Prodigiosin: A Potential Eco-Friendly Insecticide for Sustainable Crop Protection
Abstract
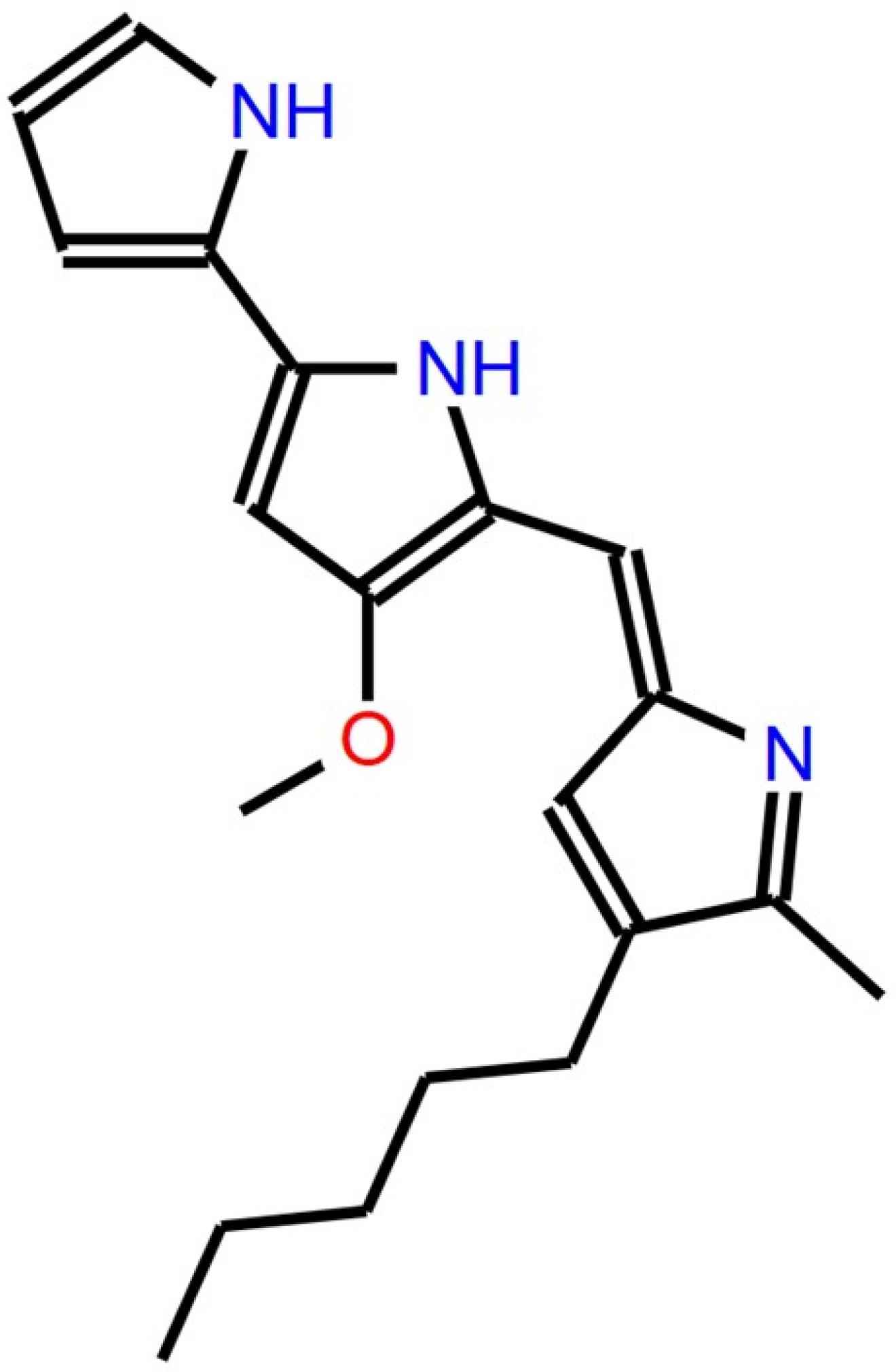
1. Introduction
2. Production, Extraction, and Purification of Prodigiosin
2.1. Production Conditions
2.2. Extraction and Purification Methods
3. Common Strategies for Insect Control
4. Insecticidal Activity of Prodigiosin and Mechanism of Action
4.1. Enzymatic Inhibition and Oxidative Stress
4.2. Effectivity as Insecticide and Working Conditions
5. Advantages and Limitations of the Use of Prodigiosin
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo, R.G.; Rodríguez-Zavala, N.; Castillo-Zacarías, C.; Barocio, M.E.; Hidalgo-Vázquez, E.; Parra-Arroyo, L.; Rodríguez-Hernández, J.A.; Martínez-Prado, M.A.; Sosa-Hernández, J.E.; Martínez-Ruiz, M.; et al. Recent advances in prodigiosin as a bioactive compound in nanocomposite applications. Molecules 2022, 27, 4982. [Google Scholar] [CrossRef] [PubMed]
- Mnif, S.; Jardak, M.; Bouizgarne, B.; Aifa, S. Prodigiosin from Serratia: Synthesis and potential applications. Asian Pac. J. Trop. Biomed. 2022, 12, 233–242. [Google Scholar] [CrossRef]
- Köksal Karayildirim, C.; Şahiner, A.; Çalişkan, S.; Soylu, F.E.; Gökhan, A.; Eroğlu, E.; Uyanikgil, Y.; Karayildirim, T. Isolation, identification, and antimicrobial evaluation of secondary metabolite from Serratia marcescens via an in vivo epicutaneous infection model. ACS Omega 2024, 9, 8397–8404. [Google Scholar] [CrossRef]
- Paul, T.; Bandyopadhyay, T.K.; Mondal, A.; Tiwari, O.N.; Muthuraj, M.; Bhunia, B. A comprehensive review on recent trends in production, purification, and applications of prodigiosin. Biomass Convers. Biorefinery 2022, 12, 1409–1431. [Google Scholar] [CrossRef]
- Islan, G.A.; Rodenak-Kladniew, B.; Noacco, N.; Duran, N.; Castro, G.R. Prodigiosin: A promising biomolecule with many potential biomedical applications. Bioengineered 2022, 13, 14227–14258. [Google Scholar] [CrossRef]
- Haddix, P.L.; Shanks, R.M.Q. Prodigiosin pigment of Serratia marcescens is associated with increased biomass production. Arch. Microbiol. 2018, 200, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.H.; Mahalingam, S.; Wan, K.L.; Nathan, S. Prodigiosin inhibits bacterial growth and virulence factors as a potential physiological response to interspecies competition. PLoS ONE 2021, 16, e0253445. [Google Scholar] [CrossRef]
- Gohil, N.; Bhattacharjee, G.; Singh, V. Synergistic bactericidal profiling of prodigiosin extracted from Serratia marcescens in combination with antibiotics against pathogenic bacteria. Microb. Pathog. 2020, 149, 104508. [Google Scholar] [CrossRef]
- Woodhams, D.C.; LaBumbard, B.C.; Barnhart, K.L.; Becker, M.H.; Bletz, M.C.; Escobar, L.A.; Flechas, S.V.; Forman, M.E.; Iannetta, A.A.; Joyce, M.D.; et al. Prodigiosin, violacein, and volatile organic compounds produced by widespread cutaneous bacteria of amphibians can inhibit two Batrachochytrium fungal pathogens. Microb. Ecol. 2018, 75, 1049–1062. [Google Scholar] [CrossRef]
- Pereira, R.F.S.; de Carvalho, C.C.C.R. Improving bioprocess conditions for the production of prodigiosin using a marine Serratia rubidaea strain. Mar. Drugs 2024, 22, 142. [Google Scholar] [CrossRef]
- Setiyono, E.; Adhiwibawa, M.A.S.; Indrawati, R.; Prihastyanti, M.N.U.; Shioi, Y.; Brotosudarmo, T.H.P. An Indonesian marine bacterium, Pseudoalteromonas rubra, produces antimicrobial prodiginine pigments. ACS Omega 2020, 5, 4626–4635. [Google Scholar] [CrossRef] [PubMed]
- Handayani, D.P.; Isnansetyo, A.; Istiqomah, I. New investigation of encoding secondary metabolites gene by genome mining of a marine bacterium, Pseudoalteromonas viridis BBR56. BMC Genom. 2024, 25, 364. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Kim, H.J.; Kim, S.; Park, S.Y.; Kim, H.R.; Jeong, S.; Lee, S.J.; Lee, M.-S. Enhanced large-scale production of Hahella chejuensis-derived prodigiosin and evaluation of its bioactivity. J. Microbiol. Biotechnol. 2021, 31, 1624–1631. [Google Scholar] [CrossRef]
- Mukhia, S.; Kumar, A.; Kumar, R. Antioxidant prodigiosin-producing cold-adapted Janthinobacterium sp. ERMR3: 09 from a glacier moraine: Genomic elucidation of cold adaptation and pigment biosynthesis. Gene 2023, 857, 147178. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Nguyen, V.B.; Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Nguyen, A.D. Production and potential applications of bioconversion of chitin and protein-containing fishery byproducts into prodigiosin: A review. Molecules 2020, 25, 2744. [Google Scholar] [CrossRef]
- Vijay, D.; Alshamsi, N.S.; Moussa, Z.; Akhtar, M.K. Extraction of the anticancer and antimicrobial agent, prodigiosin, from Vibrio gazogenes PB1 and its identification by 1D and 2D NMR. Molecules 2022, 27, 6030. [Google Scholar] [CrossRef]
- Victor, T.M.M.; Ndlovu, T.M.; Pessela, B.C.; Bull, S.; Ward, A.C. Production and evaluation of two antibiotics of Streptomyces coelicolor A3 (2), prodigiosin and actinorhodin under solid state fermentation, using micro-porous culture. Chem. Eng. Process.-Process Intensif. 2022, 170, 108685. [Google Scholar] [CrossRef]
- Tirumale, S.; Wani, N.A. Biopigments: Fungal pigments. In Fungi and Their Role in Sustainable Development: Current Perspectives; Springer: Singapore, 2018; pp. 413–426. [Google Scholar]
- Anwar, M.M.; Albanese, C.; Hamdy, N.M.; Sultan, A.S. Rise of the natural red pigment ‘prodigiosin’as an immunomodulator in cancer. Cancer Cell Int. 2022, 22, 419. [Google Scholar] [CrossRef]
- Hernandez-Velasco, P.; Morales-Atilano, I.; Rodríguez-Delgado, M.; Rodríguez-Delgado, J.M.; Luna-Moreno, D.; Ávalos-Alanís, F.G.; Villarreal-Chiu, J.F. Photoelectric evaluation of dye-sensitized solar cells based on prodigiosin pigment derived from Serratia marcescens 11E. Dye. Pigment. 2020, 177, 108278. [Google Scholar] [CrossRef]
- Jaber, G.S.; Dhaif, S.S.; Hussian, T.A.A.; Ibrahim, N.A.; Arifiyanto, A. Enhancing the prodigiosin pigment by adding Ag\TiO2 synergism for antibacterial activity. Biocatal. Agric. Biotechnol. 2023, 54, 102900. [Google Scholar] [CrossRef]
- Hossain, T.J.; Rahman, T. Prodigiosin demonstrates promising antiviral activity against Dengue Virus (DENV) and Zika Virus (ZIKV) in in-silico study. Mohammed Sajjad Hossain, Prodigiosin demonstrates promising antiviral activity against Dengue Virus (DENV) and Zika Virus (ZIKV) in in-silico study. Anal. Sci. Adv. 2024, 5, e202400039. [Google Scholar]
- Liu, W.; Yang, J.; Tian, Y.; Zhou, X.; Wang, S.; Zhu, J.; Sun, D.; Liu, C. An in situ extractive fermentation strategy for enhancing prodigiosin production from Serratia marcescens BWL1001 and its application to inhibiting the growth of Microcystis aeruginosa. Biochem. Eng. J. 2021, 166, 107836. [Google Scholar] [CrossRef]
- Sree, B.; Sagar, V.; Deepak, B.S.; Tejaswini, G.S.; Aparna, Y.; Sarada, J. Evaluation of Prodigiosin pigment for antimicrobial and insecticidal activities on selected bacterial pathogens & household pests. Int. J. Sci. Res. Biol. Sci. 2019, 6, 96–102. [Google Scholar]
- Liang, T.W.; Chen, S.Y.; Chen, Y.C.; Chen, C.H.; Yen, Y.H.; Wang, S.L. Enhancement of prodigiosin production by Serratia marcescens TKU011 and its insecticidal activity relative to food colorants. J. Food Sci. 2013, 78, M1743–M1751. [Google Scholar] [CrossRef]
- Patil, C.D.; Patil, S.V.; Salunke, B.K.; Salunkhe, R.B. Prodigiosin produced by Serratia marcescens NMCC46 as a mosquito larvicidal agent against Aedes aegypti and Anopheles stephensi. Parasitol. Res. 2011, 109, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Eski, A.; Özdemir, T. Insecticidal activity of prodigiosin pigment on Tenebrio molitor (Coleoptera: Tenebrionidae). Bilecik Şeyh Edebali Üniversitesi Fen Bilim. Derg. 2022, 9, 1035–1040. [Google Scholar] [CrossRef]
- Asano, S.; Ogiwara, K.; Nakagawa, Y.; Suzuki, K.; Hori, H.; Watanabe, T. Prodigiosin produced by Serratia marcescens enhances the insecticidal activity of Bacillus thuringiensis delta-endotoxin (CrylC) against Common Cutworm, Spodoptera litura. J. Pestic. Sci. 1999, 24, 381–385. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Patil, C.D.; Borase, H.P.; Narkhede, C.P.; Salunke, B.K.; Patil, S.V. Mosquito larvicidal and pupaecidal potential of prodigiosin from Serratia marcescens and understanding its mechanism of action. Pestic. Biochem. Physiol. 2015, 123, 49–55. [Google Scholar] [CrossRef]
- Patil, N.G.; Kadam, M.S.; Patil, V.R.; Chincholkar, S.B. Insecticidal properties of water diffusible prodigiosin produced by Serratia nematodiphila 213C. Curr. Trends Biotechnol. Pharm. 2013, 7, 773–781. [Google Scholar]
- Hu, W.; Zheng, R.; Liao, Y.; Kuang, F.; Yang, Z.; Chen, T.; Zhang, N. Evaluating the biological potential of prodigiosin from Serratia marcescens KH-001 against Asian Citrus Psyllid. J. Econ. Entomol. 2021, 114, 1219–1225. [Google Scholar] [CrossRef]
- Do, H.N.A.; Nguyen, T.H.K. Studies on the prodigiosin production from Streptomyces coelicolor in liquid media by using heated Lactobacillus rhamnosus. J. Appl. Pharm. Sci. 2014, 4, 21–24. [Google Scholar]
- Gohil, N.; Bhattacharjee, G.; Kalariya, R.; Pandya, V.; Khambhati, K.; Gohil, J.; Alzahrani, K.J.; Show, P.-L.; Maurya, R.; Singh, V. Biovalorization of agro-industrial waste soybean meal for the production of prodigiosin by Serratia marcescens. Biomass Convers. Biorefinery 2021, 1–12. [Google Scholar] [CrossRef]
- Maurya, K.K.; Tripathi, A.D.; Kumar, D.; Panesar, P.S.; Paul, V.; Agarwal, A. Standardization of process parameters for enhanced prodigiosin production from wheat bran using Taguchi methodology. Waste Biomass Valorization 2024, 15, 6159–6170. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L.; Nguyen, T.H.; Phan, T.Q.; Nguyen, T.H.; Tran, T.H.T.; Doan, M.D.; Ngo, V.A.; Nguyen, A.D. Recycling fish heads for the production of prodigiosin, a novel fungicide via experimental and molecular docking characterization. Fishes 2023, 8, 468. [Google Scholar] [CrossRef]
- Aruldass, C.A.; Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Brown sugar as a low-cost medium for the production of prodigiosin by locally isolated Serratia marcescens UTM1. Int. Biodeterior. Biodegrad. 2014, 95, 19–24. [Google Scholar] [CrossRef]
- Tran, L.T.; Techato, K.; Nguyen, V.B.; Wang, S.L.; Nguyen, A.D.; Phan, T.Q.; Doan, M.D.; Phoungthong, K. Utilization of cassava wastewater for low-cost production of prodigiosin via Serratia marcescens TNU01 fermentation and its novel potent α-glucosidase inhibitory effect. Molecules 2021, 26, 6270. [Google Scholar] [CrossRef]
- Kate, A.E.; Singh, A.; Shahi, N.C.; Pandey, J.P.; Prakash, O.; Singh, T.P. Novel eco-friendly techniques for extraction of food based lipophilic compounds from biological materials. Nat. Prod. Chem. Res. 2016, 4, 1000231. [Google Scholar]
- Sun, S.Q.; Wang, Y.J.; Xu, W.; Zhu, C.J.; Liu, X.X. Optimizing ultrasound-assisted extraction of prodigiosin by response surface methodology. Prep. Biochem. Biotechnol. 2015, 45, 101–108. [Google Scholar] [CrossRef]
- Khanam, B.; Chandra, R. Comparative analysis of prodigiosin isolated from endophyte Serratia marcescens. Lett. Appl. Microbiol. 2018, 66, 194–201. [Google Scholar] [CrossRef]
- Belluco, S.; Bertola, M.; Montarsi, F.; Di Martino, G.; Granato, A.; Stella, R.; Martinello, M.; Bordin, F.; Mutinelli, F. Insects and public health: An overview. Insects 2023, 14, 240. [Google Scholar] [CrossRef]
- Ali, M.A.; Abdellah, I.M.; Eletmany, M.R. Advances and applications of insect genetics and genomics. Chelonian Res. Found. 2022, 17, 80–87. [Google Scholar]
- Manosathiyadevan, M.; Bhuvaneshwari, V.; Latha, R. Impact of insects and pests in loss of crop production: A review. In Sustainable Agriculture Towards Food Security; Springer: Singapore, 2017; pp. 57–67. [Google Scholar]
- Mehta, V.; Kumar, S.; CS, J. Damage potential, effect on germination, and development of Sitophilus oryzae (Coleoptera: Curculionidae) on wheat grains in Northwestern Himalayas. J. Insect Sci. 2021, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, V.; Kumar, D.; Bansal, A.; Solanki, V. Recognizing wheat aphid disease using a novel parallel real-time technique based on mask scoring RCNN. In Proceedings of the 2022 2nd International Conference on Advanced Computing and Innovative Technologies in Engineering (ICACITE), Greater Noida, India, 28–29 April 2022; pp. 1372–1377. [Google Scholar]
- Dufton, S.V.; Olfert, O.O.; Laird, R.A.; Floate, K.D.; Ge, X.; Otani, J.K. A global review of orange wheat blossom midge, Sitodiplosis mosellana (Géhin)(Diptera: Cecidomyiidae), and integrated pest management strategies for its management. Can. Entomol. 2022, 154, e30. [Google Scholar] [CrossRef]
- Akbar, M.; Aleem, K.; Sandhu, K.; Shamoon, F.; Fatima, T.; Ehsan, M.; Shaukat, F. A mini review on insect pests of wheat and their management strategies. Int. J. Agric. Biosci. 2023, 12, 110–115. [Google Scholar]
- İlçin, M.; Çelik, Ş. Statistical evaluation of damage status of important grasshopper family in plants. J. Res. Agric. Anim. Sci. 2021, 8, 24–27. [Google Scholar]
- Bajpeyi, M.M.; Kumar, A.; Aman, A.S.; Kushwaha, D. Integrated management of soil dwelling pests of wheat crop. Biot. Res. Today 2023, 5, 81–83. [Google Scholar]
- Xu, Y.; Ghori, N.; Hussain, S.; Xu, X.; Su, Z.; Zhang, D.; Zhao, L.; Liu, X.; Chen, M.-S.; Bai, G. Evaluating a worldwide wheat collection for resistance to Hessian fly biotype ‘Great Plains’. Front. Plant Sci. 2024, 15, 1402218. [Google Scholar] [CrossRef]
- Zhang, Z.; Batuxi; Jiang, Y.; Li, X.; Zhang, A.; Zhu, X.; Zhang, Y. Effects of different wheat tissues on the population parameters of the Fall Armyworm (Spodoptera frugiperda). Agronomy 2021, 11, 2044. [Google Scholar] [CrossRef]
- Peirce, E.S.; Evers, B.; Winn, Z.J.; Raupp, W.J.; Guttieri, M.; Fritz, A.K.; Poland, J.; Akhunov, E.; Haley, S.; Mason, E.; et al. Identifying novel sources of resistance to wheat stem sawfly in five wild wheat species. Pest Manag. Sci. 2024, 80, 2976–2990. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Saeed, S.; Naeem-Ullah, U.; Matloob, A.; Wajid, M.; Siddique, A.; Shahid, R.; Zia, H.U.U.R.; Bilal, H.; Ramzan, M. Insect pest complex of wheat crop. In Current Trends in Wheat Research; IntechOpen: London, UK, 2021; p. 47. [Google Scholar]
- Hussain, D.; Asrar, M.; Khalid, B.; Hafeez, F.; Saleem, M.; Akhter, M.; Ahmed, M.; Ali, I.; Hanif, K. Insect pests of economic importance attacking wheat crop (Triticum aestivum L.) in Punjab, Pakistan. Int. J. Trop. Insect Sci. 2022, 42, 9–20. [Google Scholar] [CrossRef]
- Suh, P.F.; Elanga-Ndille, E.; Tchouakui, M.; Sandeu, M.M.; Tagne, D.; Wondji, C.; Ndo, C. Impact of insecticide resistance on malaria vector competence: A literature review. Malar. J. 2023, 22, 19. [Google Scholar] [CrossRef]
- Gómez, M.; Martínez, D.; Páez-Triana, L.; Luna, N.; Ramírez, A.; Medina, J.; Cruz-Saavedra, L.; Hernández, C.; Castañeda, S.; Melo, R.B.; et al. Influence of dengue virus serotypes on the abundance of Aedes aegypti insect-specific viruses (ISVs). J. Virol. 2024, 98, e01507-23. [Google Scholar] [CrossRef]
- Francis, S.; Frank, C.; Buchanan, L.; Green, S.; Stennett-Brown, R.; Gordon-Strachan, G.; Rubio-Palis, Y.; Grant, C.; Alexander-Lindo, R.L.; Nwokocha, C.; et al. Challenges in the control of neglected insect vector diseases of human importance in the Anglo-Caribbean. One Health 2021, 13, 100316. [Google Scholar] [CrossRef]
- Gabiane, G.; Yen, P.S.; Failloux, A.B. Aedes mosquitoes in the emerging threat of urban yellow fever transmission. Rev. Med. Virol. 2022, 32, e2333. [Google Scholar] [CrossRef] [PubMed]
- Tolsá-García, M.J.; Wehmeyer, M.L.; Lühken, R.; Roiz, D. Worldwide transmission and infection risk of mosquito vectors of West Nile, St. Louis encephalitis, Usutu and Japanese encephalitis viruses: A systematic review. Sci. Rep. 2023, 13, 308. [Google Scholar] [CrossRef]
- Muhoro, A.M.; Farkas, E.É. Insecticidal and antiprotozoal properties of lichen secondary metabolites on insect vectors and their transmitted protozoal diseases to humans. Diversity 2021, 13, 342. [Google Scholar] [CrossRef]
- University of Wisconsin-Madison. General Approaches to Insect Control. Wisconsin Horticulture 2025. Available online: https://hort.extension.wisc.edu/articles/general-approaches-to-insect-control/#:~:text=Most%20specific%20insect%20control%20methods,biological%20control%2C%20and%20chemical%20control (accessed on 28 January 2025).
- Rajendran, P.; Somasundaram, P.; Dufossé, L. Microbial pigments: Eco-friendly extraction techniques and some industrial applications. J. Mol. Struct. 2023, 1290, 135958. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.L.; Nguyen, V.B. Recent advances in eco-friendly and scaling-up bioproduction of prodigiosin and its potential applications in agriculture. Agronomy 2022, 12, 3099. [Google Scholar] [CrossRef]
- Chen, L.P.; Xing, X.J.; Kang, K.L.; Yang, W.Y.; Luo, L.; Wu, Y.J. Why are Drosophila larvae more sensitive to avermectin than adults? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 251, 109197. [Google Scholar] [CrossRef]
- Shete, P.; Patil, N.; Bhagat, S.; Jain, A. Comparing eco-friendly biochrome-prodigiosin yield in Serratia marcescens and Serratia rubidaea using OFAT Approach. Environ. Ecol. 2023, 41, 1620–1627. [Google Scholar] [CrossRef]
- Darshan, N.; Manonmani, H.K. Prodigiosin and its potential applications. J. Food Sci. Technol. 2015, 52, 5393–5407. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, L.; Han, Y.; Wang, S.; Liu, Z.; Xu, H. Preparation, characterization, formation mechanism, and stability studies of zein/pectin nanoparticles for the delivery of prodigiosin. Int. J. Biol. Macromol. 2025, 290, 138915. [Google Scholar] [CrossRef] [PubMed]
- Cury Camargo, P.H.; Gundappa Satyanarayana, K.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Guryanov, I.; Naumenko, E.; Akhatova, F.; Lazzara, G.; Cavallaro, G.; Nigamatzyanova, L.; Fakhrullin, R. Selective cytotoxic activity of prodigiosin@ halloysite nanoformulation. Front. Bioeng. Biotechnol. 2020, 8, 424. [Google Scholar] [CrossRef]
- Prado, G.; Mendes, E.T.; Rueda Martins, R.C.; Perdigão-Neto, L.V.; Freire, M.P.; Marchi, A.P.; Côrtes, M.F.; Lima, V.A.C.d.C.; Rossi, F.; Guimarães, T.; et al. Phenotypic and genotypic characteristics of a carbapenem-resistant Serratia marcescens cohort and outbreak: Describing an opportunistic pathogen. Int. J. Antimicrob. Agents 2022, 59, 106463. [Google Scholar] [CrossRef]
- Sebastian, L.; Paul, A.M.; Jayanthi, D. Isolation and production of prodigiosin pigments from Streptomyces spp. In Methods in Actinobacteriology; Humana: New York, NY, USA, 2022; pp. 683–693. [Google Scholar]
- Sánchez de la Nieta, R.; Santamaría, R.I.; Díaz, M. Two-component systems of Streptomyces coelicolor: An intricate network to be unraveled. Int. J. Mol. Sci. 2022, 23, 15085. [Google Scholar] [CrossRef]
| Cultural control: crop rotation, sanitation, trap cropping, selection of time of planting |
| Host resistance: improve plant characteristics that repel, tolerate, or kill pests |
| Physical controls: Use cardboard bands, glue board traps, and window screens to prevent pests from reaching hosts. |
| Mechanical control: directly remove or kill pests, like hand-picking |
| Biological control: use of beneficial organisms to control pests |
| Chemical control: use of chemicals to kill pests, repellants, irritants, synthetic pheromones, and insecticides |
| Source/Production Conditions | Target Species/Working Conditions | Effectiveness/Dose or Concentration/Mechanism of Action | Reference |
|---|---|---|---|
| Serratia marcescens ATCC274. The strain was cultivated in a stainless steel tray using a 5% agar medium containing 0.5% bactopeptone and 1% glycerol at 30°C for 48 h. The bacteria were harvested and suspended in a physiological salt solution and centrifuged at 6000 rpm. The red pigments were then extracted using 95% ethanol, concentrated, and fractionated through thin-layer chromatography (TLC) with a solvent system of chloroform, methanol, and 5 M ammonia in a ratio of 80:25:4 (V/V). | Common cutworm Spodoptera litura and diamondback moth Plutella xylostella. Synergism ocurred with delta-endotoxin Cry 1C from Bacillus thuringiensis. The lethal activity of test samples was evaluated using 50 infested neonates, administering 1 µg g−1/diet of prodigiosin and 12 µg g−1/diet of Cry 1C over a treatment period of 7 days. Additionally, assays were conducted with prodigiosin alone at a concentration of 8 µg g−1/diet. | Prodigiosin and Cry 1C achieved 100% mortality in S. litura. There was 99% mortality in Plutella xylostella and 34% in S. litura at a dose of 8 µg g−1 The mechanism of action was not specified. | [28] |
| Serratia marcescens NMCC46. A loopful of 24 h-old culture was inoculated into nutrient broth media containing mannitol and incubated for 48 h at 30 ± 2 °C with continuous shaking at 120 rpm. Following this incubation period, the broth was acidified with 1% acidified ethanol and then centrifuged at 5000 rpm for 10 min. The supernatant was extracted using a water–chloroform mixture (1:1). The concentrated pigment was then dissolved in ethanol and purified using TLC. | Mosquitoes Aedes aegypti and Anopheles stephensi. Second, third, and fourth instar larvae of A. aegypti and A. stephensi were collected in four batches of ten and placed into 99 mL of water mixed with 1.0 mL of pigment. For the dose-response assay, 100 mL of the test solution were poured into 300 mL plastic cups, each containing ten larvae from the second, third, and fourth instar stages. The larvae were not fed during the treatment period. After 48 h of exposure, the number of dead larvae was recorded. | The lethal concentration values (LC50 and LC90) for the second, third, and fourth instars of A. aegypti are LC50 = 41.65, 139.51, 103.95 ppm; and LC90 = 117.81, 213.68, and 367.82 ppm. For A. stephensi, the values are LC50 = 51.12, 105.52, 133.07 ppm; and LC90 = 134.81, 204.45, and 285.35 ppm. Mortality begins at a concentration of 500 ppm, with signs of mortality observed within the first 6 h of exposure. More than 50% of the mortality occurs within the first 24 h. The mechanism of action was not specified. | [26] |
| Serratia marcescens TKU011. The pigment was produced using a medium containing 1.5% squid pen powder (SPP), supplemented with 0.1% K2HPO4 and 0.1% FeSO4(NH4)2SO4 · 6H2O. The cultivation was conducted at 30 °C for 24 h, followed by an additional two days at 25 °C. Four autoclave cycles were implemented throughout the process. Subsequently, the pigment was purified using TLC. | Oregon R strain of Drosophila melanogaster flies were reared in plastic vials containing standard fly medium with yeast, corn syrup, and agar, maintained at 25 °C and 60% humidity under a 12 h light–dark cycle. Eggs were collected from the flies during a 6 h period and preserved at the same temperature and humidity. After 72 h, the eggs were transferred to a 96-well tissue culture plate with 30 µL of standard fly medium, covered with a moistened paper filter disk infused with 230 ppm of prodigiosin, and kept at 25 °C. The number of surviving larvae was counted on the fifth day. | Lethal concentration causing 50% mortality in Drosophila larval (LC50) of PG was identified using a 5-day exposure period at a dose of 230 ppm. The mechanism of action was not specified. | [25] |
| Serratia nematodiphila 213C. A 50 mL fresh fermentation medium containing the following concentrations (g L−1), glycerol at 0.2 and peptone at 0.5, was prepared with a pH of 7.0 in 250 mL Erlenmeyer flasks. Each flask was inoculated with an inoculum that had an absorbance of 1.0 at 600 nm, at a concentration of 1% (v/v), and then incubated at 28 °C with shaking at 180 rpm for 72 h. Prodigiosine was purified through filtration using a glass wool-tied funnel or by centrifugation at 10,000 rpm for 15 min, and it was subsequently concentrated using a rotary vacuum evaporator at 50 °C under a vacuum of 10–5 torr. | Larvae of cotton bollworm Helicoverpa armigera and cotton leafworm Spodoptera litura. Second instar larvae were positioned in 12- or 24-well flat-bottom plates, utilizing pigment as their dietary feed. The plates were sealed and incubated in a humidified growth chamber set to 28 °C. The larvae were also placed on Petri dishes containing various concentrations of pigment applied to okra pods as food. The pigment’s larvicidal effect was assessed by counting the number of deceased larvae after 72 h and evaluating their motility through needle probing. | The mortality rates of the larvae of Helicoverpa armigera and Spodoptera litura were 70 and 100% when exposed to doses of 20 and 30 mg mL−1. The mechanism of action is likely to be due to a combination of factors, including the regulation of nitrogen-activated protein kinase, DNA damage, modulation of pH, and inhibition of the cell cycle. | [30] |
| Serratia marcescens NMCC 75. An active culture was inoculated into a medium containing sucrose and peptone and incubated for 24 h at 28 ± 2 °C. Following incubation, the culture was centrifuged at 7000× g for 10 min. The resulting cell pellet was suspended in methanol and centrifuged again. The crude pigment was then collected from the supernatant and heated to 90 °C. The dried pigment was re-dissolved in 2 mL of methanol and combined with 5 mL of sterile distilled water. After allowing the mixture to stand for 5 to 6 h, the pigment was separated on a silica gel column, with the elution performed using a hexane:methanol (1:2) solution. | Larval and pupal stages of mosquitoes of Aedes aegypti and Anopheles stephensi. Experiments were conducted at a temperature of 28 ± 2 °C and a relative humidity of 75–85%, following a light–dark cycle of 14:10 h. Bioassays were performed on early second, third, and fourth instar larvae. Twenty-five larvae from each stage were placed in a beaker containing 500 mL of dechlorinated tap water. For the initial screening, a concentration of 1 mg mL−1 was tested. Throughout a period of 6 to 24 h, the larvae were monitored for movement under an insect microscope. In the dose-response assay, various concentrations of prodigiosin were added to 100 mL of dechlorinated tap water, along with 25 larvae. Larval mortality was assessed over a period of 6 to 24 h. A similar methodology was applied to the pupae. | The LC50 values for the early second, third, fourth instar and pupal stages of A. aegypti were found to be 14 ± 1.2, 15.6 ± 1.48, 18 ± 1.3, and 21 ± 0.87 μg mL−1, respectively. For A. stephensi the LC50 values against the same stages were 19.7 ± 1.12, 24.7 ± 1.47, 26.6 ± 1.67, and 32.2 ± 1.79 μg mL−1, respectively. The mechanism of action involves the inhibition of the enzymes catalase, oxidase, carbonic anhydrase, and H+-V-ATPase. | [29] |
| Serratia sp. Peanut broth was inoculated with an overnight-grown culture of Serratia and incubated at 30 °C for 72 h. Following incubation, the mixture was centrifuged at 6000 rpm for 10 min. The pigment was extracted using acetone, methanol, or ethanol, and then centrifuged at 10,000 rpm for 10 min. Purification was carried out through TLC on silica gel G-60 F25, using a chloroform: methanol (95:5; v/v) mixture as the eluent. | The insecticidal efficacy was assessed through the application of a spray formulation targeting adult specimens of common household pests, including Periplaneta americana (American cockroach), Isoptera (termites), Dorymyrmex insanus (pyramid ants), and Solenopsis geminata (tropical fire ants). | 100 % mortality was achived against cockroaches and tropical ants, while 85 to 71% effectiveness was noted with termites and pyramid ants when spraying prodigiosin (concentration not specified). The mechanism of action was not specified. | [24] |
| Serratia marcescens Se9. A 24 h culture was inoculated into 250 mL of nutrient broth medium and incubated at 30 °C for 48 h. Afterward, it was centrifuged at 1000 rpm for 15 min at 4 °C. The pellet was resuspended in acidified ethanol and vortexed for 5 min. It was then centrifuged at 10,000 rpm for 15 min, and the supernatant was transferred to a sterile 50 mL Falcon tube. Finally, the solvent was removed and the solution was concentrated using a rotary evaporator. | Larval and adult stages of yellow mealworm Tenebrio molitor. 20 mg of the dry pigment were resuspended in 10 mL of sterile 96% ethanol and subsequently filtered through a 0.20 μm sterile syringe filter. Various concentrations of the pigment were tested on both fourth instar larvae and adults of Tenebrio molitor using a leaf disk feeding assay. Disks measuring 5 cm in diameter were cut from cabbage leaves, dipped in the pigment concentrations, and allowed to dry for 30 min. Twenty larvae or adults were then placed in a Petri dish maintained at 25 °C and 60% relative humidity under a 12:12 h light-dark photoperiod for a duration of 5 days for each concentration. | A dose of 125 ppm of crude extract resulted in a 5% mortality rate, while a concentration of 2000 ppm caused a mortality rate of 68% in larvae and 30% in adults. The LC50 for the crude pigment in adults was found to be 4570 ppm. The mechanism of action was not specified. | [27] |
| Serratia marcescens KH-001. The bacterium was initially cultured in LB medium for 12 h at 28 °C and 180 rpm. Following this, a 2.5% inoculum was introduced into 50 mL of a basal medium, which contained 1.35 mg L−1 of olive oil, 500 mg L−1 of MgSO4, and 500 mg L−1 of beef extract, and fermentation was carried out for 48 h to produce prodigiosin. The mixture was then extracted using an equal volume of acetonitrile and sonicated for 30 min, after which NaCl was added. The supernatant was collected and concentrated in a rotary evaporator set at 55 °C. The crude product was harvested and stored at −80 °C. The crude product was purified by dissolving in a mixture of petroleum ether: ethyl acetate (3:1, v/v) and then passed through a column, followed by elution with the same solvent mixture. | Asian citrus psyllid Diaphorina citri Kuwayama. Toxicity assays were conducted at various temperatures with 10 mg of dried extracted pigment dissolved in 10 mL of methanol. Fresh citrus leaves were immersed in the resulting solutions for 10 s. After drying, each leaf was placed in a Petri dish containing ten fifth instar nymphs and then positioned in an illuminated incubator set to 25 °C, 30 °C, and 35 °C, with a relative humidity of 68 ± 2% and a light–dark photoperiod of 14:10 h. The impact on oviposition was assessed using one-year-old potted citrus plants sprayed with an aqueous solution of 40 mg L−1 prodigiosin. Two male and two female newly emerged adults (0–1 day old) were put into each cage for oviposition at 30 °C and 60% relative humidity, under a 14:10 h light–dark photoperiod for 7 days. Additionally, the influence of prodigiosin on egg hatching was evaluated using eggs that had not been exposed to pesticides. The leaves containing the eggs were soaked in a 40 mg L−1 prodigiosin aqueous solution for 10 s and then maintained in an illuminated incubator at 30 °C with a relative humidity of 68 ± 2% and a 14:10 h light–dark photoperiod for 4 days. Feeding impact was assessed with citrus leaves soaked in aqueous solutions of prodigiosin (12 and 40 mg L−1) for 30 s. Two leaf discs were placed into a Petri dish with a 1.5% agar solid medium at the bottom to secure them. Ten adult insects were released into each dish and maintained in an illuminated incubator at 30 °C and 68 ± 2% relative humidity with a 14:10 h light–dark photoperiods for 48 h. | At 30 °C, doses of prodigiosin at 12.64 and 40.53 mg L−1 resulted in mortalities of 50% (LC50) and 20% (LC20) of nymphs after 24 h, respectively. At 25 °C, the LC20 and LC50 values to D. citri were 40.09 and 223.79 mg L−1, respectively. At 35 °C, the LC20 and LC50 values for prodigiosin were 1.71 and 27.90 mg L−1, respectively. In experiments conducted at 30 °C using concentrations of 12 and 40 mg L−1, an oviposition inhibitory rate of 42% was observed, while egg hatching rates were found to be 65.30%. Additionally, adult feeding with 12 and 40 mg L−1 led to reductions in feeding of 28.02 and 34.66%, respectively. The mechanism of action was not specified. | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintanilla-Villanueva, G.E.; Ríos-Del Toro, E.E.; Arvizu-De León, I.C.; Luna-Moreno, D.; Rodríguez-Delgado, M.M.; Villarreal-Chiu, J.F. Prodigiosin: A Potential Eco-Friendly Insecticide for Sustainable Crop Protection. Colorants 2025, 4, 18. https://doi.org/10.3390/colorants4020018
Quintanilla-Villanueva GE, Ríos-Del Toro EE, Arvizu-De León IC, Luna-Moreno D, Rodríguez-Delgado MM, Villarreal-Chiu JF. Prodigiosin: A Potential Eco-Friendly Insecticide for Sustainable Crop Protection. Colorants. 2025; 4(2):18. https://doi.org/10.3390/colorants4020018
Chicago/Turabian StyleQuintanilla-Villanueva, Gabriela Elizabeth, Esther Emilia Ríos-Del Toro, Iris Cristina Arvizu-De León, Donato Luna-Moreno, Melissa Marlene Rodríguez-Delgado, and Juan Francisco Villarreal-Chiu. 2025. "Prodigiosin: A Potential Eco-Friendly Insecticide for Sustainable Crop Protection" Colorants 4, no. 2: 18. https://doi.org/10.3390/colorants4020018
APA StyleQuintanilla-Villanueva, G. E., Ríos-Del Toro, E. E., Arvizu-De León, I. C., Luna-Moreno, D., Rodríguez-Delgado, M. M., & Villarreal-Chiu, J. F. (2025). Prodigiosin: A Potential Eco-Friendly Insecticide for Sustainable Crop Protection. Colorants, 4(2), 18. https://doi.org/10.3390/colorants4020018







