Flaxseed Lignans and Polyphenols Enhanced Activity in Streptozotocin-Induced Diabetic Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Flaxseed Extract
2.2. Experimental Animals
2.3. Induction of Experimental DM
2.4. Experimental Design
2.5. Evaluation of Clinical Parameters
2.6. Biochemical Analysis
2.7. Histopathological Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Identified Lignans and Polyphenols Quantification
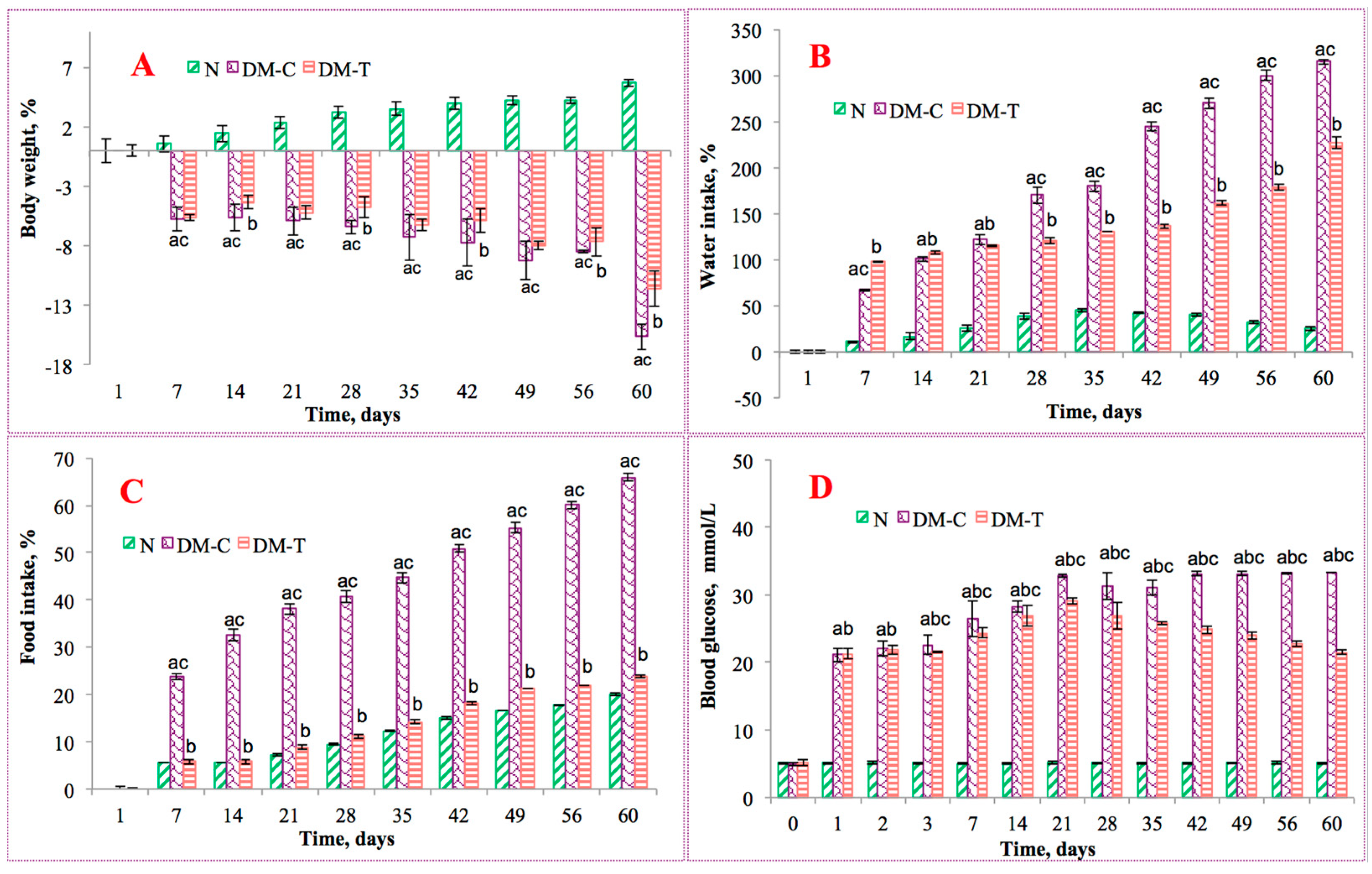
3.2. Changes in Clinical Parameters in Experimental Animals
3.3. Biochemical Analysis
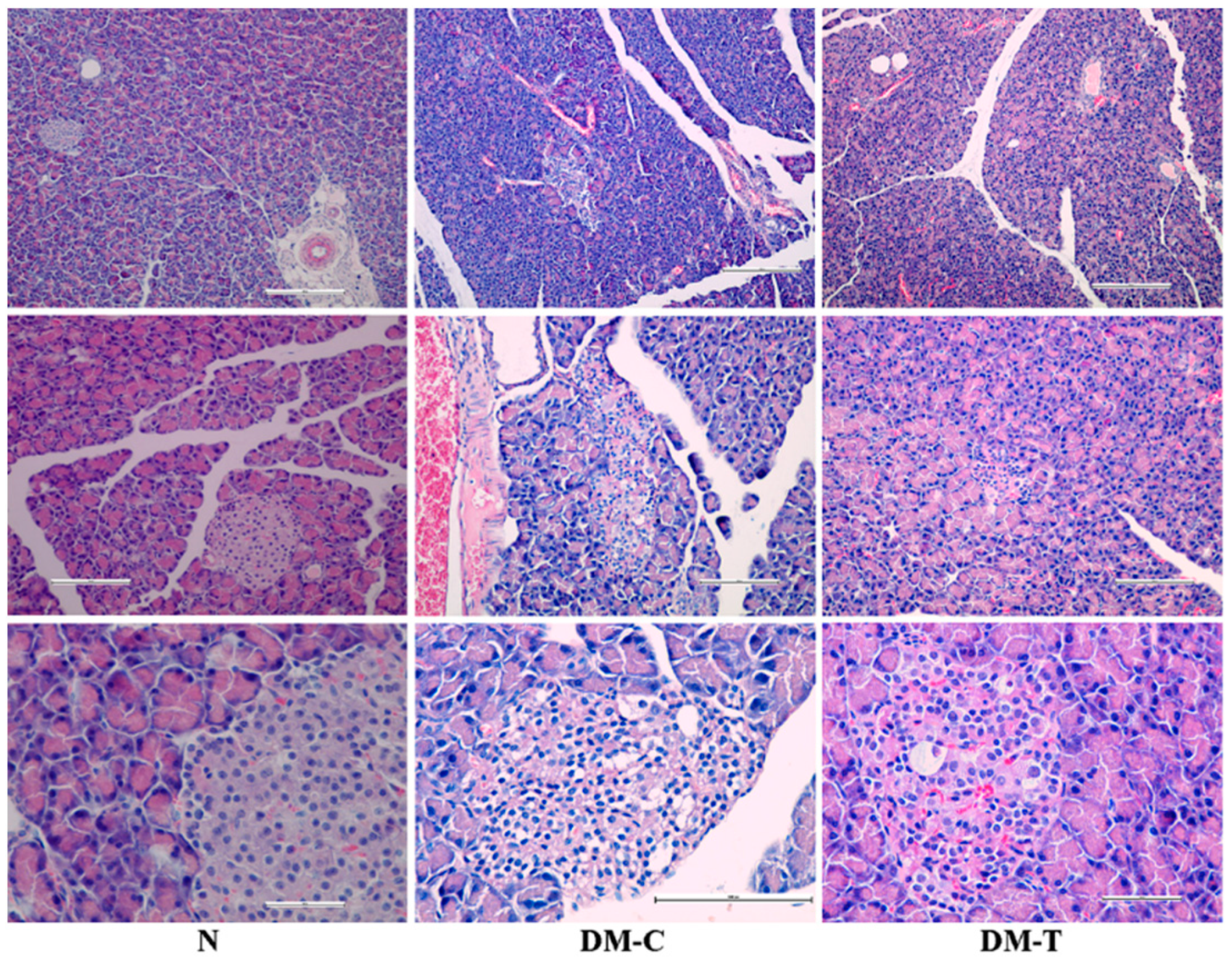
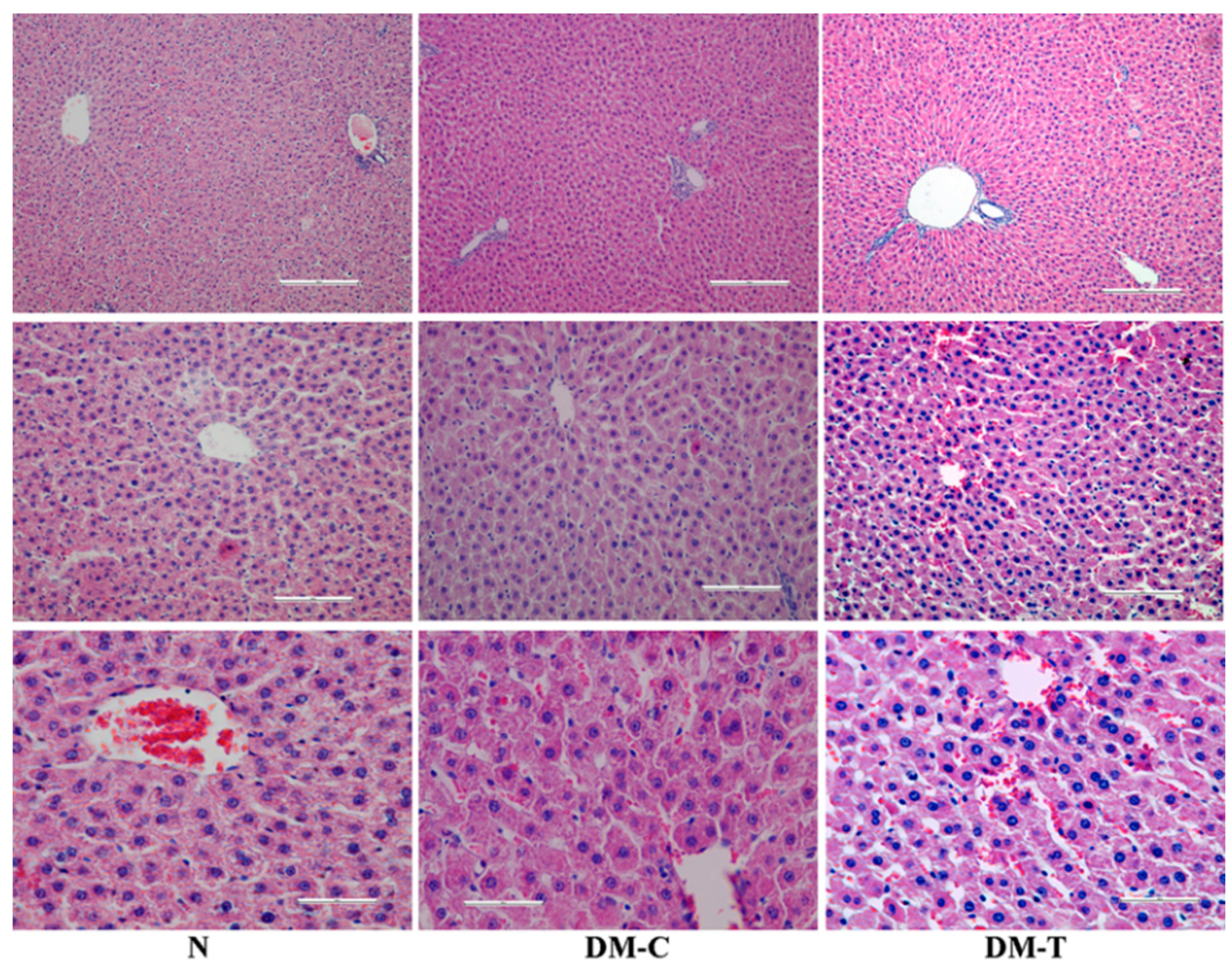
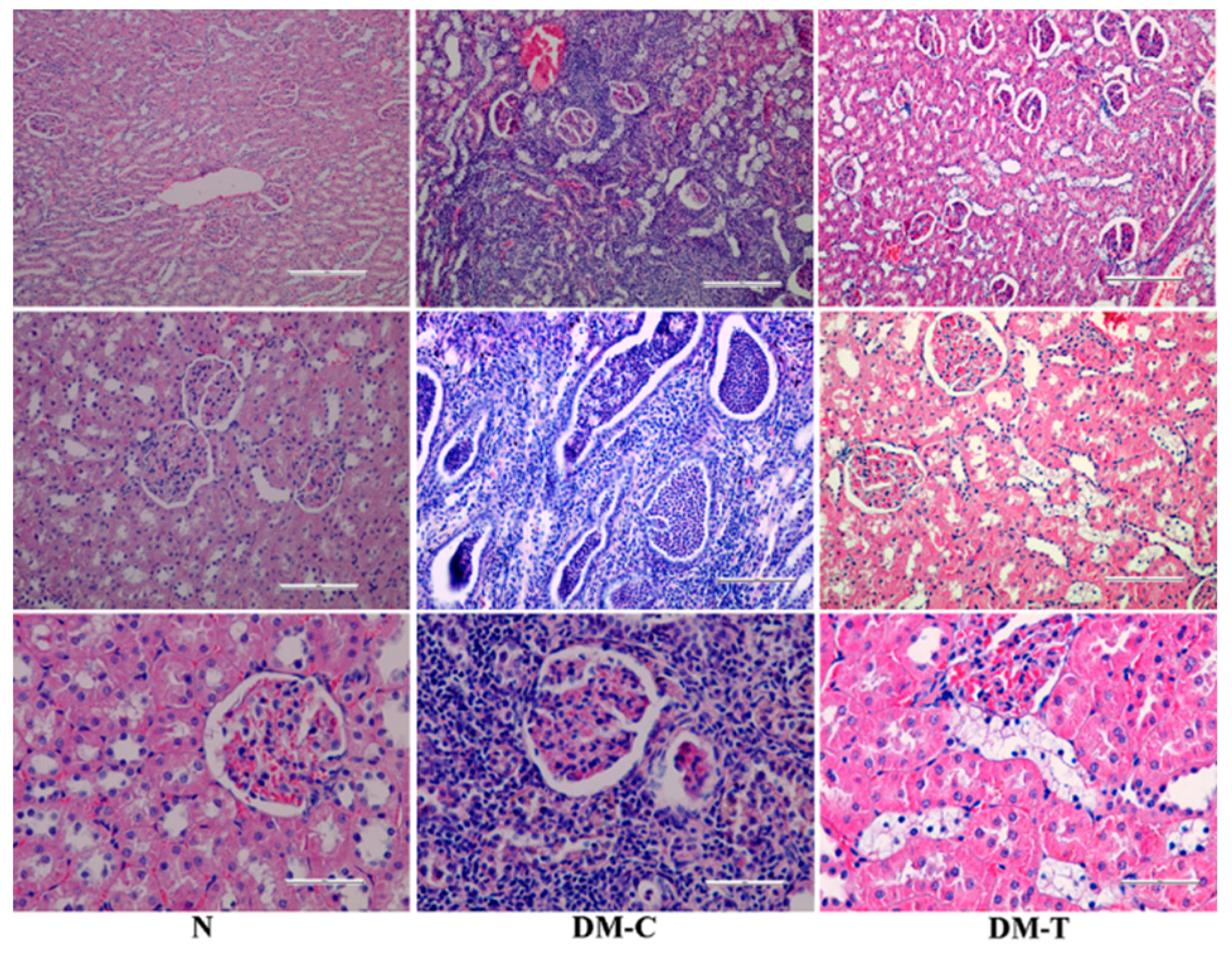
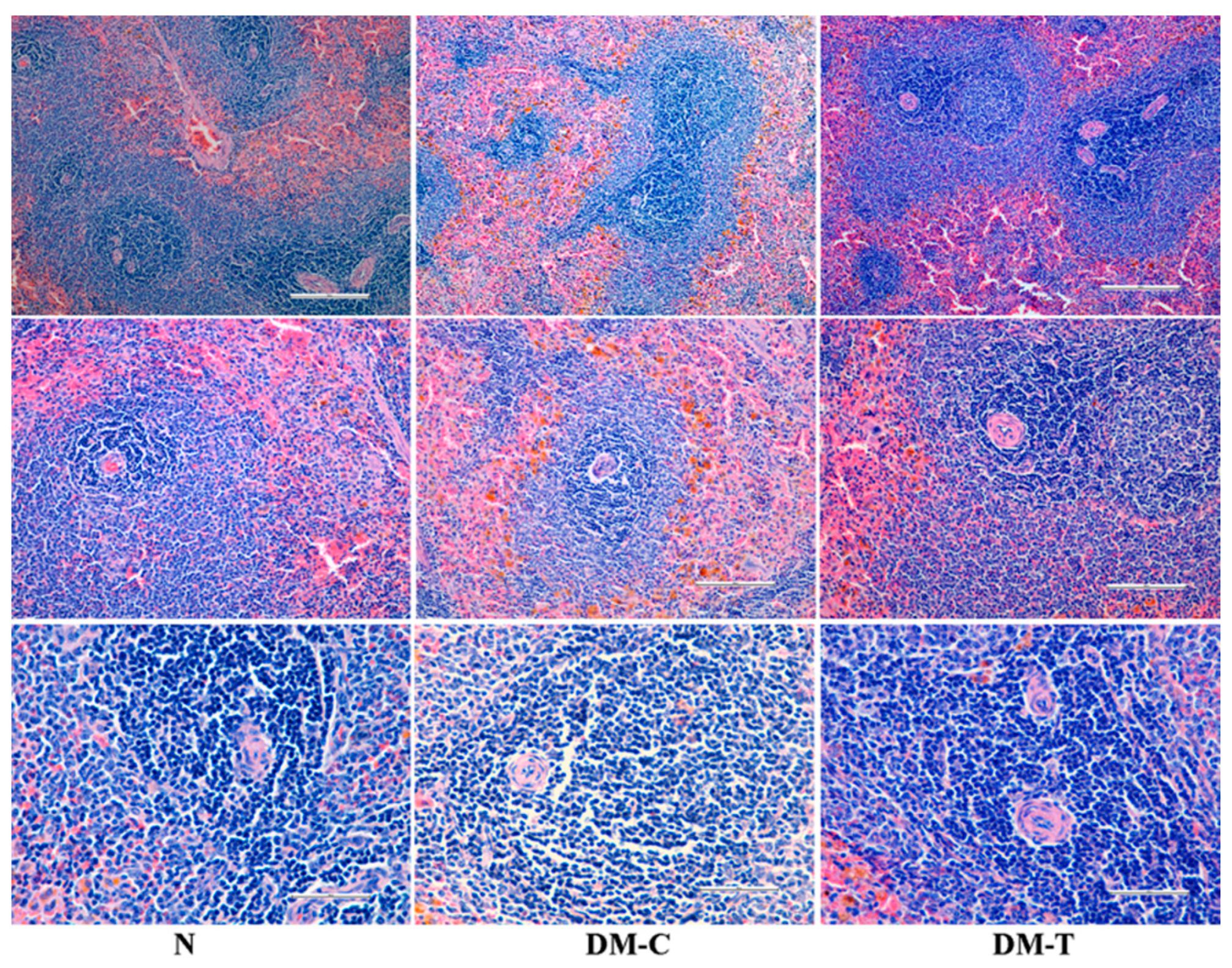
3.4. Observations of Organs
- -
- N group: Complete normal islet structure with regularly distributed and abundant pancreatic cells.
- -
- DM-C group: The pancreatic islets morphology showed pronounced endogenous damage, such as cytolysis, cell rupture, and apoptosis; the Langerhans islets number was reduced and accompanied by the cytoplasmic vacuolation of pancreatic β-cells, responsible for secreting insulin and vascular congestion; histopathology examination revealed the presence of an infiltration with lymphocytes and mastocytes located at vascular–conjunctive interlobular septum.
- -
- DM-T group: The treatment with flaxseed extract reverted the abnormal modifications in islet structure located in the STZ lesions, contributing to architectural amelioration of islet structure and a gradual increase in β-cell counts; the number of damaged pancreatic Langerhans islets decreased substantially after the flaxseed extract administration. A small percentage of the pancreatic β-cells still preserved the cytoplasmic vacuolation, accompanied by a reduced infiltration with lymphocytes and mastocytes at the vascular–conjunctive interlobular septum. The obtained architecture implies the possibility that flaxseed extract is capable of protecting pancreatic β-cells or promoting their regeneration.
- -
- N group: Intact architecture and normal hepatocytes with well-preserved cytoplasm, nucleus, nucleolus, and central vein.
- -
- DM-C group: The hepatic cords became distorted and the hepatocytes evidenced a low deposit of glycogen, in association with necrosis of the hepatic cells, degeneration, vacuolation in hepatic cells, and infiltration of the parenchyma with inflammatory cells in comparison to healthy rats.
- -
- DM-T group: Flaxseed extract administration significantly tempered the STZ-induced hepatocellular necrosis and fibrotic changes in rat liver; the mild normalization of hepatocellular architecture with nucleus, cytoplasm, and distinct hepatic layer was also observed; on the other hand, the reduced capacity of liver to produce glycogen was maintained [36].
- -
- N group: Normal structure of renal tubules, renal corpuscles, and collecting ducts without any inflammatory changes.
- -
- DM-C group: Showed infiltration of inflammatory cells (neutrophils, lymphocytes, and plasmocytes) spread as an unlimited band in the cortical region up to renal pelvis. The obliterated lumen from neutrophils accumulation of renal tubules was observed in association with multiple areas of necrosis involving glomeruli, the renal tubules, and the walls of the blood vessels. The renal pelvis exhibited edematous changes with cellular injury and moderate lymphocytes infiltration at subjacent transition epithelium region. Additionally, the necrotic changes displayed hemorrhage in renal cortex, an increased space filtering, compression atrophy of the renal glomeruli, and dilatation of the renal collecting tubules. All of these features indicate a suppurative nephritis and renal hydronephrosis.
- -
- DM-T group: The atypical modifications in renal structure were partially rescued in the STZ lesions, contributing to architectural amelioration of epithelium necrosis and displaying features of epithelium necrosis with a reduced number of renal collecting tubules.
- -
- N group: A highly vascular organ with red pulps represented by blood sinuses and white pulps indicated by oval splenic discrete nodules of lymphoid accumulations adjacent the central arteries, creating periarteriolar lymphoid sheath.
- -
- DM-C group: Displayed normal architecture comparable with the healthy rats.
- -
- DM-T group: No injuries on spleen tissue were observed for diabetic rats treated with flaxseed extract.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.B. Flaxseed secoisolariciresinol diglucoside and visceral obesity, A closer look at its chemical properties, absorption, metabolism, bioavailability, and effects on visceral fat, lipid profile, systemic inflammation, and hypertension. In Nutrition in the Prevention and Treatment of Abdominal Obesity Book, 1st ed.; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 317–328. [Google Scholar]
- Carter, J. Potential of flaxseed and flaxseed oil in baked goods and other products in human nutrition. Cer. Foods World 1993, 38, 753–759. [Google Scholar]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J.T. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: A review. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef]
- Bhatty, R.S.; Cherdkiatgumchai, P. Compositional analysis of laboratory-prepared and commercial samples of linseed meal and of hull isolated from flax. J. Am. Oil Chem. Soc. 1990, 67, 79–84. [Google Scholar] [CrossRef]
- Thompson, L.U. Experimental studies on lignans and cancer. Bailliere’s Clin. Endocrinol. Metab. 1998, 12, 691–705. [Google Scholar] [CrossRef]
- Toure, A.; Xueming, X. Flaxseed Lignans: Source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr. Rev. Food Sci. Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef]
- Prasad, N.; Strauss, D.; Reichart, G. Cyclodextrins Inclusion for Food, Cosmetics and Pharmaceuticals. Eur. Pat. 1999, 1, 625. [Google Scholar]
- Fukumitsu, S.; Aida, K.; Shimizu, H.; Toyoda, K. Flaxseed lignan lowers blood cholesterol and decreases liver disease risk factors in moderately hypercholesterolemic men. Nutr. Res. 2010, 30, 441–446. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar]
- American Diabetes Association (ADA). Diagnosis and classification of diabetes mellitus. Diabetes Care 2008, 31, S55–S60. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Hellman, R.; Korytkowski, M.T.; Kosiborod, M.; Maynard, G.A.; Montori, V.M.; Seley, J.J.; Van der Berghe, G. Management of hyperglycemia in hospitalized patients in non-critical care setting: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 16–38. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Mantha, S.V.; Muir, A.D.; Westcott, N.D. Protective effect of secoisolariciresinol diglucoside against streptozotocin-induced diabetes and its mechanism. Mol. Cell Biochem. 2000, 206, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Parimelazhagan, T. Antidiabetic activity of Ficus amplissima Smith. bark extract in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2013, 147, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Shervington, A.; Pariente, J.A.; Martinez-Burgos, M.A.; Salido, G.M.; Adeghate, E.; Singh, J. Mechanism of exocrine pancreatic insufficiency in streptozotocin-induced type 1 diabetes mellitus. Ann. N. Y. Acad. Sci. 2006, 1084, 71–88. [Google Scholar] [CrossRef]
- King, A. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.M.; Brown, B.D.; Cunnane, S.C.; Domitrovich, S.G.; Adams, E.R.; Bobowiec, C.E. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: A randomized study. Nutr. Res. 2013, 33, 367–375. [Google Scholar] [CrossRef]
- Kezimana, P.; Dmitriev, A.A.; Kudryavtseva, A.V.; Romanova, E.V.; Melnikova, N.V. Secoisolariciresinol Diglucoside of flaxseed and its metabolites: Biosynthesis and potential for nutraceuticals. Front. Genet. 2018, 9, 641. [Google Scholar] [CrossRef]
- Prasad, K. Oxidative stress as a mechanism of diabetes in diabetic BB prone rats: Effect of secoisolariciresinol diglucoside (SDG). Mol. Cell Biochem. 2000, 209, 89–96. [Google Scholar] [CrossRef]
- Hano, C.; Renouard, S.; Molinié, R.; Corbin, C.; Barakzoy, E.; Doussot, J.; Lamblin, F.; Lainé, E. Flaxseed (Linum usitatissimum L.) extract aswell as (+)-secoisolariciresinol diglucoside and its mammalian derivatives are potent inhibitors of a-amylase activity. Bioorg. Med. Chem. Lett. 2013, 23, 3007–3012. [Google Scholar] [CrossRef]
- Moree, S.S.; Kavishankar, G.B.; Rajesha, J. Antidiabetic effect of secoisolariciresinol diglucoside in streptozotocin-induced diabetic rats. Phytomedicine 2013, 20, 237–245. [Google Scholar] [CrossRef]
- Dusane, M.B.; Joshi, B.N. Beneficial effect of flax seeds in streptozotocin (STZ) induced diabetic mice: Isolation of active fraction having islet regenerative and glucosidase inhibitory properties. Can. J. Physiol. Pharmacol. 2013, 91, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kishore, L.; Singh, R. Therapeutic effect of Linum usitatissimum L. in STZ-nicotinamide induced diabetic nephropathy via inhibition of AGE’s and oxidative stress. J. Food Sci. Technol. 2017, 54, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Draganescu, D.; Ibanescu, C.; Tamba, B.I.; Andritoiu, C.V.; Dodi, G.; Popa, M.I. Flaxseed lignan wound healing formulation: Characterization and in vivo therapeutic evaluation. Int. J. Biol. Macromol. 2015, 72, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Florence, N.T.; Benoit, M.Z.; Jonas, K.; Alexandra, T.; Désiré, D.D.P.; Pierre, K.; Théophile, D. Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2014, 151, 784–790. [Google Scholar] [CrossRef]
- Doyen, A.; Udenigwe, C.C.; Mitchell, P.L.; Marette, A.; Aluko, R.E.; Bazinet, L. Anti-diabetic and antihypertensive activities of two flaxseed protein hydrolysate fractions revealed following their simultaneous separation by electrodialysis with ultrafiltration membranes. Food Chem. 2014, 145, 66–76. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Bhathena, S.J.; Ranich, T.; Schwartz, A.M.; Kardon, D.E.; Ali, A.A.; Haudenschild, C.; Hansen, C.T. Dietary flaxseed meal reduces proteinuria and ameliorates nephropathy in an animal model of type II diabetes mellitus. Kidney Int. 2003, 64, 2100–2107. [Google Scholar] [CrossRef]
- Prasad, K. Hypocholesterolemic and antiatherosclerotic effect of flax lignan complex isolated from flaxseed. Atherosclerosis 2005, 179, 269–275. [Google Scholar] [CrossRef]
- Doss, A.; Palaniswamy, M.; Angayarkanni, J.; Dhanabalan, R. Antidiabetic activity of water extract of Solanum trilobatum (Linn.) in alloxan-induced diabetes in rats. Afr. J. Biotechnol. 2009, 8, 5562–5564. [Google Scholar]
- Prasad, K. Secoisolariciresinol diglucoside from flaxseed delays the development of type 2 diabetes in Zucker rat. J. Lab. Clin. Med. 2001, 138, 32–39. [Google Scholar] [CrossRef]
- Nain, P.; Saini, V.; Sharma, S.; Nain, J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J. Ethnopharmacol. 2012, 142, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Kang, S.M.; Seo, B.I.; Choi, H.Y.; Choi, H.S.; Ku, S.K. Anti-diabetic activity of SMK001, a poly herbal formula in streptozotocin-induced diabetic rats: Therapeutic study. Biol. Pharm. Bull. 2006, 29, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Naeem-Ul-Hassan Naqvi, S. Effects of STZ-Induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: A comparative study. Int. J. Morphol. 2010, 28, 135–142. [Google Scholar] [CrossRef]
- Sundaram, R.; Naresh, R.; Shanthi, P.; Sachdanandam, P. Modulatory effect of green tea extract on hepatic key enzymes of glucose metabolism in streptozotocin and high fat diet induced diabetic rats. Phytomedicine 2013, 20, 577–584. [Google Scholar] [CrossRef]
- Ríos, J.F.; Andújar, I.S.; Schinella, G.; Francini, F. Modulation of Diabetes by Natural Products and Medicinal Plants via Incretins. Planta Med. 2019, 85, 825–839. [Google Scholar] [CrossRef]
- Bolkent, S.; Yanardag, R.; Ozsoy-Sacan, O.; Karabulut-Bulan, O. Effects of Parsley (Petroselinumcrispum) on the liver of diabetic rats: A morphological and biochemical study. Phytother. Res. 2004, 18, 996–999. [Google Scholar] [CrossRef]
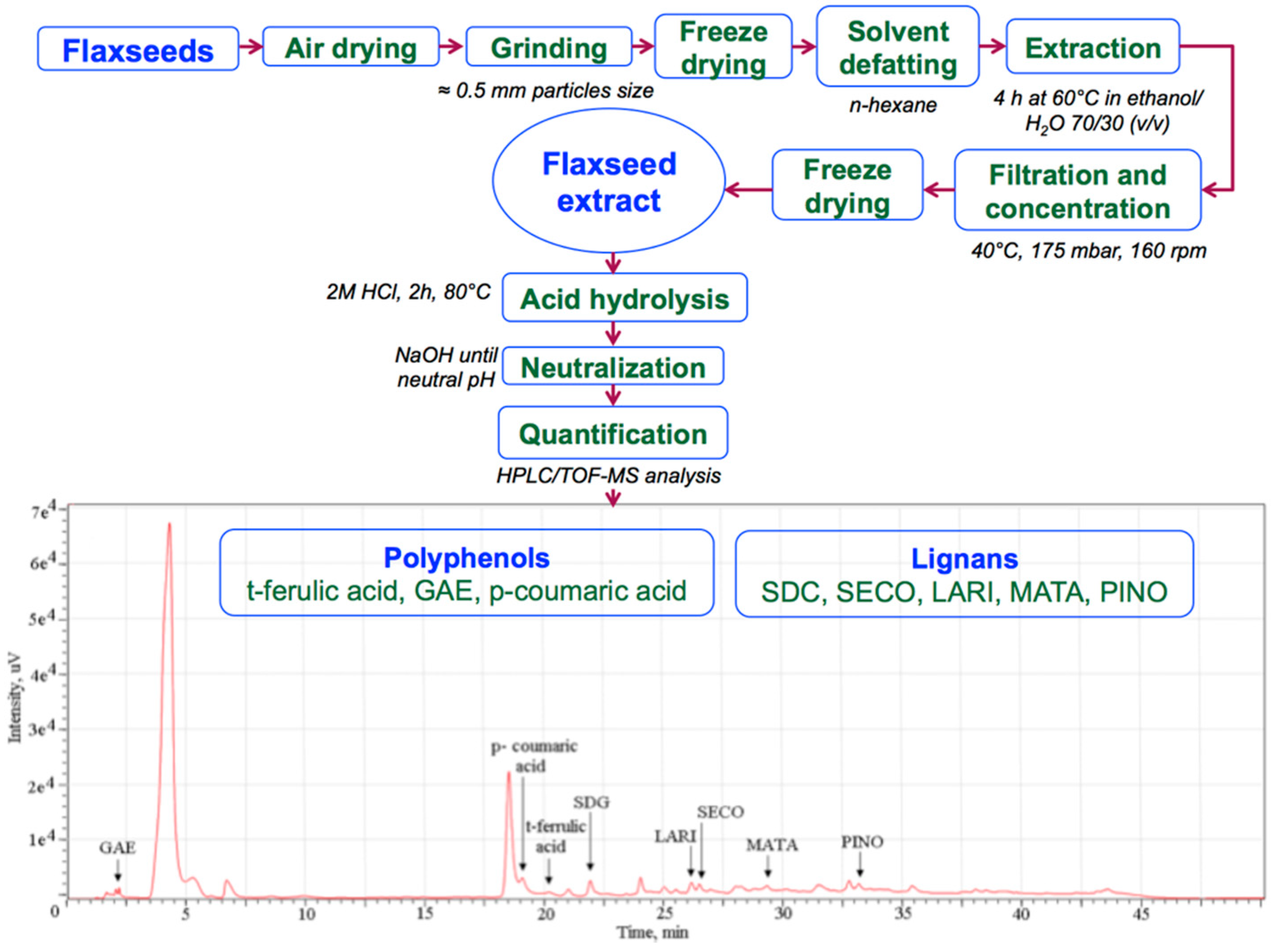
| Compound | Concentration/100 g Dry Material | Daily Administrated Concentration/0.5 g | Total Administrated Concentration/30 g | |
|---|---|---|---|---|
| Lignans | SDG | 9.3 mg | 0.774 mg | 46.44 mg |
| SECO | 110.8 mg | |||
| LARI | 4.9 mg | |||
| MATA | 6.9 mg | |||
| PINO | 22.9 mg | |||
| Polyphenols | t-ferulic acid | 4.8 mg | 0.073 mg | 4.38 mg |
| GAE | 4.6 mg | |||
| p-coumaric acid | 5.2 mg | |||
| Parameter/Group | N | DM-C | DM-T |
|---|---|---|---|
| HbA1c, mmol/mol | 18.47 ± 0.1 | 111.48 ± 0.3 ac | 103.27 ± 0.4 bc |
| AST, UI/L | 94.5 ± 4.95 | 378.5 ± 17.68 a | 374 ± 16.97 b |
| ALT, UI/L | 44.5 ± 3.54 | 176.5 ± 4.95 ac | 182.5 ± 3.53 bc |
| Total cholesterol, mg/dL | 62.5 ± 3.53 | 145.5 ± 4.95 ac | 93 ± 2.83 c |
| HDL cholesterol, mg/dL | 59 ± 1.41 | 59.5 ± 4.95 | 49.5 ± 3.53 |
| LDL cholesterol, mg/dL | 43 ± 4.24 | 67.5 ± 3.54 a | 63.5 ± 2.12 b |
| Triglyceride, mg/dL | 100.5 ± 14.85 | 235 ± 14.14 ac | 148.5 ± 4.95 c |
| Creatinine, mg/dL | 0.175 ± 0.05 | 0.13 ± 0.07 | 0.095 ± 0.02 bc |
| Serum urea, mg/dL | 53.5 ± 2.12 | 75 ± 1.41 ac | 58 ± 4.24 c |
| Uric acid, mg/dL | 1.05 ± 0.35 | 2.05 ± 0.5 ac | 0.7 ± 0.57 c |
| Blood urea nitrogen, mg/dL | 25.17 ± 0.24 | 34.97 ± 0.76 ac | 27.02 ± 1.97 c |
| Weight of pancreas, g | 0.6 ± 0.01 | 0.54 ± 0.03 ac | 0.66 ± 0.01 c |
| Weight of liver, g | 10.2 ± 0.01 | 11.2 ± 0.05 ac | 9.92 ± 0.61 c |
| Weight of kidney, g | 0.9 ± 0.005 | 1.61 ± 0.21 ac | 1.1 ± 0.07 c |
| Weight of spleen, g | 0.55 ± 0.005 | 0.53 ± 0.08 | 0.57 ± 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Draganescu, D.; Andritoiu, C.; Hritcu, D.; Dodi, G.; Popa, M.I. Flaxseed Lignans and Polyphenols Enhanced Activity in Streptozotocin-Induced Diabetic Rats. Biology 2021, 10, 43. https://doi.org/10.3390/biology10010043
Draganescu D, Andritoiu C, Hritcu D, Dodi G, Popa MI. Flaxseed Lignans and Polyphenols Enhanced Activity in Streptozotocin-Induced Diabetic Rats. Biology. 2021; 10(1):43. https://doi.org/10.3390/biology10010043
Chicago/Turabian StyleDraganescu, Dan, Calin Andritoiu, Doina Hritcu, Gianina Dodi, and Marcel Ionel Popa. 2021. "Flaxseed Lignans and Polyphenols Enhanced Activity in Streptozotocin-Induced Diabetic Rats" Biology 10, no. 1: 43. https://doi.org/10.3390/biology10010043
APA StyleDraganescu, D., Andritoiu, C., Hritcu, D., Dodi, G., & Popa, M. I. (2021). Flaxseed Lignans and Polyphenols Enhanced Activity in Streptozotocin-Induced Diabetic Rats. Biology, 10(1), 43. https://doi.org/10.3390/biology10010043







