132. [Google Scholar] [CrossRef]
- Saito, S.; Sakai, M. Th1/Th2 balance in preeclampsia. J. Reprod. Immunol. 2003, 59, 161–173. [Google Scholar] [CrossRef]
- Williams, P.J.; Bulmer, J.N.; Searle, R.F.; Innes, B.A.; Robson, S.C. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: A comparison with late normal pregnancy. Reproduction 2009, 138, 177–184. [Google Scholar] [CrossRef]
- Crespo, A.C.; Mulik, S.; Dotiwala, F.; Ansara, J.A.; Sen Santara, S.; Ingersoll, K.; Ovies, C.; Junqueira, C.; Tilburgs, T.; Strominger, J.L.; et al. Decidual NK Cells Transfer Granulysin to Selectively Kill Bacteria in Trophoblasts. Cell 2020, 182, 1125–1139.e18. [Google Scholar] [CrossRef] [PubMed]
- Sindram-Trujillo, A.P.; Scherjon, S.A.; van Hulst-van Miert, P.P.; Kanhai, H.H.; Roelen, D.L.; Claas, F.H. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J. Reprod. Immunol. 2004, 62, 125–137. [Google Scholar] [CrossRef] [PubMed]
- van Egmond, A.; van der Keur, C.; van Beelen, E.; Scherjon, S.A.; Claas, F.H.J. No direct effect of an elective caesarean section on the phenotypic and functional characteristics of maternal peripheral blood T lymphocytes. Hum. Immunol. 2016, 77, 898–904. [Google Scholar] [CrossRef] [PubMed]


| Control 1 (n = 10) | Severe Preeclampsia (n = 10) | Control 2 (n = 10) | Mild Preeclampsia (n = 10) | p-Value | |
|---|---|---|---|---|---|
| Maternal age (years) mean ± SD | 29.08 ± 5.81 | 27.50 ± 4.51 | 28.42 ± 5.22 | 31.30 ± 5.73 | 0.390 |
| Gestational age (weeks) median (IqR) | 35 (34–39) | 34 (33–39) | 39 (38–41) | 38 (34–41) | <0.001 |
| Systolic RR (mmHg) mean ± SD | 118 ± 5.23 | 173 ± 18.07 | 113 ± 5.72 | 141 ± 7.35 | <0.001 |
| Diastolic RR (mmHg) median (IqR) | 75 (66–81) | 121 (111–140) | 77 (65–80) | 89 (86–100) | <0.001 |
| Birth weight (grams) median (IqR) | 2340 (2123–3220) | 1820 (1250–2200) | 3310 (2370–4500) | 3315 (1803–3950) | <0.001 |
| Cesarean deliveries (%) | 2 (20) | 10 (100) | 1 (10) | 2 (20) | <0.001 |
| Body mass index (BMI) mean ± SD | 24.94 ± 4.86 | 23.67 ± 2.42 | 25.84 ± 4.16 | 23.52 ± 2.16 | 0.06 |
| Intrauterin growth restriction (IUGR) (%) | 1 (10) | 7 (70) | 0 (0) | 1 (10) | <0.001 |
| Postpartal complications (%) | 0 (0) | 6 (60) | 0 (0) | 1 (10) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soljic, V.; Barbaric, M.; Vukoja, M.; Curlin, M.; Orlovic Vlaho, M.; Cerni Obrdalj, E.; Lasic Arapovic, L.; Bevanda Glibo, D.; Vukojevic, K. Decreased Expression of Cytotoxic Proteins in Decidual CD8+ T Cells in Preeclampsia. Biology 2021, 10, 1037. https://doi.org/10.3390/biology10101037
Soljic V, Barbaric M, Vukoja M, Curlin M, Orlovic Vlaho M, Cerni Obrdalj E, Lasic Arapovic L, Bevanda Glibo D, Vukojevic K. Decreased Expression of Cytotoxic Proteins in Decidual CD8+ T Cells in Preeclampsia. Biology. 2021; 10(10):1037. https://doi.org/10.3390/biology10101037
Chicago/Turabian StyleSoljic, Violeta, Maja Barbaric, Martina Vukoja, Marina Curlin, Martina Orlovic Vlaho, Edita Cerni Obrdalj, Lidija Lasic Arapovic, Daniela Bevanda Glibo, and Katarina Vukojevic. 2021. "Decreased Expression of Cytotoxic Proteins in Decidual CD8+ T Cells in Preeclampsia" Biology 10, no. 10: 1037. https://doi.org/10.3390/biology10101037
APA StyleSoljic, V., Barbaric, M., Vukoja, M., Curlin, M., Orlovic Vlaho, M., Cerni Obrdalj, E., Lasic Arapovic, L., Bevanda Glibo, D., & Vukojevic, K. (2021). Decreased Expression of Cytotoxic Proteins in Decidual CD8+ T Cells in Preeclampsia. Biology, 10(10), 1037. https://doi.org/10.3390/biology10101037








