Abstract
How have animals evolved new body designs (morphological evolution)? This requires explanations both for simple morphological changes, such as differences in pigmentation and hair patterns between different Drosophila populations and species, and also for more complex changes, such as differences in the forelimbs of mice and bats, and the necks of amphibians and reptiles. The genetic changes and pathways involved in these evolutionary steps require identification. Many, though not all, of these events occur by changes in cis-regulatory (enhancer) elements within developmental genes. Enhancers are modular, each affecting expression in only one or a few tissues. Therefore it is possible to add, remove or alter an enhancer without producing changes in multiple tissues, and thereby avoid widespread (pleiotropic) deleterious effects. Ideally, for a given step in morphological evolution it is necessary to identify (i) the change in phenotype, (ii) the changes in gene expression, (iii) the DNA region, enhancer or otherwise, affected, (iv) the mutation involved, (v) the nature of the transcription or other factors that bind to this site. In practice these data are incomplete for most of the published studies upon morphological evolution. Here, the investigations are categorized according to how far these analyses have proceeded.
1. Introduction
Changes in the shape or pigmentation patterns of the body provide the most dramatic examples of morphological evolution, and these commonly occur by mutations in developmental genes. The body plan of the adult is preceded by a pre-plan of developmental gene expression in the embryo. These genes are expressed in precise patterns in time and space, and it is these that instruct the cells on how they must develop. Developmental genes code, primarily, for transcription factors and signalling components, and each of these is typically deployed at multiple tissues and times in order to specify a wide range of different developmental events. Developmental genes therefore exert pleiotropic (multiple and seemingly unrelated) effects during embryonic development [1].
Temporo-spatial variety in the expression of a developmental gene is typically mediated by variety in the range of tissue-specific cis-regulatory elements (CREs) located around the coding sequence. These include enhancers and silencers of gene expression. Regulation is therefore modular, and mutational changes within any individual CRE can affect expression in one, or a subset of, tissue(s) without affecting expression at others. This may allow evolutionary change in the morphology of part of an animal without producing multiple (pleiotropic), and likely deleterious, effects elsewhere in the body [2,3,4].
The purpose of this article is to review and categorize examples of how mutation within CREs may cause morphological evolution. It is important to stress, however, that this is not the only way in which morphological evolution can occur. In particular, changes in the coding sequence of, for example, Ubx and Antp Hox genes have modified the target specificity of their encoded transcription factor proteins. This has mediated evolutionary changes in the arrangement of arthropod legs [5,6,7]. Thus, the Hox proteins are themselves modular, with an alteration in protein sequence affecting function in one tissue but not others [8]. The argument that mutations in CREs are less likely to produce pleiotropic effects than mutations in coding sequence [2,3,4], while likely valid, should not overshadow the significance of these alternative routes to the avoidance of adverse pleiotropic effects [9,10,11]. It seems likely that evolution will exploit a multiplicity of different mechanisms in order to effect morphological change [12].
In attempting to identify the mutational events that underlie a phenotypic change, there is great benefit in choosing a species where transgenic animals can be produced, permitting analysis of the effects of enhancer mutations upon both reporter gene expression and phenotypic rescue. The importance of these techniques is apparent in the examples described below.
2. Examples of Changes in Cis-regulatory Elements during Morphological Evolution
Here, we categorize the investigations according to how far they have proceeded to relate phenotypic change, CRE identification, CRE mutations, and the resultant gain or loss of functional transcription factor binding sites. The model systems described may in future change their categories as additional data become available.
2.1. Evolutionary Change Recognized as Change in a Cis-regulatory Element of a Developmental Gene
2.1.1. Stickleback Pelvic Fin Loss
Threespine sticklebacks usually possess a robust pelvic apparatus with attached pelvic spine. However, among those living in freshwater, there has been repeated evolution of pelvic-reduced populations, which lack pelvic spines, and which may thereby become less susceptible to low calcium and to predatory arthropods which grasp the spines (Figure 1) [13]. Chan et al. noted that these fish have pelvis-specific depletion in their expression of Pitx1 [13], a gene expressed in hindlimbs but not forelimbs of many different vertebrates. These authors found that an upstream pelvis CRE is commonly deleted within the Pitx1 gene of pelvic-reduced fish populations. They showed that a 2.5 kilobase DNA fragment including this region from normal fish could, when incorporated in a Pitx1 transgene, be used to rescue normal pelvic structures in pelvic-reduced fish (Figure 1). It appeared that the Pitx1 enhancer may be particularly sensitive to loss due to its location in a fragile, flexible, sub-telomeric region, and that deletions in the pelvic enhancer region are subject to positive selection in multiple natural populations [13]. Change of Pitx1 activity may also account for pelvic reduction in a mammal, the manatee [14].

Figure 1.
Stickleback populations have evolved loss of pelvic fins by loss of a Pitx1 pelvis cis-regulatory element. Pelvic fin-negative populations (b,d) may be restored to an ancestral-like condition (a,c) (Tg1, Tg2) by expression of a Pitx1 transgene that contains 2.5 kilobases of 5′ flanking region taken from a pelvic fin-positive population linked to Pitx1 coding sequence from a pelvic-reduced population. Arrows in a and b show pelvic fin position. In c and d the pelvic apparatus is shown stained with alizarin red. Reprinted with permission from [13], Copyright 2010, AAAS, and D. Kingsley.
2.1.2. Forelimb Length in Bat and Mouse
Bats have forelimb skeletal elements proportionally longer than mice, and this difference develops mainly in late gestation. The ratio of forelimb length to crown rump length is similar for both animals at early stages of limb development but, by adult, becomes about 1:3 for mouse and 2:1 for bat (Carollia perspicillata) [15]. Cretekos et al. noted that the Prx1 gene, expressed in the embryonic forelimb, promotes forelimb bone elongation, and Prx1-null homozygous mice have forelimbs 12.5% shorter than controls at 18.5 days [15]. In particular, these authors found that mice in which an upstream 1 kilobase CRE region of the Prx1 gene is replaced with the orthologous bat sequence have forelimbs that are about 6% lengthened at late gestation, suggesting that mutational changes in the CRE may have been important in the evolution of the bat wing.
2.1.3. Vertebral Formulae of Mouse and Chicken
Mouse and chicken differ in their vertebral formulae (relative numbers of cervical, thoracic, lumbar and sacral vertebrae). In particular, the chicken has more neck vertebrae and fewer thoracic than mouse. This is due to evolutionary shifts (transpositions) of Hox gene expression boundaries along the axial series of somites [16,17]. Boundaries within neural tissue are correspondingly transposed [18]. Hoxc8 and Hoxa7 are two genes whose endogenous expression boundaries in neural and somitic tissues are shifted posteriorly in chicken relative to mouse.
Belting et al. compared mouse and chicken Hoxc8 CREs in their ability to direct expression of a lacZ reporter gene in transgenic mice [19]. They showed that the mouse 5′-located ‘early enhancer’ CRE directs anterior boundaries of lacZ expression in somitic and neural tissues at positions about four segments more anterior than those directed by the orthologous chicken CRE. These authors concluded that evolutionary transposition of Hoxc8 expression is due to changes in its CRE. The Hoxc8 enhancers from a variety of fish and other vertebrates display a range of mutational changes within putative transcription factor binding sites [19,20,21,22]. Some, though not all, of these provide novel lacZ expression boundaries when tested in the transgenic mouse reporter assay. These additional studies show further that there have been mutational changes within a Hoxc8 CRE during vertebrate evolution, and that these are associated with functional changes in the lacZ reporter assay within transgenic mice.
It might be expected that these findings are reproducible for other Hox genes that show transpositions in their expressions in mouse relative to chicken. However, mouse and chicken Hoxa7 CREs do not differ significantly in the anterior boundaries of neural lacZ expression that they generate in the transgenic mouse reporter assay [18]. This suggests that changes in CREs are unlikely to have been responsible for this difference in Hoxa7 expression in mouse versus chicken. Instead, change in trans-acting factors seems more likely.
2.1.4. Loss of Vibrissae and Penile Spines in Human
McLean et al. used database analysis to identify more than 500 regions of high sequence conservation deleted in humans (hCONDELs) but present in chimp and macaque [23]. One of these flanks the androgen receptor (AR) locus. This region includes a highly conserved CRE which, taken from chimp or mouse, directs lacZ expression in transgenic mouse embryos to two androgen responsive sites: facial vibrissae and the genital tubercle [23]. Mice with a mutation in the AR coding sequence do not have penile spines, structures present in many mammals and thought to increase tactile stimulation. Castrated primates lose their penile spines and castrated mice have shortened facial vibrissae. Both effects are reversed by testosterone administration. Humans do not have sensory vibrissae or penile spines, and this is likely due to evolutionary loss of the AR vibrissae and penile spine enhancer [23]. Strictly speaking, verification requires rescue of this phenotype in human, as performed for pelvic spines in fish, though ethics preclude this.
2.3. Evolutionary Change Understood as Acquisition, Loss, or Changed Efficiency of Defined Functional Motifs within Cis-regulatory Elements
2.3.1. Male-specific Wing Spots in Drosophila biarmipes
The yellow gene, required for production of black pigment, is expressed uniformly at low levels throughout the D. melanogaster wing, but is also expressed intensely to produce a distal anterior wing spot in D. biarmipes, a closely related species (Figure 5). Gompel et al. showed that an upstream region, the so-called ‘wing element’, regulates yellow gene expression [32]. These authors found that GFP reporter transgenes driven by the D. biarmipes wing element are expressed in D. melanogaster with a pattern rather similar to that of the wing spot in D. biarmipes. They showed that separate sequences in the wing element of D. biarmipes regulate (i) general wing expression, (ii) wing spot. Deletion and mutation studies upon the spot element showed that it contained an activator element (which promotes expression in both anterior and posterior parts of the wing) and repressor elements (which exclude the spot from the posterior wing compartment). These repressor elements, present in D. biarmipes but not D. melanogaster, have gained binding sites for the transcription factor Engrailed, which confine the spot to the anterior compartment. Therefore Engrailed has been co-opted to pattern the location of the wing spot. Gompel et al. suggest that, as a general mechanism for generating wing patterns, random mutation of ancestral CREs (including point and insertional mutations) generate transcription factor binding sites to modify wing pigment patterns by co-opting from the many transcription factors already present in spatially distinct patterns within the wing [32]. This is proposed not only as a mechanism for wing patterns but more importantly as a general mechanism to evolve novel patterns of gene expression for diverse traits in diverse organisms [32].
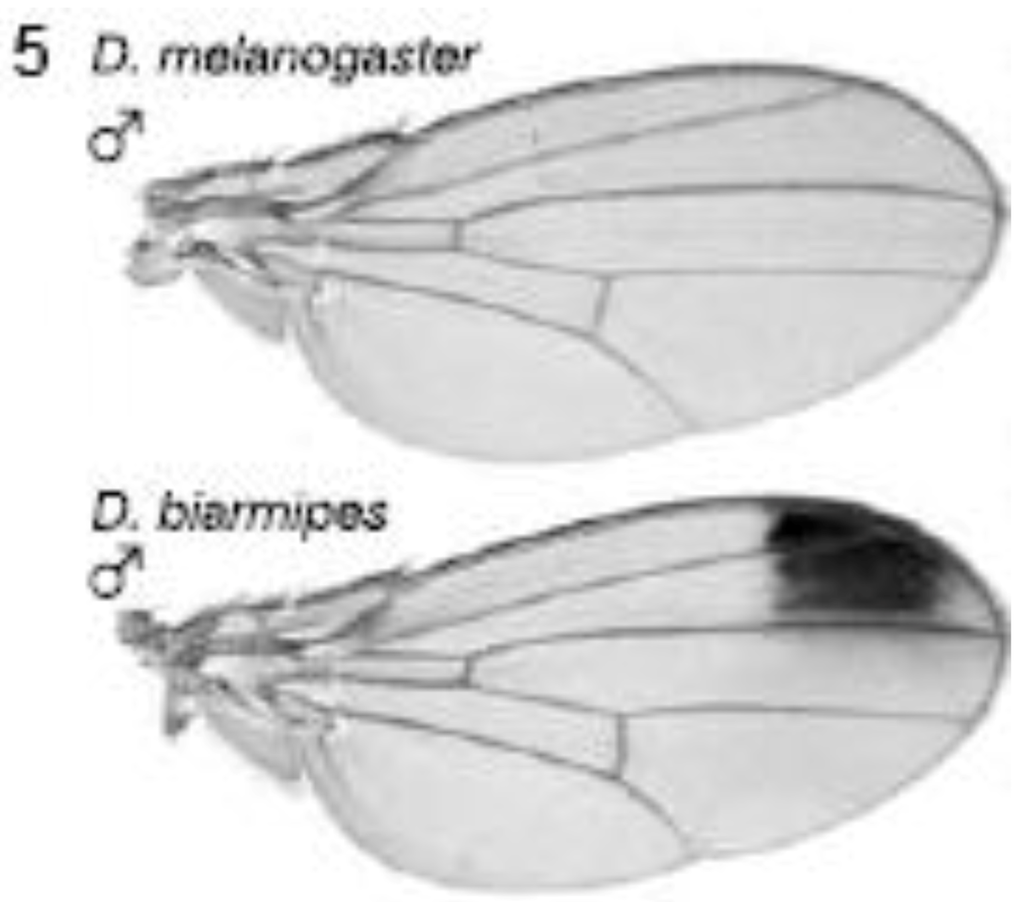
Figure 5.
Evolution of male-specific wing spot in Drosophila biarmipes involved acquisition of new Engrailed binding motifs in the yellow gene cis-regulatory element which limit expression to the anterior compartment of the wing. Reprinted with permission from [32], Copyright 2005, Macmillan Publishers Ltd.
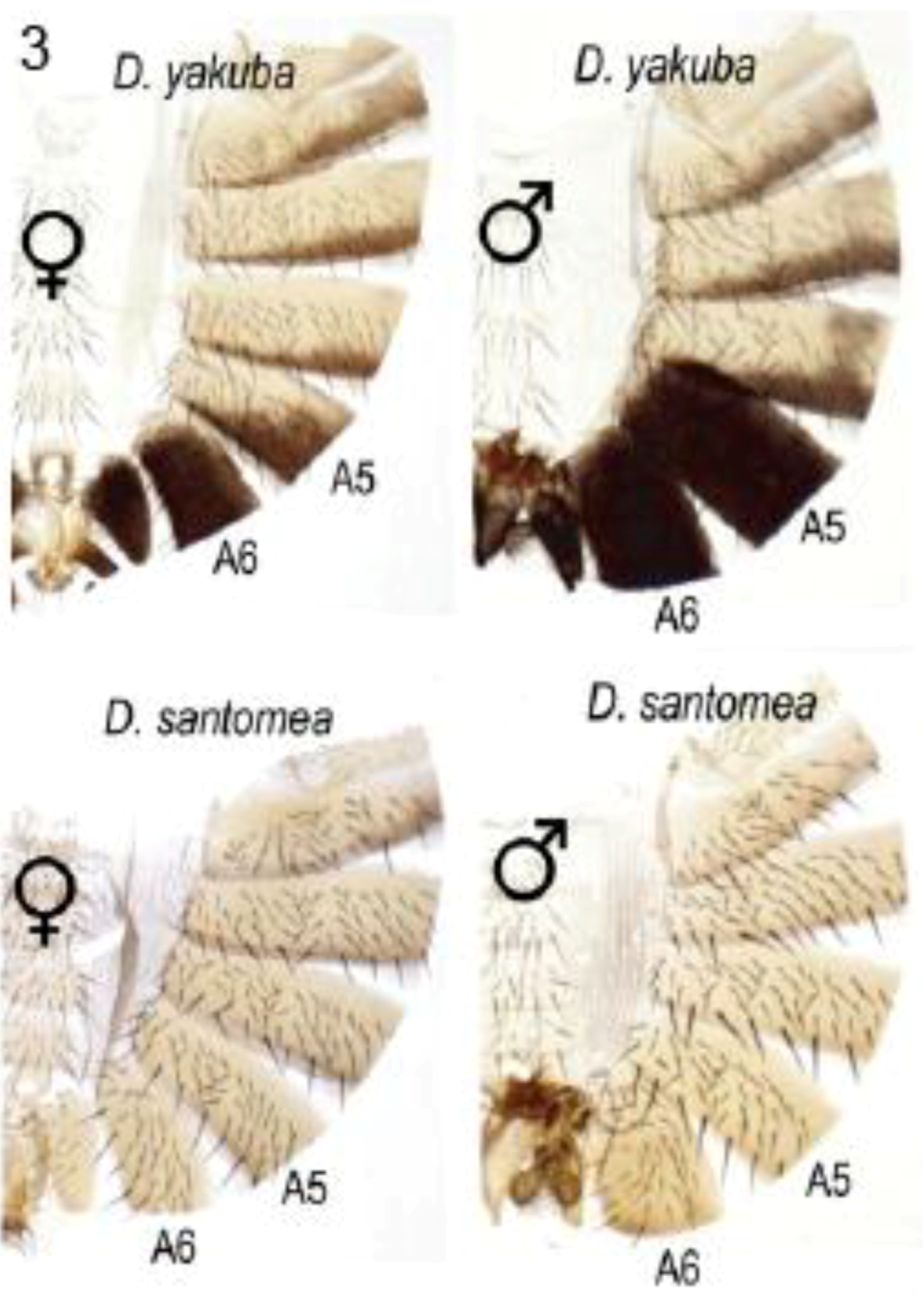
2.3.2. Male-specific Abdominal Pigmentation Differences between Drosophila melanogaster and D. willistoni
As already indicated (section 2.2.2), male abdominal-specific pigmentation is under the control of the Abd-B gene. Abd-B protein directly activates yellow to promote melanin formation and pigmentation in abdominal segments A5 and A6 of males.In females, bric à brac (bab), represses this pigmentation. The bab1 gene is regulated by Abd-B and sexually dimorphic doublesex (dsx) proteins which bind to a bab1 intron CRE [29]. The Abd-B/bab/dsx pathway forms a sexually dimorphic switch which was already present in the common ancestor of D. melanogaster and D. willistoni. However, the switch was subsequently utilized to regulate male specific abdominal pigmentation in the D. melanogaster lineage [26]. Williams et al. showed that D. willistoni and D. melanogaster differ in their male-specific pattern (Figure 6) due to changes in the effects of Abd-B and dsx proteins upon the bab1 intron CRE [26]. They examined a variety of GFP reporter transgenes driven by the bab1 intron CRE to show that this is not simply due to change in the number of binding motifs, but also to modification of their overall efficiency by change in the polarity and spacing of existing sites. They describe this as ‘molecular remodelling’ of a pre-existing Abd-B- and dsx-regulated CRE.

Figure 6.
Male-specific pigmentation in Drosophila melanogaster and D. willistoni differ by ‘molecular remodelling’ of Abd-B and dsx binding motifs within the intron cis-regulatory element of the bric à brac gene, bab1. Reprinted with permission from [26], Copyright 2008, Elsevier.
2.3.3. Abdominal Pigmentation Differences between Drosophila melanogaster and D. kikkawai Species
As already indicated above, male abdominal-specific pigmentation is under the control of the Abd-B gene. Jeong et al. showed that Abd-B protein activates yellow expression and pigmentation by direct interaction with specific binding motifs within the 5′-located CRE in the yellow gene [29]. They found that GFP reporter transgenes driven by the yellow CRE are only expressed in the male-specific pattern if they include these Abd-B binding sites. In a separate mechanism from that described in sections 2.2.2 and 2.3.2, male-specific pigmentation in D. kikkawai has been lost by mutations which include loss of the Abd-B binding motifs within the yellow gene CRE [29].
2.4. Phylogenetic Modification/Appearance/Loss of a Cis-regulatory Element which is of Suggestive but Unproven Function
2.4.1. Human Specific Mutations within a Limb Cis-regulatory Element
Prabhakar et al. showed that a rapidly evolving non-coding DNA element in human (human accelerated conserved noncoding sequence 1, HACNS1) functions as a transcriptional enhancer in mouse embryos, mediating strong expression of a lacZ reporter in the limbuds [27]. Expression is strong in the distal forelimb. In contrast, orthologous DNA elements from chimp and rhesus monkey drive only weak expression in the proximal limb. The authors propose that gain of function in HACNS1 may have influenced the evolution of human limb features, such as hand dexterity and bipedalism, by altering the expression of nearby genes during limb development. Thirteen base substitutions over an 81 base region accounted for the functional difference between chimp and human elements. More recently, it has been suggested that the changes in human HACNS1 have disrupted a repressor function rather than activated a new enhancer [33].
2.4.2. Sox Response Elements in Fezf2, a Determinant of Corticospinal Neuron Identity
Fezf2 encodes a zinc-finger transcription factor required for molecular specification within layers 5 and 6 of the mammalian neocortex and its corticospinal output. Shim et al. showed that Fezf2 expression is driven by a CRE, named E4 [34]. Mice deleted for E4 showed down-regulation of Fezf2 expression in the neocortex and anatomical changes as earlier reported in Fezf2-deficient mice. Transfection experiments in cultured cells using luciferase reporters driven by E4 showed that it contained at least one functional Sox binding motif. This Sox binding element apparently arose within the E4 CRE at the junction between fish and tetrapods. The authors propose that this event may have contributed to the evolution of the neocortex in mammals and at least some other amniotes [34].
2.4.3. Retinoic Acid Response Elements in Two Cis-regulatory Elements of the Vertebrate Homeobox Gene, Cdx1
Cdx1 expression in mice utilizes both upstream and intron CREs [35,36]. Homeotic genes including Cdx1 and several anteriorly-expressed Hox genes are directly responsive to retinoic acid (RA), and contain retinoic acid response elements (RAREs) in their CREs. RA is a morphogen in chordates, though not in pre-chordates [37]. In cell culture assays upon transfected Cdx1 reporter constructs, Gaunt and Paul found that both intron and upstream RAREs are functional in mouse DNA, yet only the intron RARE is functional in chicken [38]. Database analysis indicated that the intron RARE first appeared at the transition from amphibians to amniotes (reptiles, birds and mammals), while the upstream enhancer RARE first appeared at the transition from marsupial to eutherian mammals (Table 1) [38].
There is no direct evidence linking acquisition of Cdx1 RAREs to morphological evolution of vertebrate groups, but these authors noted that the main sites of action of Cdx1 were also important sites of distinction between vertebrate types. Cdx1 regulates homeotic patterning of the neck vertebrae in mice, up to and including the atlas/skull level, probably by its effect upon Hox genes [39]. Like most fish, extant amphibian species differ from amniotes in lacking a distinct neck (region between the skull and pectoral girdle). These amphibians possess only one neck vertebra (atlas) and lack the rotation-permitting atlas-axis joint. Fossil evidence indicates that the latter first arose at around the amphibian/amniote split [40,41]. Acquisition of the Cdx1 intron RARE at around this time might therefore have contributed to evolution of the vertebrate neck, including ability to lift and rotate the head in early tetrapods [38]. Cdx1 also regulates patterning of the ureters and Müllerian ducts (precursors to the uterus). Marsupials differ from eutherian mammals in the layout of the ureters and Müllerian ducts. Acquisition of the Cdx1 upstream RARE at the marsupial/eutherian split may therefore have facilitated morphological evolution of the eutherian reproductive tract [38]. These proposed effects of Cdx1 upon morphological evolution remain hypotheses, awaiting experimental verification.

Table 1.
Retinoic acid response elements (RAREs) in vertebrate Cdx1 and Hox gene cis-regulatory elements. Black ticks indicate RAREs described in the literature. Red ticks indicate RAREs identified from our own database analysis (supplementary Figure 1). DR2 and DR5 indicate RAREs where the paired 6-base binding motifs are separated by either 2 or 5 spacer nucleotides, respectively, and these are located either upstream (5′) or downstream (3′) of the coding region. In general, most Hox RAREs are found to be conserved from fish to mammals, although we note that (i) some species have apparently lost a RARE even though it was present in their ancestry, (ii) the fish species examined do not have an obvious Hoxa1 RARE homologue. It may be that this was lost subsequent to genome duplication in ray finned fish [42] since, as shown in zebrafish, Hoxa1 function for development of the hindbrain has become adopted by Hoxb1b [43,44]. *, likely Hoxa1a of zebrafish [45]. This 3′ DR5 may not be homologous with that in the other vertebrates shown since it is of opposite sense. **, likely Hoxb1a of zebrafish [45]. B23-biology-01-00557, likely Hoxd4a of zebrafish [46].
| Fish | Amphibian | Lizard | Bird | Marsupial | Eutherian | References | |
|---|---|---|---|---|---|---|---|
| Cdx1 upstream | ✓ | [47], [38] | |||||
| Cdx1 intron | ✓ | ✓ | ✓ | ✓ | [38] | ||
| Hoxa1 (3’ DR5) | ?* |  |  |  | ✓ | [45] | |
| Hoxa4 (5’ DR5) | ✓ |  |  |  |  | ✓ | [48] |
| Hoxb1 (3’ DR2) | ✓ |  |  | ✓ | [49] | ||
| Hoxb1 (3’ DR5) | ✓** |  | ✓ |  | ✓ | [50], [45] | |
| Hoxb1 (5’ DR2) | ✓ | ✓ | [51] | ||||
| Hoxb4 (3’ DR5) | ✓ |  |  |  | ✓ | [52] | |
| Hoxb5 (3’ DR5) |  |  |  | ✓ | [53] | ||
| Hoxd4 (5’ DR5) | ✓*** |  |  |  |  | ✓ | [46] |
| Hoxd4 (3’ DR5) | ✓*** |  |  |  |  | ✓ | [46] |
2.4.4. Human-specific Loss of a CRE in the GADD45G Tumour Suppressor Gene
McLean et al. described a conserved region deleted in human (hCONDEL) from a position next to the tumour suppressor gene GADD45G [23]. When taken from chimp or mouse, this DNA region functions as an enhancer to drive lacZ expression in subventricular zones of transgenic mouse embryo forebrain. It contains forebrain-specific p300 transcription factor binding sites. GADD45G normally suppresses the cell cycle. The authors propose that this deletion provides a plausible molecular basis for increased production of particular neuronal cell types in subventricular zone regions, a suggested requirement in evolutionary expansion of the neocortex in primates [23].
3. Discussion
3.1. Modification of Cis-regulatory Elements Revealed as a Mechanism Used in Morphological Evolution
Experiments reviewed above show how CREs have become modified in order to permit new patterns of gene expression and new morphologies [54]. This occurred by acquisition of new transcription factor binding sites (section 2.3.1), loss of transcription factor sites (section 2.3.2), molecular remodelling of sites (section 2.3.3), acquisition of multiple point mutations (sections 2.2.1, 2.2.2, 2.2.3), or loss of the entire region (section 2.1.1, 2.1.4). It is suggested that loss of the entire CRE, as opposed to multiple point mutations, may contribute to morphological evolution only rarely because many single CRE ‘modules’ do in fact drive expression in multiple domains [9], and their loss will therefore produce pleiotropic effects [28]. The studies identify more examples of evolutionary loss, rather than gain, of CRE function. This likely indicates that gain of function occurs more rarely [3].
Of the above studies, those on Drosophila in particular were facilitated by comparing closely related populations differing by relatively simple morphologies, such that significant mutations were less likely to be masked by secondary genetic changes [55]. A limitation with this approach, however, is that we often wish to compare species more distantly related and with widely divergent morphologies.
3.2. A Problem in Comparing Cis-regulatory Elements of Distantly Related Species
The studies on evolutionary transposition of Hoxc8 and Hoxa7 expression boundaries (section 2.1.3) reached different conclusions about the likely role of changes in CREs. The experiments were based upon comparing chicken and mouse regulatory regions as drivers of lacZ reporter expression in transgenic mice. The contrasting results may indeed indicate a true difference between the evolutionary histories of the two genes being studied. However, the experiments reported do not rule out problems in cross species reporter assays.
This was recognized by Morrison et al. in their studies upon Hoxb4 [56]. Endogenous Hoxb4 is expressed in somitic mesoderm to a similar anterior boundary (the presumptive axis at the level of somite 7) in both mouse and chicken [56,57]. In somitic mesoderm of transgenic mice, a lacZ reporter transgene driven by a mouse Hoxb4 CRE is expressed to this level [57], whereas a lacZ reporter driven by the corresponding chicken CRE has a boundary of expression that is more posterior by several segments [56]. CREs typically bind several different transcription factors and co-factors. As suggested by Morrison et al. it is not clear that these factors in mouse can function optimally on chicken DNA sequences since binding sites and their cognate factors may have evolved in concert within each species [56]. An effect observed in cross-species reporter assays might therefore reflect this general incompatibility rather than a specific regulatory mutation that has been important in evolution of axial morphology. This potential problem likely increases with evolutionary distance in time. Chickens and mice are separated by ~300Myr [58]; bats and mice by ~90Myr [15]. The problem with the assay of chicken CREs in mice might be alleviated if it were possible to perform the reciprocal experiment in transgenic chickens. Expression of lacZ reporter transgenes driven by mouse Hoxc8 CRE should then be located anterior to that of endogenous chicken Hoxc8.
3.3. Evolutionarily Origins of Cis-regulatory Elements Identified in Database Searches
We have reviewed, in section 2.4, studies in which CREs have been identified functionally in the mouse, and then their evolutionary origins inferred from analyses upon DNA sequence databases. New species are rapidly being added to the databases, and so it seems likely that, as for Cdx1 and Fezf2 (sections 2.4.3 and 2.4.2), many other genes will soon be found to have acquired enhancers at points of morphological evolution, and that these will provide clues about the underlying mechanisms. Newly acquired enhancers within the Hox genes would be of obvious interest with regard to morphological evolution, and we have considered this with respect to RAREs. Like Cdx1, Hox genes of paralogous groups 1 to 5 contain RARE motifs and are functional in patterning of the vertebrate neck. Table 1 summarizes findings from both the literature and our own database survey (supplementary Figure 1). For most Hox genes it is clear that RARE motifs are conserved from fish to mammals and, unlike in Cdx1, they do not originate at intermediate phylogenetic stages. Cdx1 may therefore be special in that its RAREs may have played roles in vertebrate morphological evolution [38]. Database studies have also been used to identify conserved DNA regions that have become deleted during human evolution [23]. Over 500 such deletions occur in non-coding regions, especially near genes involved in steroid hormone signalling and neural function. So far, two of these have been shown to be in CREs likely to be involved in morphological evolution (sections 2.1.4. and 2.4.4.) [23].
3.4. Problems in Elucidating the Evolutionary Basis of Widely Divergent Morphologies
For distantly-related species showing widely divergent morphologies there are substantial challenges in attempts to assess the evolutionary significance of changes in CREs. Examples discussed above include evolution of the bat wing, the human brain, the amniote neck, and the eutherian reproductive tract. It is likely that evolution proceeds through multiple small steps, or ‘successive slight modifications’ [59]. Reversal of any one of these modifying mutations may not substantially reverse the phenotype due to the multiplicity of genes involved, and because individual gene functions may be redundant due to acquisition of overlapping functions by related genes.
Replacement of the mouse Prx1 enhancer with that of the bat does not result in a mouse with bat-like wings but it does produce a slight lengthening of the forelimbs [15]. This is a valuable indication of at least one component part in the evolution of the bat wing. With regard to Cdx1, mutation of the upstream RARE in mice has not been reported to have an effect upon the female reproductive tract [36]. The intron RARE has not yet been mutated in mice, but it might be surprising if this allowed ‘reverse engineering’ of a minimal, amphibian-like neck since complete loss of Cdx1 activity does not produce this effect [60]. Cdx1 knockout mice have shown detachment of the odontoid peg from the axis [60] but it is unclear whether this relates to a similar pattern seen in fossils as a transitional state during the evolution of the amniote atlas-axis joint [41]. Overlapping gene function is a further problem in analysis of Cdx1 function. For example, Cdx2 plays similar roles to Cdx1 in patterning the mouse neck, though only as far forward as the axis/atlas joint [39], and also the female urogenital system [38]. An alternative analytical approach might lie in attempting to genetically manipulate amphibians by adding gene functions known to be involved in development of the amniote neck. For example, it may be possible to induce multiple pre-thoracic vertebrae (a neck) in this way.
4. Conclusions
There have been many successful attempts over recent years to identify changes in CREs that underlie morphological evolution. There has been great benefit in choosing species or populations which (i) are closely related, so that mutations are less likely to be obscured by secondary genetic changes, (ii) differ by relatively simple morphologies, and (iii) are amenable to transgenesis, to permit analysis of mutation effects upon gene expression and function. In particular, comparison of Drosophila melanogaster populations and different Drosophila species has provided clear links between phenotype and genotype, and provided important illustration of the mechanistic principles of morphological evolution. Comparison of distantly related species with widely divergent morphologies is more challenging, yet the rapidly increasing number of species genomes sequenced means that CREs identified in one species can readily be traced in their phylogenetic origins. Although this can provide interesting clues about how CRE evolution may have changed phenotype, there are substantial problems in verification. Three difficulties discussed are (i) problems in cross-species enhancer assays, (ii) the multi-step nature of evolution and the multiplicity of genes that may be involved in complex traits, (iii) masking effects of overlapping gene functions. Although it is uncertain how we shall finally unravel the genetic changes involved in the transition between major animal groups, the identification of newly evolved CREs will provide us with many clues.
References and Notes
- Carroll, S.B.; Grenier, J.K.; Weatherbee, S.D. From DNA to diversity: Molecular Genetics and the Evolution of Animal Design, 2nd; Sean, B.C., Jennifer, K.G., Eds.; Blackwell Science Press: Madison, WI, USA, 2005. [Google Scholar]
- Carroll, S.B. Evolution at two levels: On genes and form. PloS Biol. 2005. [Google Scholar] [CrossRef]
- Prud'homme, B.; Gompel, N.; Carroll, S.B. Emerging principles of regulatory evolution. Proc. Natl. Acad. Sci. USA 2007, 104, 8605–8612. [Google Scholar]
- Wray, G.A.; Hahn, M.W.; Abouheif, E.; Balhoff, J.P.; Pizer, M.; Rockman, M.V.; Romano, L.A. The evolution of transcriptional regulation in eukaryotes. Mol. Biol. Evol. 2003, 20, 1377–1419. [Google Scholar] [CrossRef]
- Galant, R.; Carroll, S.B. Evolution of a transcriptional repression domain in an insect hox protein. Nature 2002, 415, 910–913. [Google Scholar] [CrossRef]
- Ronshaugen, M.; McGinnis, N.; McGinnis, W. Hox protein mutation and macroevolution of the insect body plan. Nature 2002, 415, 914–917. [Google Scholar] [CrossRef]
- Shiga, Y.; Yasumoto, R.; Yamagata, H.; Hayashi, S. Evolving role of antennapedia protein in arthropod limb patterning. Development 2002, 129, 3555–3561. [Google Scholar]
- Lynch, V.J.; Wagner, G.P. Resurrecting the role of transcription factor change in developmental evolution. Evolution 2008, 62, 2131–2154. [Google Scholar] [CrossRef]
- Monteiro, A.; Podlaha, O. Wings, horns, and butterfly eyespots: How do complex traits evolve? Plos Biol. 2009. [Google Scholar] [CrossRef]
- Hoekstra, H.E.; Coyne, J.A. The locus of evolution: Evo devo and the genetics of adaptation. Evolution 2007, 61, 995–1016. [Google Scholar] [CrossRef]
- Pennisi, E. Deciphering the genetics of evolution. Science 2008, 321, 760–763. [Google Scholar] [CrossRef]
- Pick, L.; Heffer, A. Hox gene evolution: Multiple mechanisms contributing to evolutionary novelties. Ann. NY Acad. Sci. 2012, 1256, 15–32. [Google Scholar]
- Chan, Y.F.; Marks, M.E.; Jones, F.C.; Villarreal, G., Jr.; Shapiro, M.D.; Brady, S.D.; Southwick, A.M.; Absher, D.M.; Grimwood, J.; Schmutz, J.; et al. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a pitx1 enhancer. Science 2010, 327, 302–305. [Google Scholar]
- Shapiro, M.D.; Bell, M.A.; Kingsley, D.M. Parallel genetic origins of pelvic reduction in vertebrates. Proc. Natl. Acad. Sci. USA 2006, 103, 13753–13758. [Google Scholar]
- Cretekos, C.J.; Wang, Y.; Green, E.D.; Martin, J.F.; Rasweiler, J.J.T.; Behringer, R.R. Regulatory divergence modifies limb length between mammals. Genes Dev. 2008, 22, 141–151. [Google Scholar] [CrossRef]
- Gaunt, S.J. Conservation in the hox code during morphological evolution. Int. J. Dev. Biol. 1994, 38, 549–552. [Google Scholar]
- Burke, A.C.; Nelson, C.E.; Morgan, B.A.; Tabin, C. Hox genes and the evolution of vertebrate axial morphology. Development 1995, 121, 333–346. [Google Scholar]
- Gaunt, S.J.; Dean, W.; Sang, H.; Burton, R.D. Evidence that hoxa expression domains are evolutionarily transposed in spinal ganglia, and are established by forward spreading in paraxial mesoderm. Mech. Develop. 1999, 82, 109–118. [Google Scholar] [CrossRef]
- Belting, H.G.; Shashikant, C.S.; Ruddle, F.H. Modification of expression and cis-regulation of hoxc8 in the evolution of diverged axial morphology. Proc. Natl. Acad. Sci. USA 1998, 95, 2355–2360. [Google Scholar]
- Shashikant, C.S.; Bolanowsky, S.A.; Anand, S.; Anderson, S.M. Comparison of diverged hoxc8 early enhancer activities reveals modification of regulatory interactions at conserved cis-acting elements. J. Exp. Zool. B. Mol. Dev. Evol. 2007, 308, 242–249. [Google Scholar]
- Shashikant, C.S.; Kim, C.B.; Borbely, M.A.; Wang, W.C.; Ruddle, F.H. Comparative studies on mammalian hoxc8 early enhancer sequence reveal a baleen whale-specific deletion of a cis-acting element. Proc. Natl. Acad. Sci. USA 1998, 95, 15446–15451. [Google Scholar]
- Anand, S.; Wang, W.C.; Powell, D.R.; Bolanowski, S.A.; Zhang, J.; Ledje, C.; Pawashe, A.B.; Amemiya, C.T.; Shashikant, C.S. Divergence of hoxc8 early enhancer parallels diverged axial morphologies between mammals and fishes. Proc. Natl. Acad. Sci. USA 2003, 100, 15666–15669. [Google Scholar]
- McLean, C.Y.; Reno, P.L.; Pollen, A.A.; Bassan, A.I.; Capellini, T.D.; Guenther, C.; Indjeian, V.B.; Lim, X.; Menke, D.B.; Schaar, B.T.; et al. Human-specific loss of regulatory DNA and the evolution of human-specific traits. Nature 2011, 471, 216–219. [Google Scholar] [CrossRef]
- Rebeiz, M.; Pool, J.E.; Kassner, V.A.; Aquadro, C.F.; Carroll, S.B. Stepwise modification of a modular enhancer underlies adaptation in a drosophila population. Science 2009, 326, 1663–1667. [Google Scholar] [CrossRef]
- McGregor, A.P.; Orgogozo, V.; Delon, I.; Zanet, J.; Srinivasan, D.G.; Payre, F.; Stern, D.L. Morphological evolution through multiple cis-regulatory mutations at a single gene. Nature 2007, 448, 587–590. [Google Scholar]
- Williams, T.M.; Selegue, J.E.; Werner, T.; Gompel, N.; Kopp, A.; Carroll, S.B. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in drosophila. Cell 2008, 134, 610–623. [Google Scholar] [CrossRef]
- Prabhakar, S.; Visel, A.; Akiyama, J.A.; Shoukry, M.; Lewis, K.D.; Holt, A.; Plajzer-Frick, I.; Morrison, H.; Fitzpatrick, D.R.; Afzal, V.; et al. Human-specific gain of function in a developmental enhancer. Science 2008, 321, 1346–1350. [Google Scholar]
- Frankel, N.; Erezyilmaz, D.F.; McGregor, A.P.; Wang, S.; Payre, F.; Stern, D.L. Morphological evolution caused by many subtle-effect substitutions in regulatory DNA. Nature 2011, 474, 598–603. [Google Scholar]
- Jeong, S.; Rokas, A.; Carroll, S.B. Regulation of body pigmentation by the abdominal-b hox protein and its gain and loss in drosophila evolution. Cell 2006, 125, 1387–1399. [Google Scholar] [CrossRef]
- Jeong, S.; Rebeiz, M.; Andolfatto, P.; Werner, T.; True, J.; Carroll, S.B. The evolution of gene regulation underlies a morphological difference between two drosophila sister species. Cell 2008, 132, 783–793. [Google Scholar] [CrossRef]
- Sucena, E.; Stern, D.L. Divergence of larval morphology between drosophila sechellia and its sibling species caused by cis-regulatory evolution of ovo/shaven-baby. Proc. Natl. Acad. Sci. USA 2000, 97, 4530–4534. [Google Scholar] [CrossRef]
- Gompel, N.; Prud'homme, B.; Wittkopp, P.J.; Kassner, V.A.; Carroll, S.B. Chance caught on the wing: Cis-regulatory evolution and the origin of pigment patterns in drosophila. Nature 2005, 433, 481–487. [Google Scholar]
- Sumiyama, K.; Saitou, N. Loss-of-function mutation in a repressor module of human-specifically activated enhancer hacns1. Mol. Biol. Evol. 2011, 28, 3005–3007. [Google Scholar] [CrossRef]
- Shim, S.; Kwan, K.Y.; Li, M.; Lefebvre, V.; Sestan, N. Cis-regulatory control of corticospinal system development and evolution. Nature 2012, 486, 74–79. [Google Scholar]
- Gaunt, S.J.; Drage, D.; Cockley, A. Vertebrate caudal gene expression gradients investigated by use of chick cdx-a/lacz and mouse cdx-1/lacz reporters in transgenic mouse embryos: Evidence for an intron enhancer. Mech. Dev. 2003, 120, 573–586. [Google Scholar] [CrossRef]
- Houle, M.; Sylvestre, J.R.; Lohnes, D. Retinoic acid regulates a subset of cdx1 function in vivo. Development 2003, 130, 6555–6567. [Google Scholar] [CrossRef]
- Marletaz, F.; Holland, L.Z.; Laudet, V.; Schubert, M. Retinoic acid signaling and the evolution of chordates. Int. J.Biol. Sci. 2006, 2, 38–47. [Google Scholar]
- Gaunt, S.J.; Paul, Y.L. Origins of cdx1 regulatory elements suggest roles in vertebrate evolution. Int. J. Dev. Biol. 2011, 55, 93–98. [Google Scholar] [CrossRef]
- Gaunt, S.J.; Drage, D.; Trubshaw, R.C. Increased cdx protein dose effects upon axial patterning in transgenic lines of mice. Development 2008, 135, 2511–2520. [Google Scholar] [CrossRef]
- Evans, F.G. The morphology and functional evolution of the atlas-axis complex from fish to mammals. Ann. N. Y. Acad. Sci. 1939, 39, 29–104. [Google Scholar] [CrossRef]
- Sumida, S.S.; Lombard, R.E. The atlas-axis complex in the late paleozoic genus diadectes and the characteristics of the atlas-axis complex across the amphibian to amniote transition. J. Paleontol. 1991, 65, 973–983. [Google Scholar]
- Vandepoele, K.; De Vos, W.; Taylor, J.S.; Meyer, A.; Van de Peer, Y. Major events in the genome evolution of vertebrates: Paranome age and size differ considerably between ray-finned fishes and land vertebrates. Proc. Natl. Acad. Sci. USA 2004, 101, 1638–1643. [Google Scholar]
- McClintock, J.M.; Carlson, R.; Mann, D.M.; Prince, V.E. Consequences of hox gene duplication in the vertebrates: An investigation of the zebrafish hox paralogue group 1 genes. Development 2001, 128, 2471–2484. [Google Scholar]
- McClintock, J.M.; Kheirbek, M.A.; Prince, V.E. Knockdown of duplicated zebrafish hoxb1 genes reveals distinct roles in hindbrain patterning and a novel mechanism of duplicate gene retention. Development 2002, 129, 2339–2354. [Google Scholar]
- Ishioka, A.; Jindo, T.; Kawanabe, T.; Hatta, K.; Parvin, M.S.; Nikaido, M.; Kuroyanagi, Y.; Takeda, H.; Yamasu, K. Retinoic acid-dependent establishment of positional information in the hindbrain was conserved during vertebrate evolution. Dev. Biol. 2011, 350, 154–168. [Google Scholar] [CrossRef]
- Nolte, C.; Amores, A.; Nagy Kovacs, E.; Postlethwait, J.; Featherstone, M. The role of a retinoic acid response element in establishing the anterior neural expression border of hoxd4 transgenes. Mech. Dev. 2003, 120, 325–335. [Google Scholar] [CrossRef]
- Houle, M.; Prinos, P.; Iulianella, A.; Bouchard, N.; Lohnes, D. Retinoic acid regulation of cdx1: An indirect mechanism for retinoids and vertebral specification. Mol. Cell Biol. 2000, 20, 6579–6586. [Google Scholar] [CrossRef] [Green Version]
- Packer, A.I.; Crotty, D.A.; Elwell, V.A.; Wolgemuth, D.J. Expression of the murine hoxa4 gene requires both autoregulation and a conserved retinoic acid response element. Development 1998, 125, 1991–1998. [Google Scholar] [Green Version]
- Marshall, H.; Studer, M.; Popperl, H.; Aparicio, S.; Kuroiwa, A.; Brenner, S.; Krumlauf, R. A conserved retinoic acid response element required for early expression of the homeobox gene hoxb-1. Nature 1994, 370, 567–571. [Google Scholar] [Green Version]
- Langston, A.W.; Thompson, J.R.; Gudas, L.J. Retinoic acid-responsive enhancers located 3' of the hox a and hox b homeobox gene clusters. Functional analysis. J. Biol. Chem. 1997, 272, 2167–2175. [Google Scholar] [Green Version]
- Studer, M.; Popperl, H.; Marshall, H.; Kuroiwa, A.; Krumlauf, R. Role of a conserved retinoic acid response element in rhombomere restriction of hoxb-1. Science 1994, 265, 1728–1732. [Google Scholar] [Green Version]
- Gould, A.; Itasaki, N.; Krumlauf, R. Initiation of rhombomeric hoxb4 expression requires induction by somites and a retinoid pathway. Neuron 1998, 21, 39–51. [Google Scholar] [CrossRef]
- Oosterveen, T.; Niederreither, K.; Dolle, P.; Chambon, P.; Meijlink, F.; Deschamps, J. Retinoids regulate the anterior expression boundaries of 5' hoxb genes in posterior hindbrain. EMBO. J. 2003, 22, 262–269. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Kalay, G. Cis-regulatory elements: Molecular mechanisms and evolutionary processes underlying divergence. Nat. Rev. Genet. 2012, 13, 59–69. [Google Scholar]
- Simpson, P. Evolution of development in closely related species of flies and worms. Nature Rev. Genet. 2002, 3, 907–917. [Google Scholar] [CrossRef]
- Morrison, A.; Chaudhuri, C.; Ariza-McNaughton, L.; Muchamore, I.; Kuroiwa, A.; Krumlauf, R. Comparative analysis of chicken hoxb-4 regulation in transgenic mice. Mech. Dev. 1995, 53, 47–59. [Google Scholar] [CrossRef]
- Whiting, J.; Marshall, H.; Cook, M.; Krumlauf, R.; Rigby, P.W.; Stott, D.; Allemann, R.K. Multiple spatially specific enhancers are required to reconstruct the pattern of hox-2.6 gene expression. Genes Dev. 1991, 5, 2048–2059. [Google Scholar] [CrossRef]
- Gilbert, W.; Marchionni, M.; McKnight, G. On the antiquity of introns. Cell 1986, 46, 151–153. [Google Scholar] [CrossRef]
- Darwin, C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life, 5th; Murray, J., Ed.; The University of Oxford: London, UK, 1859. [Google Scholar]
- Subramanian, V.; Meyer, B.I.; Gruss, P. Disruption of the murine homeobox gene cdx1 affects axial skeletal identities by altering the mesodermal expression domains of hox genes. Cell 1995, 83, 641–653. [Google Scholar] [CrossRef]
Supplementary Materials
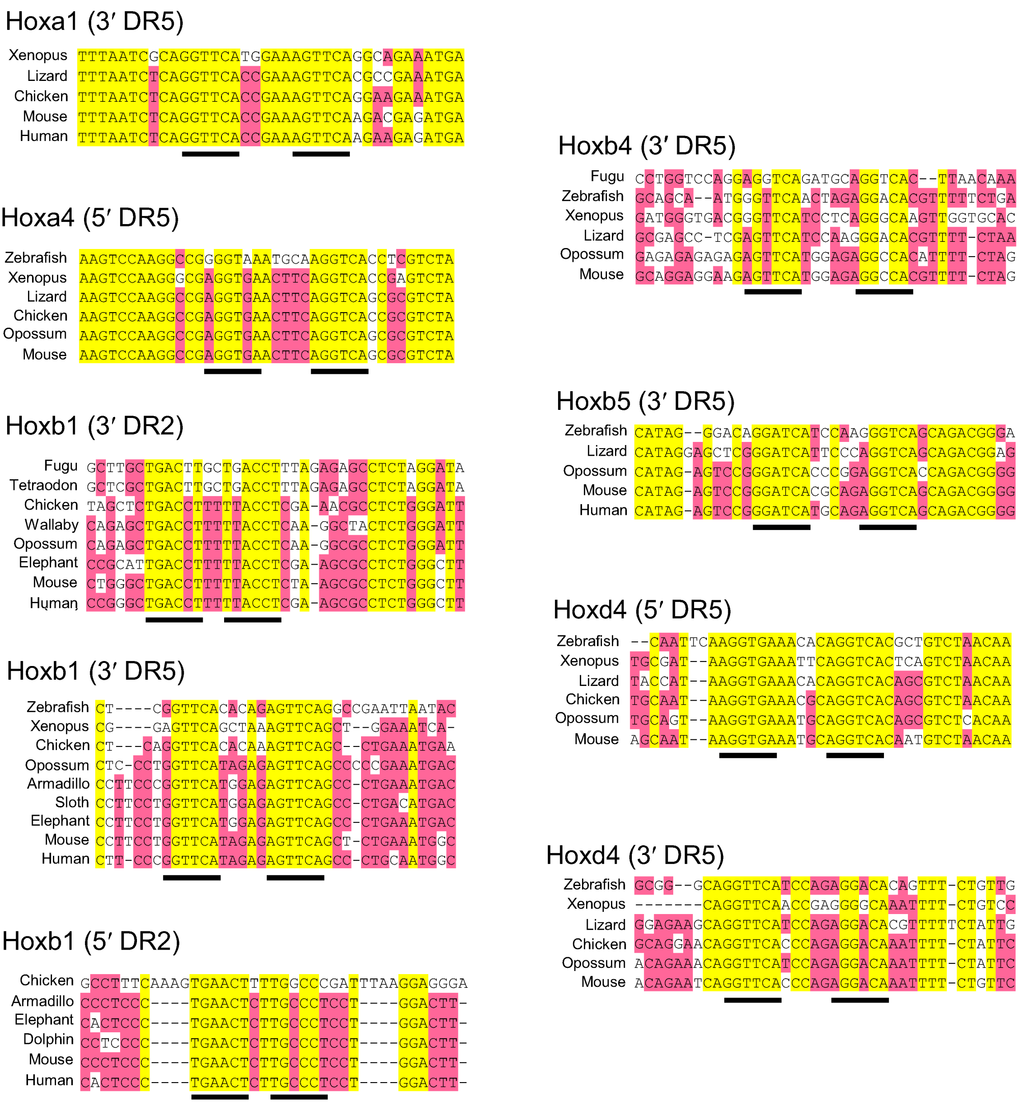
Figure S1.
Retinoic acid response elements (RAREs) in vertebrate Hox gene enhancers. The hexamer repeats are underlined. Yellow highlight, fully conserved bases; red, highly conserved. Sequences, from ENSEMBL or NCBI databases, are aligned using Vector NTI. Lizard is Anole lizard; Armadillo is nine-banded armadillo; Sloth is two-toed sloth.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).