A New Transmission Route for the Propagation of the SARS-CoV-2 Coronavirus
Abstract
:Simple Summary
Abstract
1. Background
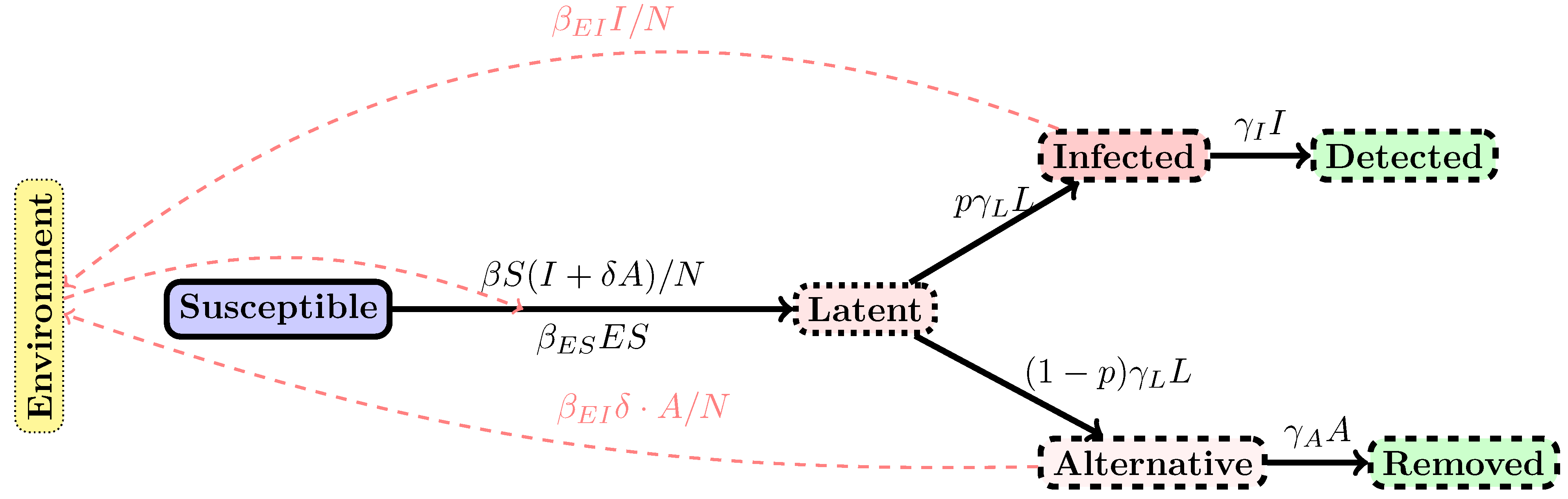
2. Methods
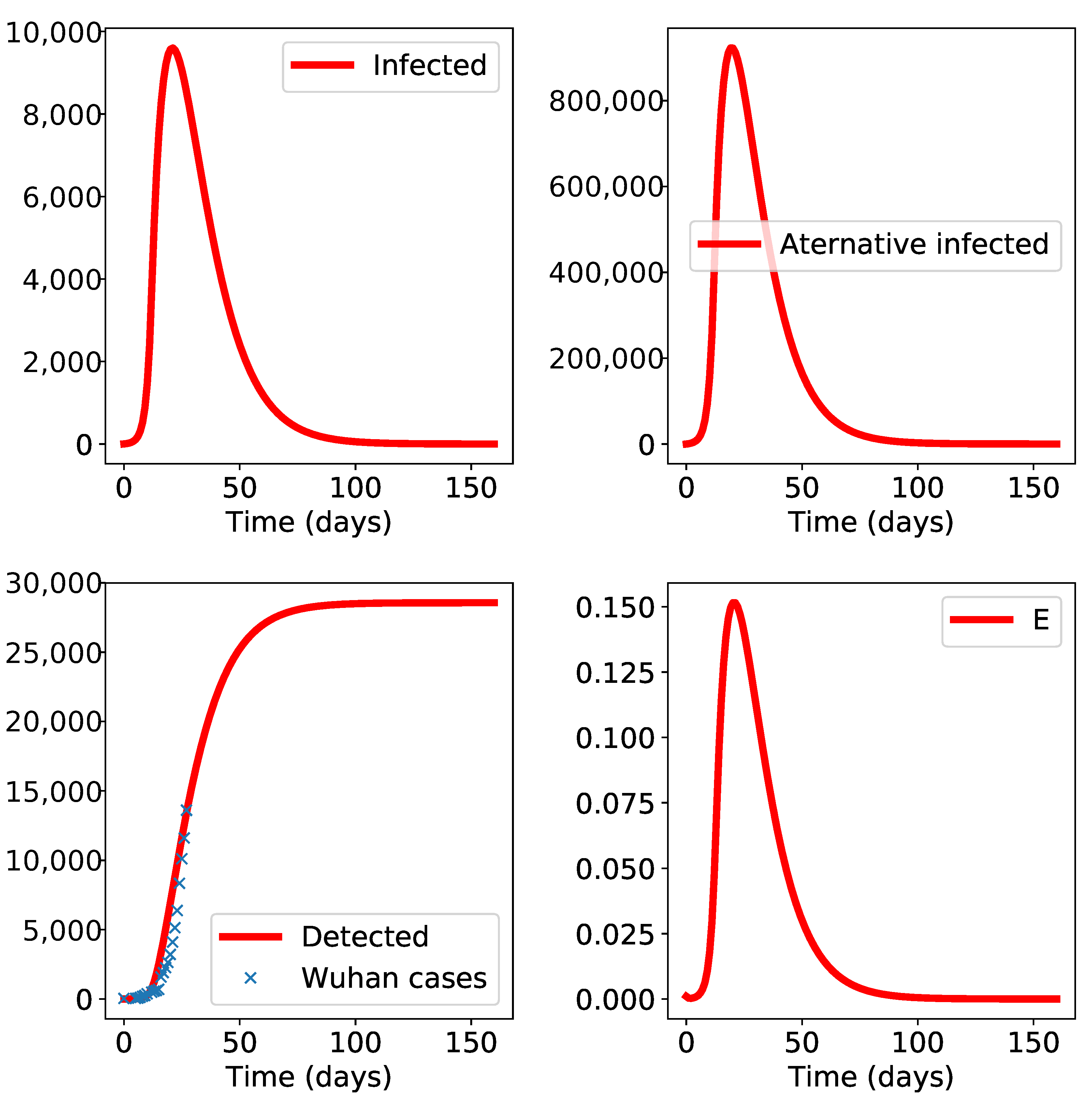
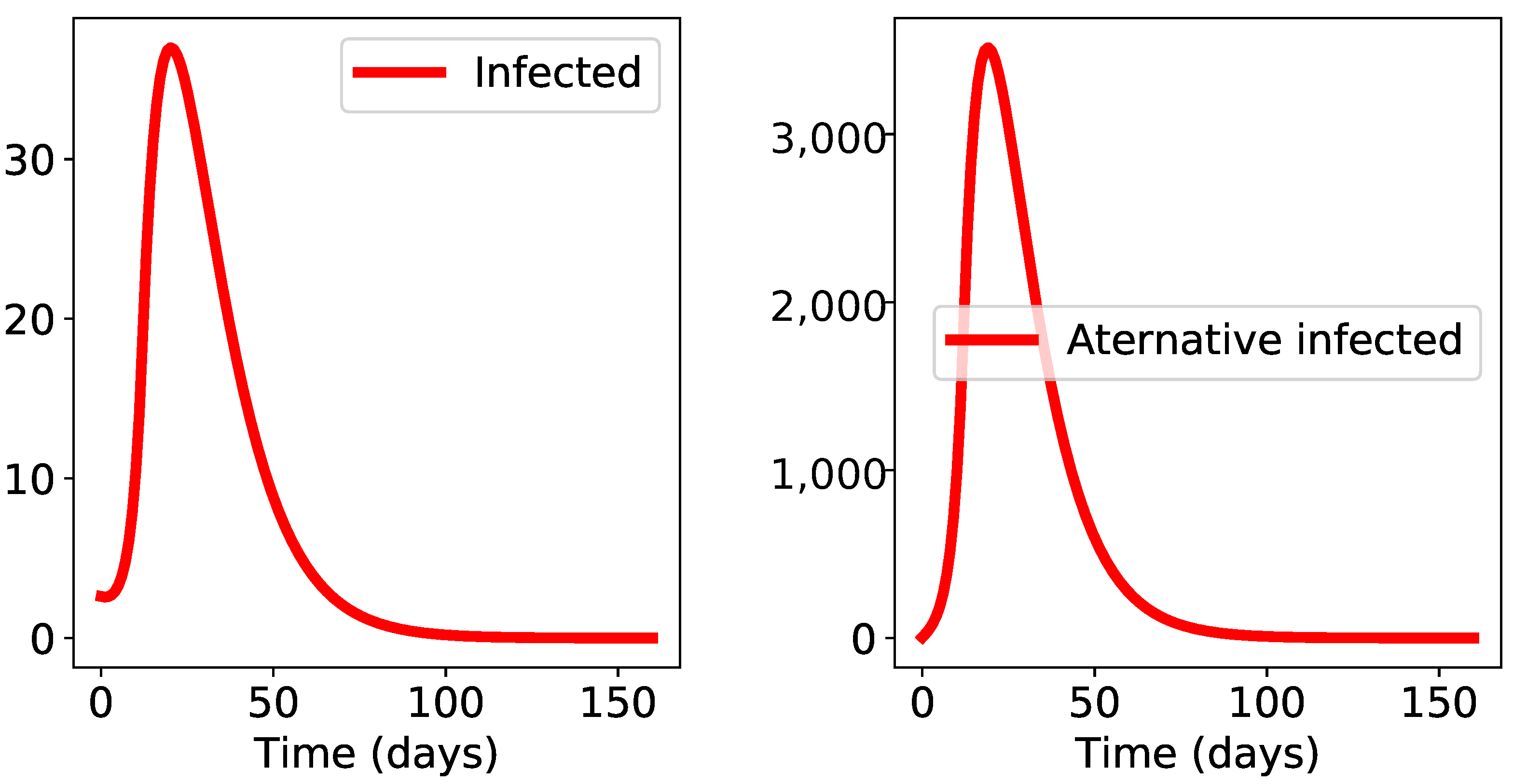
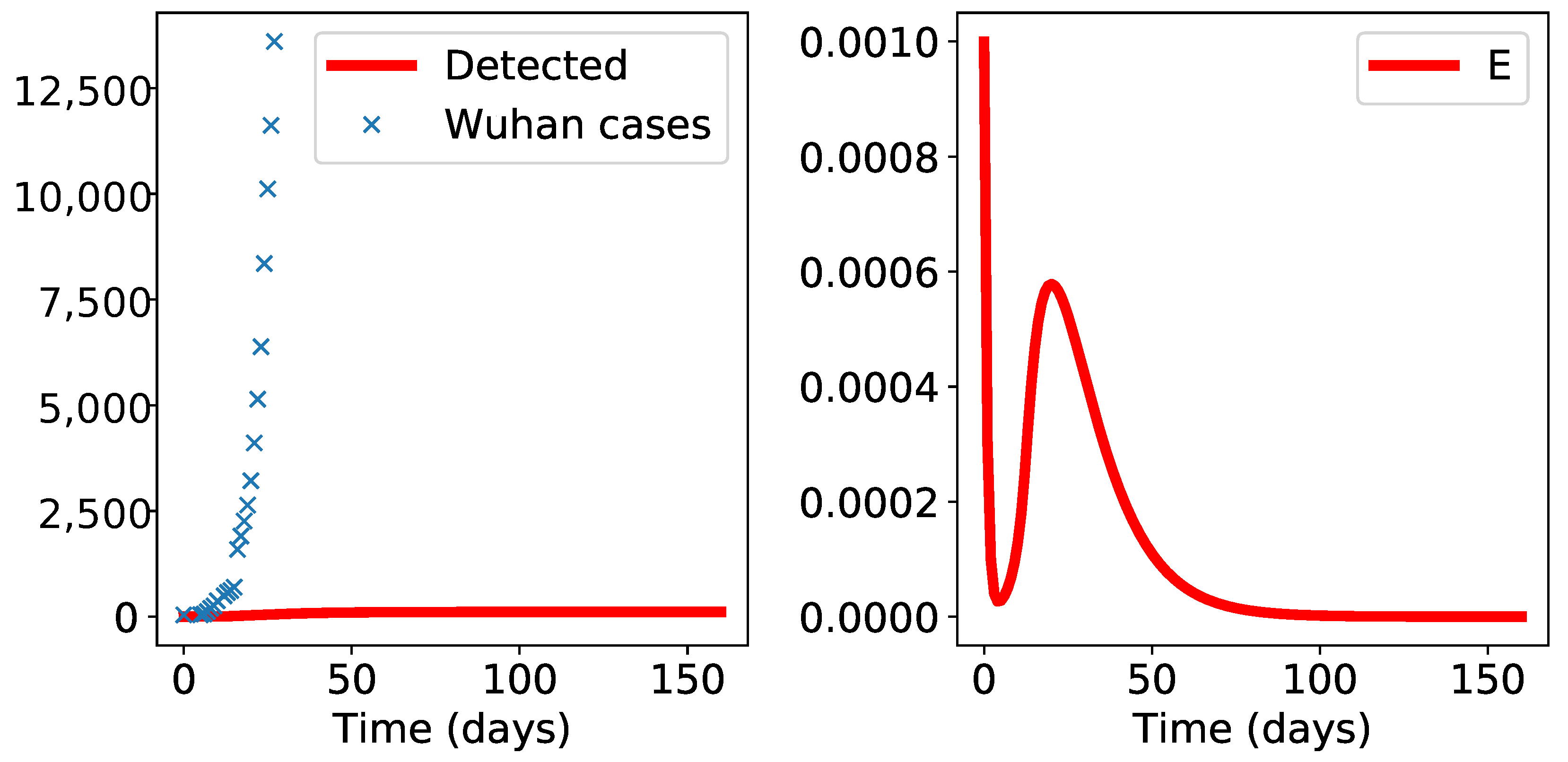
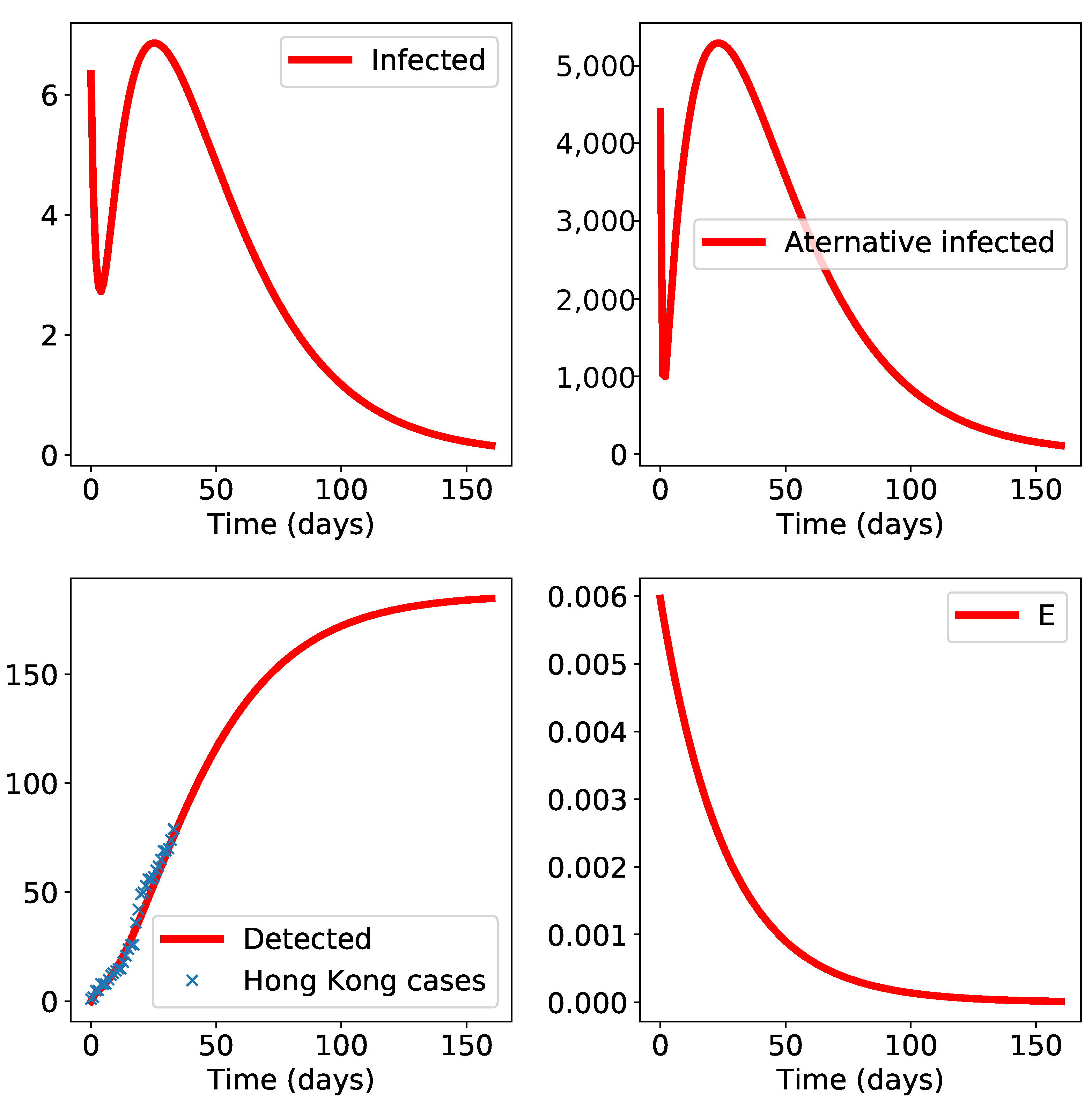
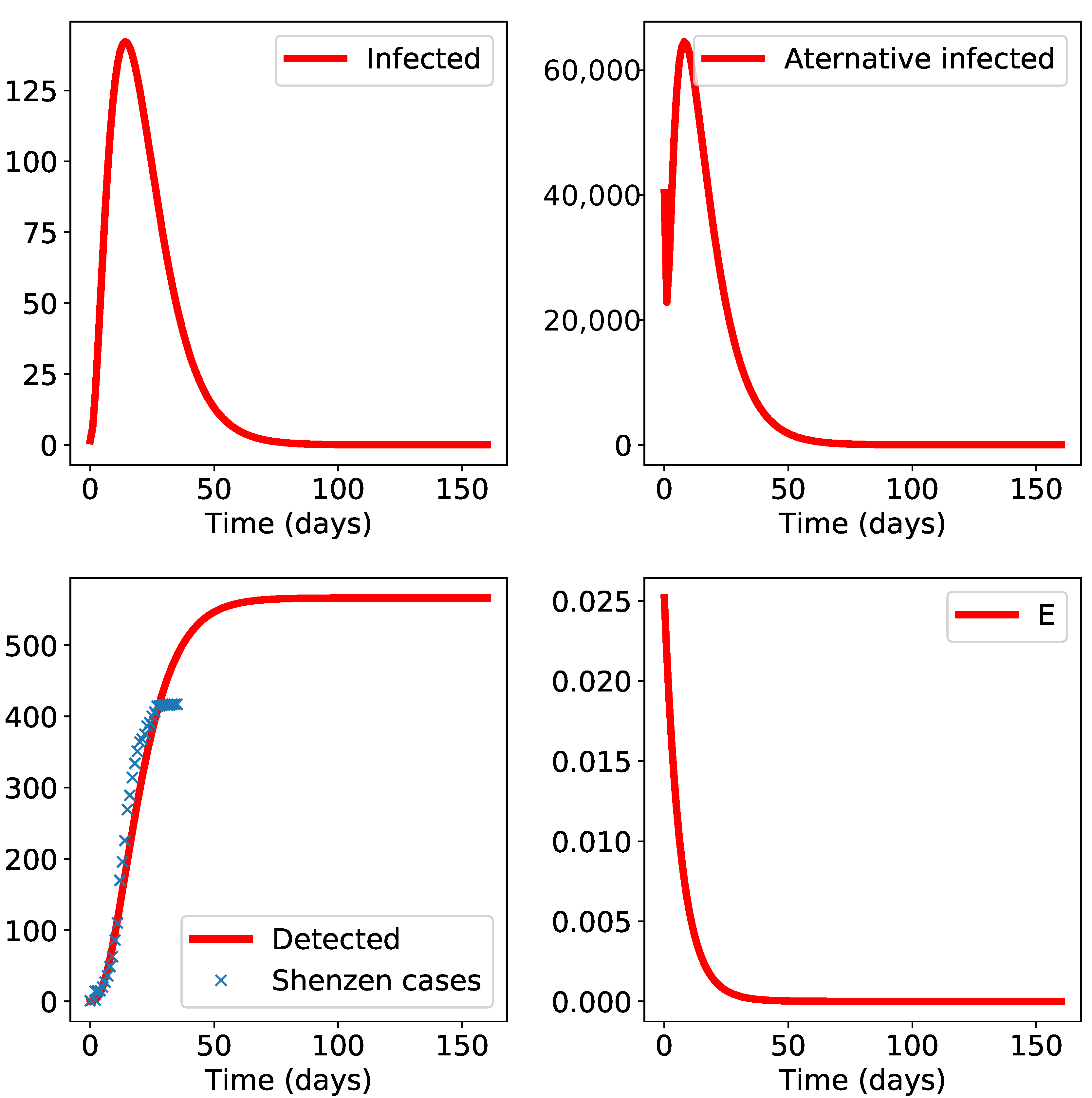
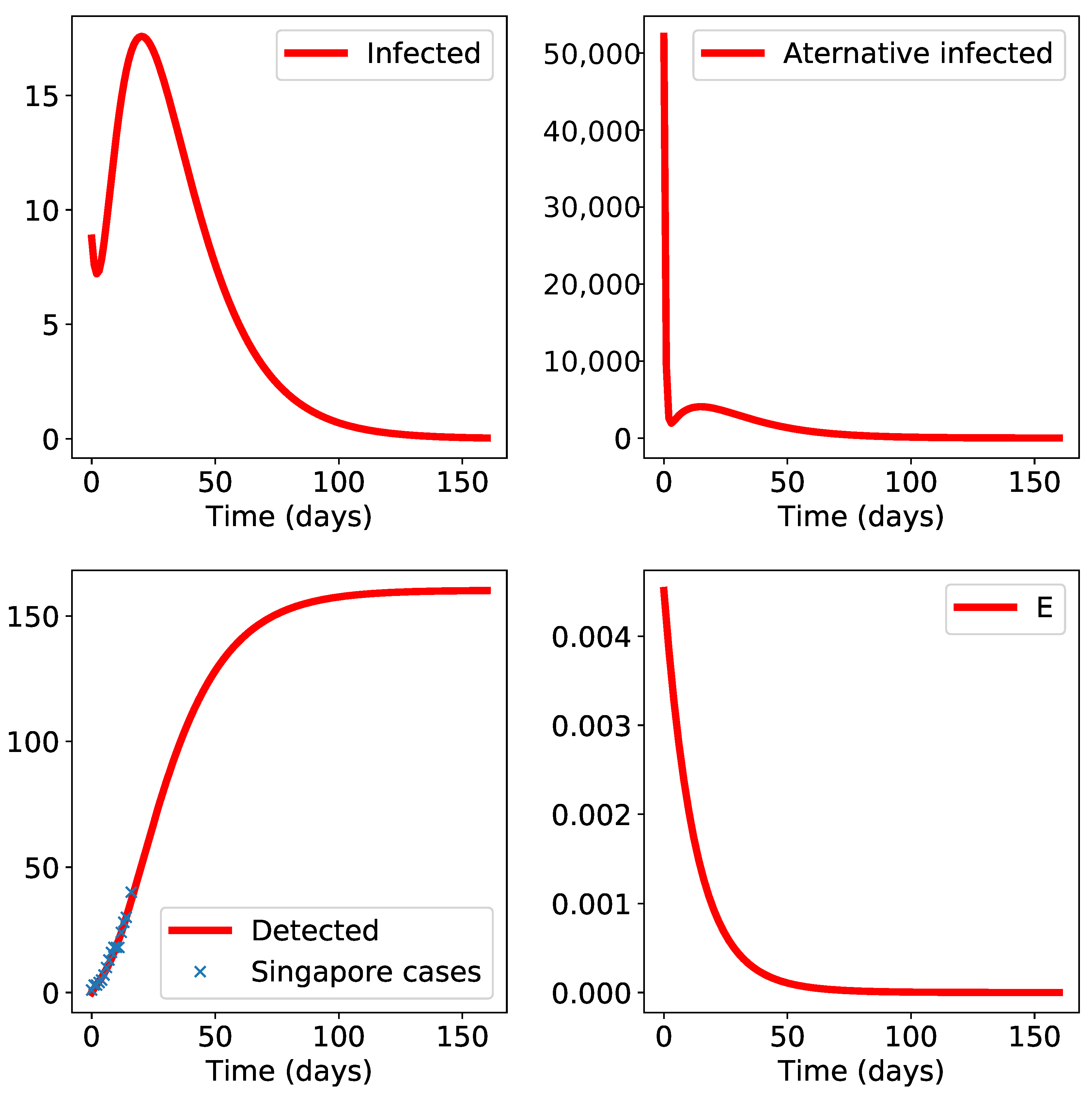
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
- (1)
- Health Commission of Hubei Province repository,http://wjw.hubei.gov.cn/fbjd/dtyw/
- (2)
- The Shenzhen Municipal Health Commission repository,http://wjw.sz.gov.cn/yqxx/
- (3)
- Department of Health, The Government of Hong Kong SAR repository,https://www.coronavirus.gov.hk/eng/index.html#Updates_on_COVID-19_Situation.
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| SARS | Severe acute respiratory syndrome |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| WHO | The World Health Organization |
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.T.; Leung, K.; Leung, G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study. Lancet 2020. [Google Scholar] [CrossRef] [Green Version]
- Rasschaert, D.; Duarte, M.; Laude, H. Porcine respiratory coronavirus differs from transmissible gastroenteritis virus by a few genomic deletions. J. Gen. Virol. 1990, 71, 2599–2607. [Google Scholar] [CrossRef]
- Laude, H.; Rasschaert, D.; Delmas, B.; Eleouët, J.F. Le coronavirus respiratoire porcin PRCV: Un virus émergent pas comme les autres. Virologie 1998, 2, 305–316. [Google Scholar]
- Garwes, D.J. Transmissible gastroenteritis. Vet. Rec. 1988, 122, 462–463. [Google Scholar] [CrossRef]
- Song, H.D.; Tu, C.C.; Zhang, G.W.; Wang, S.Y.; Zheng, K.; Lei, L.C.; Chen, Q.X.; Gao, Y.W.; Zhou, H.Q.; Xiang, H.; et al. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl. Acad. Sci. USA 2005, 102, 2430. [Google Scholar] [CrossRef] [Green Version]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef]
- Pradhan, P.; Pandey, A.K.; Mishra, A.; Gupta, P.; Tripathi, P.K.; Menon, M.B.; Gomes, J.; Vivekanandan, P.; Kundu, B. Uncanny similarity of unique inserts in the 2019-nCoV spike protein to HIV-1 gp120 and Gag. BioRxiv 2020. [Google Scholar] [CrossRef]
- Ng, T.W.; Turinici, G.; Danchin, A. A double epidemic model for the SARS propagation. BMC Infect. Dis. 2003, 3, 19. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, Z.; Gong, C.; Liu, H.; Li, B.; Li, K.; Chen, X.; Xu, C.; Jing, Q.; Liu, G.; et al. Sewage as a Possible Transmission Vehicle During a Coronavirus Disease 2019 Outbreak in a Densely populated Community: Guangzhou, China, April 2020. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Laude, H.; Charley, B.; Gelfi, J. Replication of Transmissible Gastroenteritis Coronavirus (TGEV) in Swine Alveolar Macrophages. J. Gen. Virol. 1984, 65, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Li, J.; Guo, T.; Zhen, B.; Kong, Q.; Yi, B.; Li, Z.; Song, N.; Jin, M.; Xiao, W.; et al. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital of the Chinese People’s Liberation Army. Water Sci. Technol. 2005, 52, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.S.M.; Tomlinson, B.; Cockram, C.S.; Thomas, G.N. Lessons from the severe acute respiratory syndrome outbreak in Hong Kong. Emerg. Infect. Dis. 2003, 9, 1042–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, L.S. The SARS epidemic in Hong Kong: What lessons have we learned? J. R. Soc. Med. 2003, 96, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Chen, F.; Ou, Z.; Fan, Q.; Tan, X.; Wang, Y.; Pan, Y.; Ke, B.; Li, L.; Guan, Y.; et al. A compromised specific humoral immune response against the SARS-CoV-2 receptor-binding domain is related to viral persistence and periodic shedding in the gastrointestinal tract. Cell. Mol. Immunol. 2020, 17, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Ouzounis, C.; Wang, D.; Sun, W.; Li, J.; Chen, W.; Marlière, P.; Danchin, A. A Path toward SARS-CoV-2 Attenuation: Metabolic Pressure on CTP Synthesis Rules the Virus Evolution. Genome Biol. Evol. 2020, 12, 2467–2485. [Google Scholar] [CrossRef]
- Maghool, S.; Maleki-Jirsaraei, N.; Cremonini, M. The coevolution of contagion and behavior with increasing and decreasing awareness. PLoS ONE 2019, 14, e0225447. [Google Scholar] [CrossRef]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Pierson, E.; Koh, P.W.; Gerardin, J.; Redbird, B.; Grusky, D.; Leskovec, J. Mobility network models of COVID-19 explain inequities and inform reopening. Nature 2020. [Google Scholar] [CrossRef]
- Correia, G.; Rodrigues, L.; Gameiro da Silva, M.; Gonçalves, T. Airborne route and bad use of ventilation systems as non-negligible factors in SARS-CoV-2 transmission. Med. Hypotheses 2020, 141, 109781. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans Dynamics and Control; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Murray, J.D. Mathematical Biology: I. An Introduction; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Brauer, F. Some simple epidemic models. Math. Biosci. Eng. 2006, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Arino, J.; Brauer, F.; Driessche, P.v.d.; Watmough, J.; Wu, J. A final size relation for epidemic models. Math. Biosci. Eng. 2007, 4, 159. [Google Scholar] [CrossRef] [PubMed]
- Arino, J.; Brauer, F.; van den Driessche, P.; Watmough, J.; Wu, J. Simple models for containment of a pandemic. J. R. Soc. Interface 2006, 3, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Gumel, A.B.; Ruan, S.; Day, T.; Watmough, J.; Brauer, F.; van den Driessche, P.; Gabrielson, D.; Bowman, C.; Alexander, M.E.; Ardal, S.; et al. Modelling strategies for controlling SARS outbreaks. Proc. R. Soc. Lond. Ser. Biol. Sci. 2004, 271, 2223–2232. [Google Scholar] [CrossRef] [Green Version]
- Longini, I.M.; Halloran, M.E.; Nizam, A.; Yang, Y. Containing Pandemic Influenza with Antiviral Agents. Am. J. Epidemiol. 2004, 159, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Imai, N.; Dorigatti, I.; Cori, A.; Donnelly, C.A.; Riley, S.; Ferguson, N.M. Estimating the Potential Total Number of Novel Coronavirus Cases in Wuhan City, China; Imperial College London: London, UK, 2020. [Google Scholar] [CrossRef]
- Storn, R.; Price, K. Differential Evolution—A Simple and Efficient Heuristic for global Optimization over Continuous Spaces. J. Glob. Optim. 1997, 11, 341–359. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danchin, A.; Ng, T.W.; Turinici, G. A New Transmission Route for the Propagation of the SARS-CoV-2 Coronavirus. Biology 2021, 10, 10. https://doi.org/10.3390/biology10010010
Danchin A, Ng TW, Turinici G. A New Transmission Route for the Propagation of the SARS-CoV-2 Coronavirus. Biology. 2021; 10(1):10. https://doi.org/10.3390/biology10010010
Chicago/Turabian StyleDanchin, Antoine, Tuen Wai Ng, and Gabriel Turinici. 2021. "A New Transmission Route for the Propagation of the SARS-CoV-2 Coronavirus" Biology 10, no. 1: 10. https://doi.org/10.3390/biology10010010
APA StyleDanchin, A., Ng, T. W., & Turinici, G. (2021). A New Transmission Route for the Propagation of the SARS-CoV-2 Coronavirus. Biology, 10(1), 10. https://doi.org/10.3390/biology10010010





