Squaric Acid Core Substituted Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Effect of Electron Acceptors on Their Photovoltaic Performance
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
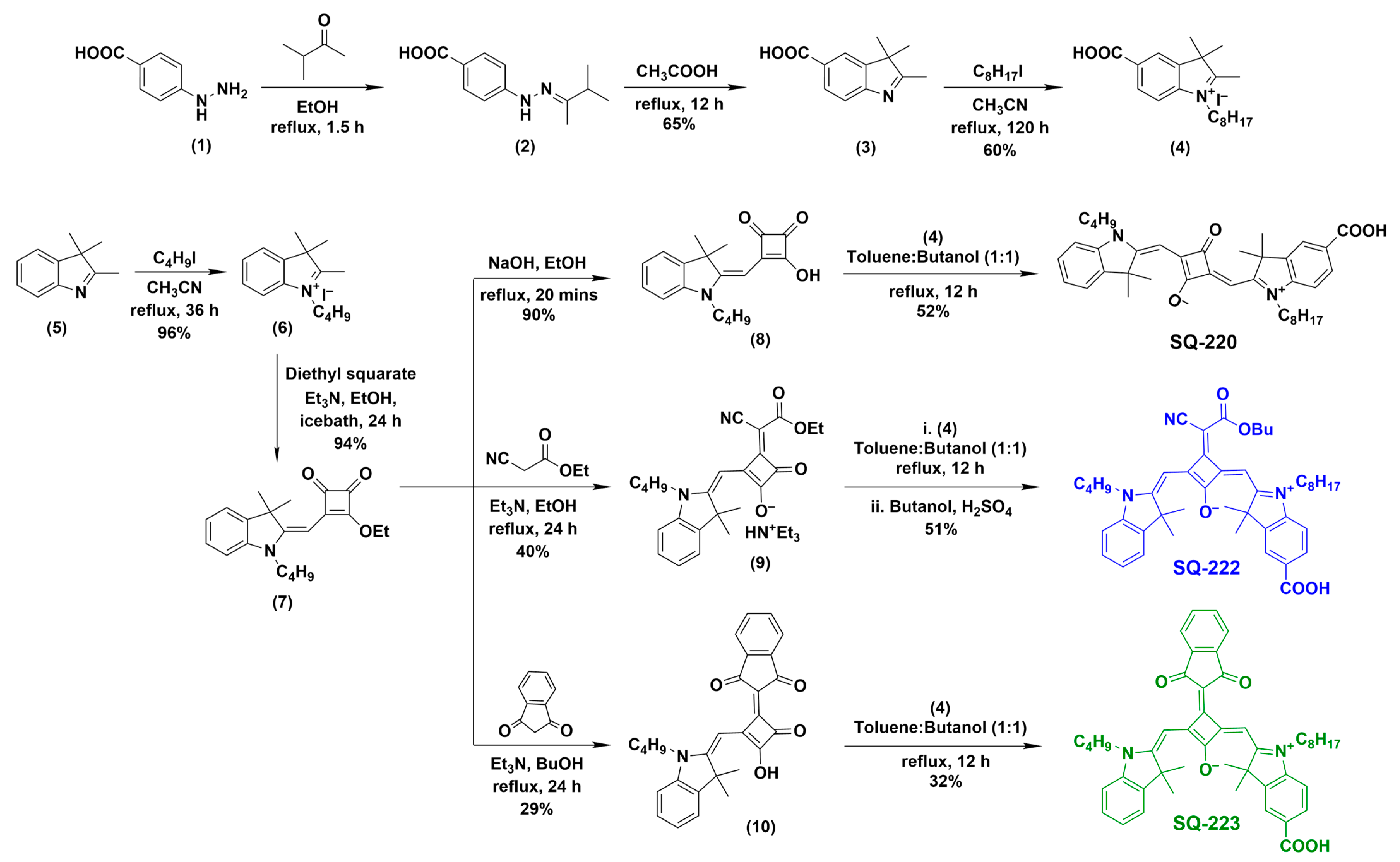
2.2. Synthesis of Far-Red-Sensitive Unsymmetrical Squaraine Sensitizers
2.3. Fabrication of DSSCs
3. Results and Discussion
3.1. Optical Characterization
3.2. Electrochemical Characterization
3.3. Energy Band Diagram
3.4. Quantum Chemical Calculations
3.5. Photovoltaic Characterizations
3.6. Electrochemical Impedance Spectroscopy (EIS)
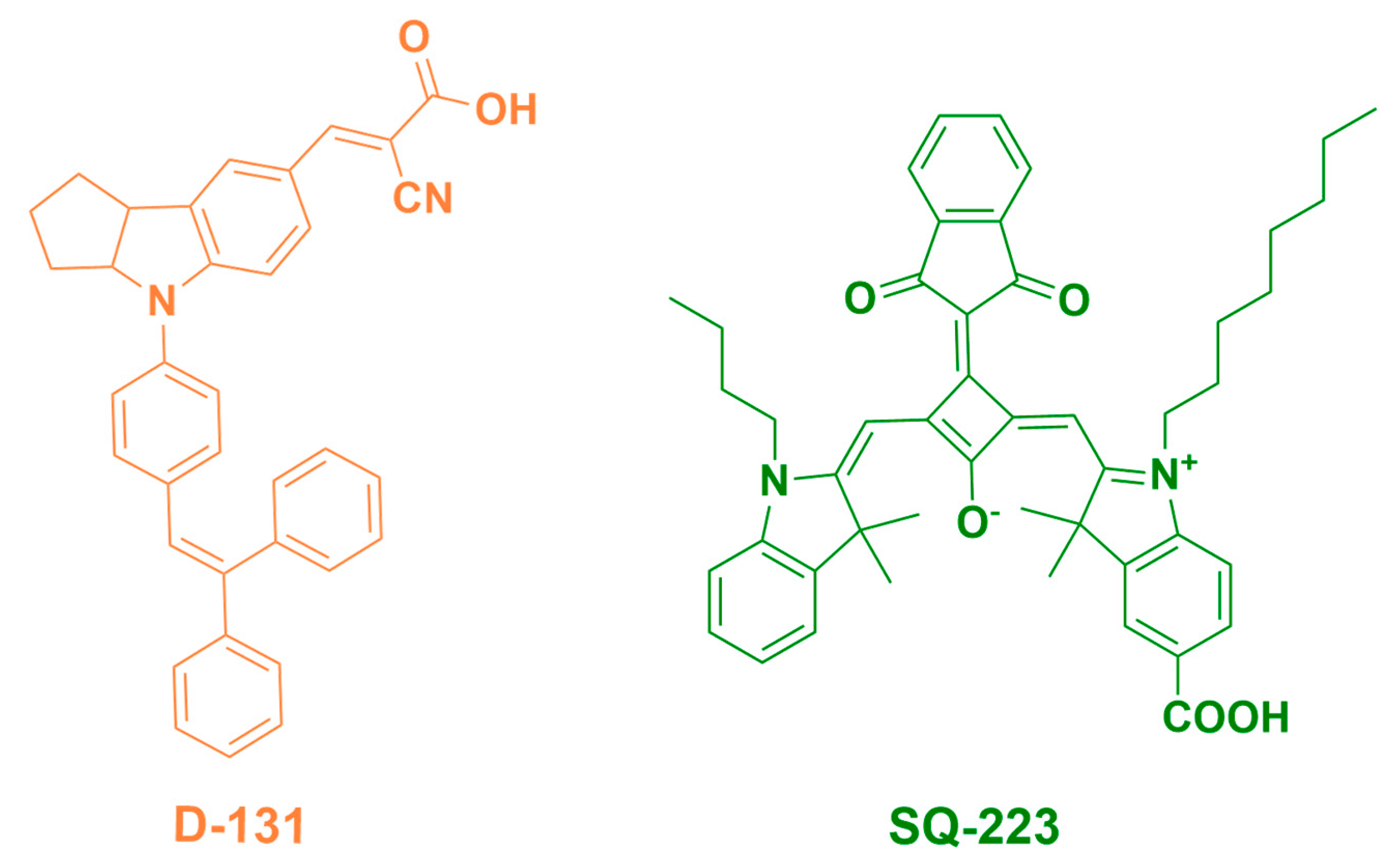
4. Enhancement of PCE Employing Co-Sensitization Using Visible Dye
4.1. Optical Characterization
4.2. Photovoltaic Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced Research Trends in Dye-Sensitized Solar Cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.U.; Mohamed, N.M.; Muhsan, A.S.; Zaine, S.N.A.; Bashiri, R.; Khatani, M.; Samsudin, A.E. Solvent Exfoliated Graphene Incorporated Mixed Phase TiO2 Transparent Photoelectrode for the Efficient and Color Transparent Dye-Sensitized Solar Cell. Sol. Energy 2020, 206, 317–329. [Google Scholar] [CrossRef]
- Yu, M.; McCulloch, W.D.; Beauchamp, D.R.; Huang, Z.; Ren, X.; Wu, Y. Aqueous Lithium–Iodine Solar Flow Battery for the Simultaneous Conversion and Storage of Solar Energy. J. Am. Chem. Soc. 2015, 137, 8332–8335. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Kumar Sinha, N.; Tiwari, S.; Khare, A. A Review on Perovskite Solar Cells: Evolution of Architecture, Fabrication Techniques, Commercialization Issues and Status. Sol Energy 2020, 198, 665–688. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-Sensitized Solar Cells for Efficient Power Generation under Ambient Lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hug, H.; Bader, M.; Mair, P.; Glatzel, T. Biophotovoltaics: Natural Pigments in Dye-Sensitized Solar Cells. Appl. Energy 2014, 115, 216–225. [Google Scholar] [CrossRef]
- Amogne, N.Y.; Ayele, D.W.; Tsigie, Y.A. Recent Advances in Anthocyanin Dyes Extracted from Plants for Dye Sensitized Solar Cell. Mater. Renew. Sustain. Energy 2020, 9, 23. [Google Scholar] [CrossRef]
- Gu, P.; Yang, D.; Zhu, X.; Sun, H.; Li, J. Fabrication and Characterization of Dye-Sensitized Solar Cells Based on Natural Plants. Chem. Phys. Lett. 2018, 693, 16–22. [Google Scholar] [CrossRef]
- Mátravölgyi, B.; Hergert, T.; Thurner, A.; Varga, B.; Sangiorgi, N.; Bendoni, R.; Zani, L.; Reginato, G.; Calamante, M.; Sinicropi, A.; et al. Synthesis and Investigation of Solar-Cell Photosensitizers Having a Fluorazone Backbone. Eur. J. Org. Chem. 2017, 2017, 1843–1854. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Sangiorgi, A.; Dessì, A.; Zani, L.; Calamante, M.; Reginato, G.; Mordini, A.; Sanson, A. Improving the Efficiency of Thin-Film Fiber-Shaped Dye-Sensitized Solar Cells by Using Organic Sensitizers. Sol. Energy Mater. Sol. Cells 2020, 204, 110209. [Google Scholar] [CrossRef]
- Gratzel, M. Conversion of Sunlight to Electric Power by Nanocrystalline Dye-Sensitized Solar Cells*1. J. Photochem. Photobiol. A Chem. 2004, 164, 3–14. [Google Scholar] [CrossRef]
- Tachibana, Y.; Moser, J.E.; Grätzel, M.; Klug, D.R.; Durrant, J.R. Subpicosecond Interfacial Charge Separation in Dye-Sensitized Nanocrystalline Titanium Dioxide Films. J. Phys. Chem. 1996, 100, 20056–20062. [Google Scholar] [CrossRef]
- Pradhan, A.; Morimoto, T.; Saikiran, M.; Kapil, G.; Hayase, S.; Pandey, S.S. Investigation of the Minimum Driving Force for Dye Regeneration Utilizing Model Squaraine Dyes for Dye-Sensitized Solar Cells. J. Mater. Chem. A 2017, 5, 22672–22682. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Wang, W.; Gurzadyan, G.G.; Li, J.; Li, X.; An, J.; Yu, Z.; Wang, H.; Cai, B.; et al. 13.6% Efficient Organic Dye-Sensitized Solar Cells by Minimizing Energy Losses of the Excited State. ACS Energy Lett. 2019, 4, 943–951. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, M.; Wu, H.; Yang, L.; Li, R.; Wang, P. Donor/Acceptor Indenoperylene Dye for Highly Efficient Organic Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2015, 137, 3799–3802. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Mueller, E.; Liska, P.; Vlachopoulos, N.; Graetzel, M. Conversion of Light to Electricity by Cis-X2bis(2,2′-Bipyridyl-4,4′-Dicarboxylate)Ruthenium(II) Charge-Transfer Sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on Nanocrystalline Titanium Dioxide Electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Péchy, P.; Renouard, T.; Zakeeruddin, S.M.; Humphry-Baker, R.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V.; et al. Engineering of Efficient Panchromatic Sensitizers for Nanocrystalline TiO2-Based Solar Cells. J. Am. Chem. Soc. 2001, 123, 1613–1624. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)–Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine Organic Dyes for Dye-Sensitized Solar Cells. Chem. Soc. Rev. 2013, 42, 3453. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef] [PubMed]
- Grisorio, R.; De Marco, L.; Baldisserri, C.; Martina, F.; Serantoni, M.; Gigli, G.; Suranna, G.P. Sustainability of Organic Dye-Sensitized Solar Cells: The Role of Chemical Synthesis. ACS Sustain. Chem. Eng. 2015, 3, 770–777. [Google Scholar] [CrossRef]
- Cid, J.-J.; Yum, J.-H.; Jang, S.-R.; Nazeeruddin, M.K.; Martínez-Ferrero, E.; Palomares, E.; Ko, J.; Grätzel, M.; Torres, T. Molecular Cosensitization for Efficient Panchromatic Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2007, 46, 8358–8362. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.Y.; Giribabu, L.; Lyness, C.; Snaith, H.J.; Vijaykumar, C.; Chandrasekharam, M.; Lakshmikantam, M.; Yum, J.-H.; Kalyanasundaram, K.; Grätzel, M.; et al. Efficient Sensitization of Nanocrystalline TiO2 Films by a Near-IR-Absorbing Unsymmetrical Zinc Phthalocyanine. Angew. Chem. Int. Ed. 2007, 46, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Higashino, T.; Kawamoto, K.; Sugiura, K.; Fujimori, Y.; Tsuji, Y.; Kurotobi, K.; Ito, S.; Imahori, H. Effects of Bulky Substituents of Push–Pull Porphyrins on Photovoltaic Properties of Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 15379–15390. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.; Li, X.; Ågren, H.; Zhu, W.-H.; Xie, Y. Porphyrins Containing a Triphenylamine Donor and up to Eight Alkoxy Chains for Dye-Sensitized Solar Cells: A High Efficiency of 10.9%. ACS Appl. Mater. Interfaces 2015, 7, 27976–27985. [Google Scholar] [CrossRef]
- Saccone, D.; Galliano, S.; Barbero, N.; Quagliotto, P.; Viscardi, G.; Barolo, C. Polymethine Dyes in Hybrid Photovoltaics: Structure-Properties Relationships. Eur. J. Org. Chem. 2016, 2016, 2244–2259. [Google Scholar] [CrossRef]
- Pandey, S.S.; Watanabe, R.; Fujikawa, N.; Shivashimpi, G.M.; Ogomi, Y.; Yamaguchi, Y.; Hayase, S. Effect of Extended π-Conjugation on Photovoltaic Performance of Dye Sensitized Solar Cells Based on Unsymmetrical Squaraine Dyes. Tetrahedron 2013, 69, 2633–2639. [Google Scholar] [CrossRef]
- Ajayaghosh, A. Chemistry of Squaraine-Derived Materials: Near-IR Dyes, Low Band Gap Systems, and Cation Sensors. Acc. Chem. Res. 2005, 38, 449–459. [Google Scholar] [CrossRef]
- Sreejith, S.; Carol, P.; Chithra, P.; Ajayaghosh, A. Squaraine Dyes: A Mine of Molecular Materials. J. Mater. Chem. 2008, 18, 264–274. [Google Scholar] [CrossRef]
- Yang, D.; Sano, T.; Sasabe, H.; Kido, J. A Novel π-D1-A-D2 Type Low Bandgap Squaraine Dye for Efficient Small Molecular Organic Solar Cells. Dye. Pigment. 2019, 163, 564–572. [Google Scholar] [CrossRef]
- Treibs, A.; Jacob, K. Cyclotrimethine Dyes Derived from Squaric Acid. Angew. Chem. Int. Ed. 1965, 4, 694. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W. Organic Sensitizers from D–π–A to D–A–π–A: Effect of the Internal Electron-Withdrawing Units on Molecular Absorption, Energy Levels and Photovoltaic Performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Park, S. Facile Preparative Redox Chemistry of Bis(4-Dialkylaminophenyl)Squaraine Dyes. Tetrahedron Lett. 2002, 43, 7669–7671. [Google Scholar] [CrossRef]
- Pandey, S.S.; Morimoto, T.; Fujikawa, N.; Hayase, S. Combined Theoretical and Experimental Approaches for Development of Squaraine Dyes with Small Energy Barrier for Electron Injection. Sol. Energy Mater. Sol. Cells 2017, 159, 625–632. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. G16_C01 2016; Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Improta, R.; Barone, V.; Scalmani, G.; Frisch, M.J. A State-Specific Polarizable Continuum Model Time Dependent Density Functional Theory Method for Excited State Calculations in Solution. J. Chem. Phys. 2006, 125, 054103. [Google Scholar] [CrossRef]
- Beverina, L.; Ruffo, R.; Mari, C.M.; Pagani, G.A.; Sassi, M.; De Angelis, F.; Fantacci, S.; Yum, J.-H.; Grätzel, M.; Nazeeruddin, M.K. Panchromatic Cross-Substituted Squaraines for Dye-Sensitized Solar Cell Applications. ChemSusChem 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Mayerhöffer, U.; Fimmel, B.; Würthner, F. Bright Near-Infrared Fluorophores Based on Squaraines by Unexpected Halogen Effects. Angew. Chem. Int. Ed. 2012, 51, 164–167. [Google Scholar] [CrossRef]
- Qin, C.; Numata, Y.; Zhang, S.; Yang, X.; Islam, A.; Zhang, K.; Chen, H.; Han, L. Novel Near-Infrared Squaraine Sensitizers for Stable and Efficient Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2014, 24, 3059–3066. [Google Scholar] [CrossRef]
- Galliano, S.; Novelli, V.; Barbero, N.; Smarra, A.; Viscardi, G.; Borrelli, R.; Sauvage, F.; Barolo, C. Dicyanovinyl and Cyano-Ester Benzoindolenine Squaraine Dyes: The Effect of the Central Functionalization on Dye-Sensitized Solar Cell Performance. Energies 2016, 9, 486. [Google Scholar] [CrossRef]
- Paternò, G.M.; Barbero, N.; Galliano, S.; Barolo, C.; Lanzani, G.; Scotognella, F.; Borrelli, R. Excited State Photophysics of Squaraine Dyes for Photovoltaic Applications: An Alternative Deactivation Scenario. J. Mater. Chem. C 2018, 6, 2778–2785. [Google Scholar] [CrossRef]
- Kulinich, A.V.; Derevyanko, N.A.; Mikitenko, E.K.; Ishchenko, A.A. Merocyanines Based on 1,3-Indanedione: Electronic Structure and Solvatochromism. J. Phys. Org. Chem. 2011, 24, 732–742. [Google Scholar] [CrossRef]
- Pigot, C.; Péralta, S.; Bui, T.-T.; Nechab, M.; Dumur, F. Push-Pull Dyes Based on Michler’s Aldehyde: Design and Characterization of the Optical and Electrochemical Properties. Dye Pigment. 2022, 202, 110278. [Google Scholar] [CrossRef]
- Barcenas, G.; Biaggne, A.; Mass, O.A.; Wilson, C.K.; Obukhova, O.M.; Kolosova, O.S.; Tatarets, A.L.; Terpetschnig, E.; Pensack, R.D.; Lee, J.; et al. First-Principles Studies of Substituent Effects on Squaraine Dyes. RSC Adv. 2021, 11, 19029–19040. [Google Scholar] [CrossRef]
- Cole, J.M.; Pepe, G.; Al Bahri, O.K.; Cooper, C.B. Cosensitization in Dye-Sensitized Solar Cells. Chem. Rev. 2019, 119, 7279–7327. [Google Scholar] [CrossRef]
- Su, B.; Girault, H.H. Absolute Standard Redox Potential of Monolayer-Protected Gold Nanoclusters. J. Phys. Chem. B 2005, 109, 11427–11431. [Google Scholar] [CrossRef]
- Mosconi, E.; Yum, J.-H.; Kessler, F.; Gómez García, C.J.; Zuccaccia, C.; Cinti, A.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. Cobalt Electrolyte/Dye Interactions in Dye-Sensitized Solar Cells: A Combined Computational and Experimental Study. J. Am. Chem. Soc. 2012, 134, 19438–19453. [Google Scholar] [CrossRef]
- Geiger, T.; Kuster, S.; Yum, J.-H.; Moon, S.-J.; Nazeeruddin, M.K.; Grätzel, M.; Nüesch, F. Molecular Design of Unsymmetrical Squaraine Dyes for High Efficiency Conversion of Low Energy Photons into Electrons Using TiO2 Nanocrystalline Films. Adv. Funct. Mater. 2009, 19, 2720–2727. [Google Scholar] [CrossRef]
- Anthonysamy, A.; Lee, Y.; Karunagaran, B.; Ganapathy, V.; Rhee, S.-W.; Karthikeyan, S.; Kim, K.S.; Ko, M.J.; Park, N.-G.; Ju, M.-J.; et al. Molecular Design and Synthesis of Ruthenium(Ii) Sensitizers for Highly Efficient Dye-Sensitized Solar Cells. J. Mater. Chem. 2011, 21, 12389–12397. [Google Scholar] [CrossRef]
- Chen, G.; Sasabe, H.; Igarashi, T.; Hong, Z.; Kido, J. Squaraine Dyes for Organic Photovoltaic Cells. J. Mater. Chem. A 2015, 3, 14517–14534. [Google Scholar] [CrossRef]
- Eisfeld, A.; Briggs, J.S. The J- and H-Bands of Organic Dye Aggregates. Chem. Phys. 2006, 324, 376–384. [Google Scholar] [CrossRef]
- Yum, J.-H.; Walter, P.; Huber, S.; Rentsch, D.; Geiger, T.; Nüesch, F.; De Angelis, F.; Grätzel, M.; Nazeeruddin, M.K. Efficient Far Red Sensitization of Nanocrystalline TiO2 Films by an Unsymmetrical Squaraine Dye. J. Am. Chem. Soc. 2007, 129, 10320–10321. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.L.; Morandeira, A.; Persson, M.; Zietz, B.; Ripaud, E.; Leriche, P.; Roncali, J.; Hagfeldt, A.; Boschloo, G. Contribution from a Hole-Conducting Dye to the Photocurrent in Solid-State Dye-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2011, 13, 20172–20177. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Dye Aggregation in Dye-Sensitized Solar Cells. J. Mater. Chem. A 2017, 5, 19541–19559. [Google Scholar] [CrossRef]
- Yum, J.H.; Moon, S.J.; Humphry-Baker, R.; Walter, P.; Geiger, T.; Nüesch, F.; Grätzel, M.; Nazeeruddin, M.D.K. Effect of Coadsorbent on the Photovoltaic Performance of Squaraine Sensitized Nanocrystalline Solar Cells. Nanotechnology 2008, 19, 424005. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Yoon, Z.S.; Shin, J.-Y.; Kim, K.S.; Yoon, M.-C.; Kim, D. The Photophysical Properties of Expanded Porphyrins: Relationships between Aromaticity, Molecular Geometry and Non-Linear Optical Properties. Chem. Commun. 2008, 45, 261–273. [Google Scholar] [CrossRef]
- Ogomi, Y.; Kato, T.; Hayase, S. Dye Sensitized Solar Cells Consisting of Ionic Liquid and Solidification. J. Photopolym. Sci. Technol. 2006, 19, 403–408. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Graetzel, M. Light-Induced Redox Reactions in Nanocrystalline Systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
- Yao, Z.; Guo, Y.; Wang, L.; Hao, Y.; Guo, Y.; Franchi, D.; Zhang, F.; Kloo, L.; Sun, L. Energy-Loss Reduction as a Strategy to Improve the Efficiency of Dye-Sensitized Solar Cells. Sol. RRL 2019, 3, 1900253. [Google Scholar] [CrossRef]
- Pastore, M.; Fantacci, S.; De Angelis, F. Modeling Excited States and Alignment of Energy Levels in Dye-Sensitized Solar Cells: Successes, Failures, and Challenges. J. Phys. Chem. C 2013, 117, 3685–3700. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, B.; Grätzel, M. Combined Experimental and DFT-TDDFT Computational Study of Photoelectrochemical Cell Ruthenium Sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.S.; Inoue, T.; Fujikawa, N.; Yamaguchi, Y.; Hayase, S. Substituent Effect in Direct Ring Functionalized Squaraine Dyes on near Infra-Red Sensitization of Nanocrystalline TiO2 for Molecular Photovoltaics. J. Photochem. Photobiol. A Chem. 2010, 214, 269–275. [Google Scholar] [CrossRef]
- Hoshikawa, T.; Yamada, M.; Kikuchi, R.; Eguchi, K. Impedance Analysis of Internal Resistance Affecting the Photoelectrochemical Performance of Dye-Sensitized Solar Cells. J. Electrochem. Soc. 2005, 152, E68. [Google Scholar] [CrossRef]
- Kapil, G.; Ohara, J.; Ogomi, Y.; Pandey, S.S.; Ma, T.; Hayase, S. Fabrication and Characterization of Coil Type Transparent Conductive Oxide-Less Cylindrical Dye-Sensitized Solar Cells. RSC Adv. 2014, 4, 22959–22963. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Cheng, S.; Yan, F. Plasmon-Induced Broadband Light-Harvesting for Dye-Sensitized Solar Cells Using a Mixture of Gold Nanocrystals. ChemSusChem 2016, 9, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wu, J.; Zheng, M.; Tu, Y.; Lan, Z. A Transparent Cobalt Sulfide/Reduced Graphene Oxide Nanostructure Counter Electrode for High Efficient Dye-Sensitized Solar Cells. Electrochim. Acta 2016, 187, 210–217. [Google Scholar] [CrossRef]
- Hwang, D.-K.; Lee, B.; Kim, D.-H. Efficiency Enhancement in Solid Dye-Sensitized Solar Cell by Three-Dimensional Photonic Crystal. RSC Adv. 2013, 3, 3017. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Bisquert, J.; Palomares, E.; Otero, L.; Kuang, D.; Zakeeruddin, S.M.; Grätzel, M. Correlation between Photovoltaic Performance and Impedance Spectroscopy of Dye-Sensitized Solar Cells Based on Ionic Liquids. J. Phys. Chem. C 2007, 111, 6550–6560. [Google Scholar] [CrossRef]
- Yella, A.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari Astani, N.; Teuscher, J.; Polander, L.E.; Mathew, S.; Moser, J.-E.; Tavernelli, I.; Rothlisberger, U.; et al. Molecular Engineering of a Fluorene Donor for Dye-Sensitized Solar Cells. Chem. Mater. 2013, 25, 2733–2739. [Google Scholar] [CrossRef]
- Karjule, N.; Munavvar Fairoos, M.K.; Nithyanandhan, J. Heterotriangulene-Based Unsymmetrical Squaraine Dyes: Synergistic Effects of Donor Moieties and out-of-Plane Branched Alkyl Chains on Dye Cell Performance. J. Mater. Chem. A 2016, 4, 18910–18921. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, C.; Yang, X.; Islam, A.; Zhang, S.; Chen, H.; Han, L. High-Performance, Transparent, Dye-Sensitized Solar Cells for See-Through Photovoltaic Windows. Adv. Energy Mater. 2014, 4, 1301966. [Google Scholar] [CrossRef]
- Punitharasu, V.; Mele Kavungathodi, M.F.; Singh, A.K.; Nithyanandhan, J. π-Extended Cis—Configured Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Panchromatic Response. ACS Appl. Energy Mater. 2019, 2, 8464–8472. [Google Scholar] [CrossRef]
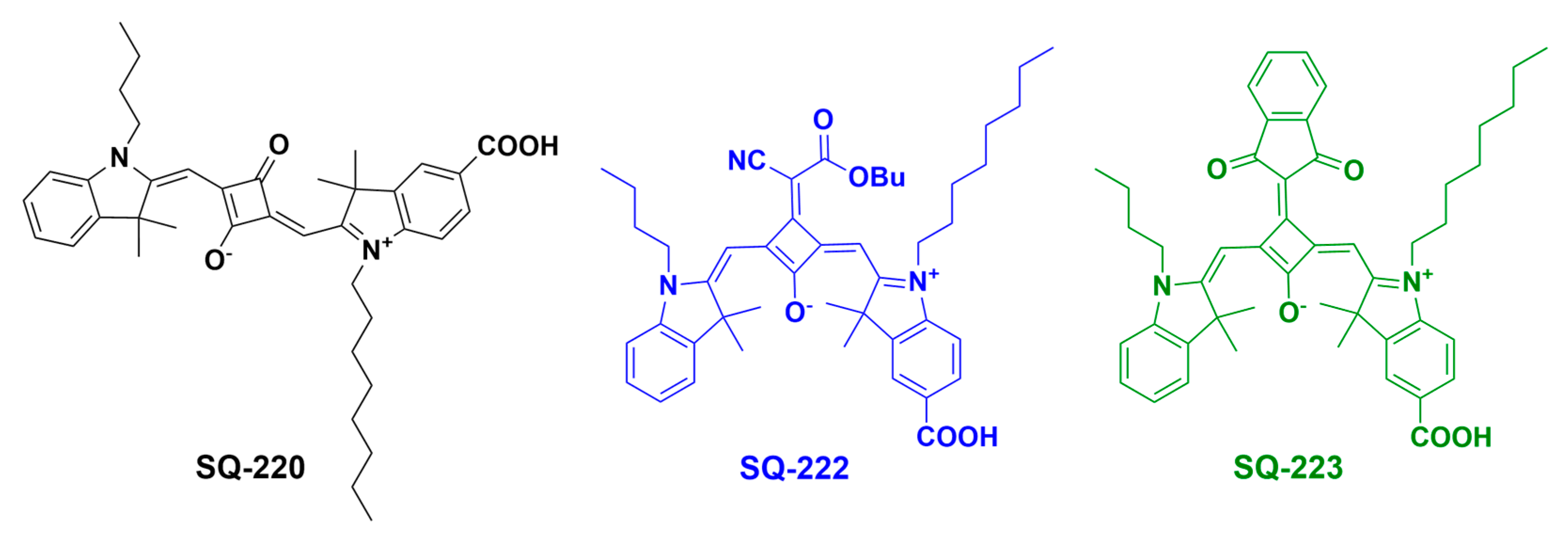
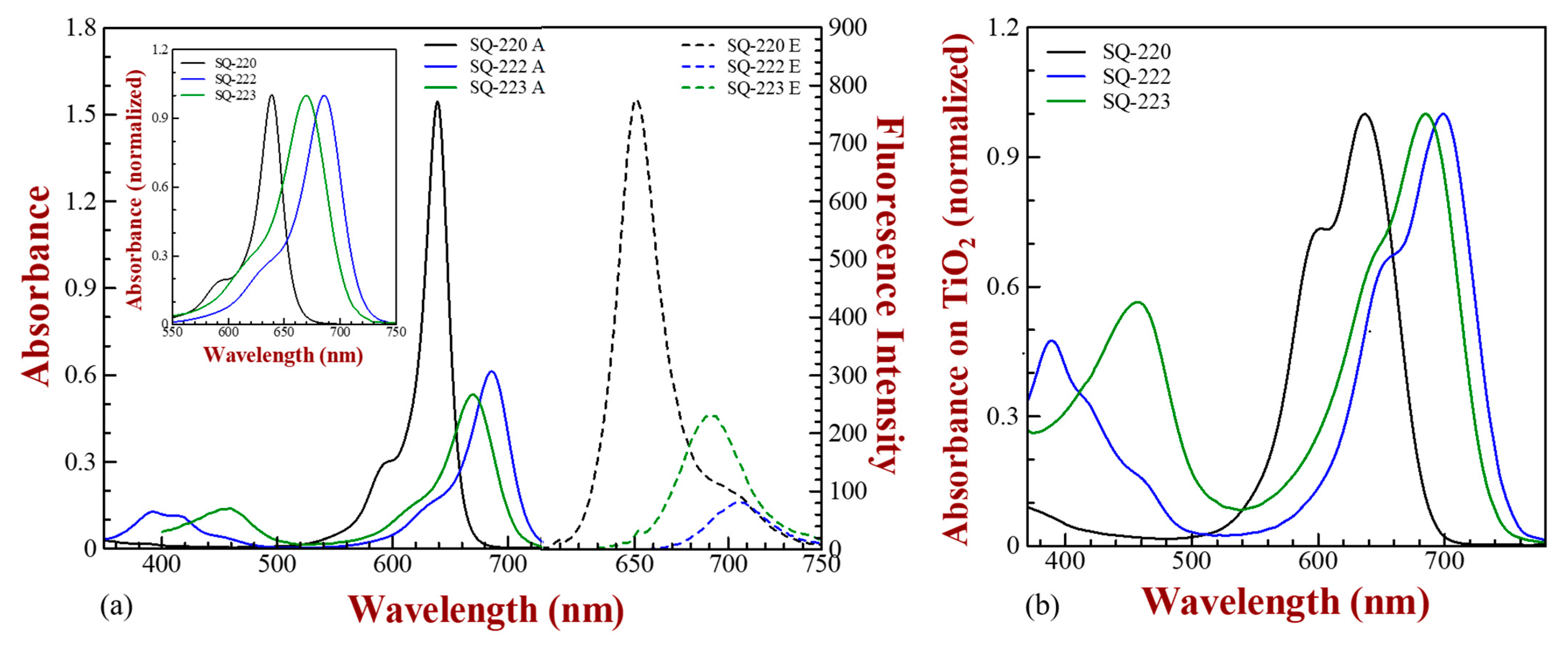






| Sensitizing Dye | ε (i) (dm3 mol−1 cm−1) | λmax (Abs) (ii) (Solution) | λmax (Em) (iii) | Stokes Shift (iv) | λmax (v) (Solid-State) | λopt (vi) | Band Gap (Eg) (vii) (eV) | Extent of Aggregation |
|---|---|---|---|---|---|---|---|---|
| SQ-220 | 3.08 × 105 | 638 nm | 650 nm | 12 nm | 636 nm | 686 nm | 1.81 | 0.73 |
| SQ-222 | 1.22 × 105 | 686 nm | 707 nm | 21 nm | 699 nm | 752 nm | 1.65 | 0.69 |
| SQ-223 | 1.12 × 105 | 670 nm | 692 nm | 22 nm | 685 nm | 740 nm | 1.68 | 0.66 |
| Sensitizing Dye | Jsc [mA/cm2] | Integrated Jsc [mA/cm2] (% Deviation) | Voc [V] | FF | PCE [%] | Dye Loading [nmol cm−2 µm−1] |
|---|---|---|---|---|---|---|
| SQ-220 | 10.04 (9.93 ± 0.23) | 9.49 (5.49) | 0.65 (0.64 ± 0.02) | 0.62 (0.63 ± 0.01) | 4.02 (4.02 ± 0.03) | 1.02 |
| SQ-222 | 10.36 (10.25 ± 0.19) | 9.63 (7.05) | 0.64 (0.63 ± 0.01) | 0.62 (0.61 ± 0.01) | 4.12 (3.94 ± 0.15) | 0.47 |
| SQ-223 | 12.44 (11.93 ± 0.46) | 11.40 (8.36) | 0.63 (0.62 ± 0.01) | 0.60 (0.60 ± 0.01) | 4.67 (4.42 ± 0.18) | 1.25 |
| Sensitizing Dye | RS (Ω) | R1 (Ω) | R2 (Ω) | R3 (Ω) |
|---|---|---|---|---|
| SQ-220 | 33.32 | 12.52 | 25.39 | 2.02 |
| SQ-222 | 33.30 | 12.07 | 23.07 | 1.92 |
| SQ-223 | 33.28 | 12.17 | 21.09 | 2.26 |
| Sensitizing Dye | Jsc [mA/cm2] | Integrated Jsc [mA/cm2] (% Deviation) | Voc [V] | FF | PCE [%] |
|---|---|---|---|---|---|
| D-131 | 10.54 (10.30 ± 0.23) | 8.64 (18.02) | 0.68 (0.68 ± 0.01) | 0.55 (0.56 ± 0.01) | 3.94 (3.81 ± 0.20) |
| SQ-223 | 12.44 (11.93 ± 0.46) | 11.40 (8.36) | 0.63 (0.62 ± 0.01) | 0.60 (0.60 ± 0.01) | 4.67 (4.42 ± 0.18) |
| D-131: SQ-223 (1:9) | 14.01 (13.48 ± 0.38) | 13.08 (6.64) | 0.63 (0.63 ± 0.01) | 0.56 (0.57 ± 0.01) | 4.93 (4.79 ± 0.17) |
| D-131: SQ-223 (9:1) | 17.40 (16.92 ± 0.47) | 14.71 (15.46) | 0.66 (0.64 ± 0.01) | 0.51 (0.52 ± 0.01) | 5.81 (5.59 ± 0.18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, S.; Kurokawa, Y.; Shaban, S.; Pandey, S.S. Squaric Acid Core Substituted Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Effect of Electron Acceptors on Their Photovoltaic Performance. Colorants 2023, 2, 654-673. https://doi.org/10.3390/colorants2040034
Pradhan S, Kurokawa Y, Shaban S, Pandey SS. Squaric Acid Core Substituted Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Effect of Electron Acceptors on Their Photovoltaic Performance. Colorants. 2023; 2(4):654-673. https://doi.org/10.3390/colorants2040034
Chicago/Turabian StylePradhan, Safalmani, Yuki Kurokawa, Suraya Shaban, and Shyam S. Pandey. 2023. "Squaric Acid Core Substituted Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Effect of Electron Acceptors on Their Photovoltaic Performance" Colorants 2, no. 4: 654-673. https://doi.org/10.3390/colorants2040034
APA StylePradhan, S., Kurokawa, Y., Shaban, S., & Pandey, S. S. (2023). Squaric Acid Core Substituted Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Effect of Electron Acceptors on Their Photovoltaic Performance. Colorants, 2(4), 654-673. https://doi.org/10.3390/colorants2040034






