Development of a Spinal Cord Injury Model Permissive to Study the Cardiovascular Effects of Rehabilitation Approaches Designed to Induce Neuroplasticity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Experimental Design
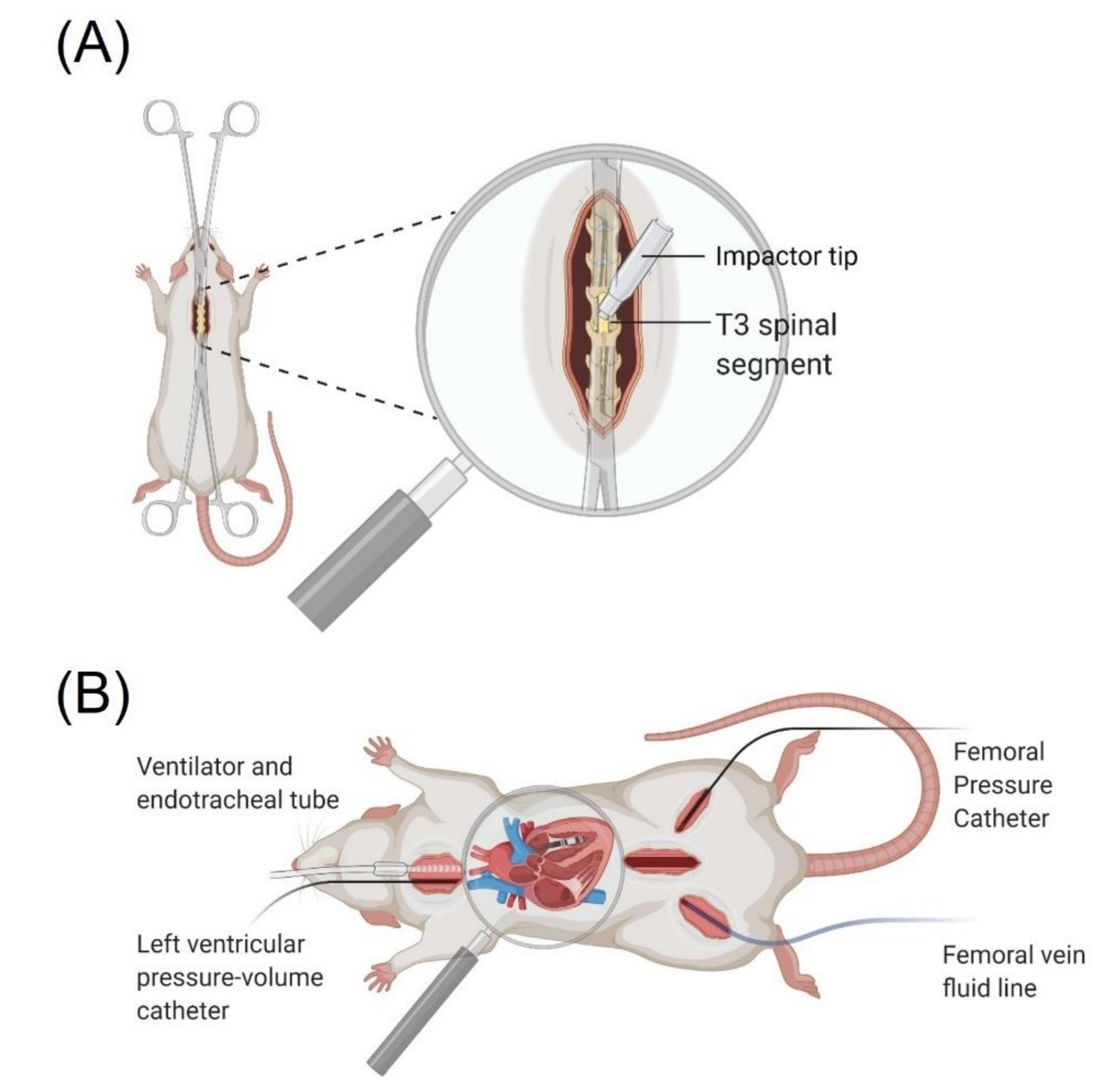
2.3. Spinal Cord Injury Surgery
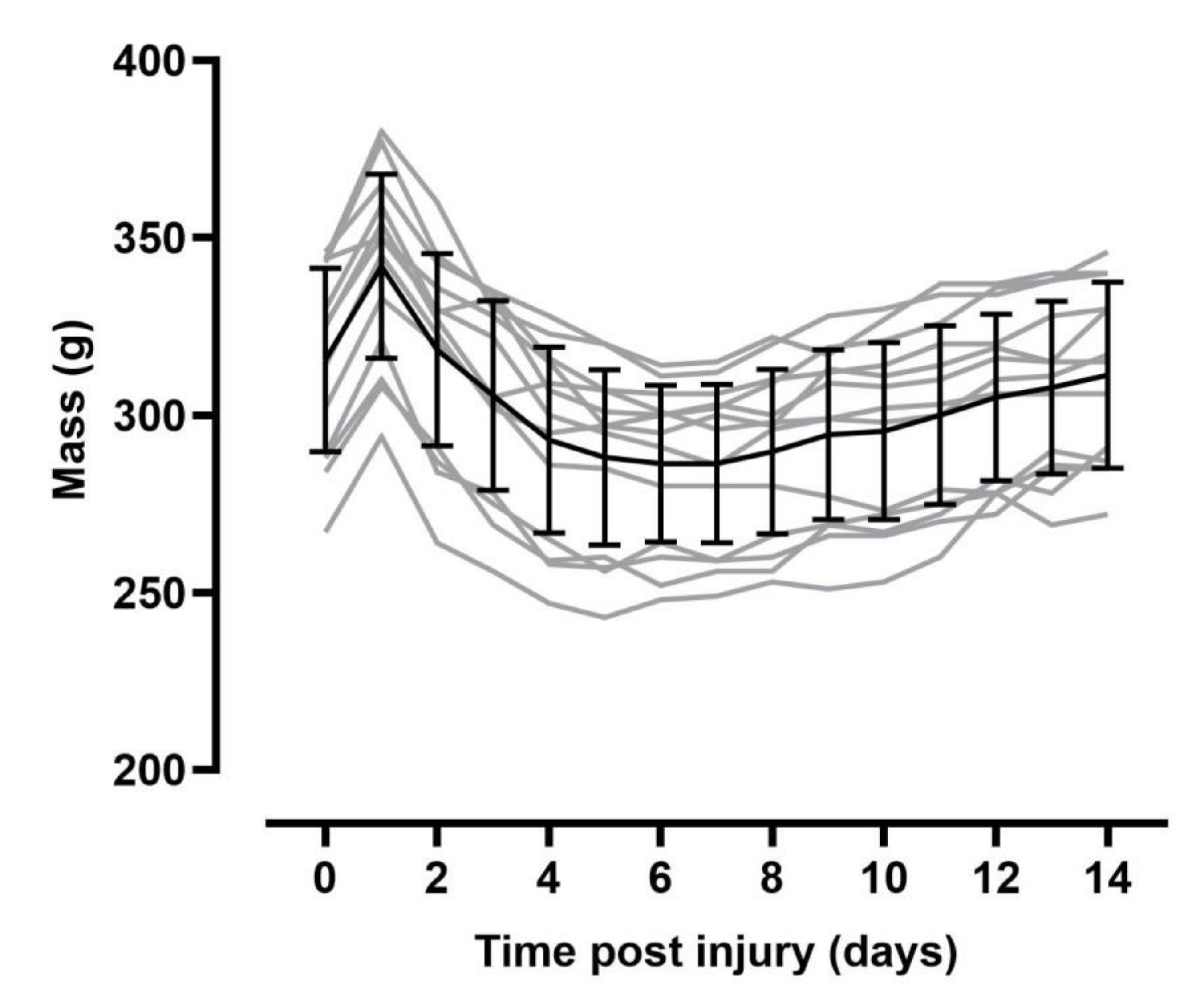
2.4. Post-Surgical Care
2.5. Outcome Surgery
2.6. Ethanasia and Tissue Processing
2.7. Data Analysis
2.8. Immunohistochemistry
2.9. Statistics
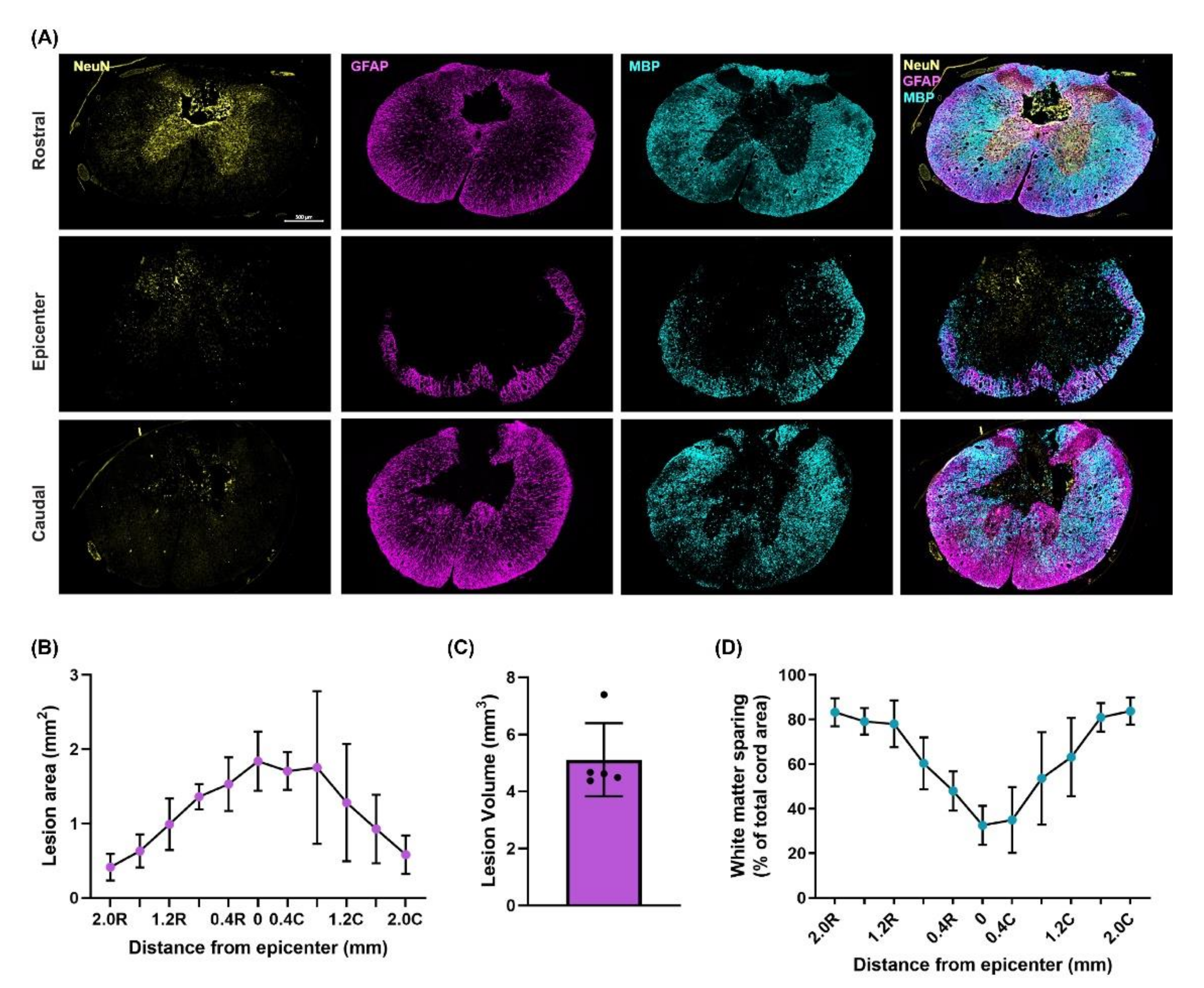
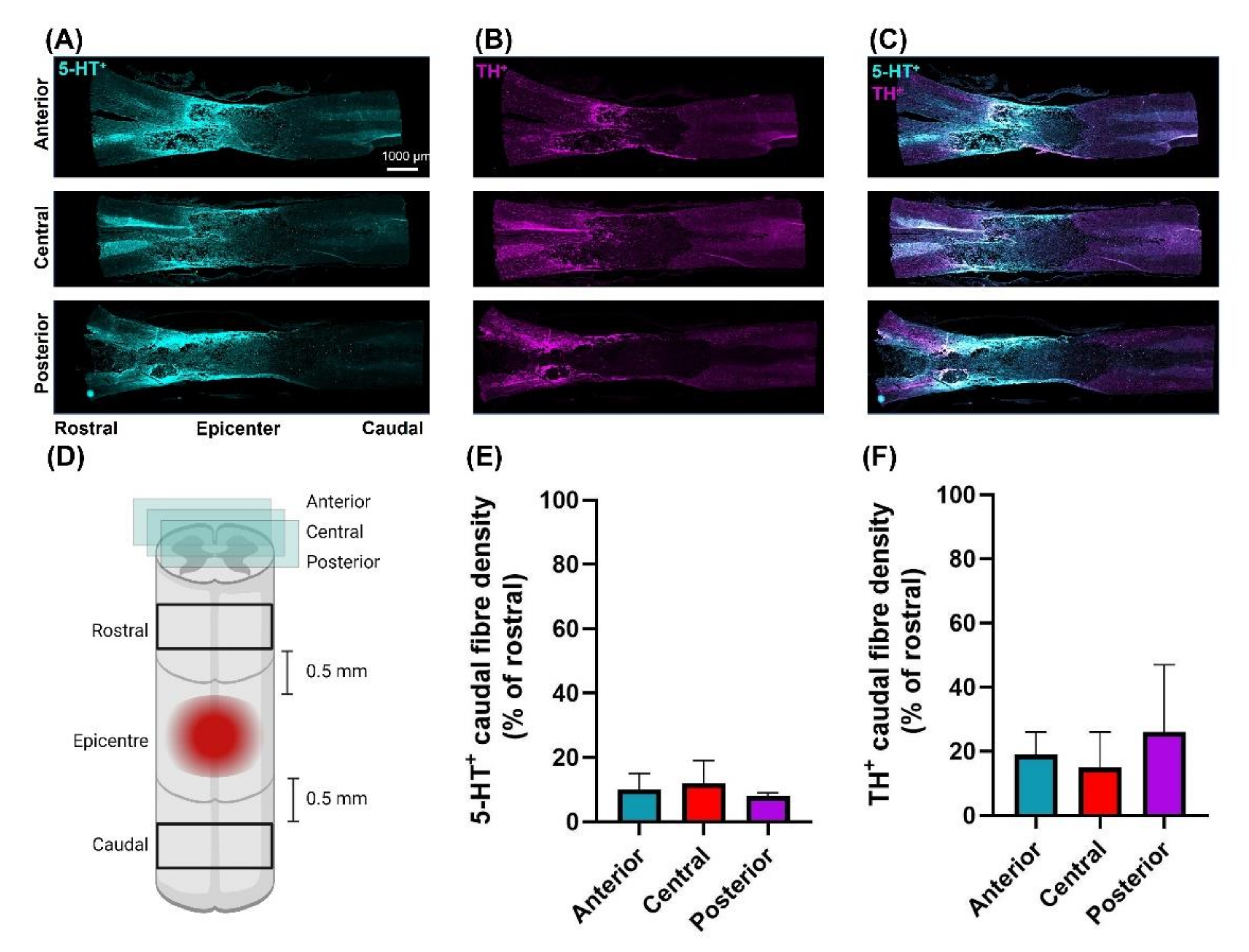
3. Results
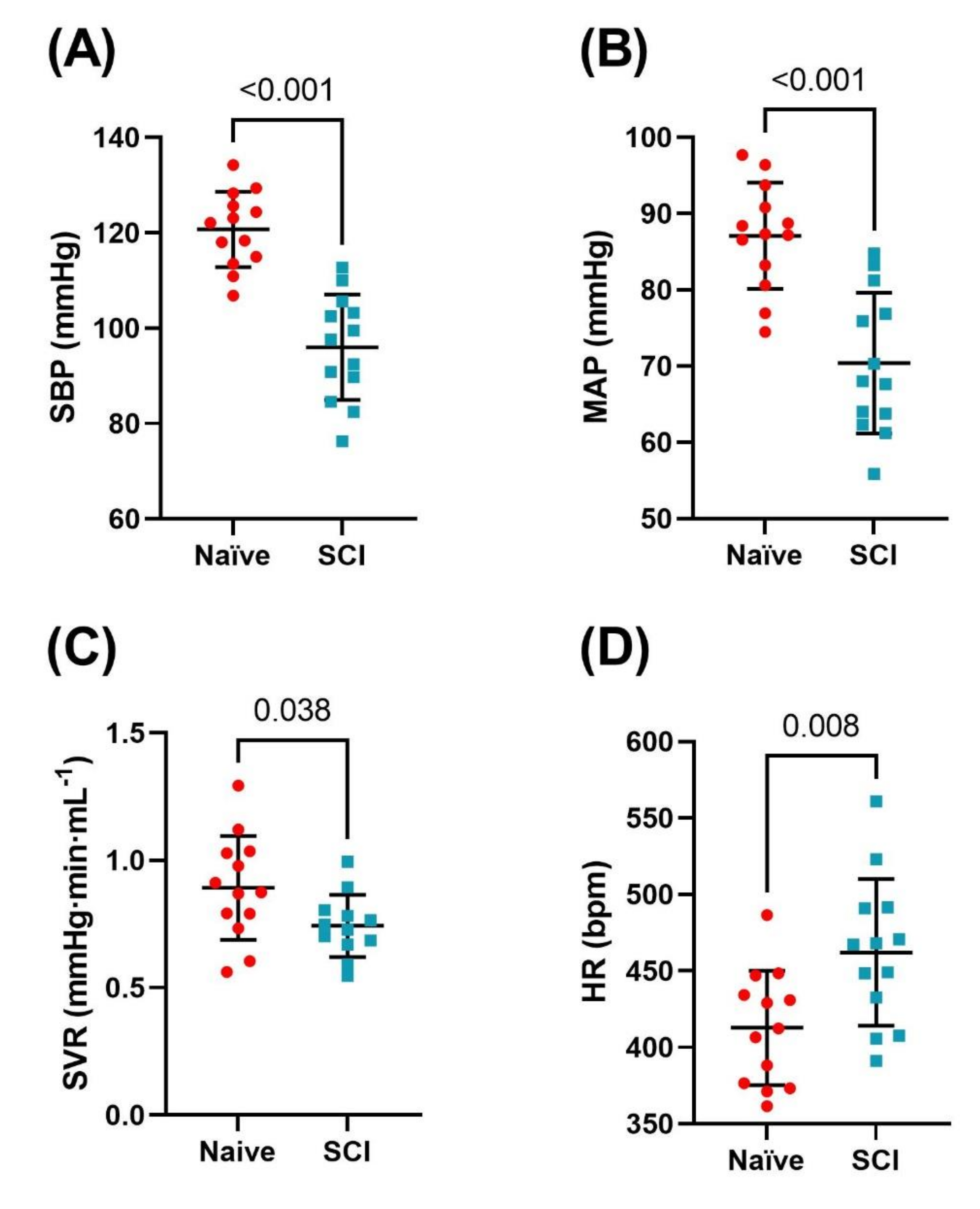
3.1. Resting Hemodynamics Are Impaired in T3 300 kdyn SCI Rats
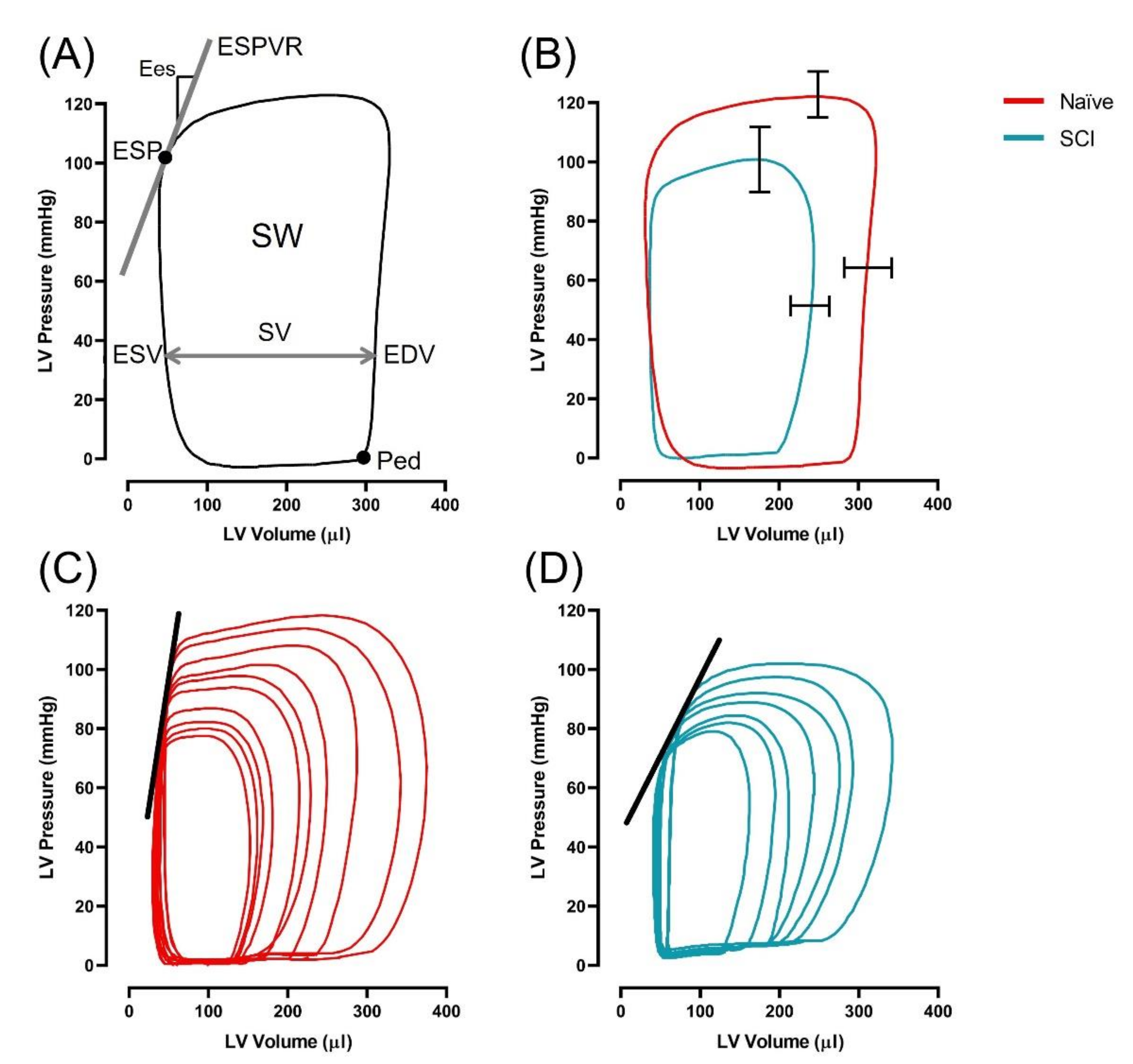
3.2. Left Ventricular Systolic Function Is Impaired in T3 300 kdyn SCI Rats
3.3. Moderately-Severe T3 Midline Injury Interrupts Descending Pathways
4. Discussion
4.1. Resting Hemodynamics Are Impaired in T3 300 kdyn SCI Rats
4.2. Left Ventricular Systolic Function Is Impaired in T3 300 kdyn SCI Rats
4.3. T3 Moderately-Severe Contusion Injury Interrupts Descending Pathways
4.4. Comparison to Other Rodent Models of CV Instability
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garshick, E.; Kelley, A.; A Cohen, S.; Garrison, A.; Tun, C.G.; Gagnon, D.; Brown, R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005, 43, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Cragg, J.J.; Noonan, V.K.; Krassioukov, A.; Borisoff, J. Cardiovascular disease and spinal cord injury: Results from a national population health survey. Neurology 2013, 81, 723–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llewellyn-Smith, I.J.; Weaver, L.C. Changes in synaptic inputs to sympathetic preganglionic neurons after spinal cord injury. J. Comp. Neurol. 2001, 435, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.H.; Noble, B.T.; Wang, Y.; Guan, Z.; Davis, H.; Mo, X.; Harris, C.; Eroglu, C.; Ferguson, A.R.; Popovich, P.G. Acute post-injury blockade of α2δ-1 calcium channel subunits prevents pathological autonomic plasticity after spinal cord injury. Cell Rep. 2021, 34, 108667. [Google Scholar] [CrossRef] [PubMed]
- Krenz, N.R.; Weaver, L.C. Changes in the morphology of sympathetic preganglionic neurons parallel the development of autonomic dysreflexia after spinal cord injury in rats. Neurosci. Lett. 1998, 243, 61–64. [Google Scholar] [CrossRef]
- Weaver, L.C.; Verghese, P.; Bruce, J.; Fehlings, M.; Krenz, N.; Marsh, D. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J. Neurotrauma 2001, 18, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Duale, H.; Cameron, A.A.; Abshire, S.M.; Lyttle, T.S.; Rabchevsky, A.G. Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J. Comp. Neurol. 2008, 509, 382–399. [Google Scholar] [CrossRef] [Green Version]
- Prüss, H.; Tedeschi, A.; Thiriot, A.; Lynch, L.; Loughhead, S.M.; Stutte, S.; Mazo, I.B.; Kopp, M.A.; Brommer, B.; Blex, C.; et al. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat. Neurosci. 2017, 20, 1549–1559. [Google Scholar] [CrossRef]
- Christensen, M.D.; Hulsebosch, C.E. Chronic central pain after spinal cord injury. J. Neurotrauma 1997, 14, 517–537. [Google Scholar] [CrossRef]
- Christiansen, L.; Urbin, M.A.; Mitchell, G.S.; Perez, M.A. Acute intermittent hypoxia enhances corticospinal synaptic plasticity in humans. eLife 2018, 7, e34304. [Google Scholar] [CrossRef]
- DeVinney, M.J.; Huxtable, A.G.; Nichols, N.L.; Mitchell, G.S. Hypoxia-induced phrenic long-term facilitation: Emergent properties. Ann. N. Y. Acad. Sci. 2013, 1279, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Baker-Herman, T.L.; Fuller, D.D.; Bavis, R.W.; Zabka, A.G.; Golder, F.J.; Doperalski, N.J.; Johnson, R.A.; Watters, J.J.; Mitchell, G.S. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat. Neurosci. 2003, 7, 48–55. [Google Scholar] [CrossRef]
- Golder, F.J. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J. Neurosci. 2005, 25, 2925–2932. [Google Scholar] [CrossRef] [Green Version]
- Lovett-Barr, M.R.; Satriotomo, I.; Muir, G.D.; Wilkerson, J.E.R.; Hoffman, M.S.; Vinit, S.; Mitchell, G.S. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J. Neurosci. 2012, 32, 3591–3600. [Google Scholar] [CrossRef]
- Prosser-Loose, E.J.; Hassan, A.; Mitchell, G.S.; Muir, G.D. Delayed Intervention with intermittent hypoxia and task training improves forelimb function in a rat model of cervical spinal injury. J. Neurotrauma 2015, 32, 1403–1412. [Google Scholar] [CrossRef]
- Magnuson, D.S.K.; Smith, R.R.; Brown, E.H.; Enzmann, G.; Angeli, C.; Quesada, P.M.; Burke, D. Swimming as a model of task-specific locomotor retraining after spinal cord injury in the rat. Neurorehabilit. Neural Repair 2009, 23, 535–545. [Google Scholar] [CrossRef]
- Smith, R.R.; Shum-Siu, A.; Baltzley, R.; Bunger, M.; Baldini, A.; Burke, D.A.; Magnuson, D.S. Effects of swimming on functional recovery after incomplete spinal cord injury in rats. J. Neurotrauma 2006, 23, 908–919. [Google Scholar] [CrossRef]
- Shah, P.K.; Garcia-Alias, G.; Choe, J.; Gad, P.; Gerasimenko, Y.; Tillakaratne, N.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Use of quadrupedal step training to re-engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain 2013, 136, 3362–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.K.; Woller, S.A.; Moreno, G.; Grau, J.W.; Hook, M.A. Exercise therapy and recovery after SCI: Evidence that shows early intervention improves recovery of function. Spinal Cord 2011, 49, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Sandrow-Feinberg, H.R.; Izzi, J.; Shumsky, J.S.; Zhukareva, V.; Houle, J.D. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J. Neurotrauma 2009, 26, 721–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenrich, K.K.; Hallworth, B.W.; Vavrek, R.; Raposo, P.J.; Misiaszek, J.E.; Bennett, D.J.; Fouad, K.; Torres-Espin, A. Self-directed rehabilitation training intensity thresholds for efficient recovery of skilled forelimb function in rats with cervical spinal cord injury. Exp. Neurol. 2020, 339, 113543. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Roy, R.R.; Edgerton, V.R.; Gómez-Pinilla, F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 2005, 193, 411–419. [Google Scholar] [CrossRef]
- West, C.; Crawford, M.; Poormasjedi-Meibod, M.-S.; Currie, K.D.; Fallavollita, A.; Yuen, V.; McNeill, J.H.; Krassioukov, A.V. Passive hind-limb cycling improves cardiac function and reduces cardiovascular disease risk in experimental spinal cord injury. J. Physiol. 2014, 592, 1771–1783. [Google Scholar] [CrossRef]
- Squair, J.W.; West, C.R.; Popok, D.; Assinck, P.; Liu, J.; Tetzlaff, W.; Krassioukov, A.V. High thoracic contusion model for the investigation of cardiovascular function after spinal cord injury. J. Neurotrauma 2017, 34, 671–684. [Google Scholar] [CrossRef]
- Trueblood, C.T.; Iredia, I.W.; Collyer, E.S.; Tom, V.J.; Hou, S. Development of cardiovascular dysfunction in a rat spinal cord crush model and responses to serotonergic interventions. J. Neurotrauma 2019, 36, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Squair, J.W.; Deveau, K.M.; Harman, K.A.; Poormasjedi-Meibod, M.-S.; Hayes, B.; Liu, J.; Magnuson, D.S.; Krassioukov, A.V.; West, C.R. Spinal cord injury causes systolic dysfunction and cardiomyocyte atrophy. J. Neurotrauma 2018, 35, 424–434. [Google Scholar] [CrossRef] [PubMed]
- DeVeau, K.M.; Harman, K.A.; Squair, J.W.; Krassioukov, A.V.; Magnuson, D.S.K.; West, C.R. A comparison of passive hindlimb cycling and active upper-limb exercise provides new insights into systolic dysfunction after spinal cord injury. Am. J. Physiol. Circ. Physiol. 2017, 313, H861–H870. [Google Scholar] [CrossRef] [Green Version]
- Squair, J.W.; Gautier, M.; Mahe, L.; Soriano, J.E.; Rowald, A.; Bichat, A.; Cho, N.; Anderson, M.A.; James, N.D.; Gandar, J.; et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature 2021, 590, 308–314. [Google Scholar] [CrossRef]
- West, C.R.; Popok, D.; Crawford, M.A.; Krassioukov, A.V. Characterizing the temporal development of cardiovascular dysfunction in response to spinal cord injury. J. Neurotrauma 2015, 32, 922–930. [Google Scholar] [CrossRef]
- Fossey, M.; Squair, J.; Poormasjedi-Meibod, M.; Hayes, B.; Erskine, E.; Ahmadian, M.; West, C. Impaired cardiac function following experimental spinal cord injury occurs due to the loss of descending sympathetic control and precedes structural remodeling. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Williams, A.M.; Manouchehri, N.; Erskine, E.; Tauh, K.; So, K.; Shortt, K.; Webster, M.; Fisk, S.; Billingsley, A.; Munro, A.; et al. Cardio-centric hemodynamic management improves spinal cord oxygenation and mitigates hemorrhage in acute spinal cord injury. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Poormasjedi-Meibod, M.-S.; Mansouri, M.; Fossey, M.; Squair, J.W.; Liu, J.; McNeill, J.H.; West, C.R. Experimental spinal cord injury causes left-ventricular atrophy and is associated with an upregulation of proteolytic pathways. J. Neurotrauma 2019, 36, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Saltos, T.M.; Mironets, E.; Trueblood, C.T.; Connors, T.M.; Tom, V.J. Grafting embryonic raphe neurons reestablishes serotonergic regulation of sympathetic activity to improve cardiovascular function after spinal cord injury. J. Neurosci. 2020, 40, 1248–1264. [Google Scholar] [CrossRef]
- Squair, J.W.; Liu, J.; Tetzlaff, W.; Krassioukov, A.V.; West, C.R. Spinal cord injury-induced cardiomyocyte atrophy and impaired cardiac function are severity dependent. Exp. Physiol. 2018, 103, 179–189. [Google Scholar] [CrossRef] [PubMed]
- DeVeau, K.M.; Martin, E.K.; King, N.T.; Shum-Siu, A.; Keller, B.B.; West, C.R.; Magnuson, D.S. Challenging cardiac function post-spinal cord injury with dobutamine. Auton. Neurosci. 2018, 209, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Nagayama, T.; Mukhopadhyay, P.; Bátkai, S.; Kass, D.A. Measurement of cardiac function using pressure–volume conductance catheter technique in mice and rats. Nat. Protoc. 2008, 3, 1422–1434. [Google Scholar] [CrossRef] [Green Version]
- Inskip, J.A.; Ramer, L.M.; Ramer, M.S.; Krassioukov, A.V.; Claydon, V.E. Spectral analyses of cardiovascular control in rodents with spinal cord injury. J. Neurotrauma 2012, 29, 1638–1649. [Google Scholar] [CrossRef]
- Claydon, V.; Steeves, J.D.; Krassioukov, A. Orthostatic hypotension following spinal cord injury: Understanding clinical pathophysiology. Spinal Cord 2006, 44, 341–351. [Google Scholar] [CrossRef] [Green Version]
- West, C.R.; Wong, S.C.; Krassioukov, A.V. Autonomic cardiovascular control in paralympic athletes with spinal cord injury. Med. Sci. Sports Exerc. 2014, 46, 60–68. [Google Scholar] [CrossRef]
- Hubli, M.; Krassioukov, A.V. Ambulatory blood pressure monitoring in spinal cord injury: Clinical practicability. J. Neurotrauma 2014, 31, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Lujan, H.L.; Dicarlo, S.E. T5 spinal cord transection increases susceptibility to reperfusion-induced ventricular tachycardia by enhancing sympathetic activity in conscious rats. Am. J. Physiol. Circ. Physiol. 2007, 293, H3333–H3339. [Google Scholar] [CrossRef] [Green Version]
- Strack, A.; Sawyer, W.; Hughes, J.; Platt, K.; Loewy, A. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989, 491, 156–162. [Google Scholar] [CrossRef]
- Suga, H.; Sagawa, K.; Shoukas, A.A. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ. Res. 1973, 32, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Minson, J.; Chalmers, J.; Drolet, G.; Kapoor, V.; Llewellyn-Smith, I.; Mills, E.; Morris, M.; Pilowsky, P. Central serotonergic mechanisms in cardiocascular regulation. Cardiovasc. Drugs Ther. 1990, 4, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.P.; Pilowsky, P.M.; Minson, J.B.; Kapoor, V.; Mills, E.; West, M.J. Central serotonergic mechanisms in hypertension. Am. J. Hypertens. 1988, 1, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Sawchenko, P.E.; Bohn, M.C. Glucocorticoid receptor-immunoreactivity in C1, C2, and C3 adrenergic neurons that project to the hypothalamus or to the spinal cord in the rat. J. Comp. Neurol. 1989, 285, 107–116. [Google Scholar] [CrossRef]
- Bruinstroop, E.; Cano, G.; Vanderhorst, V.G.; Cavalcante, J.C.; Wirth, J.; Sena-Esteves, M.; Saper, C.B. Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. J. Comp. Neurol. 2012, 520, 1985–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormier, C.M.; Mukhida, K.; Walker, G.; Marsh, D.R. Development of autonomic dysreflexia after spinal cord injury is associated with a lack of serotonergic axons in the intermediolateral cell column. J. Neurotrauma 2010, 27, 1805–1818. [Google Scholar] [CrossRef]
- Hou, S.; Lu, P.; Blesch, A. Characterization of supraspinal vasomotor pathways and autonomic dysreflexia after spinal cord injury in F344 rats. Auton. Neurosci. 2013, 176, 54–63. [Google Scholar] [CrossRef]
- Harman, K.A.; DeVeau, K.M.; Squair, J.W.; West, C.R.; Krassioukov, A.V.; Magnuson, D.S.K. Effects of early exercise training on the severity of autonomic dysreflexia following incomplete spinal cord injury in rodents. Physiol. Rep. 2021, 9, e14969. [Google Scholar] [CrossRef]
- Harman, K.A.; States, G.; Wade, A.; Stepp, C.; Wainwright, G.; Deveau, K.; King, N.; Shum-Siu, A.; Magnuson, D.S.K. Temporal analysis of cardiovascular control and function following incomplete T3 and T10 spinal cord injury in rodents. Physiol. Rep. 2018, 6, e13634. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.B.; Ramer, L.M.; Inskip, J.A.; Alan, N.; Ramer, M.S.; Krassioukov, A.V. Care of rats with complete high-thoracic Spinal cord injury. J. Neurotrauma 2010, 27, 1709–1722. [Google Scholar] [CrossRef] [PubMed]
- Fossey, M.; Poormasjedi-Meibod, M.; Hayes, B.; Erskine, E.L.; Azad, R.K.; Granville, D.J.; Ramer, M.S.; West, C.R. A reduction in cardiac function precedes structural adaptations in experimental spinal cord injury. FASEB J. 2019, 33, 831.7. [Google Scholar] [CrossRef]
| Naïve | SCI | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Hemodynamic Data | |||||||
| SBP (mmHg) | 121 | ± | 7 | 96 | ± | 11 | <0.001 |
| DBP (mmHg) | 70 | ± | 7 | 58 | ± | 9 | <0.001 |
| MAP (mmHg) | 88 | ± | 7 | 70 | ± | 9 | <0.001 |
| PP (mmHg) | 50 | ± | 4 | 38 | ± | 6 | <0.001 |
| HR (BPM) | 413 | ± | 38 | 462 | ± | 48 | 0.008 |
| SVR (mmHg·min−1µL−1) * | 0.89 | ± | 0.20 | 0.74 | ± | 0.12 | 0.038 |
| Pressure-Volume Data | |||||||
| ESV (µL) | 66 | ± | 18 | 59 | ± | 11 | 0.256 |
| EDV(µL) | 311 | ± | 47 | 261 | ± | 23 | 0.002 |
| Systolic Function | |||||||
| SW (mmHg·mL) | 33 | ± | 7 | 21 | ± | 4 | <0.001 |
| SWI (mmHg·mL−1100 g−1) | 10.90 | ± | 2.53 | 7.52 | ± | 1.64 | <0.001 |
| CO (mL/min) | 102 | ± | 20 | 93 | ± | 12 | 0.201 |
| CI (mL·min−1100 g−1) | 33.69 | ± | 7.72 | 35.22 | ± | 7.32 | 0.610 |
| SV (µL) | 245 | ± | 33 | 202 | ± | 24 | 0.001 |
| Pes (mmHg) | 98 | ± | 11 | 75 | ± | 10 | <0.001 |
| EF (%) | 79 | ± | 4 | 77 | ± | 5 | 0.272 |
| dP/dtmax (mmHg/s) | 10316 | ± | 809 | 6084 | ± | 755 | <0.001 |
| Ees (mmHg/µL) ** | 1.59 | ± | 0.23 | 0.89 | ± | 0.24 | <0.001 |
| Ea (mmHg/µL) | 0.41 | ± | 0.09 | 0.38 | ± | 0.07 | 0.296 |
| Ea/Ees ** | 0.26 | ± | 0.06 | 0.44 | ± | 0.23 | 0.021 |
| PRSW (mmHg) * | 131 | ± | 30 | 94 | ± | 17 | 0.001 |
| +dP/dtmax–EDV (mmHg·s−1 µL−1) * | 34 | ± | 7 | 27 | ± | 4 | <0.001 |
| Diastolic Function | |||||||
| dP/dtmin (mmHg/s) | −5890 | ± | 449 | −4021 | ± | 630 | <0.001 |
| Ped (mmHg) | 3 | ± | 2 | 4 | ± | 4 | 0.231 |
| τ (ms) | 7.32 | ± | 0.77 | 8.03 | ± | 3 | 0.416 |
| Injury Model | ||||||
|---|---|---|---|---|---|---|
| T3 300 kdyn Contusion | T2 400 kdyn Contusion | T2 200 kdyn Contusion | T2-3 Transection | T4 Complete Crush | T10 400 kdyn Contusion | |
| ↓ Blood pressure | ✔ | ✔ | ✔ | ✔ | ✖ | ✖ |
| ↓ Cardiac pressures | ✔ | ✔ | ? | ✔ | ? | ? |
| ↓ Cardiac output | ✖ | ✔ | ✖ | ✔ | ? | ? |
| >15% tissue sparing | ✔ | ✖ | ✖ | ✖ | ? | ? |
| >20% white matter Preservation | ✔ | ✖ | ✖ | ✖ | ? | ✖ |
| Preserved sub-lesional serotonergic/ catecholaminergic pathways | ✔ | ✖ | ✔ | ? | ✖ | N/A |
| References | [24,26,27,34] | [24,34] | [23,30,32] | [25] | [50,51] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wainman, L.; Erskine, E.L.; Ahmadian, M.; Hanna, T.M.; West, C.R. Development of a Spinal Cord Injury Model Permissive to Study the Cardiovascular Effects of Rehabilitation Approaches Designed to Induce Neuroplasticity. Biology 2021, 10, 1006. https://doi.org/10.3390/biology10101006
Wainman L, Erskine EL, Ahmadian M, Hanna TM, West CR. Development of a Spinal Cord Injury Model Permissive to Study the Cardiovascular Effects of Rehabilitation Approaches Designed to Induce Neuroplasticity. Biology. 2021; 10(10):1006. https://doi.org/10.3390/biology10101006
Chicago/Turabian StyleWainman, Liisa, Erin L. Erskine, Mehdi Ahmadian, Thomas Matthew Hanna, and Christopher R. West. 2021. "Development of a Spinal Cord Injury Model Permissive to Study the Cardiovascular Effects of Rehabilitation Approaches Designed to Induce Neuroplasticity" Biology 10, no. 10: 1006. https://doi.org/10.3390/biology10101006
APA StyleWainman, L., Erskine, E. L., Ahmadian, M., Hanna, T. M., & West, C. R. (2021). Development of a Spinal Cord Injury Model Permissive to Study the Cardiovascular Effects of Rehabilitation Approaches Designed to Induce Neuroplasticity. Biology, 10(10), 1006. https://doi.org/10.3390/biology10101006





