Biodiversity of Pathogenic and Toxigenic Seed-Borne Mycoflora of Wheat in Egypt and Their Correlations with Weather Variables
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
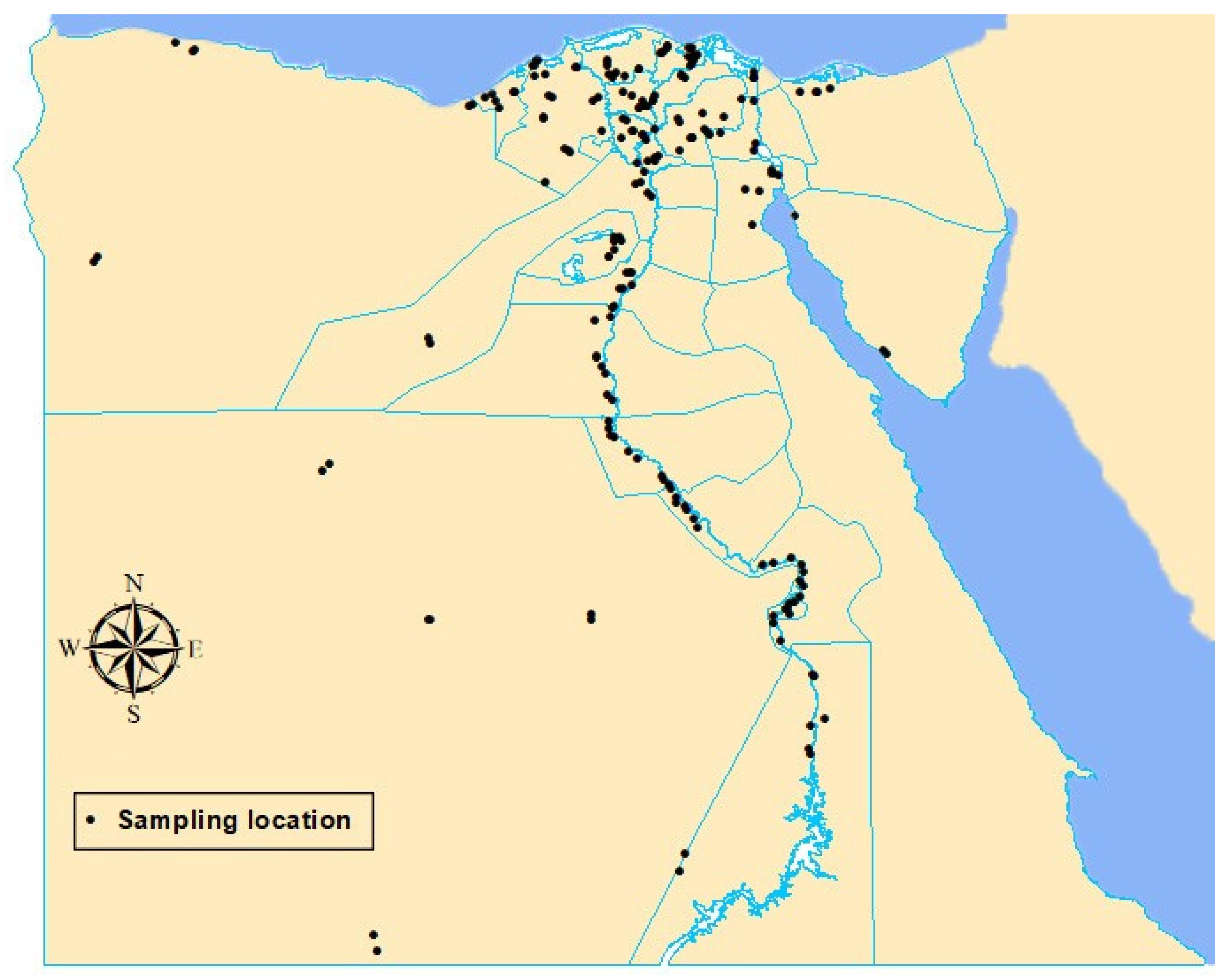
2.1. Study Area
2.2. Meteorological Conditions
2.3. Sample Collection
2.4. Detection of Seed-Borne Fungi
2.4.1. Deep-Freezing Blotter Method (DFB)
2.4.2. Washing Test
2.4.3. Embryo Count Test
2.5. Identification of Seed-Borne Fungi
2.6. Calculating Biodiversity Indicators
2.7. Pathogenicity Test
2.8. Statistical Analyses
3. Results
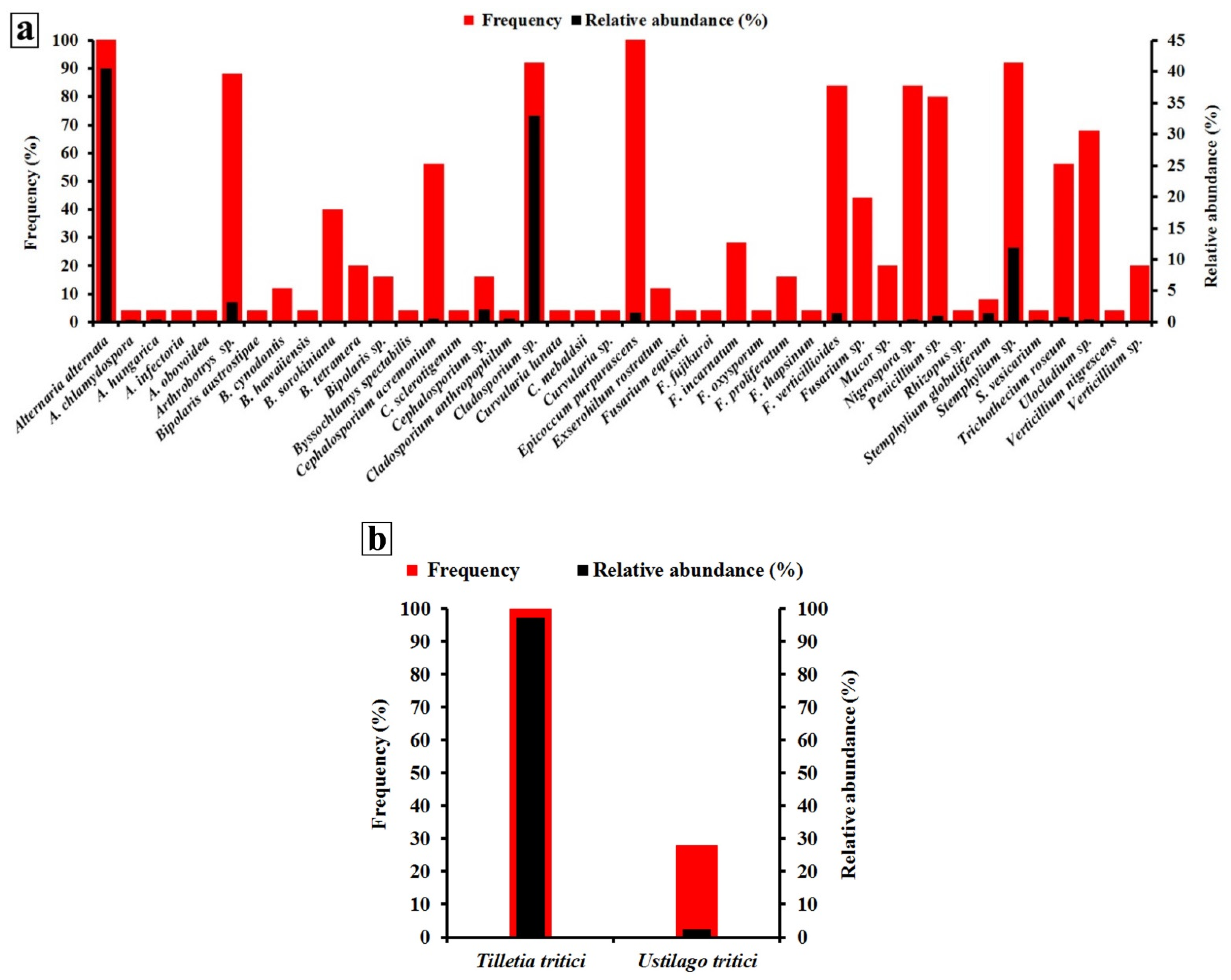
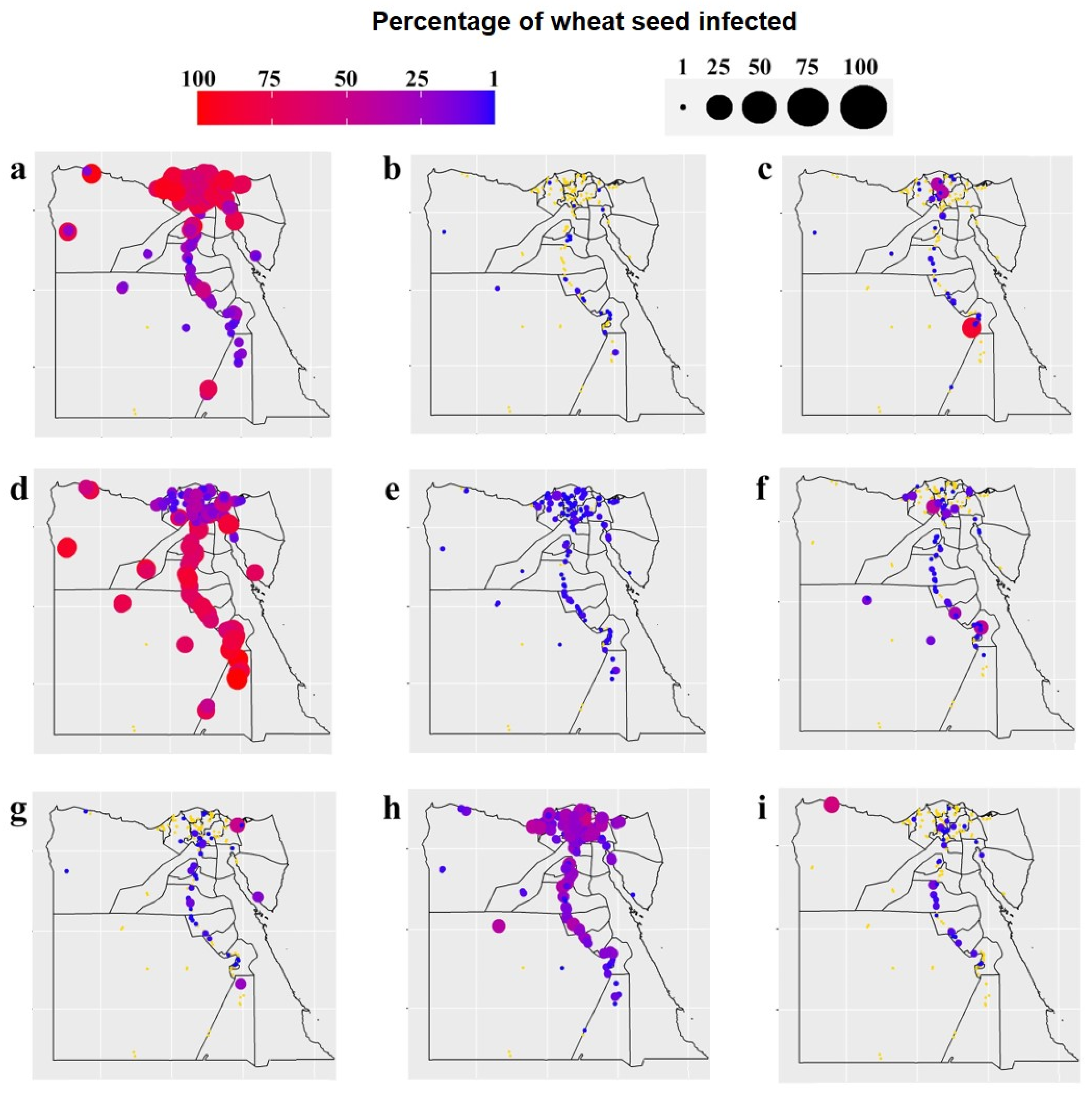
3.1. Occurrence and Distribution of Wheat Seed-Borne Fungi
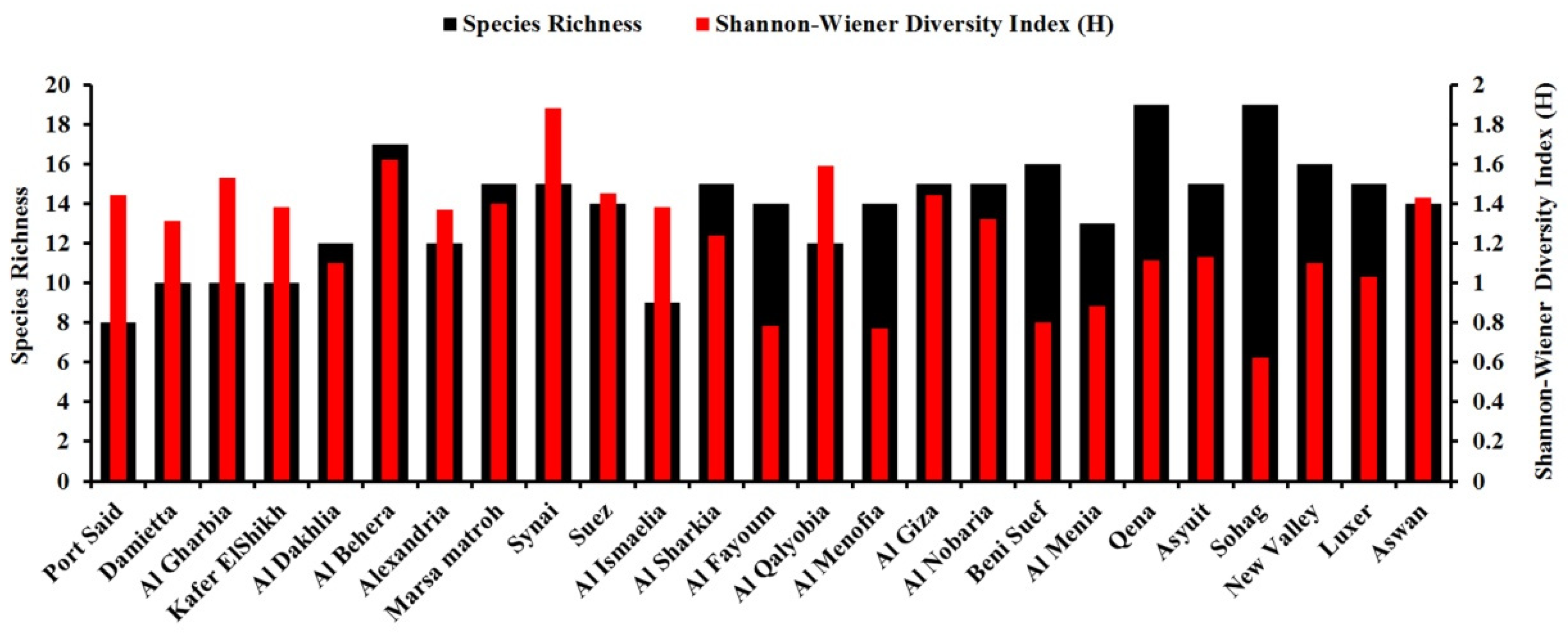
3.2. Biodiversity of Wheat Seed-Borne Fungi
3.3. Phylogenetic Analysis
3.4. Pathogenicity Test
3.5. Correlations between the Occurrence of Wheat Fungal Pathogens and Weather Variables
4. Discussion
- Release of new resistant cultivars by wheat breeders;
- Reconsidering the date/location of wheat cultivation along the wheat-growing areas to avoid the disease spread;
- Use of heat tolerant biocontrol agents;
- Utilizing new disease control measures and tillage practices.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT © FAO, Statistics Division. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 March 2021).
- Gebeyaw, M. Review on: Impact of Seed-Borne Pathogens on Seed Quality. Am. J. Plant Biol. 2020, 5, 77–81. [Google Scholar] [CrossRef]
- Singh, D.P.; Sharma, A.K.; Tewari, A.N.; Singh, K.P.; Singh, A.K.; Singh, R.N.; Singh, S.P.; Kalappanawar, I.K.; Dodan, D.S.; Singh, V.K. Assessment of losses due to leaf blight in popular varieties of wheat (Triticum aestivum) under different sowing conditions and agroclimatic zones in India. Indian J. Agric. Sci. 2004, 74, 110–113. [Google Scholar]
- Palumbo, R.; Crisci, A.; Venâncio, A.; Abrahantes, J.C.; Dorne, J.L.; Battilani, P.; Toscano, P. Occurrence and co-occurrence of mycotoxins in cereal-based feed and food. Microorganisms 2020, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Gulhane, A.R.; Khambalkar, S.V.; Giri, G.K. Histopathological Studies of Seed Infected with Seed Borne Fungi. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 4108–4112. [Google Scholar] [CrossRef]
- Pathak, N.; Zaidi, R.K. Studies on seed-borne fungi of wheat in seed health testing programme. Arch. Phytopathol. Plant Prot. 2013, 46, 389–401. [Google Scholar] [CrossRef]
- Duan, C.X.; Wang, X.M.; Zhu, Z.D.; Wu, X.F. Testing of Seedborne Fungi in Wheat Germplasm Conserved in the National Crop Genebank of China. Agric. Sci. China 2007, 6, 682–687. [Google Scholar] [CrossRef]
- Majumder, D.; Rajesh, T.; Suting, E.G.; Debbarma, A. Detection of seed borne pathogens in wheat: Recent trends. Aust. J. Crop. Sci. 2013, 7, 500–507. [Google Scholar]
- El-Kady, I.A.; Abdel-Hafez, S.I.I.; El-Maraghy, S.S. Contribution to the fungal flora of cereal grains in Egypt. Mycopathologia 1982, 77, 103–109. [Google Scholar] [CrossRef]
- Mazen, M.B.; Abdel-Hafez, S.I.I.; Shaban, G.M.M. Survey on the mycoflora of Egyptian wheat grains and their lemmae and paleae. Mycopathologia 1984, 85, 155–159. [Google Scholar] [CrossRef]
- Baka, Z.A. Plant extract control of the fungi associated with different Egyptian wheat cultivars grains. J. Plant Prot. Res. 2014, 54, 231–237. [Google Scholar] [CrossRef]
- Grulke, N.E. The nexus of host and pathogen phenology: Understanding the disease triangle with climate change. New Phytol. 2011, 189, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Perkins, L.B.; Leger, E.A.; Nowak, R.S. Invasion triangle: An organizational framework for species invasion. Ecol. Evol. 2011, 1, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Maghrawy, H.; Sayour, H.; Gummadidala, P.M.; Gomaa, O.M. Impact of Climate Change on Plant-Associated Fungi. In Climate Change Impacts on Agriculture and Food Security in Egypt; Ewis Omran, E.-S., Negm, A.M., Eds.; Springer International Publishing: Basel, Switzerland, 2020; pp. 83–96. [Google Scholar]
- Eastburn, D.M.; McElrone, A.J.; Bilgin, D.D. Influence of atmospheric and climatic change on plant-pathogen interactions. Plant Pathol. 2011, 60, 54–69. [Google Scholar] [CrossRef]
- Popovski, S.; Celar, F.A. The impact of environmental factors on the infection of cereals with Fusarium species and mycotoxin production - A review. Acta Agric. Slov. 2013, 101, 105–116. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Esmaeili, A. Future distributions of Fusarium oxysporum f. spp. in European, Middle Eastern and North African agricultural regions under climate change. Agric. Ecosyst. Environ. 2014, 197, 96–105. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [Green Version]
- AQUASTAT FAO’s Information System on Water and Agriculture, Climate Information Tool. Available online: http://www.fao.org/aquastat/en/ (accessed on 16 March 2021).
- ISTA International rules for seed testing. Seed Sci. Technol. 1999, 27, 1–333.
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi; Lubrecht & Cramer Ltd.: Port Jervis, NY, USA, 1980; Volume 1, p. 1264. [Google Scholar]
- Summerell, B.A.; Salleh, B.; Leslie, J.F. A utilitarian approach to Fusarium identification. Plant Dis. 2003, 87, 117–128. [Google Scholar] [CrossRef] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.T.J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Software, Cohort. Available online: https://www.cohort.com/costat.html (accessed on 5 March 2021).
- Al-Askar, A.A.; Ghoneem, K.M.; Rashad, Y.M.; Abdulkhair, W.M.; Hafez, E.E.; Shabana, Y.M.; Baka, Z.A. Occurrence and distribution of tomato seed-borne mycoflora in Saudi Arabia and its correlation with the climatic variables. Microb. Biotechnol. 2014, 7, 556–569. [Google Scholar] [CrossRef]
- Serfling, A.; Kopahnke, D.; Habekuß, A.; Novakazi, F.; Ordon, F. Wheat diseases: An overview. In Achieving Sustainable Cultivation of Wheat; Langridge, P., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Amatulli, M.T.; Fanelli, F.; Moretti, A.; Mule, G.; Logrieco, A.F. Alternaria species and mycotoxins associated to black point of cereals. Mycotoxins 2013, 63, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Tibola, C.S.; Fernandes, J.M.C.; Guarienti, E.M.; Nicolau, M. Distribution of Fusarium mycotoxins in wheat milling process. Food Control. 2015, 53, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Nie, D.; Fan, K.; Yang, J.; Guo, W.; Meng, J.; Zhao, Z.; Han, Z. A systematic review of plant-conjugated masked mycotoxins: Occurrence, toxicology, and metabolism. Crit. Rev. Food Sci. Nutr. 2020, 60, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Pertot, I. Climate Change Impacts on Plant Pathogens and Plant Diseases. J. Crop. Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Abdelmageed, K.; Chang, X.; Wang, D.M.; Wang, Y.; Yang, Y.; Zhao, G.; Tao, Z. Evolution of varieties and development of production technology in Egypt wheat: A review. J. Integr. Agric. 2019, 18, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Somma, S.; Amatulli, M.T.; Masiello, M.; Moretti, A.; Logrieco, A.F. Alternaria species associated to wheat black point identified through a multilocus sequence approach. Int. J. Food Microbiol. 2019, 293, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Somma, S.; Pose, G.; Pardo, A.; Mulè, G.; Pinto, V.F.; Moretti, A.; Logrieco, A.F. AFLP variability, toxin production, and pathogenicity of Alternaria species from Argentinean tomato fruits and puree. Int. J. Food Microbiol. 2011, 145, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.P.; Rotondo, F.; Gannibal, P.B. Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycol. Prog. 2016, 15, 3. [Google Scholar] [CrossRef]
- Christensen, K.B.; Van Klink, J.W.; Weavers, R.T.; Larsen, T.O.; Andersen, B.; Phipps, R.K. Novel chemotaxonomic markers of the Alternaria infectoria species-group. J. Agric. Food Chem. 2005, 53, 9431–9435. [Google Scholar] [CrossRef]
- Habib, W.; Masiello, M.; El Ghorayeb, R.; Gerges, E.; Susca, A.; Meca, G.; Quiles, J.M.; Logrieco, A.F.; Moretti, A. Mycotoxin profile and phylogeny of pathogenic Alternaria species isolated from symptomatic tomato plants in Lebanon. Toxins 2021, 13, 513. [Google Scholar] [CrossRef]
- Connally, A.; Smith, D.; Marek, S.; Wu, Y.; Walker, N. Phylogenetic evaluation of Bipolaris and Curvularia species collected from turfgrasses. Int. Turfgrass Soc. Res. J. 2021, 1–15. [Google Scholar] [CrossRef]
- Manamgoda, D.S.; Cai, L.; Bahkali, A.H.; Chukeatirote, E.; Hyde, K.D. Cochliobolus: An overview and current status of species. Fungal Divers. 2011, 51, 3–42. [Google Scholar] [CrossRef]
- Manamgoda, D.S.; Rossman, A.Y.; Castlebury, L.A.; Crous, P.W.; Madrid, H.; Chukeatirote, E.; Hyde, K.D. The genus Bipolaris. Stud. Mycol. 2014, 79, 221–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Restrepo, M.; Madrid, H.; Tan, Y.P.; da Cunha, K.C.; Gené, J.; Guarro, J.; Crous, P.W. Multi-locus phylogeny and taxonomy of exserohilum. Persoonia Mol. Phylogeny Evol. Fungi 2018, 41, 71–108. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yonezawa, T.; Lee, K.I.; Kumagai, S.; Sugita-Konishi, Y.; Goto, K.; Hara-Kudo, Y. Molecular phylogeny of the higher and lower taxonomy of the Fusarium genus and differences in the evolutionary histories of multiple genes. BMC Evol. Biol. 2011, 11, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chala, A.; Degefu, T.; Brurberg, M.B. Phylogenetically diverse Fusarium species associated with sorghum (Sorghum bicolor L. Moench) and finger millet (Eleusine coracana L. Garten) grains from Ethiopia. Diversity 2019, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Nelson, P.E.; Dignani, M.C.; Anaissie, E.J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 1994, 7, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Kim, K.D. Effect of temperature and relative humidity on growth of Aspergillus and Penicillium spp. and biocontrol activity of Pseudomonas protegens AS15 against Aflatoxigenic Aspergillus flavus in stored rice grains. Mycobiology 2018, 46, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Uddin, W.; Kaminski, J.E. Effects of relative humidity on infection, colonization and conidiation of Magnaporthe orzyae on perennial ryegrass. Plant Pathol. 2014, 63, 590–597. [Google Scholar] [CrossRef]
- Cendoya, E.; del Pilar Monge, M.; Chiacchiera, S.M.; Farnochi, M.C.; Ramirez, M.L. Influence of water activity and temperature on growth and fumonisin production by Fusarium proliferatum strains on irradiated wheat grains. Int. J. Food Microbiol. 2018, 266, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Patsa, R.; Hembram, S.; Bhattacharya, P.M.; Bandyopadhyay, S.; Dutta, S. Effect of temperature, light on germination and morphological characteristics of Bipolaris sorokiniana. Indian Phytopathol. 2018, 71, 243–248. [Google Scholar] [CrossRef]
- Félix, C.; Duarte, A.S.; Vitorino, R.; Guerreiro, A.C.L.; Domingues, P.; Correia, A.C.M.; Alves, A.; Esteves, A.C. Temperature modulates the secretome of the phytopathogenic fungus Lasiodiplodia theobromae. Front. Plant Sci. 2016, 7, 1096. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Singh, U.S.; Sharma, P.; Kumar, A.; Sharma, L. Seed treatments for sustainable agriculture-A review. J. Appl. Nat. Sci. 2015, 7, 521–539. [Google Scholar] [CrossRef]
- Golan, J.J.; Pringle, A. Long-Distance Dispersal of Fungi. Fungal Kingd. 2017, 309–333. [Google Scholar] [CrossRef]
- Ding, S.; Rouse, D.I.; Meinholz, K.; Gevens, A.J. Aerial concentrations of pathogens causing early blight and brown spot within susceptible potato fields. Phytopathology 2019, 109, 1425–1432. [Google Scholar] [CrossRef]
- Sephton-Clark, P.C.S.; Voelz, K. Spore Germination of Pathogenic Filamentous Fungi. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M.B.T.-A., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 102, pp. 117–157. [Google Scholar]
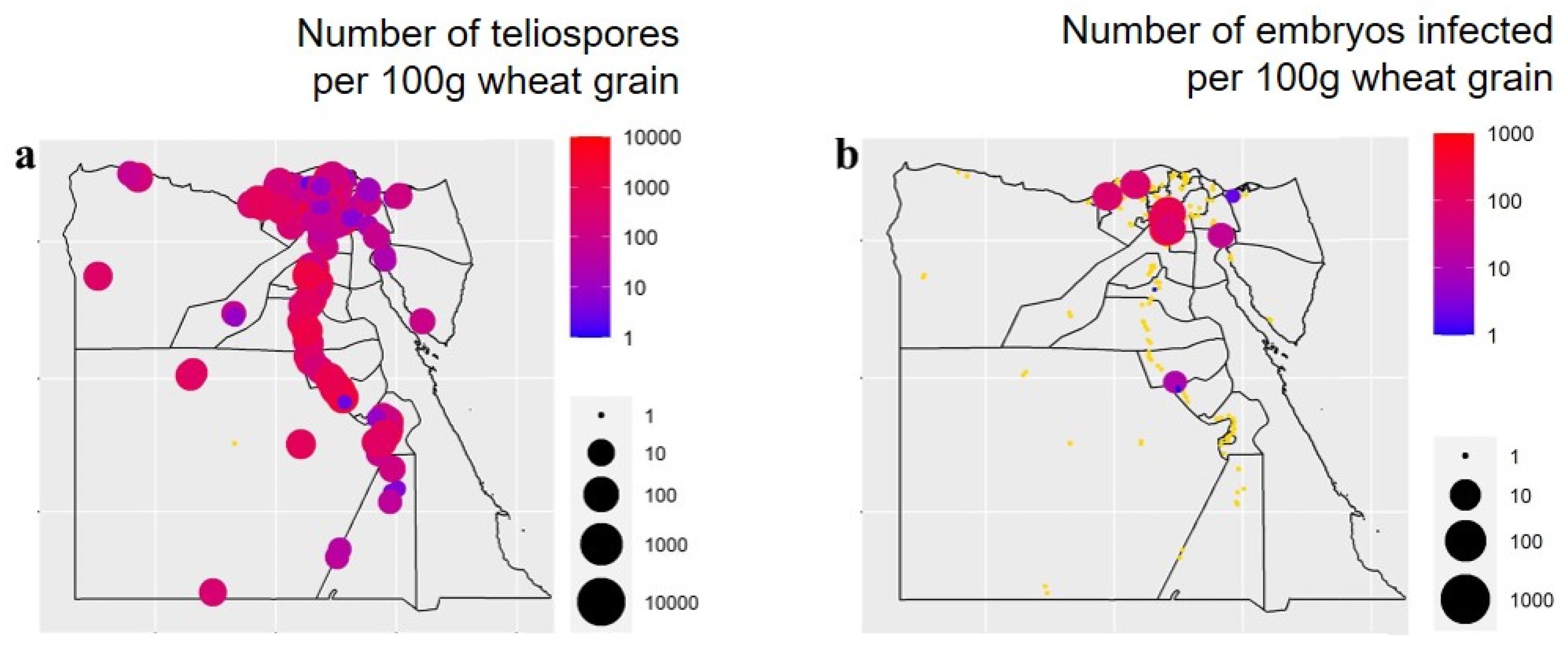
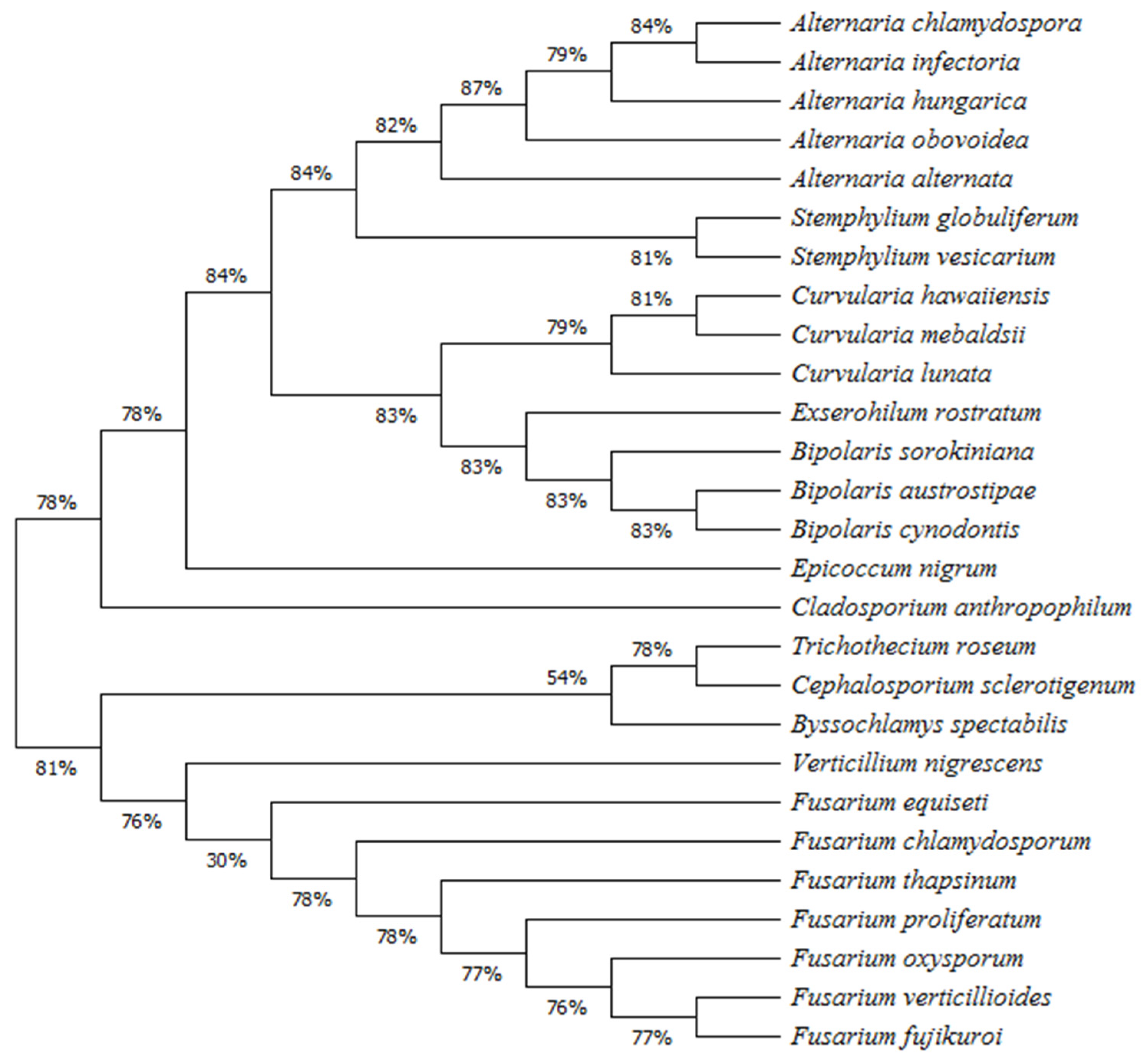
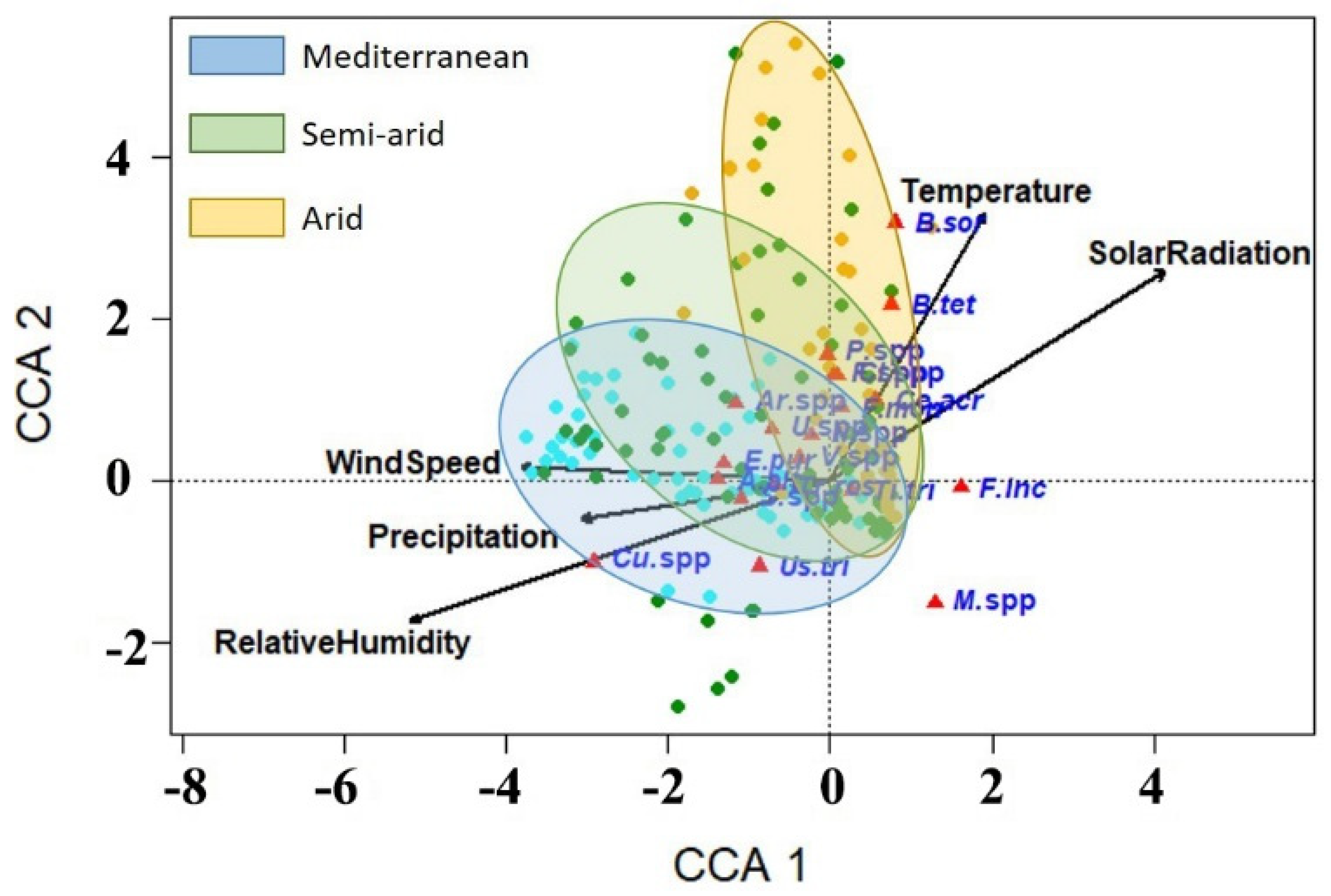
| Fungus | Governorate a | - | - | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Port Said | Damietta | Al-Gharbia | Kafr El-Sheikh | Al-Dakahlia | Al-Behera | Alexandria | Matrouh | Sinai | Suez | Al-Ismaelia | Al-Sharkia | - | - | |||||||||||||
| F% b | I% c | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | - | - | |
| Alternaria alternata | 100 | 79.8 | 100 | 65.1 | 100 | 61.4 | 100 | 83.4 | 100 | 71.8 | 75 | 55.8 | 0 | 0 | 100 | 59.5 | 100 | 53.9 | 100 | 38.8 | 100 | 80.5 | 100 | 65.7 | - | - |
| A. chlamydospora | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 8.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | |
| A. hungarica | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 10.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| A. infectoria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 43 | 6.1 | - | - |
| A. obovoidea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 0.3 | - | - |
| Arthrobotrys sp. | 80 | 79.8 | 78 | 3.4 | 63 | 1.4 | 89 | 2.2 | 83 | 8.5 | 100 | 6.4 | 86 | 3.9 | 43 | 1.4 | 38 | 0.3 | 25 | 0.13 | 63 | 0.7 | 56 | 6.2 | - | - |
| Bipolaris cynodontis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| B. sorokiniana | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.1 | 0 | 0 | 14 | 0.1 | 0 | 0 | 0 | 0 | 13 | 0.1 | 0 | 0 | - | - |
| B. tetramera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.1 | 0 | 0 | 0 | 0 | 13 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Byssochlamys spectabilis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 0.2 | - | - |
| Cephalosporium acremonium | 0 | 0 | 22 | 0.2 | 25 | 4.8 | 22 | 0.2 | 0 | 0 | 25 | 0.12 | 0 | 0 | 14 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 1.1 | - | - |
| Cephalosporium sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 18.9 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Cladosporium anthropophilum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 71 | 13.9 | - | - |
| Cladosporium spp. | 100 | 18.2 | 100 | 15.4 | 100 | 21.9 | 100 | 10.2 | 100 | 9.6 | 100 | 6.6 | 86 | 10.6 | 100 | 62.3 | 88 | 19.4 | 100 | 80.5 | 100 | 30.7 | 0 | 0 | - | - |
| Curvularia spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Epicoccum purpurascens | 80 | 1.2 | 89 | 2.1 | 100 | 0.8 | 100 | 5.2 | 92 | 2.63 | 100 | 6.3 | 86 | 1.2 | 43 | 0.9 | 88 | 2.4 | 75 | 2 | 0 | 0 | 78 | 0.9 | - | - |
| Exserohilum rostratum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0.13 | 0 | 0 | 0 | 0 | - | - |
| Fusarium equiseti | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| F. fujikuroi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0.13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| F. proliferatum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| F. verticillioides | 60 | 3.2 | 0 | 0 | 13 | 0.06 | 22 | 0.1 | 8 | 0.04 | 0 | 0 | 57 | 3.3 | 0 | 0 | 25 | 0.13 | 25 | 0.3 | 0 | 0 | 33 | 0.9 | - | - |
| Fusarium spp. | 40 | 0.6 | 33 | 0.2 | 0 | 0 | 11 | 0.06 | 0 | 0 | 13 | 0.2 | 0 | 0 | 0 | 0 | 13 | 0.1 | 0 | 0 | 13 | 0.1 | 0 | 0 | - | - |
| Mucor spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Nigrospora spp. | 0 | 0 | 11 | 0.3 | 13 | 0.06 | 0 | 0 | 8 | 0.04 | 25 | 0.2 | 29 | 0.3 | 29 | 0.4 | 0 | 0 | 50 | 0.3 | 38 | 0.3 | 22 | 0.17 | - | - |
| Penicillium spp. | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0.4 | 25 | 0.2 | 13 | 0.1 | 0 | 0 | 29 | 0.3 | 63 | 11.3 | 25 | 0.13 | 0 | 0 | 22 | 0.11 | - | - |
| Stemphylium globuliferum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 30.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 56 | 11.3 | - | - |
| Stemphylium spp. | 100 | 35.9 | 100 | 15.8 | 100 | 24.1 | 100 | 34.1 | 0 | 0 | 88 | 13.6 | 100 | 23 | 100 | 12.4 | 88 | 13 | 100 | 6.13 | 100 | 22.4 | 0 | 0 | - | - |
| S. vesicarium | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 44 | 10.2 | - | - |
| Trichothecium roseum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 0.3 | 0 | 0 | 0 | 0 | 14 | 7.9 | 13 | 0.3 | 25 | 0.13 | 0 | 0 | 22 | 0.4 | - | - |
| Ulocladium spp. | 0 | 0 | 11 | 0.06 | 25 | 0.1 | 0 | 0 | 33 | 0.2 | 50 | 0.3 | 14 | 0.1 | 29 | 0.6 | 50 | 2.9 | 50 | 0.4 | 0 | 0 | 0 | 0 | - | - |
| Verticillium spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0.3 | 0 | 0 | 13 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Fungus | Governorate | |||||||||||||||||||||||||
| Al-Fayoum | Al-Qalyobia | Al-Menofia | Al-Giza | Al-Nobaria | Bani Suef | Al-Menia | Qena | Assiut | Sohag | New Valley | Luxor | Aswan | ||||||||||||||
| F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | F% | I% | |
| Alternaria alternata | 100 | 52 | 100 | 70.7 | 100 | 67.9 | 100 | 22 | 100 | 80.1 | 100 | 22.6 | 100 | 15.8 | 100 | 17.3 | 100 | 30.8 | 100 | 14.9 | 100 | 30.7 | 100 | 7.8 | 100 | 25.7 |
| Arthrobotrys spp. | 38 | 0.7 | 75 | 4 | 75 | 1.8 | 43 | 0.3 | 88 | 26.1 | 0 | 0 | 0 | 0 | 13 | 0.1 | 25 | 1 | 38 | 1.4 | 25 | 1.9 | 0 | 0 | 25 | 11.8 |
| Bipolaris austrostipae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B. cynodontis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.13 | 0 | 0 | 0 | 0 | 25 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B. hawaiiensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0.13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B. sorokiniana | 0 | 0 | 13 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0.3 | 0 | 0 | 0 | 0 | 50 | 0.9 | 25 | 0.13 | 13 | 0.3 | 13 | 0.2 | 13 | 0.6 |
| B. tetramera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.1 | 0 | 0 | 38 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.1 | 0 | 0 |
| Bipolaris spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0.2 | 0 | 0 | 0 | 0 | 13 | 0.13 | 0 | 0 | 13 | 0.1 | 25 | 0.13 |
| Cephalosporium acremonium | 13 | 0.1 | 0 | 0 | 25 | 0.4 | 43 | 1.3 | 13 | 0.7 | 0 | 0 | 63 | 0.9 | 50 | 0.7 | 13 | 0.1 | 50 | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. sclerotigenum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0.3 | 0 | 0 | 0 | 0 |
| Cephalosporium spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 63 | 23.3 | 13 | 0.1 |
| Cladosporium spp. | 100 | 39.9 | 100 | 25.8 | 100 | 26.7 | 100 | 73.9 | 88 | 18.3 | 100 | 69.7 | 100 | 66.7 | 100 | 72.6 | 100 | 70.3 | 100 | 69.6 | 100 | 63.8 | 75 | 65.6 | 100 | 84.6 |
| Curvularia lunata | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0.14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cu. mebaldsii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.2 | 0 | 0 | 0 | 0 |
| Epicoccum purpurascens | 63 | 1.4 | 75 | 2.6 | 50 | 1.1 | 71 | 0.8 | 63 | 2.1 | 75 | 0.9 | 50 | 0.5 | 63 | 0.7 | 100 | 3 | 63 | 1.1 | 50 | 0.5 | 63 | 1.3 | 50 | 1.6 |
| Exserohilum rostratum | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fusarium incarnatum | 13 | 0.1 | 0 | 0 | 13 | 0.3 | 0 | 0 | 0 | 0 | 25 | 0.2 | 0 | 0 | 25 | 0.13 | 13 | 0.13 | 38 | 0.5 | 0 | 0 | 50 | 0.7 | 0 | 0 |
| F. oxysporum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F. proliferatum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0.4 | 13 | 1.9 | 0 | 0 | 0 | 0 |
| F. thapsinum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F. verticillioides | 50 | 0.6 | 50 | 2.4 | 38 | 1.8 | 29 | 1.9 | 13 | 5.4 | 50 | 0.9 | 75 | 1.5 | 88 | 6.8 | 75 | 2.13 | 63 | 5.13 | 88 | 5.6 | 50 | 0.6 | 13 | 0.1 |
| Fusarium spp. | 38 | 0.3 | 0 | 0 | 25 | 0.25 | 14 | 0.2 | 13 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.1 | 0 | 0 | 13 | 0.3 | 0 | 0 | 0 | 0 |
| Mucor spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.3 | 25 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.1 | 0 | 0 | 13 | 0.1 | 0 | 0 |
| Nigrospora spp. | 50 | 0.5 | 25 | 0.13 | 38 | 0.4 | 29 | 0.4 | 13 | 0.13 | 25 | 0.13 | 63 | 1.1 | 50 | 0.3 | 38 | 0.7 | 13 | 0.1 | 25 | 0.5 | 0 | 0 | 25 | 0.13 |
| Penicillium spp. | 63 | 2.4 | 63 | 2.6 | 38 | 1.2 | 14 | 0.2 | 13 | 0.13 | 38 | 0.3 | 75 | 2.9 | 38 | 0.2 | 50 | 0.5 | 25 | 0.8 | 13 | 0.1 | 25 | 0.13 | 13 | 3.1 |
| Rhizopus spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 38 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stemphylium spp. | 88 | 16.3 | 88 | 14.8 | 88 | 21.9 | 100 | 11.1 | 100 | 16.6 | 88 | 18.4 | 100 | 13.4 | 100 | 10.2 | 100 | 19.1 | 88 | 11.4 | 63 | 5.1 | 100 | 4.1 | 63 | 2.3 |
| Trichothecium roseum | 25 | 0.3 | 13 | 0.2 | 63 | 1.3 | 14 | 0.6 | 0 | 0 | 0 | 0 | 75 | 4.2 | 13 | 0.8 | 13 | 0.1 | 63 | 2.4 | 0 | 0 | 25 | 0.2 | 0 | 0 |
| Ulocladium spp. | 38 | 0.3 | 13 | 0.13 | 38 | 0.3 | 29 | 0.2 | 13 | 0.1 | 0 | 0 | 25 | 0.13 | 13 | 0.1 | 0 | 0 | 25 | 0.13 | 38 | 0.5 | 0 | 0 | 0 | 0 |
| Verticillium nigrescens | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0.2 | 0 | 0 |
| Verticillium spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0.4 | 0 | 0 | 0 | 0 | 13 | 0.13 | 0 | 0 | 0 | 0 | 13 | 0.1 | 0 | 0 | 0 | 0 |
| Fungus | Governorate a | - | - | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Port Said | Damietta | Al-Gharbia | Kafr El-Sheikh | Al-Dakahlia | Al-Behera | Alexandria | Matrouh | Sinai | Suez | Al-Ismaelia | Al-Sharkia | - | - | |||||||||||||
| F% b | D c | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | - | - | |
| Tilletia tritici | 100 | 29 | 100 | 12 | 87.5 | 60 | 100 | 125 | 100 | 487 | 100 | 221 | 100 | 232 | 100 | 263 | 85.7 | 40 | 100 | 52 | 87.5 | 82 | 100 | 398 | - | - |
| Ustilago tritici | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12.5 | 10 | 14.3 | 11 | 0 | 0 | 12.5 | 1 | 50 | 8 | 0 | 0 | 0 | 0 | - | - |
| Fungus | Al-Fayoum | Al-Qalyobia | Al-Menofia | Al-Giza | Al-Nobaria | Bani Suef | Al-Menia | Qena | Assiut | Sohag | New Valley | Luxor | Aswan | |||||||||||||
| F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | F% | D | |
| Tilletia tritici | 100 | 914 | 100 | 214 | 100 | 1000 | 100 | 89 | 100 | 399 | 100 | 837 | 100 | 660 | 87.5 | 419 | 100 | 464 | 100 | 1552 | 100 | 442 | 100 | 446 | 100 | 50 |
| Ustilago tritici | 0 | 0 | 87.5 | 19.6 | 0 | 0 | 0 | 0 | 0 | 0 | 12.5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 37.5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fungus | Pre-Emergence Damping Off ** (%) | Post-Emergence Damping Off ** (%) | Survival ** (%) |
|---|---|---|---|
| Control (fungus-free) | 1.0 e | 0.0 d | 99.0 a |
| Alternaria alternata | 2.5 e | 0.0 d | 97.5 ab |
| Bipolaris austrostipae | 7.5 c–e | 7.5 a–d | 75.0 c–f |
| B. cynodontis | 25.0 a | 12.5 ab | 62.5 f |
| B. sorokiniana | 12.5 b–e | 2.5 cd | 85.0 a–e |
| B. tetramera | 17.5 a–c | 5.0 b–d | 77.5 c–f |
| Cladosporium anthropophilum | 15.0 a–d | 2.5 cd | 82.5 b–e |
| Curvularia mebaldsii | 15.0 a–d | 0.0 d | 85.0 a–e |
| Exerohilum rostratum | 15.0 a–d | 0.0 d | 85.0 a–e |
| Fusarium chlamydosporum | 17.5 a–c | 10.0 a–c | 72.5 d–f |
| F. equiseti | 20.0 ab | 10.0 a–c | 70.0 ef |
| F. fujikuroi | 12.5 b–e | 5.0 b–d | 82.5 b–e |
| F. oxysporum | 2.5 e | 0.0 d | 97.5 ab |
| F. proliferatum | 22.5 ab | 15.0 a | 65.0 f |
| F. verticillioides | 5.0 de | 5.0 b–d | 90.0 a–c |
| Stemphylium globuliferum | 7.5 c–e | 5.0 b–d | 87.5 a–d |
| Axis | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Eigenvalue | 0.104 | 0.037 | 0.015 | 0.009 |
| Species–weather correlation | 0.569 | 0.422 | 0.227 | 0.218 |
| Cumulative percentage variance of species–weather relation | 62.0 | 83.8 | 92.7 | 98.2 |
| Temperature | Relative Humidity | Precipitation | Wind Speed | Solar Radiation | |
|---|---|---|---|---|---|
| Temperature | 1 a | ||||
| Relative humidity | −0.574 *** | 1 | |||
| Precipitation | −0.511 *** | 0.533 *** | 1 | ||
| Wind speed | −0.520 *** | 0.550 *** | 0.275 ** | 1 | |
| Solar radiation | 0.609 *** | −0.874 *** | −0.336 *** | −0.456 *** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabana, Y.M.; Rashad, Y.M.; Ghoneem, K.M.; Arafat, N.S.; Aseel, D.G.; Qi, A.; Richard, B.; Fitt, B.D.L. Biodiversity of Pathogenic and Toxigenic Seed-Borne Mycoflora of Wheat in Egypt and Their Correlations with Weather Variables. Biology 2021, 10, 1025. https://doi.org/10.3390/biology10101025
Shabana YM, Rashad YM, Ghoneem KM, Arafat NS, Aseel DG, Qi A, Richard B, Fitt BDL. Biodiversity of Pathogenic and Toxigenic Seed-Borne Mycoflora of Wheat in Egypt and Their Correlations with Weather Variables. Biology. 2021; 10(10):1025. https://doi.org/10.3390/biology10101025
Chicago/Turabian StyleShabana, Yasser M., Younes M. Rashad, Khalid M. Ghoneem, Nehal S. Arafat, Dalia G. Aseel, Aiming Qi, Benjamin Richard, and Bruce D. L. Fitt. 2021. "Biodiversity of Pathogenic and Toxigenic Seed-Borne Mycoflora of Wheat in Egypt and Their Correlations with Weather Variables" Biology 10, no. 10: 1025. https://doi.org/10.3390/biology10101025
APA StyleShabana, Y. M., Rashad, Y. M., Ghoneem, K. M., Arafat, N. S., Aseel, D. G., Qi, A., Richard, B., & Fitt, B. D. L. (2021). Biodiversity of Pathogenic and Toxigenic Seed-Borne Mycoflora of Wheat in Egypt and Their Correlations with Weather Variables. Biology, 10(10), 1025. https://doi.org/10.3390/biology10101025








