Revolutionizing Wastewater Treatment: Harnessing Metal–Organic Frameworks for Exceptional Photocatalytic Degradation of Azo-Type Dyes
Abstract
1. Introduction
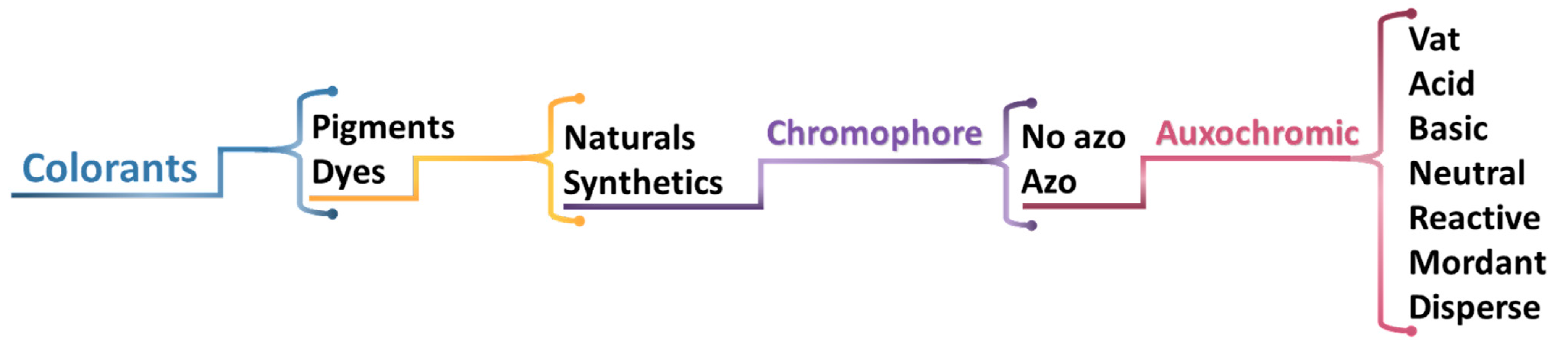
1.1. Colorants
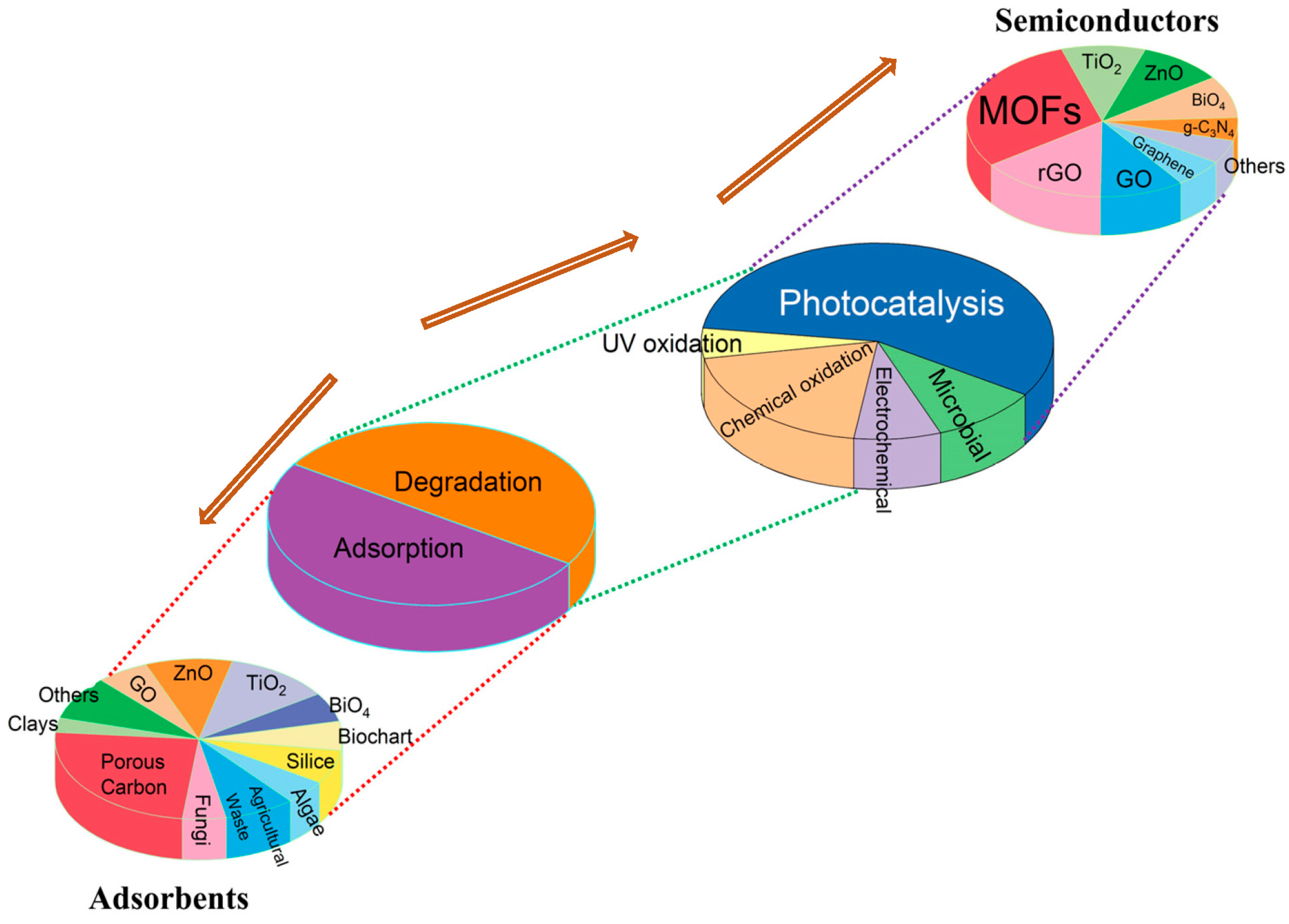
1.2. Main Degradation Methods of Dyes in Wastewater
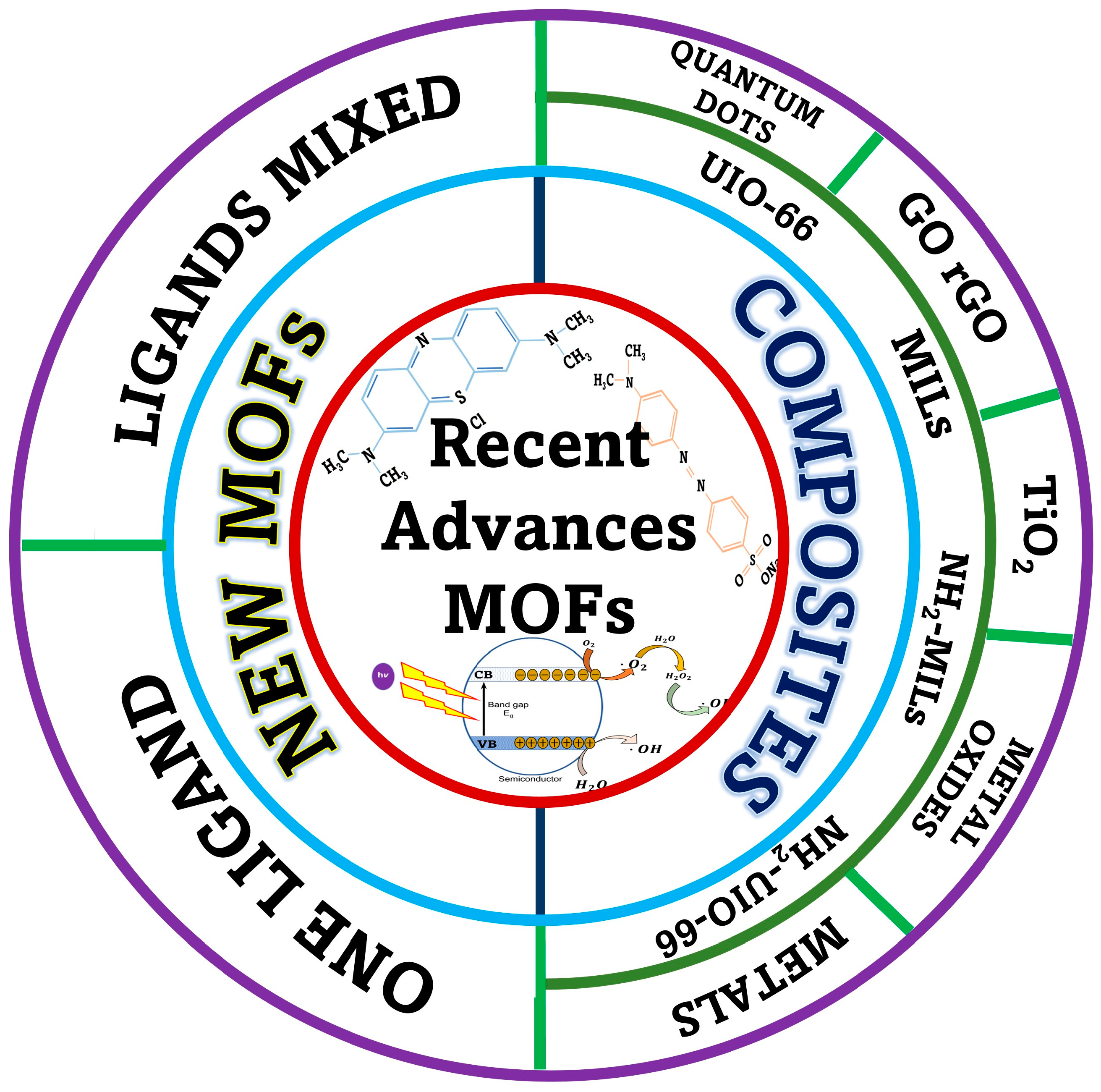
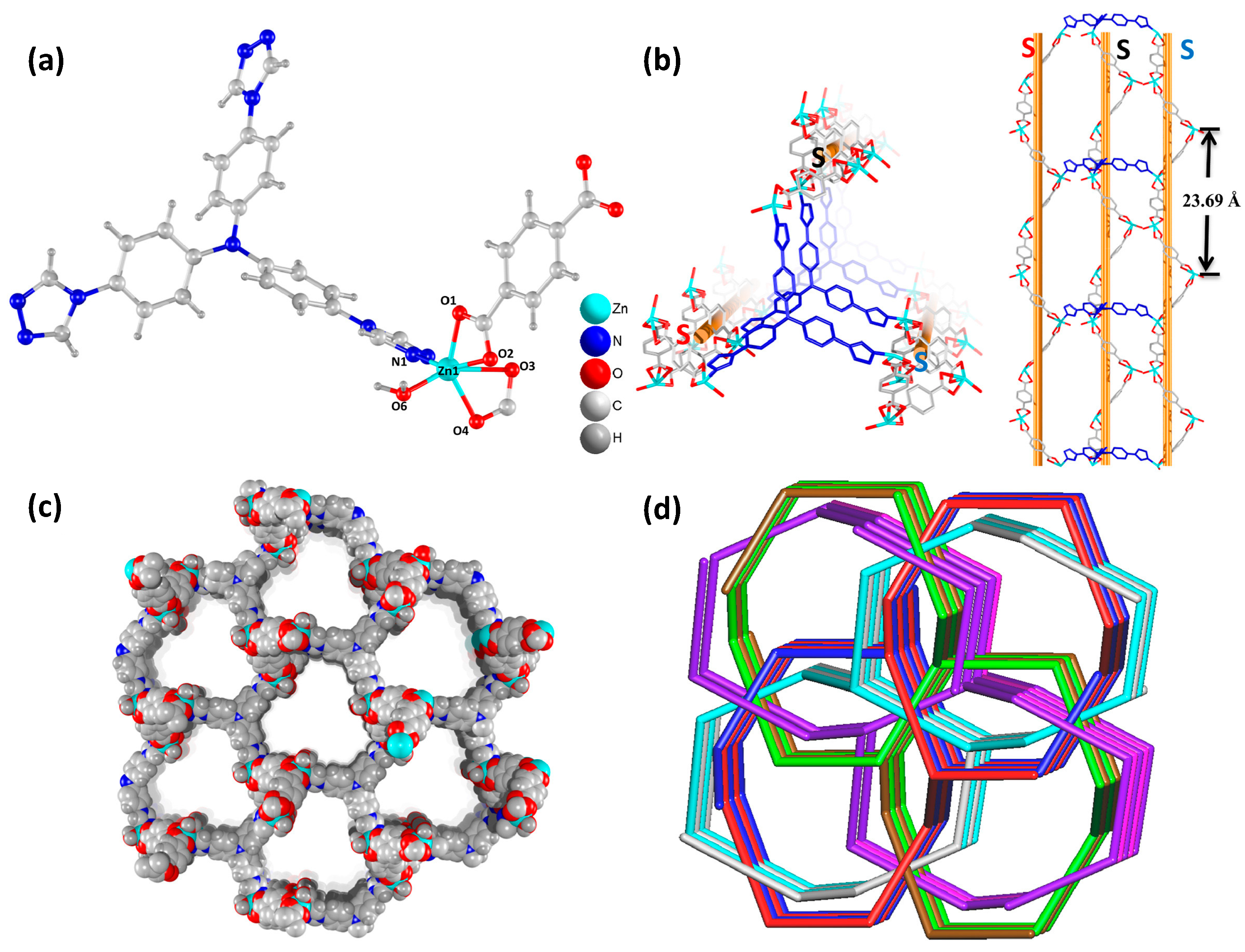
1.3. Fundamentals of Metal–Organic Frameworks (MOFs) as Semiconductors
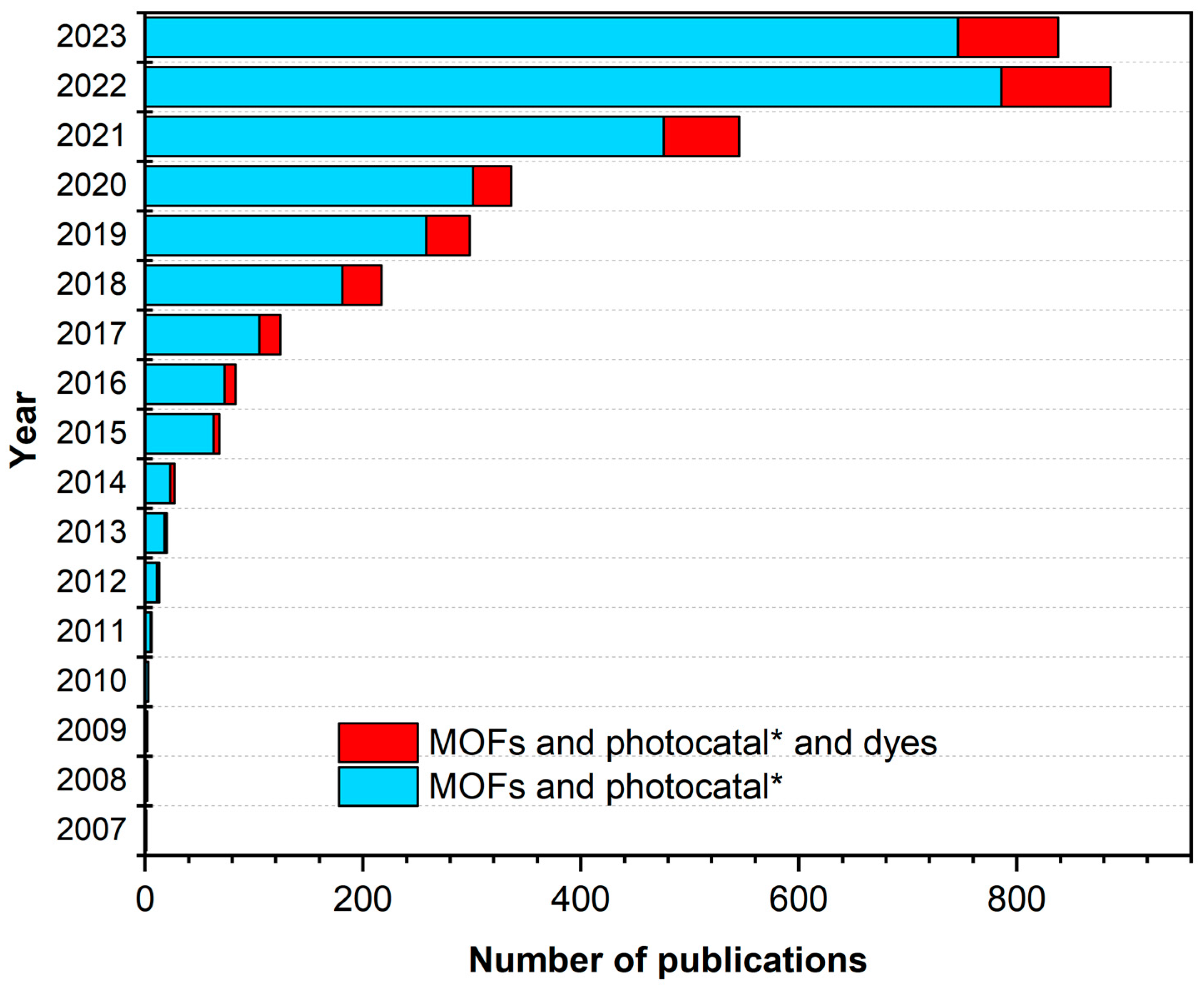
1.4. Paragraph in the Degradation of Dyes Using MOFs
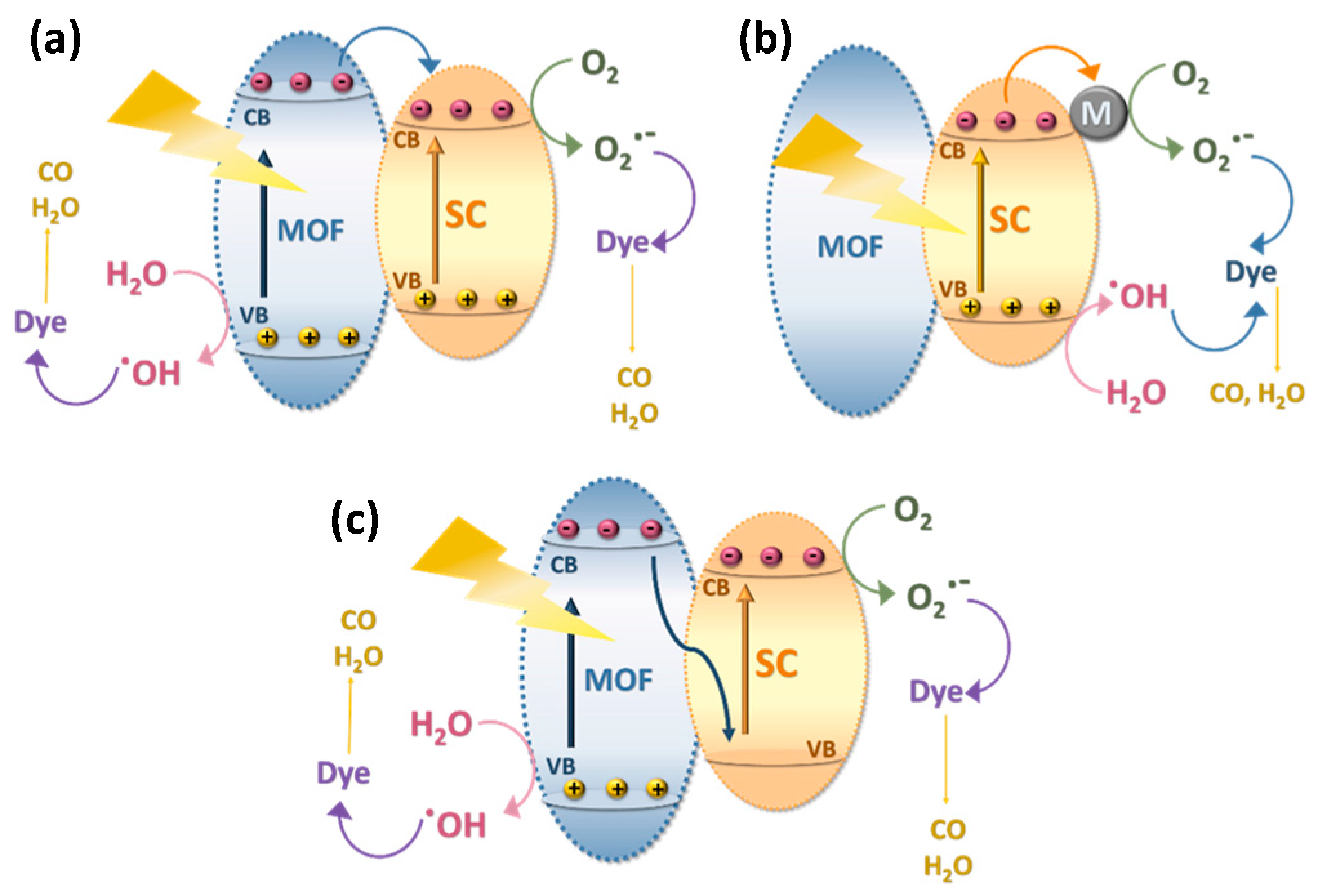
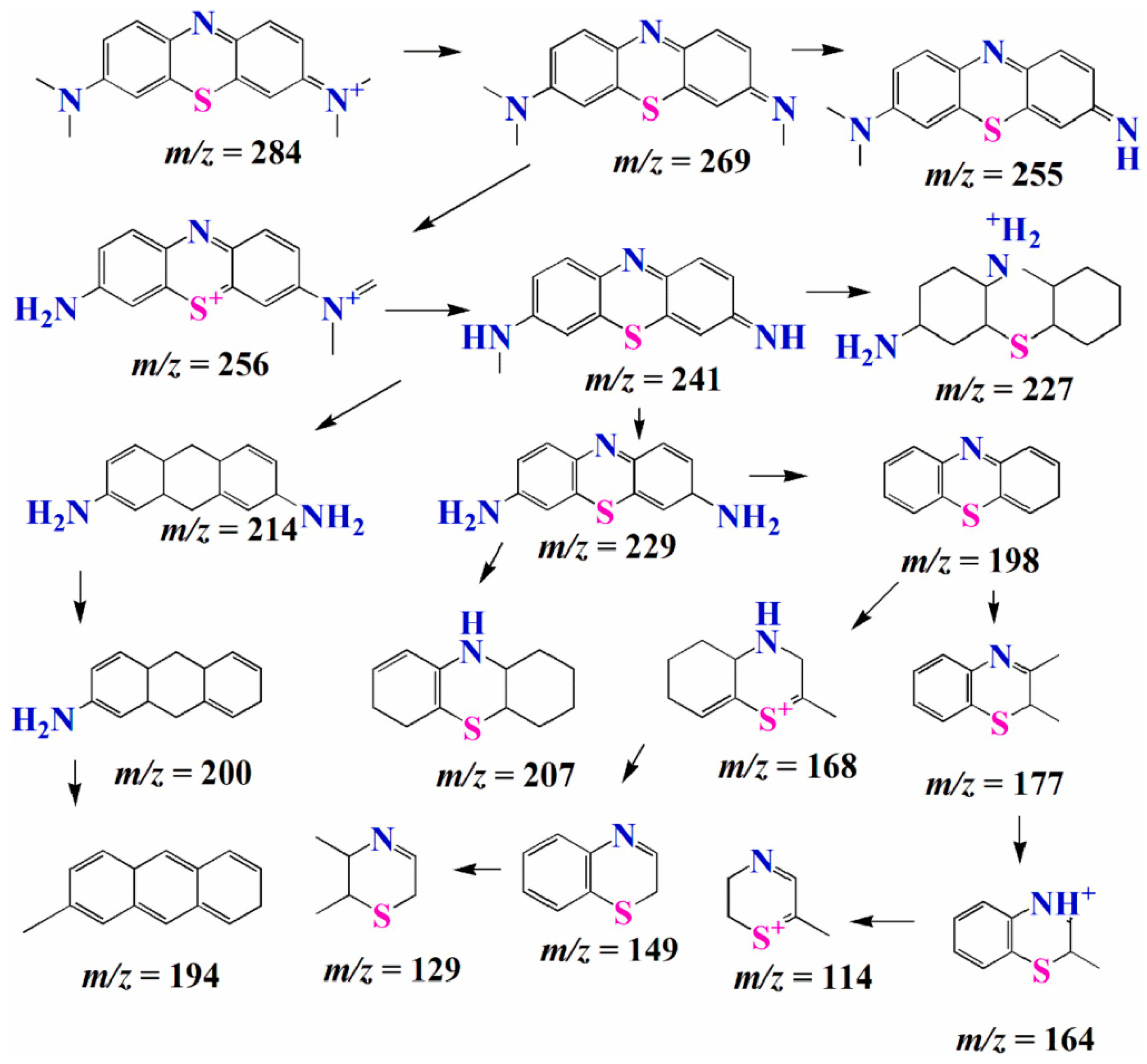
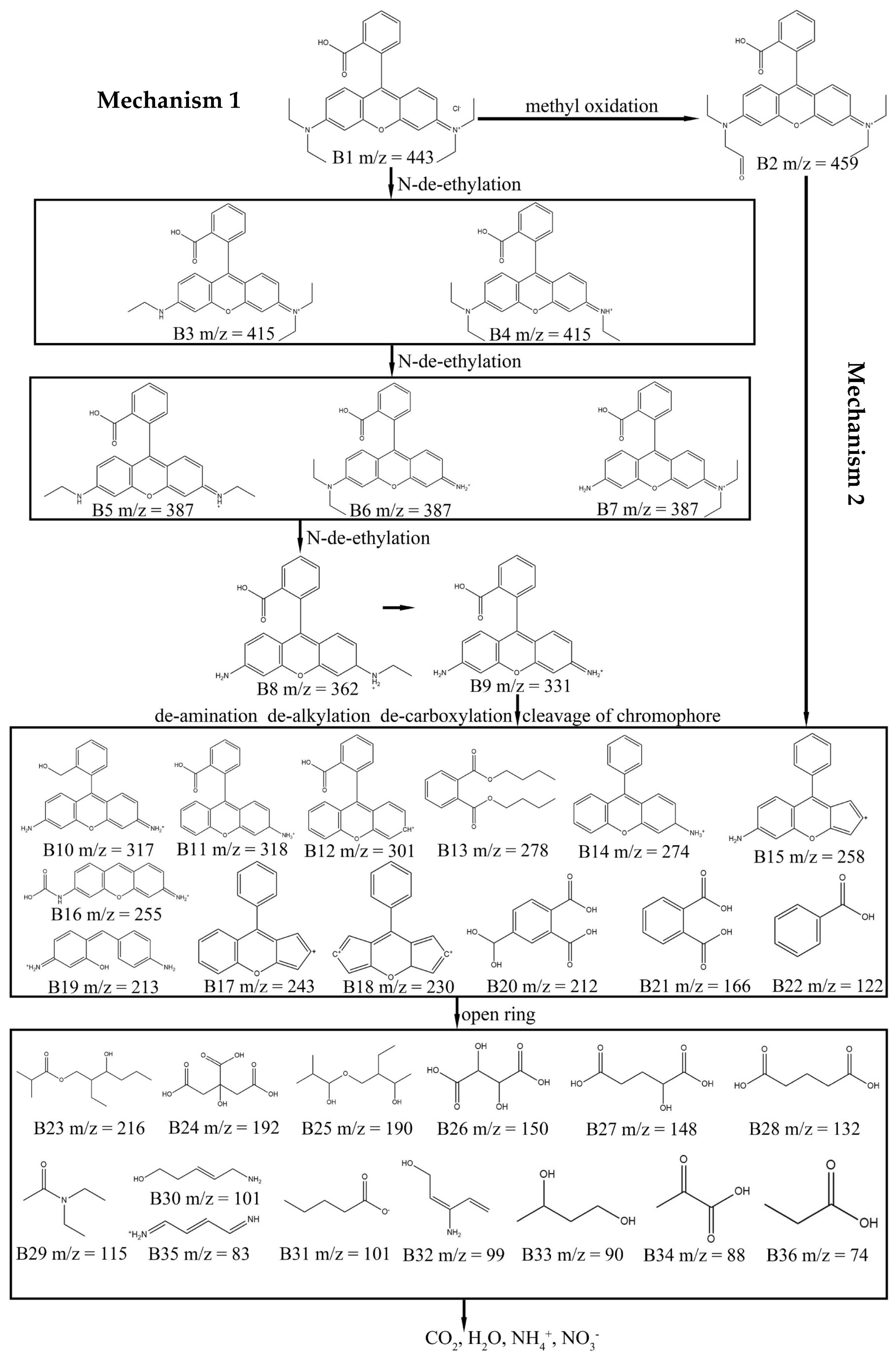
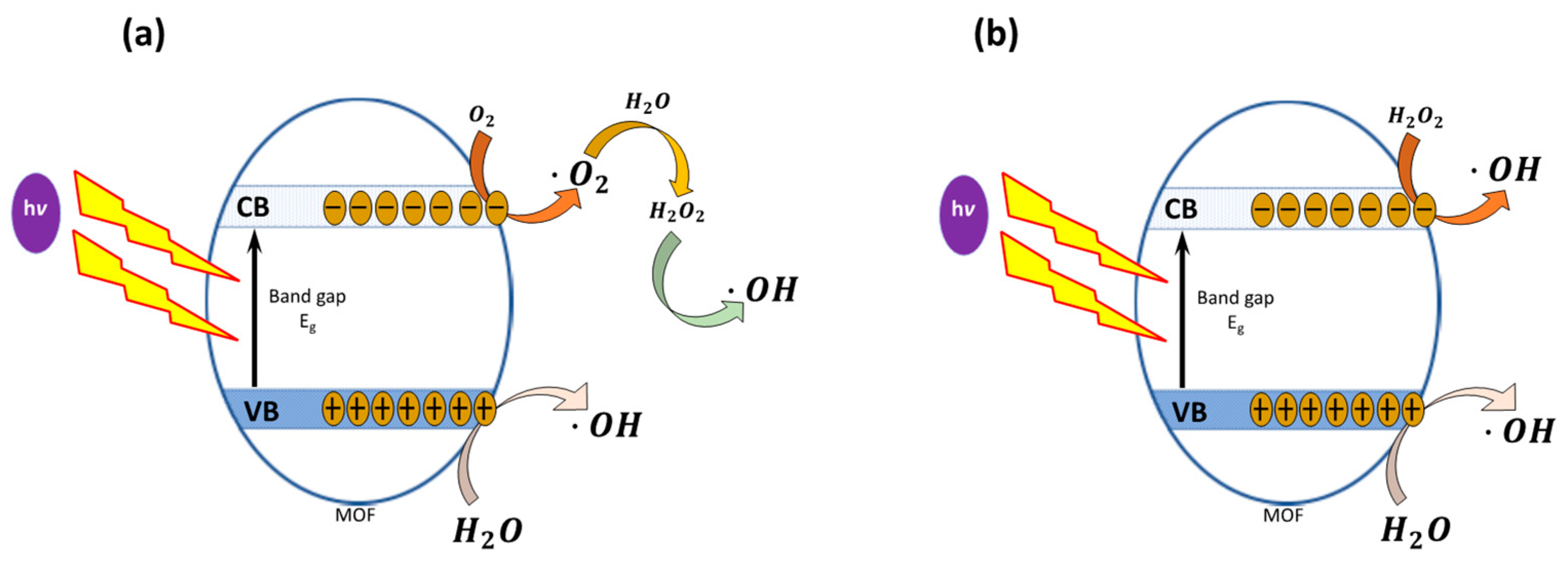
1.5. Possible Degradation Mechanism of Dyes
2. Recent Advances in the Photocatalytic Degradation of Dyes According to Classification
2.1. Acid Dyes
2.2. Basic-Dyes
2.3. Reactive Dyes
2.4. Other Dyes
| Dye Common Name/Color Index | Sample | Band Gap (eV) | Time (min) | Degradation Efficiency (%) | Reaction Conditions | Stability | Ref. |
|---|---|---|---|---|---|---|---|
| Acid Red 1 | Cl-M88A (MIL-88A (Fe)) (1) NIT-M88A (MIL-88A (Fe)) (2) | 2.17 2.19 | 120 90 | 100 100 | 20 mg/L dye: pH = 7; conc. catalyst = 1 g/L; 0.03 mL of H2O2; Xe lamp | stable | [40] |
| PBS/Acid Red 66 RhB/Basic Violet 10 | Ag/Ag2WO4/UiO-66 | 2.06 | 25 (PBS) 60 (RhB) | 95 (PBS) 90 (RhB) | 60 mg/L dye, pH = 7; conc. catalyst = 0.2 g/L; Halogen Lamp | stable | [50] |
| Acid Black 1 | MIL-53 (Cr)/polymer (1) HKUST-1 (Cu) (2) | 2.1–2.4 | 30 | 96 90 | UV lamp | stable | [114] |
| MB/Basic Blue 9 MV/Basic Violet 3 RhB/Basic Violet 10 | [Co4(bbibp)5(HCOO)8(H2O)2]n (1) [Co(bimmb)(NO3)2]n (2) | 2.50 3.07 | 75 | (1): 100 (MB); 50.8 (MV); 61.0 (RhB) (2) 94 (MB); 68.7 (MV); 64.4 (RhB) | 6 mg/L dye; pH = 7; conc. catalyst = 0.2 g/L; H2O2 = 0.5 mL; UV high pressure lamp | - | [57] |
| RhB/Basic Violet 10 CB/Crome blue MB/Basic Blue 9 | [Mn4(NDC)2(bb)4] | 2.53 | 100 | 94.33 (RhB) 34.91 (CB) 29.35 (MB) | 10 mg/L dye; pH = 7; conc. catalyst = 0.8 g/L; Hg Lamp | stable | [102] |
| AO7/AO7 MB/Basic Blue 9 | CF/MoS2/NH2-MIL-125(Ti) | 2.5 | 120 | 67.9 (AO7) 94.3 (MB) | 10 mg/L dye; pH = 7; conc. catalyst = 1.5 g/L; visible light | stable | [49] |
| MB/Basic Blue 9 | SrAl2O4:Eu2+,Dy3+/g-C3N4@NH2-UiO-66 | 2.77 | 30 | 95 | 20 mg/L dye; pH = 7; conc. catalyst = 0.4 g/L; Xe lamp | stable | [51] |
| MV/Basic Violet 3 | [Co2(μ3–OH) (L)(H2O)3·2•75H2O]n (1) [Co2 (μ3 –OH)(L)(bib)(H2O)2]n (2) | 3.02 1.91 | 40 | 55.3 (1) 61.3 (2) | 10 mg/L dye; pH = 7; conc. catalyst = 0.1 g/L; Hg lamp | stable | [115] |
| MV/Basic Violet 3 | [Zn2(1,4- BDC)2(bmp)•DMF] (1) [Cd3(H2O)2(bmp)2(DMF)(BTC)2•3•5H2O•1•5DMF](2) | - - | 40 | 70.1 (1) 37.83 (2) | - | stable | [100] |
| MV/Methyl Violet CV/Basic Red 5 NR/Basic Red 5 CR/Direct Red 28 | {[Co3 (BTC)2 (Bimb)2.5]·2H2O}n | 3.94 | 135 | 68.7 (MV) 77.5 (CV) 19.5 (NR) 15.8 (CR) | 10 mg/L dye; pH = 7; 5 mL of methanolic solution (0.01 mmol powder in 50 mL methanol); UV lamp | - | [113] |
| MB/Basic Blue 9 MV/Basic Violet 3 | [CoL (bimb)0.5]n (1) {[CoL (bimmb)] DMF}n (2) [CoL (bimmb)0.5]n (3) [CoL (bbibp)]n (4) | - - | 120 | MV: 85.5 (1); 78.4 (2); 84.2 (3); 96.2 (4) MB: 45.8 (1); 65.0 (2); 82.9 (3); 84.0 (4) | 6 mg/L dye; pH = 7; conc. catalyst = 0.2 g/L; Hg lamp | stable (4) | [58] |
| RhB/Basic Violet 10 | {[Cu8Cl5(CPT)8(H2O)4](HSiW12) (H2O)20(CH3CN)4}n | 2.69 | 70 | 97 | 10 mg/L dye; pH = 7; conc. catalyst = 0.375 g/L; H2O2 = 1 mL; visible light | stable | [94] |
| CV/Basic Violet 3 AzI/52010 NR/Basic Red 5 | Mgtptc based on MgO6 octahedral (Mgtptc) | 2.84 | 55 50 55 | 90.94 (CV) 100 (Az1) 94.39 (NR) | - | stable | [92] |
| MB/Basic Blue 9 MV/Basic Violet 3 MG/Basic Green 4 | {[Mn2(L)(tib)(H2O)] 3DMA 4H2O}n (1) {[Co2(L)(bipd)2] 3H2O}n (2) {[Co(L)0.5(bimb)] 3H2O 0.5O2}n (3) | 3.35 3.37 3.22 | 120 | (1): 59.6 (MB); 63.6 (MV); 55.2 (MG); (2) 85.8 (MB); 88.8 (MV); 82.3 (MG) (3) 91.9 (MB); 93.8 (MV); 92.8 (MG) | 6 mg/L dye; pH = 7; conc. catalyst = 0.2 g/L; UV light | stable (compound 2,3) | [116] |
| MV/Basic Violet 3 MB/Basic Blue 9 | Co(L)(tib)(-H2O)2]n (1) [Co(L)(bip)0.5]n (2) | 2.63 2.80 | 120 | (1): 92.1 (MV); 75.2 (MB) (2): 89.7 (MV); 57.7 (MB) | 10 mg/L dye; pH = 7; conc. catalyst = 0.2 g/L; Hg lamp | stable | [59] |
| MB/Basic Blue 9 MV/Basic Violet 3 RhB/Basic Violet 10 | Cu-tipe/GO composite | 1.35 | 30 45 60 | 90.4 (MB) 92.2 (MV) 91.3 (RhB) | 10 mg/L dye; pH = 7; conc. catalyst = 0.4 g/L; H2O2 = 0.5 mL; visible light | - | [78] |
| MB/Basic Blue 9 | MIL-53(Al)@TiO2 | 3.05 | 240 | 95 | 20 mg/L dye; pH = 7; conc. catalyst = 0.1 g/L; visible light | - | [55] |
| MB/Basic Blue 9 | Cu(ttpa)-1@GO | - | 30 | 93.3 | visible light | - | [79] |
| RhB/Basic Violet 10 | [EuCd(pbbp)2(SO4)2Cl2(H2O)3] | - | 120 | 92 | 1 × 10−5 M dye; pH = 7; conc. catalyst = 1 g/L; H2O2 = 1 mL; Xe lamp | stable | [117] |
| CR/Direct Red 28 MB/Basic Blue 9 RhB/Basic Violet 10 | Co-doped MIL-53(Al) | 2.63 | 16 (CR) 12 (MB) 16 (RhB) | 99 (CR) 99 (MB) 99 (RhB) | 30 mg/L dye; pH = 7; conc. catalyst = 0.3 g/L; Sunlight | stable | [54] |
| RhB/Basic Violet 10 MB/Basic Blue 9 MO/Acid Orange 52 | {[Zn3(L)(BDC)3(H2O)3] 3.5H2O}n | 2.94 | 70 | 90 (RhB) 90 (MB) 90 (MO) | 15 mg/L dye; pH = 7; conc. catalyst = 0.33 g/L; UV light | - | [94] |
| MB/Basic Blue 9 | [Cd(4- Hptz)2(H2O)2Cl2] (1) [Cu(btx)2(ClO4)2]n (2) [Cu(btx)(ClO4)]n (3) | 4.12 3.58 2.59 | 100 | 93.43 (1) 47.32 (2) 14.60 (3) | 10 mg/L dye; pH = 7; conc. catalyst = 0.175 g/L; Hg lamp | stable | [118] |
| MB/Basic Blue 9 MO/Acid Orange 52 RhB/Basic Violet 10 | [Cu3(L)2(4,4′ -bipy)] | 2.82 | 100 | 23.19 (RhB) 9.26 (MO) 81.17 (MB) | 10 mg/L dye; pH = 7; catalyst: 40 mg | stable | [89] |
| RhB/Basic Violet 10 | bimetallic MOF anchored corncob calcined derived activated carbon (CCAC) | 2.55 | 75 | 100 | 20 mg/L dye; pH = 7;conc. Catal yst = 0.05 g/L; Xe lamp | stable | [119] |
| RhB/Basic Violet 10 | Ni-MOF (1) NH2-MIL-101(Fe) (2) Ti-MOF (3) | 2.77 1.63 2.55 | 90 | 9 (1) 90 (2) 50 (3) | 20 mg/L dye; pH = 7;conc. Catal yst = 1 g/L; Solar light | stable | [56] |
| RhB/Basic Violet 10 | Zr6O4(OH)4 (ABDC)6/natural sepiolite (Sp) clay | 2.85 | 120 | 64 | 20 mg/L dye; pH = 5.9; conc. catal yst = 0.5 g/L visible light | - | [120] |
| NR/Basic Red 5 | {[Co(bcpt)(bib)3/2(H2O)] H2O}n (1) {[Ni(bcpt)(bib)3/2(H2O)] H2O}n (2) | 3.6 2.9 | 100 | See information supplementary | [121] | ||
| MV/Basic Violet 3 | [Co4(μ3-OH)2(H2O)2(L)2 (bib)1.5(CH3CN) 3H2O CH3CN] (1) [Co8(μ3-OH)4(L)4 (bib)4 8CH3CN] (2) | 3.64 2.88 | 100 | 67.99 78.22 | UV light | - | [91] |
| MV/Basic Violet 3 | [NH2(CH3)2][Zn2O(bmp)(BTC)] (1) [NH2(CH3)2][Zn2O(bmp)(BTC)]·1·5DMF (2) | 3.63 3.96 | 40 | 38.25 55.95 | 10 mg/L dye; pH = 7; conc. catal yst = 0.8 g/L; UVlamp | Stable | [122] |
| MB/Basic Blue 9 | CdS/Cd-MOF | 2.36 | 100 | 20 mg/L dye; pH = 7; conc. catal yst = 0.1 g/L; Xe lamp | Stable | [123] | |
| MB/Basic Blue 9 CR/Direct Red 28 MO/Acid Orange 52 RhB/Basic Violet 10 | CuCd-BMOF/GO-x | 2.7 | 40 | 90.1 (MB) 85.4 (CR) 79.6 (MO) 87.6 (RhB) | 20 mg/L dye; pH = 7; conc. catal yst = 0.6 g/L; Xe lamp | Stable | [41] |
| MV/Basic Violet 3 MG/ RhB/Basic Violet 10 | NH2-UiO-66/FeOOH quantum dots | 2.53 | 180 | 82.2 (MV) 94.3 (MG) 81.5 (RhB) | 20 mg/L dye; pH = 7; conc. catal yst = 0.6 g/L; 30 mM H2O2; LED lamp | stable | [80] |
| MB/Basic Blue 9 | NH2-MIL-125(Ti)/polysulfone membrane | - | 300 | 88 | 10 mg/L dye; pH = 7; conc. catalyst = 0.2 g/L; visible light | - | [48] |
| RhB/Basic Violet 10 | Bi-TATB | 3.21 | 180 | 99.1 | 3 × 10−5 M dye; pH = 7; conc. catalyst = 0.1 g/L; LED light | stable | [124] |
| MB/Basic Blue 9 | Ce-MOF/g-C3N4–TS | 2.45 | 120 | 100 | 10 mg/L dye; pH = 7; conc. catalyst = 0.25 g/L; UVlight | stable | [125] |
| MB/Basic Blue 9 MV/Basic Violet 3 RhB/Basic Violet 10 | {[Ni(L)(bpt)(H2O)] 2H2O}n | - | 90 | 89.5 (MB) 84.7 (MV) 76.4 (RhB) | 6 mg/L dye; pH = 7; conc. catalyst = 0.2 g/L; UV light | - | [104] |
| MO/Acid Orange 52 | STA-12Fe | 2.6 | 40 | 12 | 10 mg/L dye; pH = 7; conc. catalyst = 0.2 g/L; Sun light | stable (Fe) | [126] |
| MB/Basic Blue 9 CR/Direct Red 28 MO/Acid Orange 52 RhB/Basic Violet 10 | CuCd-BMOF/GO-x | 2.7 | 40 | 92 (Mb) 90.1 (CR) 85.4 (MO) 79.6 (RhB) | 10 mg/L dye; pH = 7; conc. catalyst = 0.6 g/L; Xe lamp | stable | [41] |
| MB/Basic Blue 9 MV/Basic Violet 3 RhB/Basic Violet 10 | {[Cd(HL)(tib)(H2O)] 1.5H2O}}n (1) {[Co3(L)2(tib)2] 4H2O}n (2) [Cu(L)(3,5-bibp)]n (3) | 3.13 3.32 3.37 | 90 | (1): 87.4 (MB); 67.3 (MV); 66.5 (RhB) (2): 83.7 (MB); 48.4 (MV); 33.5 (RhB) (3) 86.9 (MB); 55 (MV); 53.4 (RhB) | 6 mg/L dye; pH = 7; conc. catalyst = 0.2 g/L; UV light | stable | [112] |
| RhB/Basic Violet 10 | [Mn(L)0.5(H2O)] (1) [Cd(L)0.5(H2O)] (2) | 2.84 3.05 | 150 | 90.5 (1) 70.5 (2) | 10 mg/L dye; pH = 7; conc. catalyst = 1 g/L; UV light | stable | [105] |
| RhB/Basic Violet 10 | Ag/AgCl@CoFe2O4/NH2-MIL-125(Ti) | 2.73 | 10 | 97 | 20 mg/L dye; pH = 9; conc. catalyst = 0.8 g/L; H2O2 = 20 μL; Visible light | stable | [47] |
| MB/Basic Blue 9 RhB/Basic Violet 10 | MoS2@MIL-88(Fe) | 2.75 | 60 | 98.5 (MB) 97.4 (RhB) | 50 mg/L RhB; pH = 7; conc. catalyst = 1 g/L; Xe lamp | stable | [127] |
| MB/Basic Blue 9 | Ag3PO4/UiO-66(Zr)/g-C3N4 | 2.9 | 240 | 61.7 | 10 mg/L dye; pH = 7; conc. catalyst = 0.7 g/L; Visible light | [128] | |
| MB/Basic Blue 9 MV/Basic Violet 3 | [Cd(bimd)(Hbipa)(H2O)]n (1) {[Zn(bipd)(Hbipa)] H2O}n (2) [Zn(bimb)(Hbipa)]n (3) | 2.24 2.55 2.36 | 120 | (1): 90.3 (MB); 85.7 (MV) (2): 89.8 (MB); 89 (MV) (3): 92.9 (MB);95.3 (MV) | 6mg/L dye; pH = 7; conc. catalyst = 0.2 g/L; UV light | - | [111] |
| AO7/Acid Orange 7 MB/Basic Blue 9 CR/Direct Red 28 | Ag2CrO4/MIL-53(Fe) | 2.22 | 140 | 95.5 (AO7) ~60 (MB) ~80 (CR) | 20mg/L dye; pH = 6; conc. catalyst = 1 g/L; Halogen lamp | stable | [98] |
| MB/Basic Blue 9 MV/Basic Violet 3 | NH2-MIL-101(Fe)@CuCoNi | 2.56 | 120 | 99.8 (MB) 93.8 (CV) | 25mg/L dye; pH = 6; conc. catalyst = 1 g/L; 20 μL H2O2; Visible light | stable | [129] |
| MG/Basic Green 4 MO/Acid Orange 52 | {[Zn2(DIPA)2(bimp)5]·DMF 2H2O}n (1) {[Zn2(HBPPA)2(bibp)2]·2H2O}n (2) | 2.08 2.26 | 120 | (1): 96.6 (MB); 78.6 (MV) (2): 98.3 (MB); 96.9 (MV) | 6mg/L dye; pH = 7; conc. catalyst = 0.2 g/L; Hg light | stable | [130] |
| RhB/Basic Violet 10 | g-C3N4/ZnFe2O4/UiO-66 | 2.8 | 60 | 98 | visible light | stable | [83] |
| RhB/Basic Violet 10 | NH2-MIL-101(Fe)/copper oxide | 50 | 100 | 50 mg/L dye; pH = 6.5; conc. catalyst = 0.5 g/L; LED | - | [131] | |
| MB/Basic Blue 9 MO/Acid Orange 52 | Al-PcCl@NH2-MIL-125 (1) CoPc@NH2-MIL-125 (2) CoPcPA@NH2-MIL-125 (3) | 2.47 2.42 2.54 | 120 | (1): 85(MB); 19 (MO) (2): 79 (MB); 25 (MO) (3): 99 (MB); 18 (MO) | 30 mg/L dye; pH = 7; conc. catalyst = 0.5 g/L; Visible light | Stable | [46] |
| RhB/Basic Violet 10 | NH2-UiO-66/BiOBr/Bi2S3 | 60 | 92 | 20 mg/L dye; pH = 7; conc. catalyst = 0.5 g/L | stable | [52] | |
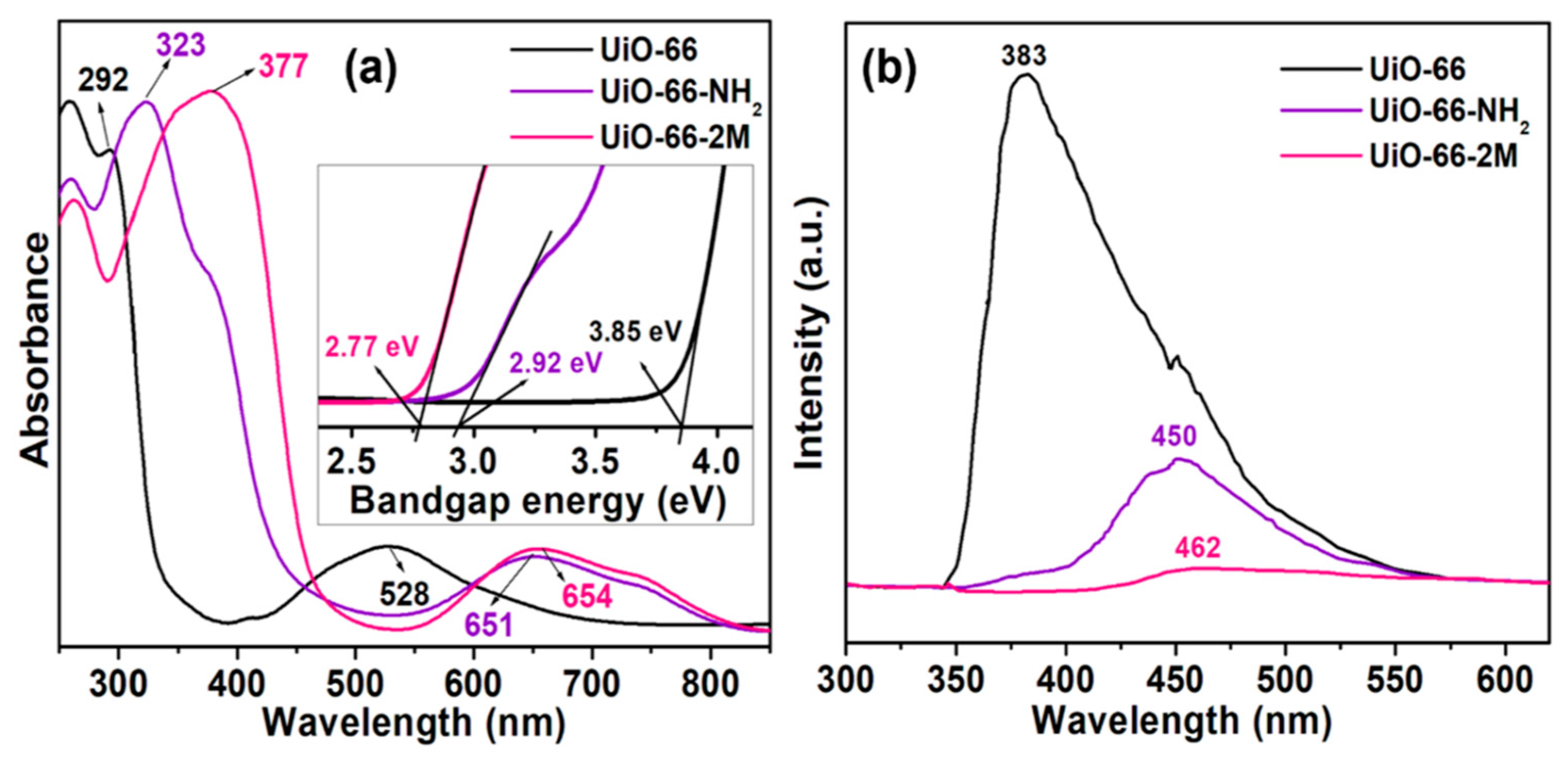
| RhB/Basic Violet 10 CR/Direct Red 28 | UiO-66 (1) UiO-66-NH2 (2) UiO-66-2M (3) | 3.85 2.92 2.77 | 120 | (1): 48 (RhB); 52 (CR) (2): 95 (RhB); 64 (CR) (3): 69 (RhB); 48 (CR) | 10 mg/L dye; pH = 7; conc. catalyst = 0.4 g/L | stable (3) | [53] |
| BG/Green Basic 4 | Cu4(O) (OH)3(bb)4(NO3)30.3 H2O 3CH3CN]n | 2.32 | 120 | 96.8 | 40 mg/L dye; pH = 5; mass catalyst = 30 mg | - | [132] |
| MB/Basic Blue 9 RhB/Basic Violet 10 | Ag3VO4@MIL-125-NH2 (1) Ag2WO4/MIL-125-NH2 (2) | 2.27 2.57 | 60 | (1): 75 (MB); 75.3 (RhB) (2): 65 (MB); 50.3 (RhB) | 5 mg/L dye; pH = 7; conc. catalyst = 1 g/L; visible light | stable | [45] |
| RhB/Basic Violet 10 | FCAU-17 (flakes) (Bi) (1) CAU-17 (rods) (Bi) (2) | 3.75 3.83 | 80 | 78.6 (1) 96.1 (2) | 5 mg/L dye; pH = 7; conc. catalyst = 0.1 g/L | [133] | |
| MB/Basic Blue 9 | amino acid-based carbon quantum dot/MIL-53 (Fe) binary composite | 2.63 | 120 | 98 | 35 mg/L dye; pH = 9; conc. catalyst = 0.1 g/L; Xe lamp | stable | [81] |
| RhB/Basic Violet 10 | NH2-MIL-125 (Ti)/Ag/NiFe layered double hydroxide (Ti-MOF/Ag/NiFeLDH) | 50 | 95 | 15 mg/L dye; pH = 7; conc. catalyst = 0.5 g/L; Xe lamp | stable | [134] | |
| RhB/Basic Violet 10 | MIL-125/g-C3N4/sodium alginate | - | 120 | 99.41 | 310 mg/L dye; pH = 5; Xe lamp | stable | [82] |
| MB/Basic Blue 9 | Ag@{[Zn2(bta)(bpy)(H2O)2] 2H2O}n) | 2.86 | 160 | 86 | 10 mg/L dye; pH = 6; conc. catalyst = 1 g/L; Xe lamp | - | [77] |
| MB/Basic Blue 9 | [Zn4(tmlb)4(H2O)]n (1) [Ni4(tmlb)4(H2O)]n (2) [Cu(tmlb)]n (3) [Co(tmlb)]n (4) [Pb(tmlb) (H2O)]n (5) | 3.92 3.89 3.9 3.75 3.8 | 125 | 87.1 (1) 69.6 (2) 94.4 (3) 75.4 (4) 74.2 (5) | 6 mg/L dye; pH = 6; conc. catalyst = 0.2 g/L; Hg lamp | stable | [110] |
| MV/Basic Violet 3 | {[Co2(bdc)2(bmp)1.5]·DMF·1.5H2O}n | 40 | 68 | 10 mg/L dye; pH = 6; conc. catalyst = 0.8 g/L; Hg lamp | stable | [101] | |
| MV/Basic Violet 3 | [Co2(μ2- H2O)(L)(2,2′-bipy)2]n (1) [Co2(μ2-H2O)(L)(phen)2]n (2) | - - | 40 | 69 (1) 61 (2) | 10 mg/L dye; pH = 6; conc. catalyst = 0.8 g/L; UV lamp | - | [135] |
| AO7/Acid Orange 7 MB/Basic Blue 9 RhB/Basic Violet 10 | Ag2CrO4/Cu (BDC) | 2.92 | 100 | 98 (AO7) 68 (MB) 46 (RhB) | 20 mg/L RhB; pH = 6; mass catalyst = 15 mg; Halogen lamp | stable | [99] |
| MB/Basic Blue 9 | AmCoPc/UiO-66-NH2 amino cobalt phthalocyanine (AmCoPc) | 2.21 | 180 | 80.17 | 10 mg/L RhB; pH = 6; conc. catalyst = 1 g/L; Xe lamp | stable | [136] |
| MB/Basic Blue 9 | Ce/Co bimetallic MOFs with the mixed ligands | 3.2 | 120 | 82.3 | 10 mg/L RhB; pH = 6; conc. catalyst = 1 g/L; Xe lamp | stable | [137] |
3. Challenges, Perspectives, and Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pregi, E.; Kun, D.; Faludi, G.; Móczó, J.; Pukánszky, B. Modeling the Mechanical Properties of Polypropylene/Lignin/Flax Hybrid Composites. Mater. Des. 2022, 220, 110833. [Google Scholar] [CrossRef]
- Varzakas, T.; Tzia, C. Handbook of Food Processing: Food Safety, Quality, and Manufacturing Processes; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-4987-2178-3. [Google Scholar]
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Gürses, A.; Karaca, S.; Doğar, Ç.; Bayrak, R.; Açıkyıldız, M.; Yalçın, M. Determination of Adsorptive Properties of Clay/Water System: Methylene Blue Sorption. J. Colloid Interface Sci. 2004, 269, 310–314. [Google Scholar] [CrossRef]
- Lipskikh, O.I.; Korotkova, E.I.; Khristunova, Y.P.; Barek, J.; Kratochvil, B. Sensors for Voltammetric Determination of Food Azo Dyes—A Critical Review. Electrochim. Acta 2018, 260, 974–985. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile Finishing Dyes and Their Impact on Aquatic Environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef] [PubMed]
- Sekar, N. 15—Acid Dyes. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2011; Volume 1, pp. 486–514. ISBN 978-1-84569-695-5. [Google Scholar]
- El Qada, E.N.; Allen, S.J.; Walker, G.M. Adsorption of Basic Dyes from Aqueous Solution onto Activated Carbons. Chem. Eng. J. 2008, 135, 174–184. [Google Scholar] [CrossRef]
- Hunger, K.; Mischke, P.; Rieper, W.; Raue, R.; Kunde, K.; Engel, A. Azo Dyes. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; ISBN 978-3-527-30673-2. [Google Scholar]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; Karthikeyan, D.; Lee, Y.R. Effective Photocatalytic Degradation of Anthropogenic Dyes Using Graphene Oxide Grafting Titanium Dioxide Nanoparticles under UV-Light Irradiation. J. Photochem. Photobiol. Chem. 2017, 333, 92–104. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent Advances Based on the Synergetic Effect of Adsorption for Removal of Dyes from Waste Water Using Photocatalytic Process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Ahn, D.-H.; Chang, W.-S.; Yoon, T.-I. Dyestuff Wastewater Treatment Using Chemical Oxidation, Physical Adsorption and Fixed Bed Biofilm Process. Process Biochem. 1999, 34, 429–439. [Google Scholar] [CrossRef]
- Soloman, P.A.; Basha, C.A.; Velan, M.; Ramamurthi, V.; Koteeswaran, K.; Balasubramanian, N. Electrochemical Degradation of Remazol Black B Dye Effluent. CLEAN-Soil Air Water 2009, 37, 889–900. [Google Scholar] [CrossRef]
- Kornaros, M.; Lyberatos, G. Biological Treatment of Wastewaters from a Dye Manufacturing Company Using a Trickling Filter. J. Hazard. Mater. 2006, 136, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Banat, F.; Al-Asheh, S.; Al-Rawashdeh, M.; Nusair, M. Photodegradation of Methylene Blue Dye by the UV/H2O2 and UV/Acetone Oxidation Processes. Desalination 2005, 181, 225–232. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; ul Haq, A.; Akram, N. Photocatalysis: An Effective Tool for Photodegradation of Dyes—A Review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef]
- Chen, J.; Hu, H.; Yang, J.; Xue, H.; Tian, Y.; Fan, K.; Zeng, Z.; Yang, J.; Wang, R.; Liu, Y. Removal Behaviors and Mechanisms for Series of Azo Dye Wastewater by Novel Nano Constructed Macro-Architectures Material. Bioresour. Technol. 2021, 322, 124556. [Google Scholar] [CrossRef]
- García, E.R.; Medina, R.L.; Lozano, M.M.; Hernández Pérez, I.; Valero, M.J.; Franco, A.M.M. Adsorption of Azo-Dye Orange II from Aqueous Solutions Using a Metal-Organic Framework Material: Iron- Benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef]
- Ayati, A.; Shahrak, M.N.; Tanhaei, B.; Sillanpää, M. Emerging Adsorptive Removal of Azo Dye by Metal–Organic Frameworks. Chemosphere 2016, 160, 30–44. [Google Scholar] [CrossRef]
- Beydaghdari, M.; Hooriabad Saboor, F.; Babapoor, A.; Karve, V.V.; Asgari, M. Recent Advances in MOF-Based Adsorbents for Dye Removal from the Aquatic Environment. Energies 2022, 15, 2023. [Google Scholar] [CrossRef]
- Liu, W.; Fan, C.; Zong, Z.; Li, N.; Ma, K.; Zhu, B.; Zhang, X.; Fan, Y. Two Co(Ⅱ)-Based Metal Organic Frameworks for Highly Efficient Removal of Azo Dyes from Aqueous Environment: Synthesis, Selective Adsorption and Adsorption Mechanism. Colloids Surf. Physicochem. Eng. Asp. 2020, 603, 125236. [Google Scholar] [CrossRef]
- Timpua, T.; Jasman, J.; Pianaung, R.; Pongoh, D.; Pongoh, E.; Syafaruddin, S. Simple design of dye water filtering system for clean and healthy water quality. MATTER Int. J. Sci. Technol. 2017, 3, 546–559. [Google Scholar] [CrossRef][Green Version]
- Soliman, E.S.; Hassan, R.A.; Farid, D.S. The Efficiency of Natural-Ecofriendly Clay Filters on Water Purification for Improving Performance and Immunity in Broiler Chickens. Open Vet. J. 2021, 11, 483–499. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of Dyes by Nanomaterials: Recent Developments and Adsorption Mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Kamoun, E.A.; El-Shanshory, A.A.; Soliman, H.M.A.; Aly, H.F. Evaluation of Graphene Oxide-Activated Carbon as Effective Composite Adsorbent toward the Removal of Cationic Dyes: Composite Preparation, Characterization and Adsorption Parameters. J. Mol. Liq. 2019, 279, 530–539. [Google Scholar] [CrossRef]
- Cuenca Vargas, J.; David, J.; Werneck, M.; Franceschini, D.; Camargo, S., Jr. Síntese e caracterização de filmes de carbono para inibição de incrustações de carbonato de cálcio. In Proceedings of the Congresso Brasileiro de Carbono, São Paulo, Brazil, 10–12 December 2021. [Google Scholar]
- Wang, B.; Jin, C.; Shao, S.; Yue, Y.; Zhang, Y.; Wang, S.; Chang, R.; Zhang, H.; Zhao, J.; Li, X. Electron-Deficient Cu Site Catalyzed Acetylene Hydrochlorination. Green Energy Environ. 2023, 8, 1128–1140. [Google Scholar] [CrossRef]
- Ganjoo, R.; Sharma, S.; Kumar, A.; Daouda, M.M. Activated Carbon: Fundamentals, Classification, and Properties. In Activated Carbon: Progress and Applications; Royal Society of Chemistry: London, UK, 2023; pp. 1–22. ISBN 978-1-83916-780-5. [Google Scholar]
- Çeçen, F.; Aktaş, Ö. Activated Carbon for Water and Wastewater Treatment; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. i–xxvii. ISBN 978-3-527-63944-1. [Google Scholar]
- Faria, J.L.; Wang, W. Carbon Materials in Photocatalysis. In Carbon Materials for Catalysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 481–506. ISBN 978-0-470-40370-9. [Google Scholar]
- Robinson, T.; Chandran, B.; Nigam, P. Effect of Pretreatments of Three Waste Residues, Wheat Straw, Corncobs and Barley Husks on Dye Adsorption. Bioresour. Technol. 2002, 85, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Sanromán, M.A.; Pazos, M.; Ricart, M.T.; Cameselle, C. Electrochemical Decolourisation of Structurally Different Dyes. Chemosphere 2004, 57, 233–239. [Google Scholar] [CrossRef]
- Dutta, S.; Bhattacharjee, J. Chapter 1—A Comparative Study between Physicochemical and Biological Methods for Effective Removal of Textile Dye from Wastewater. In Development in Wastewater Treatment Research and Processes; Shah, M.P., Rodriguez-Couto, S., Kapoor, R.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–21. ISBN 978-0-323-85657-7. [Google Scholar]
- Meghwal, K.; Agrawal, R.; Kumawat, S.; Jangid, N.K.; Ameta, C. Chemical and Biological Treatment of Dyes. In Impact of Textile Dyes on Public Health and the Environment; IGI Global: Hershey, PA, USA, 2020; pp. 170–204. ISBN 978-1-79980-311-9. [Google Scholar]
- Chen, Y.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.; Ho, S.-H. Advanced Oxidation Processes for Water Disinfection: Features, Mechanisms and Prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations: A Review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Tan, C.-E.; Su, E.-C.; Wey, M.-Y. Mixed Imidazole Ligand MIL-88A for Enhanced Photo-Fenton Decomposition of Azo Dye. Sol. Energy 2022, 246, 89–103. [Google Scholar] [CrossRef]
- Chen, J.; Wei, J.; Zhang, H.; Wang, X.; Fu, L.; Yang, T.-H. Construction of CuCd-BMOF/GO Composites Based on Phosphonate and Their Boosted Visible-Light Photocatalytic Degradation. Appl. Surf. Sci. 2022, 594, 153493. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, D.; Shen, T.; Hou, X.; Zhu, M.; Liu, S.; Hu, Q. Titanium Dioxide/Magnetic Metal-Organic Framework Preparation for Organic Pollutants Removal from Water under Visible Light. Colloids Surf. Physicochem. Eng. Asp. 2020, 589, 124484. [Google Scholar] [CrossRef]
- Ioannidou, T.; Anagnostopoulou, M.; Papoulis, D.; Christoforidis, K.C.; Vasiliadou, I.A. UiO-66/Palygorskite/TiO2 Ternary Composites as Adsorbents and Photocatalysts for Methyl Orange Removal. Appl. Sci. 2022, 12, 8223. [Google Scholar] [CrossRef]
- Andrade, P.H.M.; Palhares, H.; Volkringer, C.; Loiseau, T.; Hureau, M.; Nunes, E.; Moissette, A. State of the Art in Visible-Light Photocatalysis of Aqueous Pollutants Using Metal-Organic Frameworks. J. Photochem. Photobiol. C Photochem. Rev. 2023, 57, 100635. [Google Scholar] [CrossRef]
- Emam, H.E.; Ahmed, H.B.; Gomaa, E.; Helal, M.H.; Abdelhameed, R.M. Doping of Silver Vanadate and Silver Tungstate Nanoparticles for Enhancement the Photocatalytic Activity of MIL-125-NH2 in Dye Degradation. J. Photochem. Photobiol. Chem. 2019, 383, 111986. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; El-Shahat, M.; Abd El-Ghaffar, M.A. Boosting the Photocatalytic Activity of Ti-MOF via Emerging with Metal Phthalocyanine to Degrade Hazard Textile Pigments. J. Alloys Compd. 2022, 896, 162992. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, A.; Taghizadeh, M.; Abdi, J. In Situ Deposition of Ag/AgCl on the Surface of Magnetic Metal-Organic Framework Nanocomposite and Its Application for the Visible-Light Photocatalytic Degradation of Rhodamine Dye. J. Hazard. Mater. 2019, 378, 120741. [Google Scholar] [CrossRef]
- Ahmadi, A.; Sarrafzadeh, M.-H.; Hosseinian, A.; Ghaffari, S.-B. Foulant Layer Degradation of Dye in Photocatalytic Membrane Reactor (PMR) Containing Immobilized and Suspended NH2-MIL125(Ti) MOF Led to Water Flux Recovery. J. Environ. Chem. Eng. 2022, 10, 106999. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, W.; Zhu, B.; Cai, J.; Li, X.; Liu, J.; Chen, Z.; Li, M.; Zhang, L. Fabrication of NH2-MIL-125(Ti) Nanodots on Carbon Fiber/MoS2-Based Weavable Photocatalysts for Boosting the Adsorption and Photocatalytic Performance. J. Colloid Interface Sci. 2022, 611, 706–717. [Google Scholar] [CrossRef]
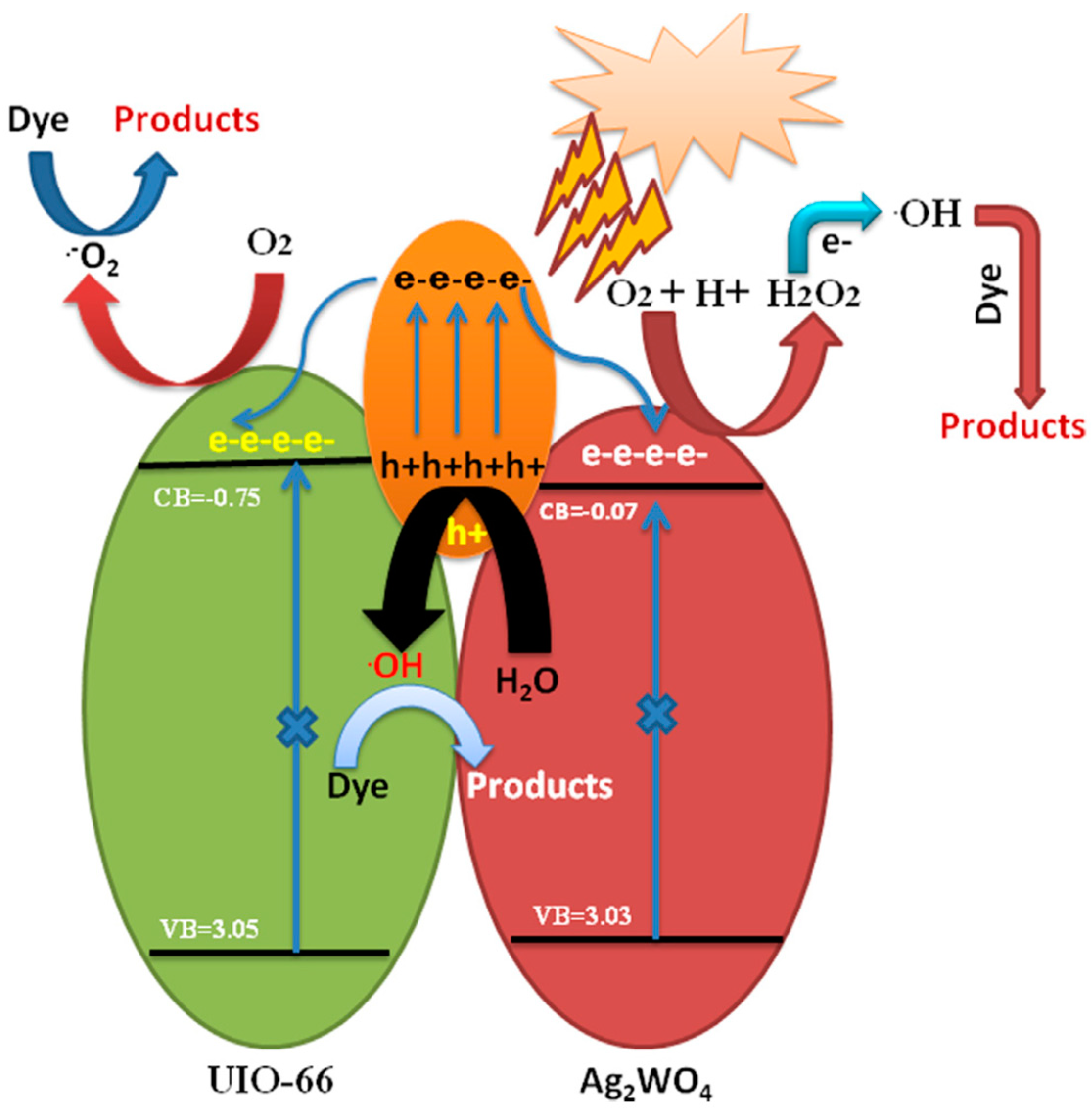
- Sofi, F.A.; Majid, K. Plasmon Induced Interfacial Charge Transfer across Zr-Based Metal-Organic Framework Coupled Ag2WO4 Heterojunction Functionalized by Ag NPs: Efficient Visible Light Photocatalyst. Chem. Phys. Lett. 2019, 720, 7–14. [Google Scholar] [CrossRef]
- Chen, M.-L.; Li, S.-S.; Wen, L.; Xu, Z.; Li, H.-H.; Ding, L.; Cheng, Y.-H. Exploration of Double Z-Type Ternary Composite Long-Afterglow/Graphitic Carbon Nitride@metal–Organic Framework for Photocatalytic Degradation of Methylene Blue. J. Colloid Interface Sci. 2023, 629, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, Z.; Li, Y.; Zhao, C.; Cai, L.; Yu, J.; Yang, Z.; Jiang, J. A Novel Visible-Light-Induced Double Z-Scheme Photocatalytic System: NH2-UiO-66/BiOBr/Bi2S3 for Degradation of Tetracycline Hydrochloride and Rhodamine B. Colloids Surf. Physicochem. Eng. Asp. 2022, 649, 129350. [Google Scholar] [CrossRef]
- Gayathri, K.; Vinothkumar, K.; Teja, Y.N.; Al-Shehri, B.M.; Selvaraj, M.; Sakar, M.; Balakrishna, R.G. Ligand-Mediated Band Structure Engineering and Physiochemical Properties of UiO-66 (Zr) Metal-Organic Frameworks (MOFs) for Solar-Driven Degradation of Dye Molecules. Colloids Surf. Physicochem. Eng. Asp. 2022, 653, 129992. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, C.; Wang, H.; Xie, S.; Zhou, J.; Fang, H. Synergistic Removal of Organic Pollutants by Co-Doped MIL-53(Al) Composite through the Integrated Adsorption/Photocatalysis. J. Solid State Chem. 2022, 316, 123582. [Google Scholar] [CrossRef]
- Zokaee, Z.; Mahmoodi, N.M.; Rahimpour, M.R.; Shariati, A. Synthesis of Visible Light Activated Metal-Organic Framework Coated on Titania Nanocomposite (MIL-53(Al)@TiO2) and Dye Photodegradation. J. Solid State Chem. 2022, 307, 122747. [Google Scholar] [CrossRef]
- Pattappan, D.; Vargheese, S.; Kavya, K.V.; Kumar, R.T.R.; Haldorai, Y. Metal-Organic Frameworks with Different Oxidation States of Metal Nodes and Aminoterephthalic Acid Ligand for Degradation of Rhodamine B under Solar Light. Chemosphere 2022, 286, 131726. [Google Scholar] [CrossRef]
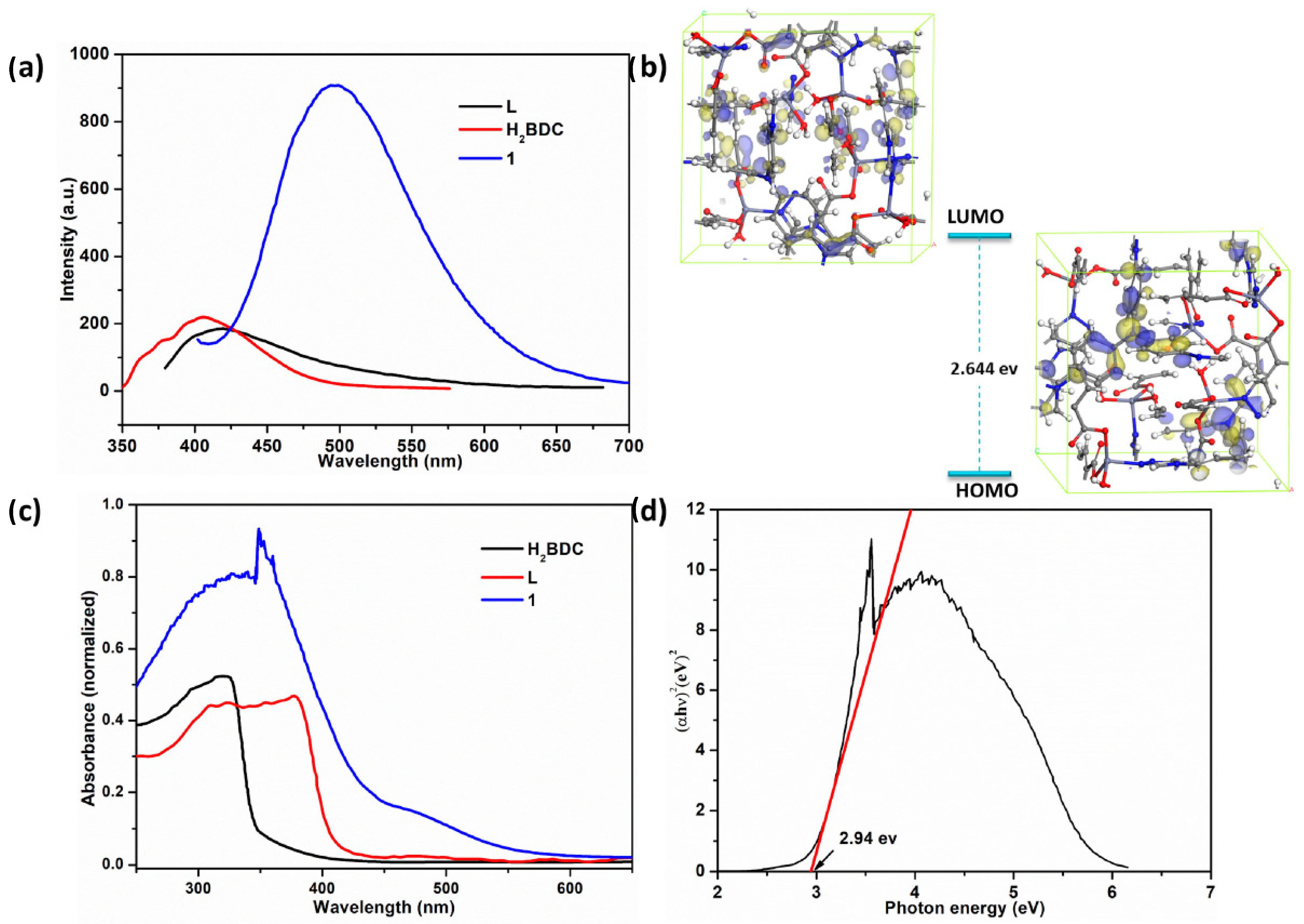
- Zhang, X.; Meng, X.; Wang, J.; Ji, Z.; Lu, P.; Wang, X.; Chen, F. Rational Design of Two Novel Metal–Organic Frameworks as Photocatalysts for Degradation of Organic Dyes and Their Derived Materials as Electrocatalysts for OER. Inorganica Chim. Acta 2021, 523, 120416. [Google Scholar] [CrossRef]
- Ma, K.; Bi, C.; Zhang, X.; Zong, Z.; Xu, C.; Li, N.; Zhang, X.; Zhang, D.; Fan, Y. Four Co(II)—MOFs Based on 3,3′-Azodibenzoic Acid Ligand: Syntheses, Crystal Structure, Photodegradation Properties. J. Solid State Chem. 2019, 277, 115–122. [Google Scholar] [CrossRef]
- Cui, G.; Liu, W.; Wang, L.; Wu, R.; Bi, C.; Zhang, D.; Fan, Y. Two Novel Co (II) Bifunctional MOFs: Syntheses and Applications in Photocatalytic Degradation of Dyes and Electrocatalytic Water Oxidation. J. Solid State Chem. 2021, 304, 122562. [Google Scholar] [CrossRef]
- Laurier, K.G.M.; Vermoortele, F.; Ameloot, R.; De Vos, D.E.; Hofkens, J.; Roeffaers, M.B.J. Iron(III)-Based Metal–Organic Frameworks As Visible Light Photocatalysts. J. Am. Chem. Soc. 2013, 135, 14488–14491. [Google Scholar] [CrossRef]
- Nasalevich, A.; Becker, R.; Ramos-Fernandez, V.; Castellanos, S.; Veber, L.V.; Fedin, M.; Kapteijn, F.; Reek, H.J.N.; van der Vlugt, J.I.; Gascon, J. Co@NH 2 -MIL-125(Ti): Cobaloxime-Derived Metal–Organic Framework-Based Composite for Light-Driven H 2 Production. Energy Environ. Sci. 2015, 8, 364–375. [Google Scholar] [CrossRef]
- Baldovi, H.G.; Krüger, M.; Reinsch, H.; Alvaro, M.; Stock, N.; Garcia, H. Transient Absorption Spectroscopy and Photochemical Reactivity of CAU-8. J. Mater. Chem. C 2015, 3, 3607–3613. [Google Scholar] [CrossRef]
- Kent, C.A.; Liu, D.; Ma, L.; Papanikolas, J.M.; Meyer, T.J.; Lin, W. Light Harvesting in Microscale Metal–Organic Frameworks by Energy Migration and Interfacial Electron Transfer Quenching. J. Am. Chem. Soc. 2011, 133, 12940–12943. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, L.; Zhang, N.; Liu, L.; Ren, X.; Ma, H.; Luo, C.; Li, Y.; Wei, Q. Zinc-Based Metal–Organic Framework with MLCT Properties as an Efficient Electrochemiluminescence Probe for Trace Detection of Trenbolone. Anal. Chem. 2022, 94, 14054–14060. [Google Scholar] [CrossRef]
- Sinha, N.; Wenger, O.S. Photoactive Metal-to-Ligand Charge Transfer Excited States in 3d6 Complexes with Cr0, MnI, FeII, and CoIII. J. Am. Chem. Soc. 2023, 145, 4903–4920. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Liu, W.; Qiu, M.; Zhang, Y.; Li, Z. Introduction of a Mediator for Enhancing Photocatalytic Performance via Post-Synthetic Metal Exchange in Metal–Organic Frameworks (MOFs). Chem. Commun. 2015, 51, 2056–2059. [Google Scholar] [CrossRef]
- Subalakshmi, K.; Senthilselvan, J.; Kumar, K.A.; Kumar, S.A.; Pandurangan, A. Solvothermal Synthesis of Hexagonal Pyramidal and Bifrustum Shaped ZnO Nanocrystals: Natural Betacyanin Dye and Organic Eosin Y Dye Sensitized DSSC Efficiency, Electron Transport, Recombination Dynamics and Solar Photodegradation Investigations. J. Mater. Sci. Mater. Electron. 2017, 28, 15565–15595. [Google Scholar] [CrossRef]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Llabrés i Xamena, F.X.; Garcia, H. Semiconductor Behavior of a Metal-Organic Framework (MOF). Chem.-Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Zhang, W.; Zhang, S.; Tian, Y.; Chen, Z.; Fang, W.; Ma, J. Intraligand Charge Transfer Boosts Visible-Light-Driven Generation of Singlet Oxygen by Metal-Organic Frameworks. Appl. Catal. B Environ. 2020, 273, 119087. [Google Scholar] [CrossRef]
- Huo, P.; Chen, T.; Hou, J.-L.; Yu, L.; Zhu, Q.-Y.; Dai, J. Ligand-to-Ligand Charge Transfer within Metal–Organic Frameworks Based on Manganese Coordination Polymers with Tetrathiafulvalene-Bicarboxylate and Bipyridine Ligands. Inorg. Chem. 2016, 55, 6496–6503. [Google Scholar] [CrossRef]
- Chen, Y.; Sakata, O.; Nanba, Y.; Kumara, L.S.R.; Yang, A.; Song, C.; Koyama, M.; Li, G.; Kobayashi, H.; Kitagawa, H. Electronic Origin of Hydrogen Storage in MOF-Covered Palladium Nanocubes Investigated by Synchrotron X-rays. Commun. Chem. 2018, 1, 61. [Google Scholar] [CrossRef]
- Yang, L.-M.; Ganz, E.; Svelle, S.; Tilset, M. Computational Exploration of Newly Synthesized Zirconium Metal–Organic Frameworks UiO-66, -67, -68 and Analogues. J. Mater. Chem. C 2014, 2, 7111–7125. [Google Scholar] [CrossRef]
- Andrade, P.H.M.; Henry, N.; Volkringer, C.; Loiseau, T.; Vezin, H.; Hureau, M.; Moissette, A. Iodine Uptake by Zr-/Hf-Based UiO-66 Materials: The Influence of Metal Substitution on Iodine Evolution. ACS Appl. Mater. Interfaces 2022, 14, 29916–29933. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Song, D. A Luminescent Metal–Organic Framework as a Turn-On Sensor for DMF Vapor. Angew. Chem. Int. Ed. 2013, 52, 710–713. [Google Scholar] [CrossRef]
- Zhang, X.; da Silva, I.; Fazzi, R.; Sheveleva, A.M.; Han, X.; Spencer, B.F.; Sapchenko, S.A.; Tuna, F.; McInnes, E.J.L.; Li, M.; et al. Iodine Adsorption in a Redox-Active Metal–Organic Framework: Electrical Conductivity Induced by Host−Guest Charge-Transfer. Inorg. Chem. 2019, 58, 14145–14150. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-Based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X.-L.; Cai, M.; Yan, R.-K.; Cui, H.-L.; Yang, H.; Wang, J.-J. Zn-MOFs Composites Loaded with Silver Nanoparticles Are Used for Fluorescence Sensing Pesticides, Trp, EDA and Photocatalytic Degradation of Organic Dyes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 289, 122228. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.-X.; Zha, M.; Ding, J.-G.; Zhu, L.-M.; Li, B.-L.; Wu, B. Visible-Light-Driven and Ultrasonic-Assisted Copper Metal-Organic Frameworks and Graphene Oxide Nanocomposite for Decolorization of Dyes. J. Solid State Chem. 2021, 304, 122627. [Google Scholar] [CrossRef]
- Zha, M.; Zhou, W.-J.; Ma, L.-X.; Li, L.-Y.; Li, B.-L.; Li, H.-Y.; Hu, C.-J. Syntheses of Two Copper Metal-Organic Frameworks with Tri(1,2,4-Triazole) and Biscarboxylate and Graphene Oxide Composites for Decomposition of Dye by Visible-Light Driven and Ultrasonic Assisted. J. Solid State Chem. 2022, 307, 122864. [Google Scholar] [CrossRef]
- Hosseini, M.-S.; Abbasi, A.; Masteri-Farahani, M. Decoration of NH2-UiO-66 with FeOOH Quantum Dots for Improving Photo-Degradation of Organic Dyes upon Visible Light Irradiation. Appl. Surf. Sci. 2022, 604, 154514. [Google Scholar] [CrossRef]
- Rezaei, H.; Zinatizadeh, A.A.; Joshaghani, M.; Zinadini, S. Amino Acid-Based Carbon Quantum Dot Modified MIL-53 (Fe): Investigation of Its Visible-Driven Photocatalytic Activity Provided by a Highly Efficient Photoreactor. Mater. Sci. Semicond. Process. 2023, 154, 107190. [Google Scholar] [CrossRef]
- Zhao, J.; Li, B.; Liu, Z.; Dai, D.; Li, Y.; Shi, R.; Zhang, H. A Novel Solar-Triggered MIL-125(Ti)/g-C3N4/SA Composite Aerogel with High Catalytic Activity for Degradation of Organic Contaminants. Sep. Purif. Technol. 2021, 279, 119696. [Google Scholar] [CrossRef]
- Sharafinia, S.; Farrokhnia, A.; Lemraski, E.G.; Rashidi, A. Decoration of ZnFe2O4 and UiO-66 over g-C3N4 as Magnetically Novel Reusable Visible Light Photocatalyst for Degradation of Rh–B. Opt. Mater. 2022, 132, 112838. [Google Scholar] [CrossRef]
- Nordin, N.A.; Mohamed, M.A.; Salehmin, M.N.I.; Mohd Yusoff, S.F. Photocatalytic Active Metal–Organic Framework and Its Derivatives for Solar-Driven Environmental Remediation and Renewable Energy. Coord. Chem. Rev. 2022, 468, 214639. [Google Scholar] [CrossRef]
- Mukherjee, D.; Van der Bruggen, B.; Mandal, B. Advancements in Visible Light Responsive MOF Composites for Photocatalytic Decontamination of Textile Wastewater: A Review. Chemosphere 2022, 295, 133835. [Google Scholar] [CrossRef]
- Xia, T.; Lin, Y.; Li, W.; Ju, M. Photocatalytic Degradation of Organic Pollutants by MOFs Based Materials: A Review. Chin. Chem. Lett. 2021, 32, 2975–2984. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent Advances in MOF-Based Photocatalysis: Environmental Remediation under Visible Light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Healy, C.; Patil, K.M.; Wilson, B.H.; Hermanspahn, L.; Harvey-Reid, N.C.; Howard, B.I.; Kleinjan, C.; Kolien, J.; Payet, F.; Telfer, S.G.; et al. The Thermal Stability of Metal-Organic Frameworks. Coord. Chem. Rev. 2020, 419, 213388. [Google Scholar] [CrossRef]
- Shi, C.; Zhou, X.; Liu, D.; Li, L.; Xu, M.; Sakiyama, H.; Muddassir, M.; Wang, J. A New 3D High Connection Cu-Based MOF Introducing a Flexible Tetracarboxylic Acid Linker: Photocatalytic Dye Degradation. Polyhedron 2021, 208, 115441. [Google Scholar] [CrossRef]
- Li, Y.; Feng, J.; Zhang, Y.; Wang, C.; Hao, J.; Wang, Y.; Xu, Y.; Cheng, X. Covalent Organic frameworks@ZIF-67 Derived Novel Nanocomposite Catalyst Effectively Activated Peroxymonosulfate to Degrade Organic Pollu-tants. Chemosphere 2023, 311, 137038. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, S.; Chen, C.; Lu, L.; Li, B.; Hu, W.; Kumar, A.; Muddassir, M. Two New Uncommon 3D Cobalt-Based Metal Organic Frameworks: Temperature Induced Syntheses and Enhanced Photocatalytic Properties against Aromatic Dyes. Dye. Pigment. 2021, 187, 109068. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Du, J.-M. An Efficiently Heterogeneous Photocatalyst for Degradation of Cation and Neutral Dyes under UV Light Based on Size-Dependent Effects of Tetracarboxyate Complex. J. Solid State Chem. 2020, 292, 121681. [Google Scholar] [CrossRef]
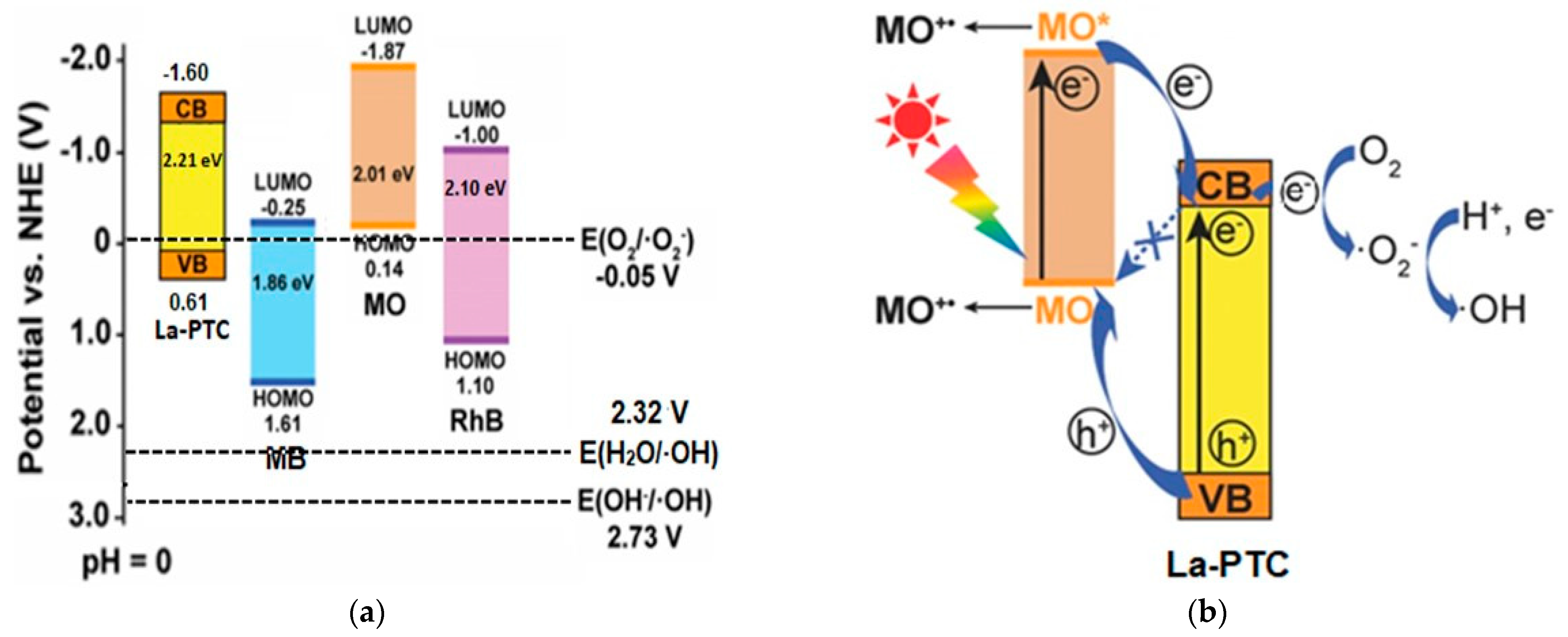
- Zulys, A.; Adawiah, A.; Gunlazuardi, J.; Yudhi, M.D.L. Light-Harvesting Metal-Organic Frameworks (MOFs) La-PTC for Photocatalytic Dyes Degradation. Bull. Chem. React. Eng. Catal. 2021, 16, 170–178. [Google Scholar] [CrossRef]
- Chen, D.-M.; Zhang, X.-J. A 8-Fold Interpenetrated Metal-Organic Framework: Luminescent Property and Photocatalytic Dye Degradation Performance. J. Solid State Chem. 2023, 321, 123919. [Google Scholar] [CrossRef]
- Jin, X.; Tang, T.; Tao, X.; Huang, L.; Xu, D. A Novel Dual-Ligand Fe-Based MOFs Synthesized with Dielectric Barrier Discharge (DBD) Plasma as Efficient Photocatalysts. J. Mol. Liq. 2021, 340, 117290. [Google Scholar] [CrossRef]
- Jin, X.; Tang, T.; Tao, X.; Huang, L.; Shang, S. Regulating N Content to Anchor Fe in Fe-MOFs: Obtaining Multiple Active Sites as Efficient Photocatalysts. J. Taiwan Inst. Chem. Eng. 2022, 132, 104133. [Google Scholar] [CrossRef]
- Murtaza, S.Z.M.; Shomal, R.; Sabouni, R.; Ghommem, M. Facile Metal Organic Framework Composites as Photocatalysts for Lone/Simultaneous Photodegradation of Naproxen, Ibuprofen and Methyl Orange. Environ. Technol. Innov. 2022, 27, 102751. [Google Scholar] [CrossRef]
- Najafidoust, A.; Abdollahi, B.; Sarani, M.; Darroudi, M.; Vala, A.M. MIL-(53)Fe Metal-Organic Framework (MOF)-Based Ag2CrO4 Hetrostructure with Enhanced Solar-Light Degradation of Organic Dyes. Opt. Mater. 2022, 125, 112108. [Google Scholar] [CrossRef]
- Abdollahi, B.; Farshnama, S.; Abbasi Asl, E.; Najafidoust, A.; Sarani, M. Cu(BDC) Metal–Organic Framework (MOF)-Based Ag2CrO4 Heterostructure with Enhanced Solar-Light Degradation of Organic Dyes. Inorg. Chem. Commun. 2022, 138, 109236. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, W.; Rao, C.; Zhou, D.; Hu, Y.; Jin, J.; Muddassir, M. Fast Photocatalytic Organic Dye by Two Metal-Organic Frameworks with 3D Two-Fold Interpenetrated Feature. J. Mol. Struct. 2021, 1227, 129538. [Google Scholar] [CrossRef]
- Zhou, E.-H.; Hu, W.; Ding, Q.; Li, L.; Liao, Z.; Jin, J.-C. Photocatalytic Decontamination of Methyl Violet by a 3D Twofold Interpenetrating Metal–Organic Framework. Inorg. Chem. Commun. 2020, 120, 108155. [Google Scholar] [CrossRef]
- Zhong, X.-L.; Wang, J.; Shi, C.; Lu, L.; Srivastava, D.; Kumar, A.; Afzal, M.; Alarifi, A. Photocatalytic Applications of a New 3D Mn(II)-Based MOF with Mab Topology. Inorg. Chim. Acta 2022, 540, 121063. [Google Scholar] [CrossRef]
- Chen, D.-M.; Zheng, Y.-P.; Shi, D.-Y.; Fang, S.-M. An Acid-Base Resistant Polyoxometalate-Based Metal–Organic Framework Constructed from {Cu4Cl}7+ and {Cu2(CO2)4} Clusters for Photocatalytic Degradation of Organic Dye. J. Solid State Chem. 2020, 287, 121384. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Fan, C.; Zong, Z.; Zhang, D.; Luo, Q.; Bi, C.; Fan, Y. A Novel Metal–Organic Frameworks Assembled by One Angular Ligand and 5-Aminoisophthalic Acid: Synthesis, Structure, Electrochemical and Photocatalytic Properties. Polyhedron 2019, 168, 21–27. [Google Scholar] [CrossRef]
- Wu, Y.; Long, J.; Wang, L.; Chen, C.; Xie, B.; Wang, J.; Wang, X.; Shi, C. Two New Coordination Polymers with Tetracarboxylate as Photocatalysts for Dye Degradation. Polyhedron 2021, 203, 115216. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Sy, D.T.; Thoa, V.T.K.; Hong, N.T.; Doan, H.V. Bimetallic Co-Fe-BTC/CN Nanocomposite Synthesised via a Microwave-Assisted Hydrothermal Method for Highly Efficient Reactive Yellow 145 Dye Photodegradation. J. Taiwan Inst. Chem. Eng. 2022, 140, 104543. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Younesi, H.; Bahramifar, N.; Tamunaidu, P.; Karimi-Maleh, H. A Novel Route to the Synthesis of α-Fe2O3@C@SiO2/TiO2 Nanocomposite from the Metal-Organic Framework as a Photocatalyst for Water Treatment. Chemosphere 2022, 297, 133992. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Taghizadeh, M.; Taghizadeh, A.; Hayati, B.; Shekarchi, A.A.; Vossoughi, M. Activated Carbon/Metal-Organic Framework Nanocomposite: Preparation and Photocatalytic Dye Degradation Mathematical Modeling from Wastewater by Least Squares Support Vector Machine. J. Environ. Manag. 2019, 233, 660–672. [Google Scholar] [CrossRef]
- Saedi, Z.; Hajinia, N. Concurrent First- and Second-Order Photodegradation of Azo Dyes Using TMU-16 Pillared-Layer Microporous Metal Organic Framework under Visible Light. J. Solid State Chem. 2021, 300, 122210. [Google Scholar] [CrossRef]
- Zong, Z.; Fan, C.; Zhang, X.; Meng, X.; Jin, F.; Fan, Y. Synthesis, Crystal Structures and Dye Removal Properties of a Series of Metal-Organic Frameworks Based on N-Heterocyclic Carboxylic Acid Ligands. Microporous Mesoporous Mater. 2019, 282, 82–90. [Google Scholar] [CrossRef]
- Wu, R.; Bi, C.; Wang, L.; Wang, J.; Fan, C.; Li, N.; Wang, M.; Shao, F.; Chen, G.; Fan, Y. Five Functional Cd/Zn-Based MOFs Constructed from V-Shaped Tricarboxylate Ligand for Rapidly Selective Adsorption and Efficiently Photocatalytic Degradation of Hazardous Aromatic Dyes. Synth. Met. 2021, 277, 116786. [Google Scholar] [CrossRef]
- Zhang, X.; Zong, Z.; Zhang, X.; Zhang, D.; Luo, Q.; Bi, C.; Fan, Y. Rational Design of Three Bifunctional MOFs for Photocatalysis Degradation and Selective Adsorption of Wastewater Organic Dyes Removal. Polyhedron 2020, 191, 114816. [Google Scholar] [CrossRef]
- Somnath; Ahmad, M.; Siddiqui, K.A. Synthesis of Mixed Ligand 3D Cobalt MOF: Smart Responsiveness towards Photocatalytic Dye Degradation in Environmental Contaminants. J. Mol. Struct. 2022, 1265, 133399. [Google Scholar] [CrossRef]
- Brahmi, C.; Benltifa, M.; Vaulot, C.; Michelin, L.; Dumur, F.; Millange, F.; Frigoli, M.; Airoudj, A.; Morlet-Savary, F.; Bousselmi, L.; et al. New Hybrid MOF/Polymer Composites for the Photodegradation of Organic Dyes. Eur. Polym. J. 2021, 154, 110560. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, S.; Wang, G.; Hu, W.; Kumar, A.; Muddassir, M. Modular Construction and Photocatalytic Properties of Two Co(II) Metal-Organic Frameworks. J. Mol. Struct. 2021, 1223, 129218. [Google Scholar] [CrossRef]
- Xu, C.; Luo, R.; Zhang, D.; Zhang, X.; Zong, Z.; Fan, C.; Zhu, B.; Fan, Y. Mn(II)/Co(II)-Based Metal-Organic Frameworks Assembled by 5,5′-(1,4-Xylylenediamino) Diisophthalic Acid and Various Nitrogen-Containing Ligands for Photocatalytic and Magnetic Properties. J. Solid State Chem. 2021, 303, 122535. [Google Scholar] [CrossRef]
- Liu, J.-J.; Fu, J.-J.; Liu, T.; Cheng, F.-X. Europium-Cadmium Organic Framework with Zwitterionic Ligand Exhibiting Tunable Luminescence, CO2 Adsorption and Dye Degradation. J. Solid State Chem. 2022, 313, 123346. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, T.; Liao, G.; Cheng, Q.; Pan, Z. Syntheses, Structures and Catalytic Mechanisms of Three New MOFs for Aqueous Cr(VI) Reduction and Dye Degradation under UV Light. Polyhedron 2019, 157, 152–162. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, X.; Qu, K.; Huang, Z.; Liu, S.; Dong, M.; Guo, Z. Bimetallic Metal-Organic Frameworks Anchored Corncob-Derived Porous Carbon Photocatalysts for Synergistic Degradation of Organic Pollutants. Chemosphere 2020, 259, 127389. [Google Scholar] [CrossRef]
- Pawar, R.R.; Chuaicham, C.; Sekar, K.; Rajendran, S.; Sasaki, K. Synthesis, Characterization, and Application of MOF@clay Composite as a Visible Light-Driven Photocatalyst for Rhodamine B Degradation. Chemosphere 2022, 291, 132922. [Google Scholar] [CrossRef]
- Qin, L.; Hu, Q.; Zou, Y.-P.; Fu, W.-P.; Ye, T.-Q.; Zhang, M.-D. Two Co(II)/Ni(II) Isostructural Metal-Organic Frameworks with Bnn Topology for Photocatalysis and Electrocatalysis. Microporous Mesoporous Mater. 2021, 312, 110813. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Rao, C.; Wang, G.-L.; Jiang, F.; Singh, A.; Kumar, A.; Liu, J. Two 3D Supramolecular Isomeric Zn(II)-MOFs as Photocatalysts for Photodegradation of Methyl Violet Dye. Dye. Pigment. 2021, 190, 109285. [Google Scholar] [CrossRef]
- Jing, C.; Zhang, Y.; Zheng, J.; Ge, S.; Lin, J.; Pan, D.; Naik, N.; Guo, Z. In-Situ Constructing Visible Light CdS/Cd-MOF Photocatalyst with Enhanced Photodegradation of Methylene Blue. Particuology 2022, 69, 111–122. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Pham, A.L.H.; Nguyen, V.-H.; Lee, T.; Nguyen, T.D. Facile Synthesis of Bismuth(III) Based Metal-Organic Framework with Difference Ligands Using Microwave Irradiation Method. Chem. Eng. Res. Des. 2022, 177, 321–330. [Google Scholar] [CrossRef]
- Durmus, Z.; Köferstein, R.; Lindenberg, T.; Lehmann, F.; Hinderberger, D.; Maijenburg, A.W. Preparation and Characterization of Ce-MOF/g-C3N4 Composites and Evaluation of Their Photocatalytic Performance. Ceram. Int. 2023, 49, 24428–24441. [Google Scholar] [CrossRef]
- Farrokhi, A.; Jafarpour, M.; Alipour, M. Solar-Driven Advanced Oxidation Process Catalyzed by Metal–Organic Frameworks for Water Depollution. Polyhedron 2019, 170, 325–333. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Kim, W.; Alfarraj, S.; Alharbi, S.A. Facile Fabrication of (2D/2D) MoS2@MIL-88(Fe) Interface-Driven Catalyst for Efficient Degradation of Organic Pollutants under Visible Light Irradiation. J. Hazard. Mater. 2021, 414, 125522. [Google Scholar] [CrossRef]
- Yassin, J.M.; Taddesse, A.M.; Sánchez-Sánchez, M. Sustainable Synthesis of Semicrystalline Zr-BDC MOF and Heterostructural Ag3PO4/Zr-BDC/g-C3N4 Composite for Photocatalytic Dye Degradation. Catal. Today 2022, 390–391, 162–175. [Google Scholar] [CrossRef]
- Chen, J.; Xing, Z.; Han, J.; Su, M.; Li, Y.; Lu, A. Enhanced Degradation of Dyes by Cu-Co-Ni Nanoparticles Loaded on Amino-Modified Octahedral Metal–Organic Framework. J. Alloys Compd. 2020, 834, 155106. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, X.; Wang, J.; Wang, L.; Zhu, B.; Xu, C.; Cui, G.; Zhang, D.; Fan, Y. Two Novel Zn (II)-Based Metal–Organic Frameworks for Rapidly Selective Adsorption and Efficient Photocatalytic Degradation of Hazardous Aromatic Dyes in Aqueous Phase. Adv. Powder Technol. 2022, 33, 103665. [Google Scholar] [CrossRef]
- Hoseinzadeh, H.; Bakhtiari, M.; Seifpanahi-Shabani, K.; Oveisi, M.; Hayati, B.; Rabeie, B.; Shahmoradi Ghaheh, F.; Salmani, R.; Ullah, H.; Mahmoodi, N.M. Synthesis of the Metal-Organic Framework—Copper Oxide Nanocomposite and LED Visible Light Organic Contaminants (Dye and Pharmaceutical) Destruction Ability in the Water. Mater. Sci. Eng. B 2021, 274, 115495. [Google Scholar] [CrossRef]
- Jin, J.-C.; Wang, J.; Guo, J.; Yan, M.-H.; Wang, J.; Srivastava, D.; Kumar, A.; Sakiyama, H.; Muddassir, M.; Pan, Y. A 3D Rare Cubane-like Tetramer Cu(II)-Based MOF with 4-Fold Dia Topology as an Efficient Photocatalyst for Dye Degradation. Colloids Surf. Physicochem. Eng. Asp. 2023, 656, 130475. [Google Scholar] [CrossRef]
- Dong, S.; Wang, L.; Lou, W.; Shi, Y.; Cao, Z.; Zhang, Y.; Sun, J. Bi-MOFs with Two Different Morphologies Promoting Degradation of Organic Dye under Simultaneous Photo-Irradiation and Ultrasound Vibration Treatment. Ultrason. Sonochem. 2022, 91, 106223. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.-M.; Yu, L. Construction of Z-Scheme Titanium-MOF/Plasmonic Silver Nanoparticle/NiFe Layered Double Hydroxide Photocatalysts with Enhanced Dye and Antibiotic Degradation Activity under Visible Light. Sep. Purif. Technol. 2021, 278, 119525. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, L.; Wang, J.; Tan, X.; An, B.; Singh, A.; Kumar, A.; Sakiyama, H.; Wang, J. Photocatalytic and Magnetic Properties of Two New Co(II) Cluster-Based Metal-Organic Frameworks. Inorg. Chem. Commun. 2020, 111, 107563. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, Y.; Xin, Z.; Xu, G.; Feng, Y.; Jiang, B.; He, X.; Zhang, H.; Ma, J. A New Type of Composite Catalyst AmCoPc/UiO-66-NH2 Synergistic Photocatalytic Degradation of Dyes. Inorg. Chem. Commun. 2022, 145, 109914. [Google Scholar] [CrossRef]
- Ma, S.; Shi, Y.; Xia, X.; Song, Q.; Yang, J. Cerium-Cobalt Bimetallic Metal–Organic Frameworks with the Mixed Ligands for Photocatalytic Degradation of Methylene Blue. Inorg. Chem. Commun. 2023, 152, 110664. [Google Scholar] [CrossRef]
| Acid Dye | |||
|---|---|---|---|
MO, Methyl Orange, C.I. Acid Orange 52 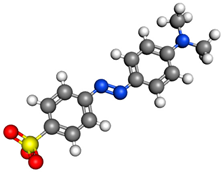 | Acid Orange, C.I. Acid Orange 7 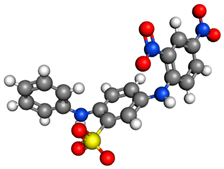 | AB92, Acid Blue, C.I. Acid Blue 92  | |
Ponceau BS, C.I. Acid Red 66  | New Coccine, NC, C.I. Acid Red 18 = 1  | Tartrazine, C.I. Acid Yellow 23  | |
Acid Black, C.I. Acid Black 1  | Acid Red 1, C.I. Acid Red 1  | ||
| Basic dye | |||
MB, Methylene Blue, C.I. Basic Blue 9  | Rhodamine B, RhB, C.I. Basic Violet 10  | Methyl violet, MV, CV, C.I. Basic Violet 3  | |
Malachite Green (MG), Green Basic, C.I. Basic Green 4  | Basic Violet (BV14), C.I. Basic Violet 14  | Neutral Red, NR, C.I. Basic Red 5  | |
| Reactive dye | |||
Reactive Red (RR)198, C.I. Reactive Red 198  | Reactive Yellow (RY), C.I. Reactive Yellow 145  | ||
| Direct dye | Mordant dye | ||
Congo Red, CR, C.I. Direct Red 28  | Eriochrome Black T, C.I. Mordant Black  | ||
| Treatment | Advantage | Disadvantage |
|---|---|---|
| Adsorption | -Able to adsorb all the concentration of the dye. -One step to recover the absorbent with the dye. | The dye only changes the medium; the environmental problem persisted throughout the time using this treatment. |
| Chemical oxidation | -Oxidation can be achieved in most of the dyes. -One or more oxidant substances can be used to oxidize the dye. | -In many cases, the sub-products are more toxic than the molecule to be degraded -This treatment can be expensive because it uses reactive in huge quantities, and they will have a high concentration. |
| Electrochemical | -The use of electric power can break down the dyes. -This method can treat vast quantities of effluents with dyes. | After applying this treatment, the formation of a massive amount of sludge that has the sub-products of the degradation of the dye |
| Biological | -Use more than one kind of bacteria. -The system does not need the addition of more reactive because the dye can be used as food for the bacteria. | -This methodology can apply only to a few quantities of dyes. -The biodegradation process is slow. |
| UV oxidation | -Using UV-C light to break the links of dye molecules. -The treatment can be applied for a short time. | -The high energy of the light can produce a health problem for the people around it. -High consumption of electricity. |
| Photocatalysis | -Require only a semiconductor and light. -This methodology can eliminate a considerable volume with a low concentration. -The system can be activated using solar radiation. -Generally, the system does not generate more toxic compounds. -The method can be achieved in many cases via the mineralization process of the dye. | -Some semiconductors must be modified to achieve a high percentage of dye degradation. -In some cases, the separation process of the semiconductor from the water. |
| Charge Transfer | Characteristics | Applications | Reference |
|---|---|---|---|
| Metal-to-Ligand Charge Transfer (MLCT) | In MLCT, electrons transfer from the metal center to the ligand. This typically involves a transition metal cation donating electrons to ligand molecules. | MLCT can enhance the photocatalytic activity of MOFs, making them useful for light-driven reactions. | [63,64,65] |
| Ligand-to-Metal Charge Transfer (LMCT) or Ligand-to- Cluster Charge Transfer (LCCT) | In LMCT, electrons transfer from the ligand to the metal center. This occurs when ligands act as donors to the metal ions. | LMCT can affect MOFs’ electronic and optical properties, making them suitable for sensors and photoluminescent materials. | [66,67,68,69] |
| Ligand-to-Ligand Charge Transfer (LLCT) | LLCT involves electron transfer between two or more ligands within the MOF structure. This can lead to changes in the color and electronic properties of the MOF. | LLCT is often associated with color changes and can be utilized in chemical sensing and colorimetric indicators. | [70] |
| Guest-to-Host Charge Transfer (GHCT) | GHCT occurs when guest molecules or ions transfer electrons to or from the MOF framework. This type of charge transfer is essential in gas adsorption and separation. | GHCT is essential in gas storage and sensing applications, where guest molecules interact with the MOF structure. | [71] |
| Linker-Based Transition (LBT) | linker orbitals form the VB and CB. | This is valuable in applications like water and pollutant degradation | [72,73] |
| Metal-Based Transition (MBT) | In MBT, electrons transfer between metal centers within the MOF structure. | This is valuable in applications like pollutant degradation | [74] |
| Host-to-Guest Charge Transfer (HGCT) | HGCT occurs when the MOF’s host structure transfers electrons to guest molecules or particles. | HGTC is significant in applications like gas sensing and photocatalytic degradation of dyes. | [73,75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera-Andrade, J.M.; de la Fuente-Maldonado, N.; Lopez-Medina, R.; Maubert-Franco, A.M.; Rojas-Garcia, E. Revolutionizing Wastewater Treatment: Harnessing Metal–Organic Frameworks for Exceptional Photocatalytic Degradation of Azo-Type Dyes. Colorants 2023, 2, 674-704. https://doi.org/10.3390/colorants2040035
Barrera-Andrade JM, de la Fuente-Maldonado N, Lopez-Medina R, Maubert-Franco AM, Rojas-Garcia E. Revolutionizing Wastewater Treatment: Harnessing Metal–Organic Frameworks for Exceptional Photocatalytic Degradation of Azo-Type Dyes. Colorants. 2023; 2(4):674-704. https://doi.org/10.3390/colorants2040035
Chicago/Turabian StyleBarrera-Andrade, Jose Manuel, Natali de la Fuente-Maldonado, Ricardo Lopez-Medina, Ana Marisela Maubert-Franco, and Elizabeth Rojas-Garcia. 2023. "Revolutionizing Wastewater Treatment: Harnessing Metal–Organic Frameworks for Exceptional Photocatalytic Degradation of Azo-Type Dyes" Colorants 2, no. 4: 674-704. https://doi.org/10.3390/colorants2040035
APA StyleBarrera-Andrade, J. M., de la Fuente-Maldonado, N., Lopez-Medina, R., Maubert-Franco, A. M., & Rojas-Garcia, E. (2023). Revolutionizing Wastewater Treatment: Harnessing Metal–Organic Frameworks for Exceptional Photocatalytic Degradation of Azo-Type Dyes. Colorants, 2(4), 674-704. https://doi.org/10.3390/colorants2040035






