Barriers and Facilitators for Implementation of a Computerized Clinical Decision Support System in Lung Cancer Multidisciplinary Team Meetings—A Qualitative Assessment
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Participants—Sampling and Recruitment
2.3. Data Collection-Methods
2.4. Data Collection-Instrument
2.5. Data Analysis
3. Results
3.1. Demographics
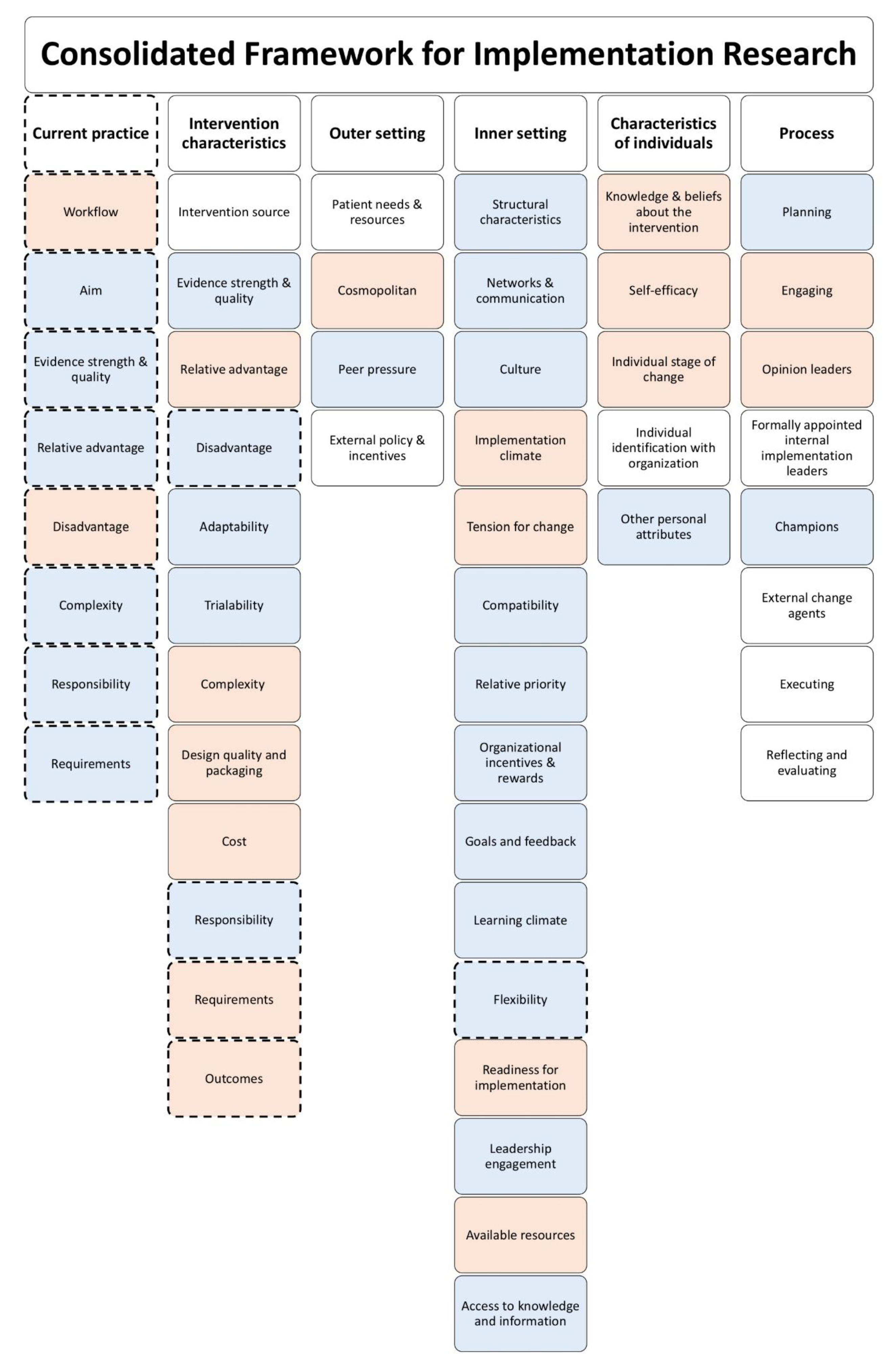
3.2. Identification of Potential Barriers and Facilitators
3.3. Current Practice—MDTMs
3.4. Intervention Characteristics—CCDSS
3.5. Outer Setting
3.6. Inner Setting
3.7. Characteristics of Individuals
3.8. Process
4. Discussion
4.1. Summary of Evidence
4.2. Implication of Results
4.3. Relation to Other Studies
4.4. Strengths and Weaknesses of This Study
4.5. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. Fact Sheet Cancer. 2018. Available online: www.who.int/news-room/fact-sheets/detail/cancer (accessed on 20 May 2020).
- Vrijens, F.; Kohn., L.; Dubois, C.; Leroy, R.; Vinck, I.; Stordeur, S.; De Schutter, H.; Schillemans, V.; Bossens, M.; Vanwaeyenbergh, L.; et al. Ten Years of Multidisciplinary Team Meetings in Oncology: Current Situation and Perspectives; Belgian Health Care Knowledge Centre: Brusells, Belgium, 2015. [Google Scholar]
- Conron, M.; Denton, E. Improving outcomes in lung cancer: The value of the multidisciplinary health care team. J. Multidiscip. Health 2016, 9, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Rosell, L.; Alexandersson, N.; Hagberg, O.; Nilbert, M. Benefits, barriers and opinions on multidisciplinary team meetings: A survey in Swedish cancer care. BMC Health Serv. Res. 2018, 18, 249. [Google Scholar] [CrossRef] [PubMed]
- Sesen, M.B.; Peake, M.D.; Banares-Alcantara, R.; Tse, D.; Kadir, T.; Stanley, R.; Gleeson, F.; Brady, M. Lung Cancer Assistant: A hybrid clinical decision support application for lung cancer care. J. R. Soc. Interface 2014, 11, 20140534. [Google Scholar] [CrossRef]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.M.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat. Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef]
- Hoinville, L.; Taylor, C.; Zasada, M.; Warner, R.; Pottle, E.; Green, J. Improving the effectiveness of cancer multidisciplinary team meetings: Analysis of a national survey of MDT members’ opinions about streamlining patient discussions. BMJ Open Qual. 2019, 8, e000631. [Google Scholar] [CrossRef] [PubMed]
- Pawloski, P.A.; Brooks, G.A.; Nielsen, M.E.; Olson-Bullis, B.A. A Systematic Review of Clinical Decision Support Systems for Clinical Oncology Practice. J. Natl. Compr. Cancer Netw. 2019, 17, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Klarenbeek, S.E.; Weekenstroo, H.H.A.; Sedelaar, J.; Futterer, J.; Prokop, M.; Tummers, M. The Effect of Higher Level Computerized Clinical Decision Support Systems on Oncology Care: A Systematic Review. Cancers 2020, 12, 1032. [Google Scholar] [CrossRef]
- Bright, T.J.; Wong, A.; Dhurjati, R.; Bristow, E.; Bastian, L.; Coeytaux, R.R.; Samsa, G.; Hasselblad, V.; Williams, J.W.; Musty, M.D.; et al. Effect of clinical decision-support systems: A systematic review. Ann. Intern. Med. 2012, 157, 29–43. [Google Scholar] [CrossRef]
- Roshanov, P.S.; Misra, S.; Gerstein, H.C.; Garg, A.X.; Sebaldt, R.J.; Mackay, J.A.; Weise-Kelly, L.; Navarro, T.; Wilczynski, N.L.; Haynes, R.B.; et al. Computerized clinical decision support systems for chronic disease management: A decision-maker-researcher partnership systematic review. Implement. Sci. 2011, 6, 92. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Hernandez, B.; Charani, E.; Castro-Sanchez, E.; Herrero, P.; Hayhoe, B.; Hope, W.; Georgiou, P.; Holmes, A.H. A systematic review of clinical decision support systems for antimicrobial management: Are we failing to investigate these interventions appropriately? Clin. Microbiol. Infect. 2017, 23, 524–532. [Google Scholar] [CrossRef]
- Coiera, E. Clinical decision support systems. In Guide to Health Informatics, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 331–344. [Google Scholar]
- Trinkley, K.E.; Blakeslee, W.W.; Matlock, D.D.; Kao, D.P.; Van Matre, A.G.; Harrison, R.; Larson, C.L.; Kostman, N.; Nelson, J.A.; Lin, C.-T.; et al. Clinician preferences for computerised clinical decision support for medications in primary care: A focus group study. BMJ Health Care Inform. 2019, 26, e000015. [Google Scholar] [CrossRef] [PubMed]
- Khairat, S.; Marc, D.; Crosby, W.; Al Sanousi, A. Reasons For Physicians Not Adopting Clinical Decision Support Systems: Critical Analysis. JMIR Med. Inform. 2018, 6, e24. [Google Scholar] [CrossRef] [PubMed]
- Van De Velde, S.; Heselmans, A.; Delvaux, N.; Brandt, L.; Marco-Ruiz, L.; Spitaels, D.; Cloetens, H.; Kortteisto, T.; Roshanov, P.; Kunnamo, I.; et al. A systematic review of trials evaluating success factors of interventions with computerised clinical decision support. Implement. Sci. 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, W. Kwaliteitscriteria Multidisciplinair Overleg (mdo), Adviesrapport Integraal Kankercentrum Nederland, Versie 1. 2016. Available online: https://www.iknl.nl/getmedia/4dea4687-6c96-42cb-8860-72d1adb0e9f7/Kwaliteitscriteria_multidisciplinair_overleg_2016_IKNL.pdf (accessed on 6 December 2019).
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An overview of clinical decision support systems: Benefits, risks, and strategies for success. NPJ Digit. Med. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Khalifa, M. Clinical Decision Support: Strategies for Success. Procedia Comput. Sci. 2014, 37, 422–427. [Google Scholar] [CrossRef]
- Shahsavarani, A.M.; Abadi, E.A.M.; Kalkhoran, M.H.; Jafari, S.; Qaranli, S. Clinical Decision Support Systems (CDSSs): State of the art Review of Literature. Int. J. Med. Rev. 2015, 2, 299–308. [Google Scholar]
- Verberne, C.J.; Nijboer, C.H.; De Bock, G.H.; Grossmann, I.; Wiggers, T.; Havenga, K. Evaluation of the use of decision-support software in carcino-embryonic antigen (CEA)-based follow-up of patients with colorectal cancer. BMC Med. Inform. Decis. Mak. 2012, 12, 14. [Google Scholar] [CrossRef]
- Castillo, R.S.; Kelemen, A. Considerations for a Successful Clinical Decision Support System. CIN Comput. Inform. Nurs. 2013, 31, 319–326. [Google Scholar] [CrossRef]
- Huryk, L.A. Information systems and decision support systems. Am. J. Nurs. 2012, 112, 62–65. [Google Scholar] [PubMed]
- Evans, E.L.; Whicher, D. What Should Oversight of Clinical Decision Support Systems Look Like? AMA J. Ethics 2018, 20, 857–863. [Google Scholar] [CrossRef][Green Version]
- Lugtenberg, M.; Pasveer, D.; Van Der Weijden, T.; Westert, G.P.; Kool, R.B. Exposure to and experiences with a computerized decision support intervention in primary care: Results from a process evaluation. BMC Fam. Pr. 2015, 16, 141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horlait, M.; Van Belle, S.; Leys, M. Input of Psychosocial Information During Multidisciplinary Team Meetings at Medical Oncology Departments: Protocol for an Observational Study. JMIR Res. Protoc. 2018, 7, e64. [Google Scholar] [CrossRef] [PubMed]
- Senteio, C.R.; Veinot, T.C.; Adler-Milstein, J.; Richardson, C. Physicians’ perceptions of the impact of the EHR on the collection and retrieval of psychosocial information in outpatient diabetes care. Int. J. Med. Inform. 2018, 113, 9–16. [Google Scholar] [CrossRef]
- Leo, F.; Venissac, N.; Poudenx, M.; Otto, J.; Mouroux, J. Multidisciplinary Management of Lung Cancer: How to Test Its Efficacy? J. Thorac. Oncol. 2007, 2, 69–72. [Google Scholar] [CrossRef]
- Storrar, W.; Laws, D. 57 Effectiveness of decision making at lung cancer multidisciplinary team meetings. Lung Cancer 2011, 71, S20. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Jassem, J. Multidisciplinary team care in advanced lung cancer. Transl. Lung Cancer Res. 2020, 9, 1690–1698. [Google Scholar] [CrossRef]
- Pluyter, J.R.; Jacobs, I.; Langereis, S.; Cobben, D.; Williams, S.; Curfs, J.; Borne, B.V.D. Looking through the eyes of the multidisciplinary team: The design and clinical evaluation of a decision support system for lung cancer care. Transl. Lung Cancer Res. 2020, 9, 1422–1432. [Google Scholar] [CrossRef]
- Press, A.L.; Mccullagh, L.; Khan, S.; Schachter, A.; Pardo, S.; McGinn, T.; Fossum, M.; Yuan, M. Usability Testing of a Complex Clinical Decision Support Tool in the Emergency Department: Lessons Learned. JMIR Hum. Factors 2015, 2, e14. [Google Scholar] [CrossRef]
- Kushniruk, A.W.; Borycki, E.M.; Kuwata, S.; Kannry, J. Emerging approaches to usability evaluation of health information systems: Towards in-situ analysis of complex healthcare systems and environments. Stud. Health Technol. Inform. 2011, 169, 915–919. [Google Scholar] [PubMed]
- Borycki, E.M.; Kushniruk, A.W.; Kuwata, S.; Kannry, J. Use of Simulation Approaches in the Study of Clinician Workflow. AMIA Annu. Symp. Proc. 2006, 2006, 61–65. [Google Scholar]
| Stakeholder | Reason for Inclusion |
|---|---|
| Pulmonologist | Pulmonologists subscribe patients with a suspicion of lung cancer for MDTMs and play a central role in the diagnostic trajectory, therapeutic decision-making, and the administration of systemic treatment. Besides, they also chair the MDTM and have a key role in ensuring that this meeting proceeds at an appropriate pace to finish at the scheduled time. |
| Cardiothoracic surgeon | Cardiothoracic surgeons assess resectability of tumors and operability of patients. |
| Radiologist | Radiologists discuss and explain radiological findings and provide input for clinical staging. |
| Radiotherapist | Radiotherapists assess and discuss radiotherapeutic treatment options. |
| Nuclear medicine physician | Nuclear medicine physicians assess and interpret nuclear medicine and hybrid imaging results. |
| Pathologist | Pathologists discuss pathology results, interpret predictive biomarkers, and provide pathological staging of the disease. |
| Specialist nurses | Nurses provide input about the social and psychological condition of the patient, important in therapeutic decision-making. |
| MDTM secretary | Secretaries are involved in planning of the meetings and recording of results. |
| Professional ID | Specialty | Hospital (Academic/General) | Gender (Female/Male) | Age Group (Years) | Seniority (Years) | Frequency of MDTM Visits |
|---|---|---|---|---|---|---|
| 1 | Radiologist in training | Academic | Male | 20–40 | Resident | Non-frequent |
| 2 | Pulmonologist | Academic | Male | 41–60 | 18 | Weekly |
| 3 | Cardiothoracic surgeon | Academic | Male | 41–60 | 10 | Weekly |
| 4 | Radiologist | Academic | Male | 20–40 | 5 | Weekly |
| 5 | Radiologist | Academic | Female | 41–60 | 6 | Weekly |
| 6 | Pulmonologist | Academic | Male | 41–60 | 14 | Weekly |
| 7 | Radiologist | Academic | Male | 41–60 | 11 | Weekly |
| 8 | Nuclear medicine physician | Academic | Male | 20–40 | 4 | Weekly |
| 9 | Pulmonologist | Academic | Female | 41–60 | 15 | Weekly |
| 10 | Radiologist | Academic | Female | 41–60 | 12 | Weekly |
| 11 | Nursing specialist pulmonary oncology | Academic | Female | 20–40 | 20 | Weekly |
| 12 | Radiologist | Academic | Male | 41–60 | 11 | Weekly |
| 13 | Radiation oncologist | Academic | Male | 41–60 | 23 | Weekly |
| 14 | Nursing specialist pulmonary oncology | Academic | Female | 20–40 | 12 | Weekly |
| 15 | Cardiothoracic surgeon | Academic | Male | 41–60 | 23 | Weekly |
| 16 | Secretary MDTM | Academic | Female | 20–40 | 10 | Monthly |
| 17 | Radiologist in training | Academic | Female | 20–40 | Resident | Non-frequent |
| 18 | Pathologist | Academic | Female | 20–40 | 1 | Weekly |
| 19 | Radiologist | Academic | Female | 41–60 | 20 | Weekly |
| 20 | Pulmonologist | Academic | Female | 41–60 | 17 | Weekly |
| 21 | Pathologist | Academic | Male | 41–60 | 30 | Weekly |
| 22 | Pulmonologist | Academic | Male | 20–40 | 9 | Weekly |
| 23 | Pathologist | Academic | Male | 41–60 | 15 | Weekly |
| 24 | Pulmonologist | General | Female | 20–40 | 8 | Weekly |
| 25 | Radiologist | Academic | Female | 41–60 | 10 | Weekly |
| 26 | Radiologist | General | Male | 20–40 | 8 | Weekly |
| Domain and Construct | Potential Barriers | Potential Facilitators |
|---|---|---|
| Current practice | ||
| Workflow | A structured way of working in clinical practice, e.g. use of consistent terminology by all medical specialists and disciplines and storage of information at dedicated locations within the EMR | |
| Disadvantage | Inconsistent storage of relevant patient data at specified locations in the EMR, making it difficult for the CCDSS to recognize and locate all data essential for decision-making | |
| Intervention characteristics | ||
| Relative advantage | Easily accessible and well-structured patient data | |
| Insight into whether data essential for MDTM decision-making is complete or entries are missing | ||
| Complexity | Implementation of an extra clinical system on top of an EMR | Intuitive user-interface |
| Incomplete or incorrect output of the CCDSS | ||
| The CCDSS may expose discrepancies between formal guidelines and contextualized decisions, making professionals legally more vulnerable for criticism from patients | ||
| Design quality and packaging | Poor readability of the data displayed by the CCDSS during MDTMs | |
| Costs | Unfavorable cost-benefit ratio | |
| Outcome | Reduced MDTM preparation time and duration of MDTMs | |
| Outer setting | ||
| Cosmopolitanism | Adaptability to various types of EMR | |
| Inner setting | ||
| Implementation climate | ||
| Tension for change | Needs for change in current MDTMs:
| |
| Readiness for implementation | ||
| Available resources | Limited additional resources and technical facilities during MDTMs | |
| Characteristics of individuals | ||
| Knowledge & beliefs | Dutch national lung cancer guidelines are considered outdated, which makes guideline adherence not important | Prioritize development and implementation of the first component (structured overview of patient variables) because this component was expected to improve MDTM workflows most |
| Lack of trust among professionals in accuracy of system’s algorithms | Perform a usability test and validation of the prototype CCDSS in real-life setting prior to roll-out | |
| Self-efficacy | Willingness of professionals to change current workflows in order to benefit from CCDSS | |
| Process | ||
| Engaging | Provide insight into purpose and potential benefits of the CCDSS | |
| Involve end users and external partners during development and implementation | ||
| Create opportunities for users to familiarize themselves with the CCDSS | ||
| Opinion leaders | Lack of acceptance by key opinion leaders | Institute pulmonologists as superusers who lead implementation |
| Engage management to arrange internal and external financial and contractual agreements |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klarenbeek, S.E.; Schuurbiers-Siebers, O.C.J.; van den Heuvel, M.M.; Prokop, M.; Tummers, M. Barriers and Facilitators for Implementation of a Computerized Clinical Decision Support System in Lung Cancer Multidisciplinary Team Meetings—A Qualitative Assessment. Biology 2021, 10, 9. https://doi.org/10.3390/biology10010009
Klarenbeek SE, Schuurbiers-Siebers OCJ, van den Heuvel MM, Prokop M, Tummers M. Barriers and Facilitators for Implementation of a Computerized Clinical Decision Support System in Lung Cancer Multidisciplinary Team Meetings—A Qualitative Assessment. Biology. 2021; 10(1):9. https://doi.org/10.3390/biology10010009
Chicago/Turabian StyleKlarenbeek, Sosse E., Olga C. J. Schuurbiers-Siebers, Michel M. van den Heuvel, Mathias Prokop, and Marcia Tummers. 2021. "Barriers and Facilitators for Implementation of a Computerized Clinical Decision Support System in Lung Cancer Multidisciplinary Team Meetings—A Qualitative Assessment" Biology 10, no. 1: 9. https://doi.org/10.3390/biology10010009
APA StyleKlarenbeek, S. E., Schuurbiers-Siebers, O. C. J., van den Heuvel, M. M., Prokop, M., & Tummers, M. (2021). Barriers and Facilitators for Implementation of a Computerized Clinical Decision Support System in Lung Cancer Multidisciplinary Team Meetings—A Qualitative Assessment. Biology, 10(1), 9. https://doi.org/10.3390/biology10010009





