Dye-Sensitized Solar Cells Application of TiO2 Using Spirulina and Chlorella Algae Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Assembly for the Cell
2.2. Characterization of Dyes and DSSC
3. Results and Discussion
3.1. UV–VIS
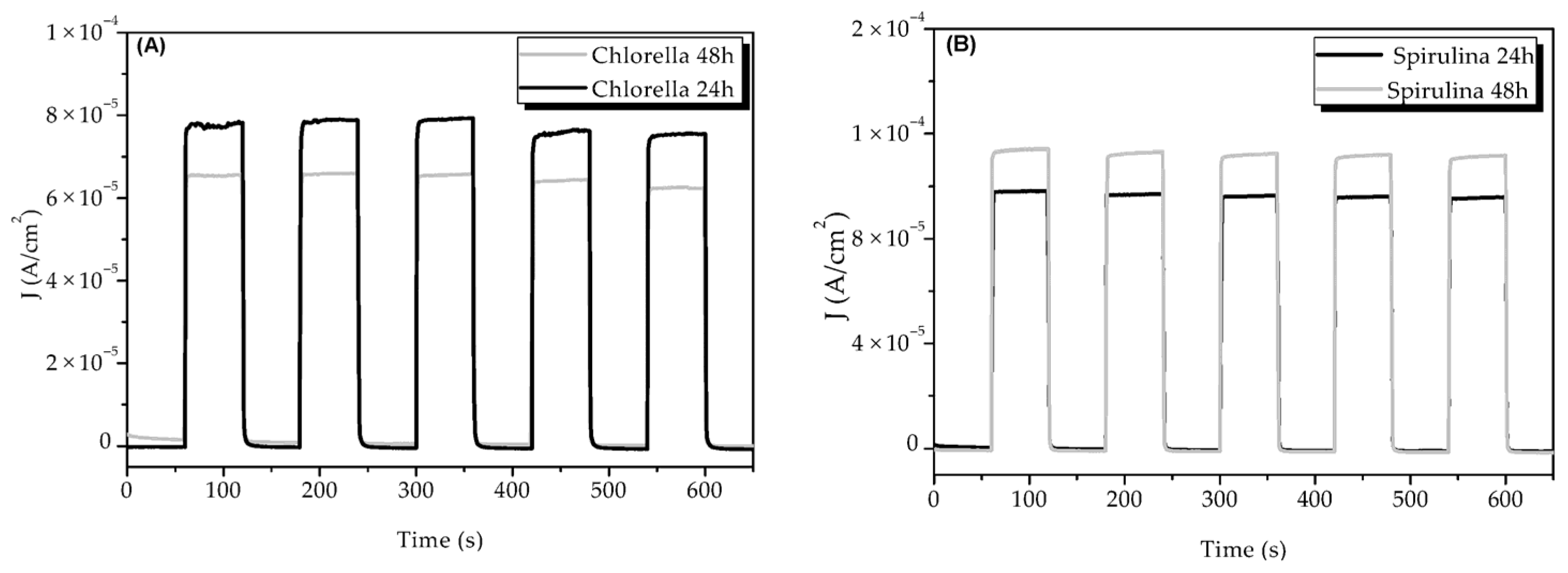
3.2. Photochronoamperometry
3.3. Open-Circuit Potential
3.4. Current Density Curve as a Function of Potential (j-V)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CB | Conduction band |
| DSSCs | Dye-sensitized solar cells |
| FF | Fill factor |
| Jsc | Short circuit current |
| OCP | Open-circuit potential |
| Pin | Incident power |
| TiO2 | Titanium dioxide |
| VB | Valence band |
| Voc | Open-circuit potential |
References
- Jaiswal, K.K.; Chowdhuey, C.R.; Yadavc, D.; Verma, R.; Duttae, S.; Jaiswal, K.S.; Sangmeshb; Karuppasamy, K.S.K. Renewable and sustainable clean energy development and impact on social, economic, and environmental health. Energy Nexus 2022, 7, 100118. [Google Scholar] [CrossRef]
- Sonai, G.; Melo, M.A., Jr.; Nunes, J.H.B.; Megiatto, J.D., Jr.; Nogueira, A.F. Células solares sensibilizadas por corantes naturais: Um experimento introdutório sobre energia renovável para alunos de graduação. Química Nova 2015, 38, 1357–1365. [Google Scholar] [CrossRef]
- Ghose, T.K.; Islam, M.R.; Aruga, K.; Jannat, A.; Islam, M.M. Disaggregated Impact of Non-Renewable Energy Consumption on the Environmental Sustainability of the United States: A Novel Dynamic ARDL Approach. Sustainability 2024, 16, 8434. [Google Scholar] [CrossRef]
- Raphael, E.; Silva, M.N.; Szostak, R.; Schiavon, M.A.; Nogueira, A.F. Células solares de perovskitas: Uma nova tecnologia emergente. Química Nova 2018, 41, 61–74. [Google Scholar] [CrossRef]
- Oliveira, A.T.E.; Sobreira, A.A.; Costa, H.F.; Ferreira, J.S.; Perez, C.A.S. A energia solar fotovoltaica: Transformação, evolução, aspectos ambientais e abordagens na sala de aula. Res. Soc. Dev. 2022, 11, e25811932533. [Google Scholar] [CrossRef]
- Lincot, D. The new paradigm of photovoltaics: From powering satellites to powering humanity. Comptes Rendus Phys. 2017, 18, 381–390. [Google Scholar] [CrossRef]
- Agnaldo, J.S.; Bastos, J.B.V.; Cressoni, J.C.; Viswanathan, G.M. Células solares de TiO2 sensibilizado por corante. Rev. Bras. Ensino Física 2006, 28, 77–84. [Google Scholar] [CrossRef]
- Tractz, G.T.; Maia, G.A.R.; Dias, B.V.; Ignachewski, F.; Rodrigues, P.R.P. Evaluation of adsorption and electrochemical study of solar cells produced with TiO2 and dye extracted from Hibiscus. Química Nova 2018, 41, 5. [Google Scholar] [CrossRef]
- Ammar, A.M.; Mohamed, H.S.H.; Yousef, M.M.K.; Abdel-Hafez, G.M.; Hassanien, A.S.; Khalil, A.S.G. Dye-sensitized solar cells (DSSCs) based on extracted natural dyes. J. Nanomater. 2019, 2019, 1867271. [Google Scholar] [CrossRef]
- Pathak, C.; Surana, K.; Shukla, V.K.; Singh, P.K. Fabrication and characterization of dye sensitized solar cell using natural dyes. Mater. Today Proc. 2019, 12, 665–670. [Google Scholar] [CrossRef]
- Bertoldi, F.C.; Sant’anna, H.; Oliveira, J.L.B. Teor de clorofila e perfil de sais minerais de Chlorella vulgaris cultivada em solução hidropônica residual. Ciência Rural. 2008, 38, 54–58. [Google Scholar] [CrossRef]
- Armendariz, E.E.; Arriaga, A.C.; Rodriguez, W.P.; Robles, A.C.; Rangel, E.R. Alternative sources of natural photosensitizers: Role of algae in dye-sensitized solar cell. Colorants 2023, 2, 137–150. [Google Scholar] [CrossRef]
- Ferreira, M.M. Spirulina: Uma Revisão. Universidade Federal de Uberlândia. 2020. Available online: https://repositorio.ufu.br/handle/123456789/29490 (accessed on 21 July 2024).
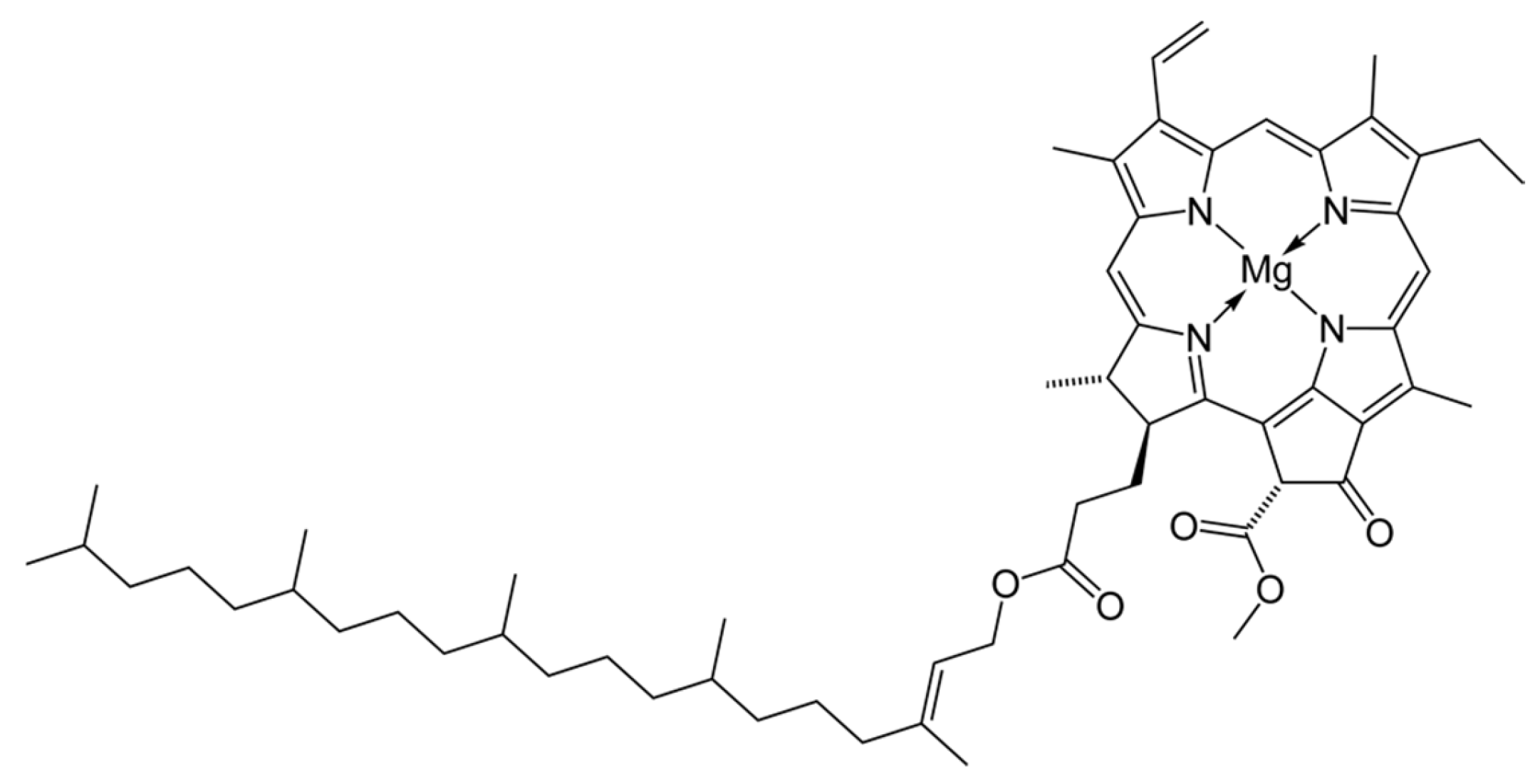
- Streit, N.M.; Canterle, L.P.; Canto, M.W.; Hecktheuer, L.H.H. As clorofilas. Ciência Rural 2005, 35, 748–755. [Google Scholar] [CrossRef]
- Structure of Chlorophyll. Available online: https://upload.wikimedia.org/wikipedia/commons/c/c4/Chlorophyll-a-2D-skeletal.png (accessed on 21 July 2024).
- Parussulo, A.L.A. Conceitos Supramoleculares e Morfologia Interfacial em Células Solares de TiO2. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2013. [Google Scholar] [CrossRef]
- Viomar, A.; Maia, G.A.R.; Scremin, F.R.; Khalil, N.M.; Cunha, M.T.; Antunes, A.C. Influência do método de obtenção de partículas de Nb2O5 empregadas em células solares sensibilizadas por corantes compostos de TiO2/Nb2O5. Rev. Virtual De Química 2016, 8, 889–900. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Sheffler, M.L.; Mercali, G.D.; Marczak, L.D.F. Extração de ficocianinas da Spirulina Platensis assistida por ultrassom. In Proceedings of the 7º Simpósio de Segurança alimentar, Online, 27–29 October 2020; Available online: http://schenautomacao.com.br/ssa7/envio/files/trabalho3_181.pdf (accessed on 29 June 2025).
- Hidayah, Q.; Kusuma, D.Y.; Aji, O.R.; Izziyah, A.N.; Purnama, B. Solid state organic photovoltaic devices using spirulina sp thylakoid membrane films as active material. J. Phys. Conf. Series 2020, 1563, 012013. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.M.; Ludin, N.A.; Mohamad, A.B.; Kadhum, A.A.H.; Baabbad, M.M.; Sopian, K. Optimization of dye extraction from Cordyline fruticose via response surface methodology to produce a natural sensitizer for dye-sensitized solar cells. Results Phys. 2016, 6, 520–529. [Google Scholar] [CrossRef]
- Pandey, A.K.; Ahmad, M.S.; Rahim, N.A.; Tyagi, V. Natural Sensitizers and their Applications in Dye-Sensitized Solar Cell. In Environmental Biotechnology: For Sustainable Future; Springer Nature: Cham, Switzerland, 2019; pp. 375–401. [Google Scholar] [CrossRef]
- Ranjitha, S.; Aroulmoji, V.; Selvankumar, T.; Sudhakar, C.; Hariharan, V. Synthesis and development of novel sensitizer from Spirulina pigment with silver doped TiO2 nano particles for bio-sensitized solar cells. Biomass Bioenergy 2020, 141, 105733. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Kühl, M. Chlorophyll d: The puzzle resolved. Trends Plant Sci. 2005, 10, 355–357. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, L.; Gao, Y.; Ma, T. Dye-sensitized solar cells usings 20 natural dyes as sensitizers. J. Photochem. Photobiol. A Chem. 2011, 219, 188–194. [Google Scholar] [CrossRef]
- Buene, A.F.; Almenningen, D.M.; Hagfeldt, A.; Gautun, O.R.; Hoff, B.H. First report of chenodeoxycholic acid-substituted dyes improving the dye monolayer quality in dye-sensitized solar cells. Sol. RRL 2020, 4, 1900569. [Google Scholar] [CrossRef]
- Manrich, A.; Mermejo, B.C.; Moraes, J.C.; Oliveira, J.E.; Mattoso, L.H.C.; Martins, M.A. Uso de espectroscopia de UV-VIS e difratometria de Raios X na identificação de ficocianina em microalgas. In Proceedings of the Simpósio Nacional de Instrumentação Agropecuária, São Carlos, Brazil, 18–20 November 2014; Available online: https://www.alice.cnptia.embrapa.br/alice/bitstream/doc/1005835/1/423siagro2014print01.pdf (accessed on 28 June 2025).
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P.K. Purification and characterization of C-phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.; Zheng, W. Purification and characterization of selenium-containing phycocyanin from selenium-enriched Spirulina platensis. Phytochemistry 2006, 67, 2424–2430. [Google Scholar] [CrossRef]
- Park, W.S.; Kim, H.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.; Kang, C.; Ferruzzi, M.G.; Ahn, M. Two classes of pigments, carotenoids and C-Phycocyanin, in spirulina powder and their antioxidant activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef]
- Orona-Navar, A.; Aguilar-Hernández, I.; Nigam, K.P.D.; Cérdan-Pasarán, A.; Ornelas-Soto, N. Alternative sources of natural pigments for dye-sensitized solar cells: Algae, cyanobacteria, bacteria, archaea and fungi. J. Biotechnol. 2021, 332, 29–53. [Google Scholar] [CrossRef]
- Ganta, D.; Combrink, K.; Villanueva, R. Natural Dye-Sensitized Solar Cells: Fabrication, Characterization, and Challenges. In Advances in Solar Energy Research; Tyagi, H., Agarwal, A.K., Chakraborty, P.R., Powar, S., Eds.; Environment and Sustainability; Springer Nature: Cham, Switzerland, 2019; pp. 129–155. [Google Scholar] [CrossRef]
- Cincárová, D.; Hájek, J.; Dobrichovský, M.; Lukes, M.; Hrouzek, P. Recommendations on the quantitive analysis of pheophorbides, photosensitizers present in algal biomass intended as food supplement. Algal Res. 2021, 56, 102298. [Google Scholar] [CrossRef]
- Tractz, G.T.; Antunes, S.R.M.; Rodrigues, P.R.P. Nb/TiO2 oxides: A study of synthesis and electron transport mechanism as an ETl in a solar device. J. Photochem. Photobiol. A Chem. 2023, 444, 114899. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Sohani, A.; Pierro, M.; Moser, D.; Cornaro, C. Comparison of physical models for bifacial PV power estimation. Energy Convers. Manag. 2025, 327, 119515. [Google Scholar] [CrossRef]
- Malavika, K. Studies on the Potential of Chlorophyll from Chlorella Vulgaris Beijerinck as a Photosensitizer in Dye Sensitized Solar Cell. Master’s Thesis, Sr. Teresa’s College, Kerala State, India, 2023. Available online: http://117.239.78.102:8080/jspui/bitstream/123456789/2593/1/Malavika%20project.pdf (accessed on 18 April 2025).




| Jsc mA/cm2 | E (V) | FF | η (%) | |
|---|---|---|---|---|
| Chlorella 24 h | 0.0910 ± 0.0119 | 0.313 ± 0.0170 | 0.327 ± 0.013 | 0.0093 ± 0.0021 |
| Chlorella 48 h | 0.1385 ± 0.0098 | 0.3637 ± 0.0059 | 0.365 ± 0.004 | 0.0184 ± 0.0015 |
| Spirulina 24 h | 0.1095 ± 0.0300 | 0.2970 ± 0.0735 | 0.320 ± 0.029 | 0.0104 ± 0.0300 |
| Spirulina 48 h | 0.1034 ± 0.0349 | 0.2990 ± 0.0858 | 0.338 ± 0.050 | 0.0105 ± 0.0349 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa, M.V.F.; Tractz, G.T.; Maia, G.A.R.; Fonseca, H.E.S.; Berbel, L.O.; de Almeida, L.J.; do Prado Banczek, E. Dye-Sensitized Solar Cells Application of TiO2 Using Spirulina and Chlorella Algae Extract. Colorants 2025, 4, 25. https://doi.org/10.3390/colorants4030025
Corrêa MVF, Tractz GT, Maia GAR, Fonseca HES, Berbel LO, de Almeida LJ, do Prado Banczek E. Dye-Sensitized Solar Cells Application of TiO2 Using Spirulina and Chlorella Algae Extract. Colorants. 2025; 4(3):25. https://doi.org/10.3390/colorants4030025
Chicago/Turabian StyleCorrêa, Maria Vitória França, Gideã Taques Tractz, Guilherme Arielo Rodrigues Maia, Hagata Emmanuely Slusarski Fonseca, Larissa Oliveira Berbel, Lucas José de Almeida, and Everson do Prado Banczek. 2025. "Dye-Sensitized Solar Cells Application of TiO2 Using Spirulina and Chlorella Algae Extract" Colorants 4, no. 3: 25. https://doi.org/10.3390/colorants4030025
APA StyleCorrêa, M. V. F., Tractz, G. T., Maia, G. A. R., Fonseca, H. E. S., Berbel, L. O., de Almeida, L. J., & do Prado Banczek, E. (2025). Dye-Sensitized Solar Cells Application of TiO2 Using Spirulina and Chlorella Algae Extract. Colorants, 4(3), 25. https://doi.org/10.3390/colorants4030025






