Evaluation of Solar Conversion Efficiency in Dye-sensitized Solar Cells Using Natural Dyes Extracted from Alpinia purpurata and Alstroemeria Flower Petals as Novel Photosensitizers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
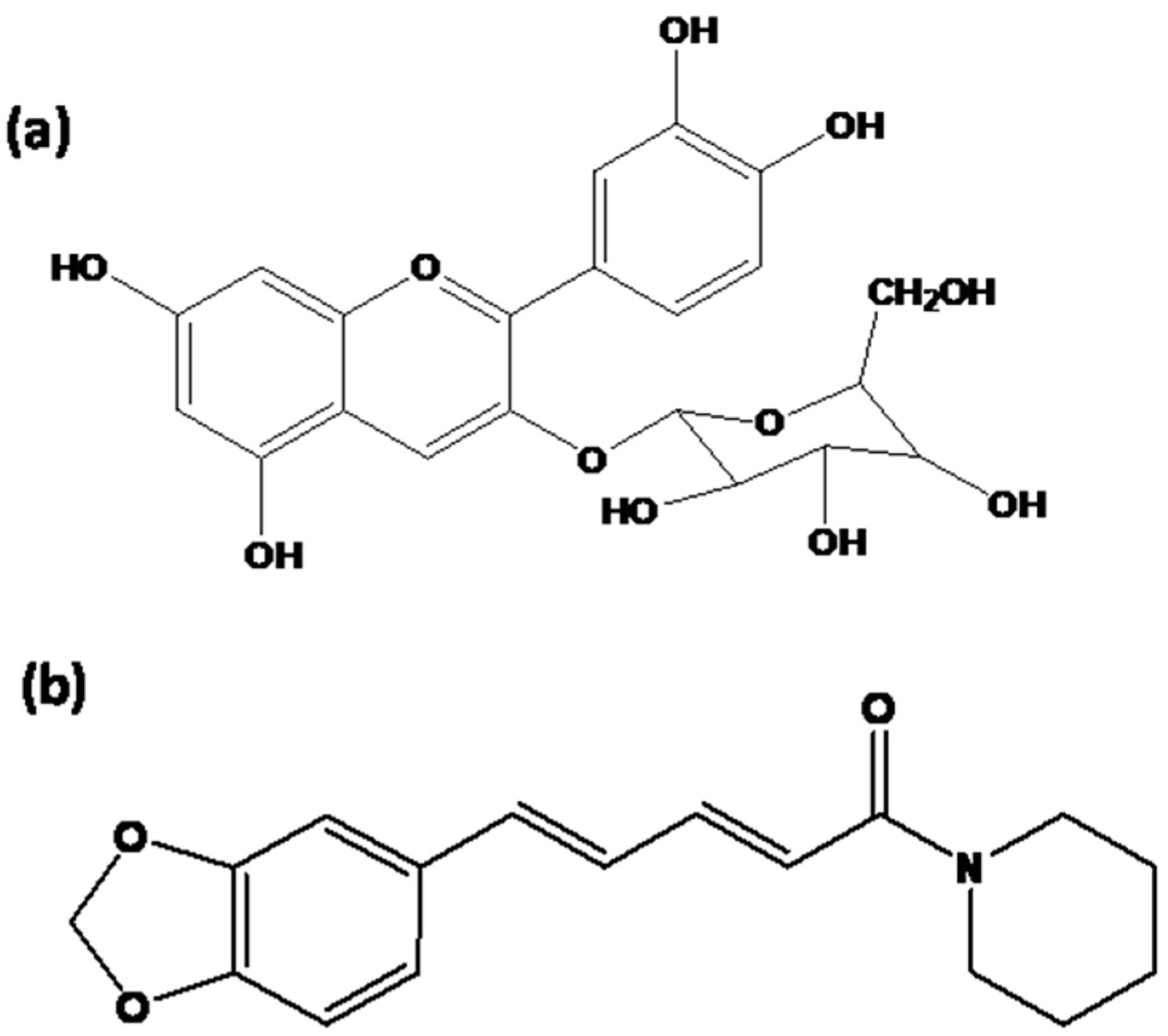
2.2. Extraction of Dye
2.3. Photoanode Fabrication
2.4. Photocathode Fabrication
2.5. Electrolyte Preparation
2.6. Construction of DSSCs
3. Results and Discussion
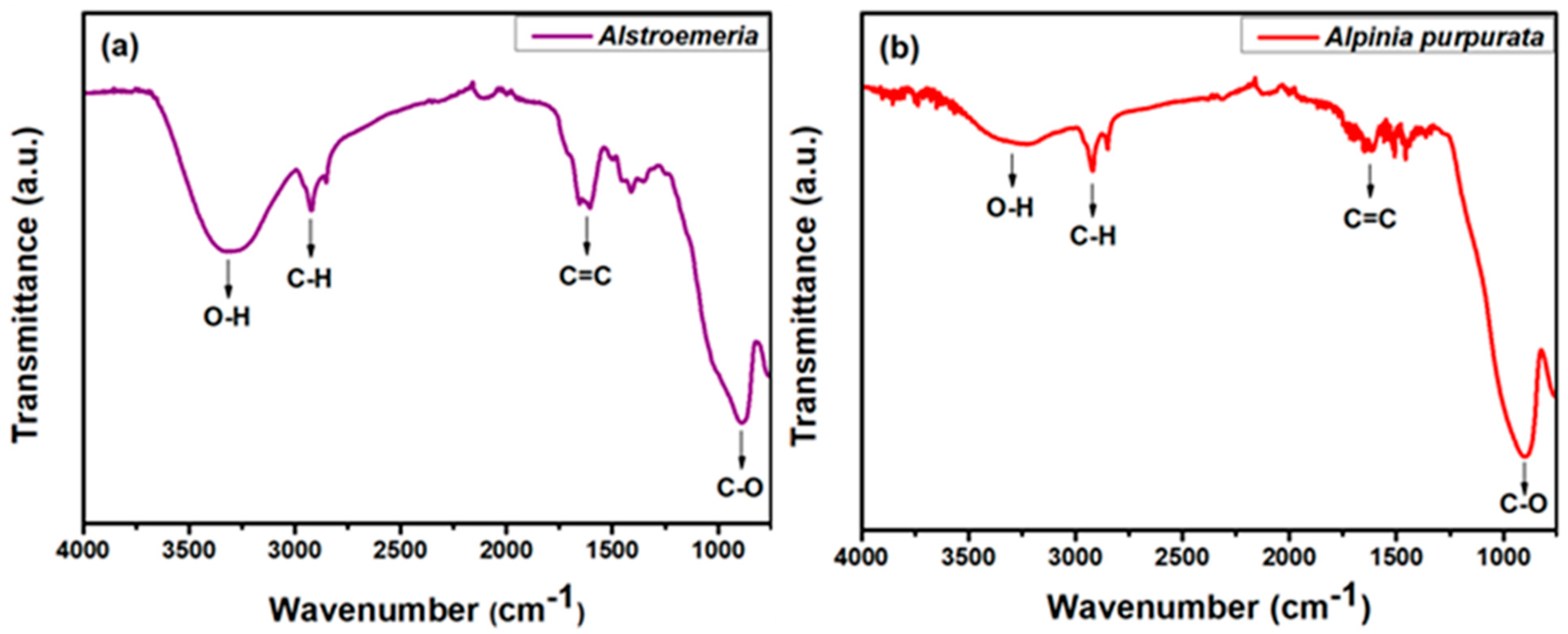
3.1. FTIR Spectroscopy
3.2. UV-Vis Spectroscopy
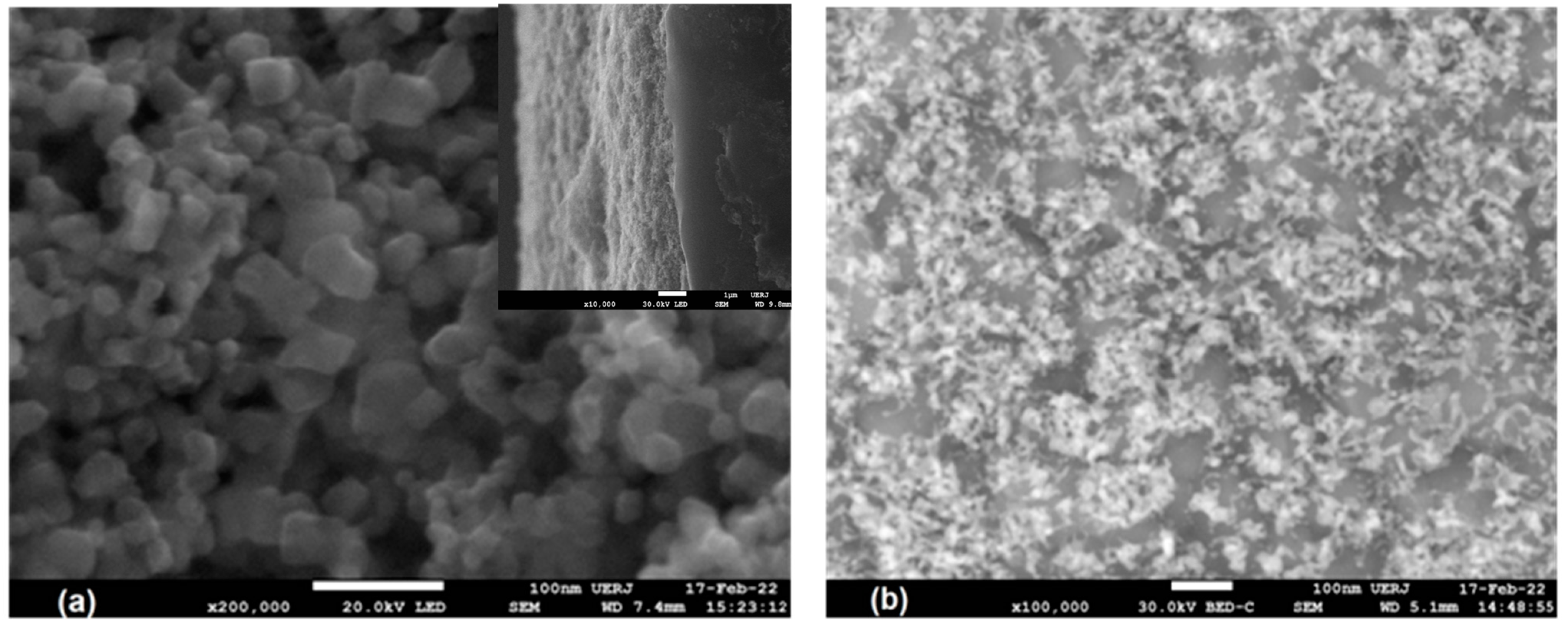
3.3. FESEM
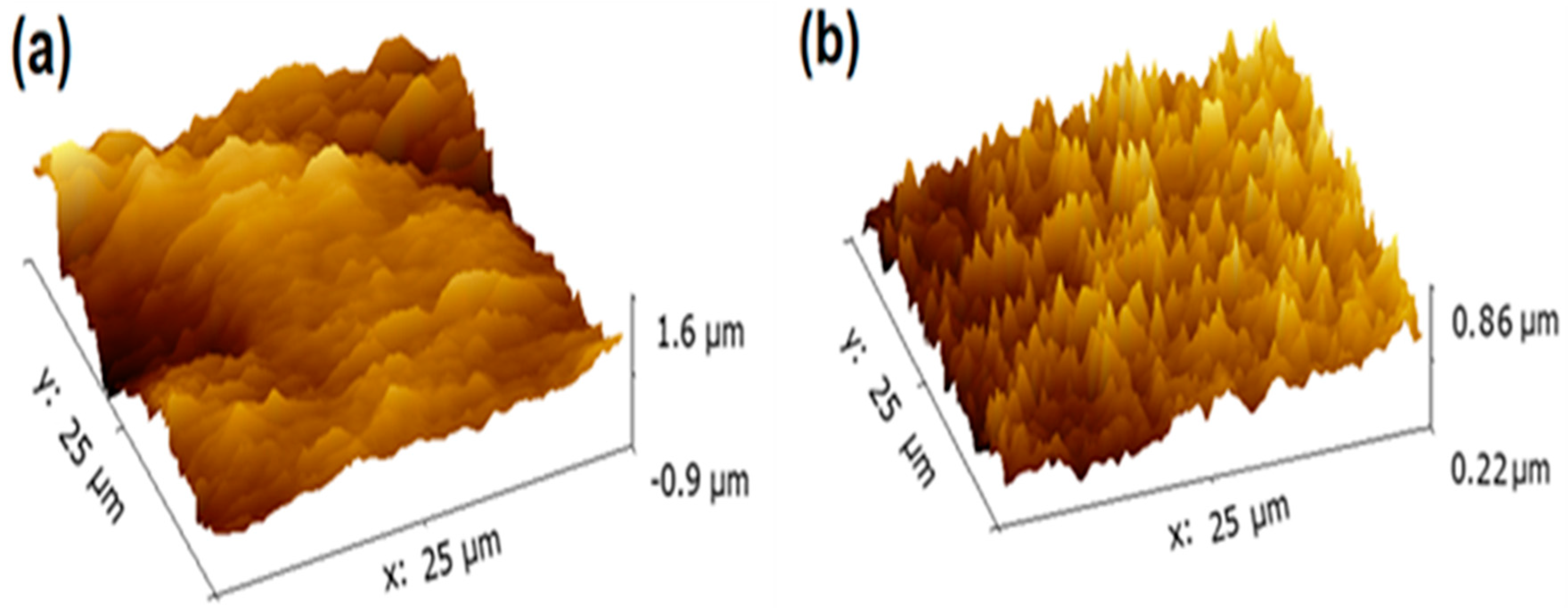
3.4. AFM
3.5. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogdanov, D.; Ram, M.; Aghahosseini, A.; Gulagi, A.; Oyewo, A.S.; Child, M.; Caldera, U.; Sadovskaia, K.; Farfan, J.; Barbosa, L.D.S.N.S.; et al. Low-cost renewable electricity as the key driver of the global energy transition towards sustainability. Energy 2021, 227, 120467. [Google Scholar] [CrossRef]
- Yashwantrao, G.; Saha, S. Perspective on the rational design strategies of quinoxaline derived organic sensitizers for dye-sensitized solar cells (DSSC). Dyes Pigm. 2022, 199, 110093. [Google Scholar] [CrossRef]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Maurya, I.C.; Singh, S.; Srivastava, P.; Maiti, B.; Bahadur, L. Natural dye extract from Cassia fistula and its application in dye-sensitized solar cell: Experimental and density functional theory studies. Opt. Mater. 2019, 90, 273–280. [Google Scholar] [CrossRef]
- Noor, S.; Sajjad, S.; Leghari, S.A.K.; Shaheen, S.; Iqbal, A. ZnO/TiO2 nanocomposite photoanode as an effective UV-Vis responsive dye sensitized solar cell. Mater. Res. Express 2018, 5, 095905. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Amao, Y.; Komori, T. Bio-photovoltaic conversion device using chlorine-e6 derived from chlorophyll from Spirulina adsorbed on a nanocrystalline TiO2 film electrode. Biosens. Bioelectron. 2004, 19, 843–847. [Google Scholar] [CrossRef]
- Ludin, N.A.; Mahmoud, A.M.A.-A.; Mohamad, A.B.; Kadhum, A.A.H.; Sopian, K.; Karim, N.S.A. Review on the development of natural dye photosensitizer for dye-sensitized solar cells. Renew. Sust. Energy Rev. 2014, 31, 386–396. [Google Scholar] [CrossRef]
- Hao, S.; Wu, J.; Huang, Y.; Lin, J. Natural dyes as photosensitizers for dye-sensitized solar cell. Sol. Energy 2006, 80, 209–214. [Google Scholar] [CrossRef]
- Polo, A.S.; Iha, N.Y.M. Blue sensitizers for solar cells: Natural dyes from Calafate and Jaboticaba. Sol. Energy Mater. Sol. Cells 2006, 90, 1936–1944. [Google Scholar] [CrossRef]
- Zainudin, S.N.F.; Abdullah, H.; Markom, M. Electrochemical studies of tin oxide based-dye-sensitized solar cells (DSSC): A review. J. Mater. Sci. Mater. Electron. 2019, 30, 5342–5356. [Google Scholar] [CrossRef]
- García-Salinas, M.J.; Ariza, M.J. Optimizing a simple natural dye production method for dye-sensitized solar cells: Examples for Betalain (Bougainvillea and Beetroot Extracts) and anthocyanin dyes. Appl. Sci. 2019, 9, 2515. [Google Scholar] [CrossRef]
- Ambapuram, M.; Maddala, G.; Simhachalam, N.B.; Sripada, S.; Kalvapalli, S.; Pedda, V.S.Y.; Mitty, R. Highly effective SnS composite counter electrode sandwiched bi-function CeO2:Er3+/Yb3+ assisted surface modified photoelectroded dye sensitized solar cell exceeds 9.5% efficiency. Sol. Energy 2020, 207, 1158–1164. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-doped TiO2: Visible light assisted photocatalytic antimicrobial activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Subalakshmi, K.; Senthilselvan, J. Effect of fluorine-doped TiO2 photoanode on electron transport, recombination dynamics and improved DSSC efficiency. Sol. Energy 2018, 171, 914–928. [Google Scholar] [CrossRef]
- Langmar, O.; Fazio, E.; Schol, P.; de la Torre, G.; Costa, R.D.; Torres, T.; Guldi, D.M. Controlling interfacial charge transfer and fill factors in CuO-based tandem dye-sensitized solar cells. Angew. Chem. Int. Ed. 2019, 58, 4056–4060. [Google Scholar] [CrossRef]
- Samanta, P.N.; Majumdar, D.; Roszak, S.; Leszczynski, J. First-principles approach for assessing cold electron injection efficiency of dye-sensitized solar cell: Elucidation of mechanism of charge injection and recombination. J. Phys. Chem. C 2020, 124, 2817–2836. [Google Scholar] [CrossRef]
- Shanmugam, V.; Manoharan, S.; Anandan, S.; Murugan, R. Performance of dye-sensitized solar cells fabricated with extracts from fruits of ivy gourd and flowers of red frangipani as sensitizers. Spectrochim. Acta Part A 2013, 104, 35–40. [Google Scholar] [CrossRef]
- Kimpa, M.I.; Momoh, M.; Isah, K.U.; Yahya, H.N.; Ndamitso, M.M. Photoelectric characterization of dye sensitized solar cells using natural dye from pawpaw leaf and flame tree flower as sensitizers. Mater. Sci. Appl. 2012, 3, 281–286. [Google Scholar]
- Maabong, K.; Muiva, C.M.; Monowe, P.; Sathiaraj, S.T.; Hopkins, M.; Nguyen, L.; Malungwa, K.; Thobega, M. Natural pigments as photosensitizers for dye-sensitized solar cells with TiO2 thin films. Int. J. Renew. Energy Res. 2015, 5, 54–60. [Google Scholar]
- Teoli, F.; Lucioli, S.; Nota, O.; Frattarelli, A.; Matteocci, F.; Di Carlo, A.; Caboni, E.; Forni, C. Role of pH and pigment concentration for natural dye-sensitized solar cells treated with anthocyanin extracts of common fruits. J. Photochem. Photobiol. A Chem. 2016, 316, 24–30. [Google Scholar] [CrossRef]
- Calogero, G.; Marco, G.D. Red Sicilian orange and purple eggplant fruits as natural sensitizers for dye-sensitized solar cells. Sol. Energy Mater Sol. Cells 2008, 92, 1341–1346. [Google Scholar] [CrossRef]
- Chang, H.; Lo, Y.J. Pomegranate leaves and mulberry fruit as natural sensitizers for dye-sensitized solar cells. Sol. Energy 2010, 84, 1833–1837. [Google Scholar] [CrossRef]
- Mozaffari, S.A.; Saeidi, M.; Rahmanian, R. Photoelectric characterization of fabricated dye-sensitized solar cell using dye extracted from red Siahkooti fruit as natural sensitizer. Spectrochim. Acta Part A 2015, 142, 226–231. [Google Scholar] [CrossRef]
- Singh, L.K.; Karlo, T.; Pandey, A. Performance of fruit extract of Melastoma malabathricum L. as sensitizer in DSSCs. Spectrochim. Acta Part A 2014, 118, 938–943. [Google Scholar] [CrossRef]
- Calogero, G.; Marco, G.D.; Cazzanti, S.; Caramori, S.; Argazzi, R.; Carlo, A.D.; Bignozzi, C.A. Efficient dye-sensitized solar cells using red turnip and purple wild sicilian prickly pear fruits. Int. J. Mol. Sci. 2010, 11, 254–267. [Google Scholar] [CrossRef]
- Rosana, N.T.M.; Amarnath, D.J.; Joseph, K.L.V.; Anandan, S. Mixed dye from nerium oleander and hibiscus flowers as a photo sensitizer in dye sensitized solar cells. Int. J. Chem. Tech. Res. 2014, 6, 5022–5026. [Google Scholar]
- Tian, H.; Chen, K.; Ye, X.; Yang, S.; Gu, Q. Hydrothermal growth of Bi2Ti2O7/TiO2 and Bi4Ti3O12/TiO2 heterostructures on highly ordered TiO2-nanotube arrays for dye-sensitized solar cells. Ceram. Int. 2019, 45, 20750–20757. [Google Scholar] [CrossRef]
- Idris, A.; Linatoc, A.C.; Bakar, M.F.A.; Takai, Z.I.; Audu, Y. Effect of light quality and quantity on the accumulation of flavonoid in plant species. J. Sci. Technol. 2018, 10, 32–45. [Google Scholar] [CrossRef]
- Yousefi, F.; Jabbarzadeh, Z.; Amiri, J.; Rasouli-Sadaghiani, M.; Shaygan, A. Foliar application of polyamines improve some morphological and physiological characteristics of rose. Folia Hortic. 2021, 33, 147–156. [Google Scholar] [CrossRef]
- Tomar, N.; Agrawal, A.; Dhaka, V.S.; Surolia, P.K. Ruthenium complexes based dye sensitized solar cells: Fundamentals and research trends. Sol Energy 2020, 207, 59–76. [Google Scholar] [CrossRef]
- Sampaio, D.M.; Babu, R.S.; Costa, H.R.M.; de Barros, A.L.F. Investigation of nanostructured TiO2 thin film coatings for DSSCs application using natural dye extracted from jabuticaba fruit as photosensitizers. Ionics 2019, 25, 2893–2902. [Google Scholar] [CrossRef]
- Ferreira, B.C.; Sampaio, D.M.; Babu, R.S.; de Barros, A.L.F. Influence of nanostructured TiO2 film thickness in dye-sensitized solar cells using naturally extracted dye from Thunbergia erecta flowers as a photosensitizer. Opt. Mater. 2018, 86, 239–246. [Google Scholar] [CrossRef]
- Karim, N.A.; Mehmood, U.; Zahid, H.F.; Asi, T. Nanostructured photoanode and counter electrode materials for efficient Dye-Sensitized Solar Cells (DSSCs). Sol. Energy 2019, 185, 165–188. [Google Scholar] [CrossRef]
- Ferreira, F.C.; Babu, R.S.; de Barros, A.L.F.; Raja, S.; da Conceição, L.R.B.; Mattoso, L.H.C. Photoelectric performance evaluation of DSSCs using the dye extracted from different color petals of Leucanthemum vulgare flowers as novel sensitizers. Spectrochim. Acta A 2020, 233, 118198. [Google Scholar] [CrossRef]
- Kuppu, S.V.; Jeyaraman, A.R.; Guruviah, P.K.; Thambusamy, S. Preparation and characterizations of PMMA-PVDF based polymer composite electrolyte materials for dye sensitized solar cell. Curr. Appl. Phys. 2018, 18, 619–625. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, P.; Cheng, H.; Tan, G.; Wu, J.; Zheng, J.; Zhou, X.; Fang, S.; Dai, Y.; Lin, Y. Synthesis of low-viscosity ionic liquids for application in dye-sensitized solar cells. Chem. Asian J. 2019, 14, 4201–4206. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, C.; Wang, S.; Wen, S. Polydopamine-modified electrospun polyvinylidene fluoride nanofiber based flexible polymer gel electrolyte for highly stable dye-sensitized solar cells. ACS Omega 2021, 6, 28663–28670. [Google Scholar] [CrossRef]
- dos Santos, F.M.M.; Leite, A.M.B.; da Conceição, L.R.B.; Sasikumar, Y.; Atchudan, R.; Pinto, M.F.; Babu, R.S.; de Barros, A.L.F. Effect of bandgap energies by various color petals of Gerbera Jamesonii flower dyes as a photosensitizer on enhancing the efficiency of dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2022, 33, 20338–20352. [Google Scholar] [CrossRef]
- Lee, K.E.; Gomez, M.A.; Elouatik, S.; Demopoulos, G.P. Further understanding of the adsorption mechanism of N719 sensitizer on anatase TiO2 Films for DSSC applications using vibrational spectroscopy and confocal Raman imaging. Langmuir 2010, 26, 9575–9583. [Google Scholar] [CrossRef] [PubMed]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of cellulosic fibers by FTIR spectroscopy for their further implementation to building materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef]
- Akila, Y.; Muthukumarasamy, N.; Agilan, S.; Mallick, T.K.; Senthilarasu, S.; Velauthapillai, D. Enhanced performance of natural dye sensitized solar cells fabricated using rutile TiO2 nanorods. Opt. Mater. 2016, 58, 76–83. [Google Scholar] [CrossRef]
- Sirat, H.M.; Liamen, M.R. Chemical constituents of Alpinia purpurata. Pertanika J. Sci. Technol. 1995, 3, 67–71. [Google Scholar]
- Patni, N.; Pillai, S.G.; Sharma, P. Effect of using betalain, anthocyanin and chlorophyll dyes together as a sensitizer on enhancing the efficiency of dye sensitized solar cell. Int. J. Energy Res. 2020, 44, 10846–10859. [Google Scholar] [CrossRef]
- Pathak, C.; Surana, K.; Shukla, V.K.; Singh, P.K. Fabrication and characterization of dye sensitized solar cell using natural dyes. Mater. Today Proc. 2019, 12, 665–670. [Google Scholar]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Das, A.; Nair, R.G. Fabrication of In2O3 functionalized ZnO based nano heterojunction photoanode for improved DSSC performance through effective interfacial charge carrier separation. Opt. Mater. 2021, 122 Pt B, 111784. [Google Scholar] [CrossRef]
- Jose, R.; Thavasi, V.; Ramakrishna, S. Metal oxides for dye-sensitized solar cells. J. Am. Ceram. Soc. 2009, 92, 289–301. [Google Scholar] [CrossRef]
- de Angelis, F.; Fantacci, S.; Selloni, A.; Gratzel, M.; Nazeeruddin, M.K. Influence of the sensitizer adsorption mode on the open-circuit potential of dye-sensitized solar cells. Nano Lett. 2007, 7, 3189–3195. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Ren, J.; Kaxiras, E. Natural Dyes Adsorbed on TiO2 Nanowire for photovoltaic applications: Enhanced light absorption and ultrafast electron injection. Nano Lett. 2008, 8, 3266–3272. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.; Murayama, M.; Nishikawa, N.; Hashimoto, N.; Shoyama, M.; Kurita, O. Utilization of natural carotenoids as photosensitizers for dye-sensitized solar cells. Sol. Energy 2007, 81, 512–516. [Google Scholar] [CrossRef]
- Gómez-Ortíz, N.M.; Vázquez-Maldonado, I.A.; Pérez-Espadas, A.R.; Mena-Rejón, G.J.; Azamar-Barrios, J.A.; Oskam, G. Dye-sensitized solar cells with natural dyes extracted from achiote seeds. Sol. Energy Mater. Sol. Cells 2010, 94, 40–44. [Google Scholar] [CrossRef]
- Sowmya, S.; Prakash, P.; Ruba, N.; Janarthanan, B.; Prabu, A.N.; Chandrasekaran, J. A study on the fabrication and characterization of dye-sensitized solar cells with Amaranthus red and Lawsonia inermis as sensitizers with maximum absorption of visible light. J. Mater. Sci. Mater. Electron. 2020, 31, 6027–6035. [Google Scholar] [CrossRef]
- Sanda, M.D.A.; Badu, M.; Awudza, J.A.M.; Boadi, N.O. Development of TiO2-based dye-sensitized solar cells using natural dyes extracted from some plant-based materials. Chem. Int. 2021, 7, 9–20. [Google Scholar]
- Purushothamreddy, N.; Dileep, R.K.; Veerappan, G.; Kovendhan, M.; Joseph, D.P. Prickly pear fruit extract as photosensitizer for dye-sensitized solar cell. Spectrochim. Acta A 2020, 228, 117686. [Google Scholar] [CrossRef]
- Ossai, A.N.; Ezike, S.C.; Timtere, P.; Ahmed, A.D. Enhanced photovoltaic performance of dye-sensitized solar cells-based Carica papaya leaf and black cherry fruit co-sensitizers. Chem. Phys. Impact 2021, 2, 100024. [Google Scholar] [CrossRef]
- Leite, A.M.B.; da Cunha, H.O.; Rodrigues, J.A.F.C.R.; Babu, R.S.; de Barros, A.L.F. Construction and characterization of organic photovoltaic cells sensitized by Chrysanthemum based natural dye. Spectrochim. Acta A 2023, 284, 121780. [Google Scholar] [CrossRef]
- Singh, S.; Maurya, I.C.; Sharma, S.; Kushwaha, P.S.; Srivastava, P.; Bahadur, L. Application of new natural dyes extracted from Nasturtium flowers (Tropaeolum majus) as photosensitizer in dye-sensitized solar cells. Optik 2021, 243, 167331. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Kathiravan, I.; Balasundaram, J.; Shkir, M.; Alfaify, S. An analysis of the dye-sensitized solar cells fabricated with the dyes extracted from the leaves and flowers of Amaranthus cruentus. Environ. Sci. Poll. Res. 2022, 29, 44271–44281. [Google Scholar] [CrossRef] [PubMed]
- Al-Alwani, M.A.M.; Hassimi, A.H.; Al-Shorgani, N.K.N.; Al-Mashaan, A.B.S.A. Natural dye extracted from Areca catechu fruits as a new sensitizer for dye-sensitized solar cell fabrication: Optimization using D-Optimal design. Mater. Chem. Phys. 2020, 240, 122204. [Google Scholar] [CrossRef]
- Ferreira, B.C.; Babu, R.S.; da Conceição, L.R.B.; da Cunha, H.O.; Sampaio, D.M.; Samyn, L.M.; de Barros, A.L.F. Performance evaluation of DSSCs using naturally extracted dyes from petals of Lantana repens and Solidago canadensis flowers as light-harvesting units. Ionics 2022, 28, 5233–5242. [Google Scholar] [CrossRef]
- Rudra, S.; Seo, H.W.; Sarker, S.; Kim, D.M. Simulation and electrochemical impedance spectroscopy of dye-sensitized solar cells. J. Indus. Eng. Chem. 2021, 97, 574–583. [Google Scholar] [CrossRef]
- Sinha, D.; De, D.; Ayaz, A. Photo sensitizing and electrochemical performance analysis of mixed natural dye and nanostructured ZnO based DSSC. Sādhanā 2020, 45, 175. [Google Scholar] [CrossRef]
- Chou, J.C.; Lu, C.C.; Liao, Y.H.; Lai, C.H.; Nien, Y.H.; Kuo, C.H.; Ko, C.C. Fabrication and electrochemical impedance analysis of dye-sensitized solar cells with titanium dioxide compact layer and graphene oxide dye absorption layer. IEEE Trans. Nanotechnol. 2019, 18, 461–466. [Google Scholar] [CrossRef]
- Faraz, S.M.; Mazhar, M.; Shah, W.; Noor, H.; Awan, Z.H.; Sayyad, M.H. Comparative study of impedance spectroscopy and photovoltaic properties of metallic and natural dye based dye sensitized solar cells. Phys. B Condens. Matter 2021, 602, 412567. [Google Scholar] [CrossRef]
- da Conceição, L.R.B.; de Barros, A.L.F.; Haddad, D.B.; Babu, R.S. Passiflora edulis and Cocos nucifera extracts as light-harvesters for efficient dye-sensitized solar cells. In Proceedings of the 2020 IEEE ANDESCON, Quito, Ecuador, 13–16 October 2020; pp. 1–5. [Google Scholar]


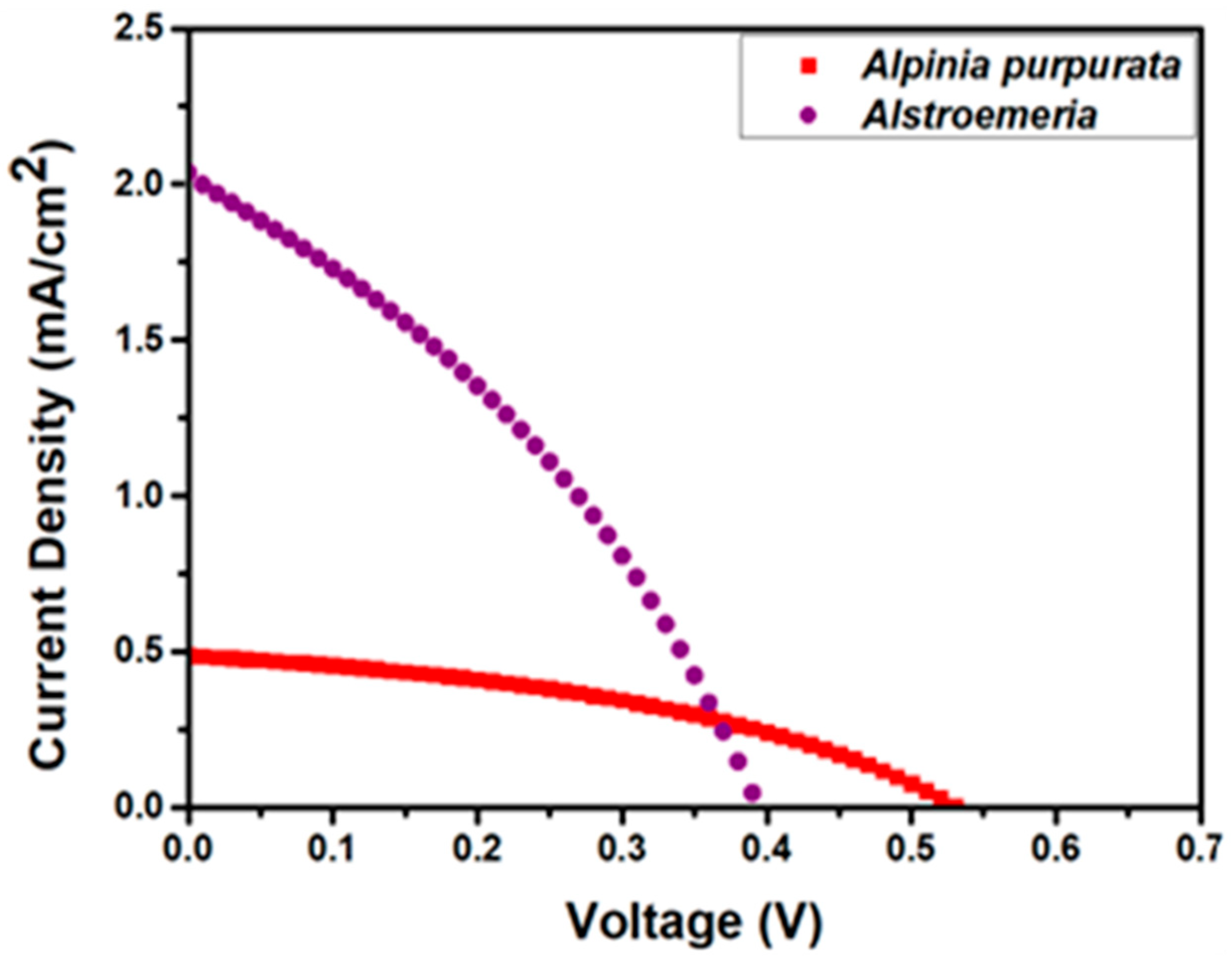
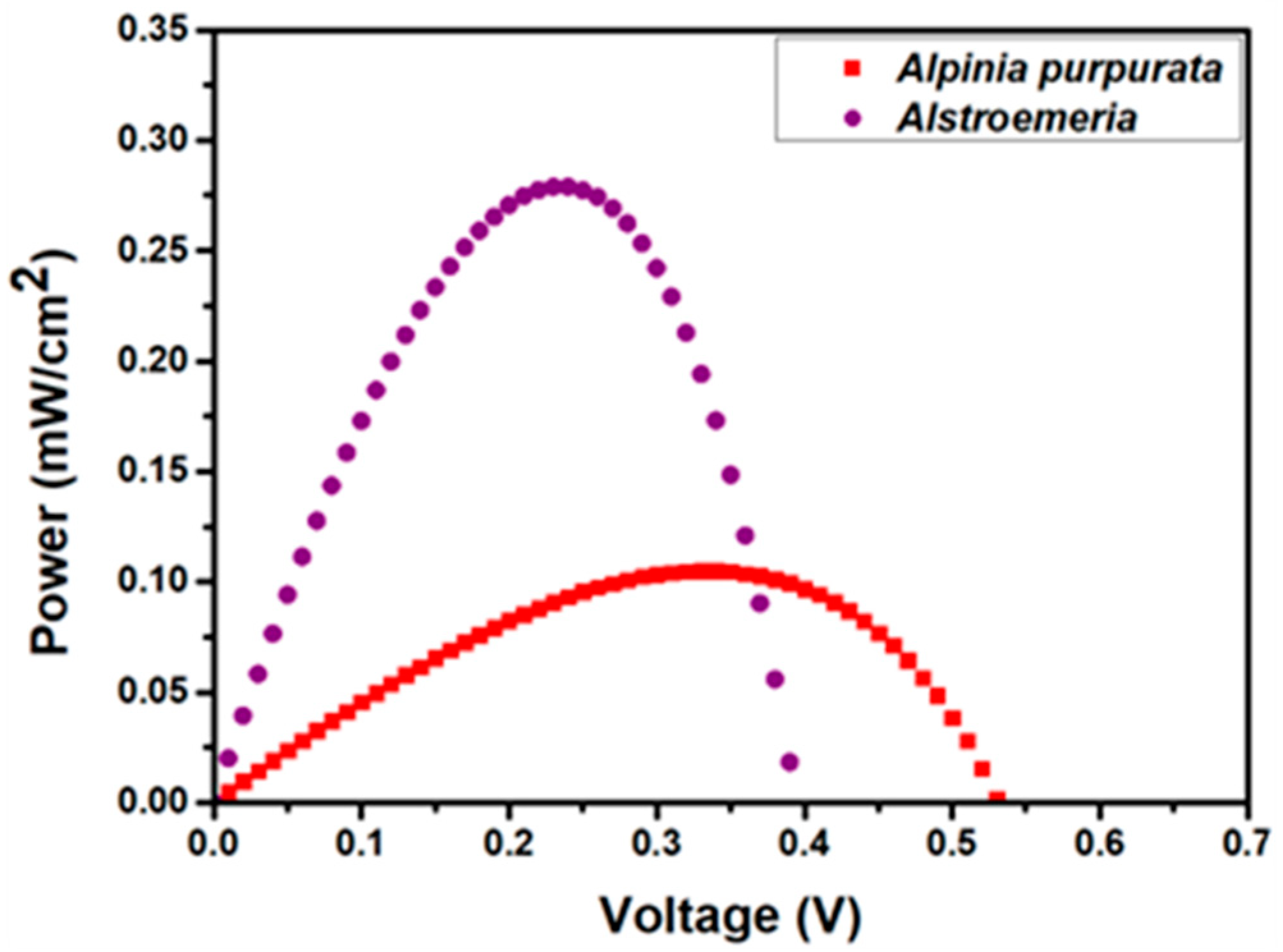
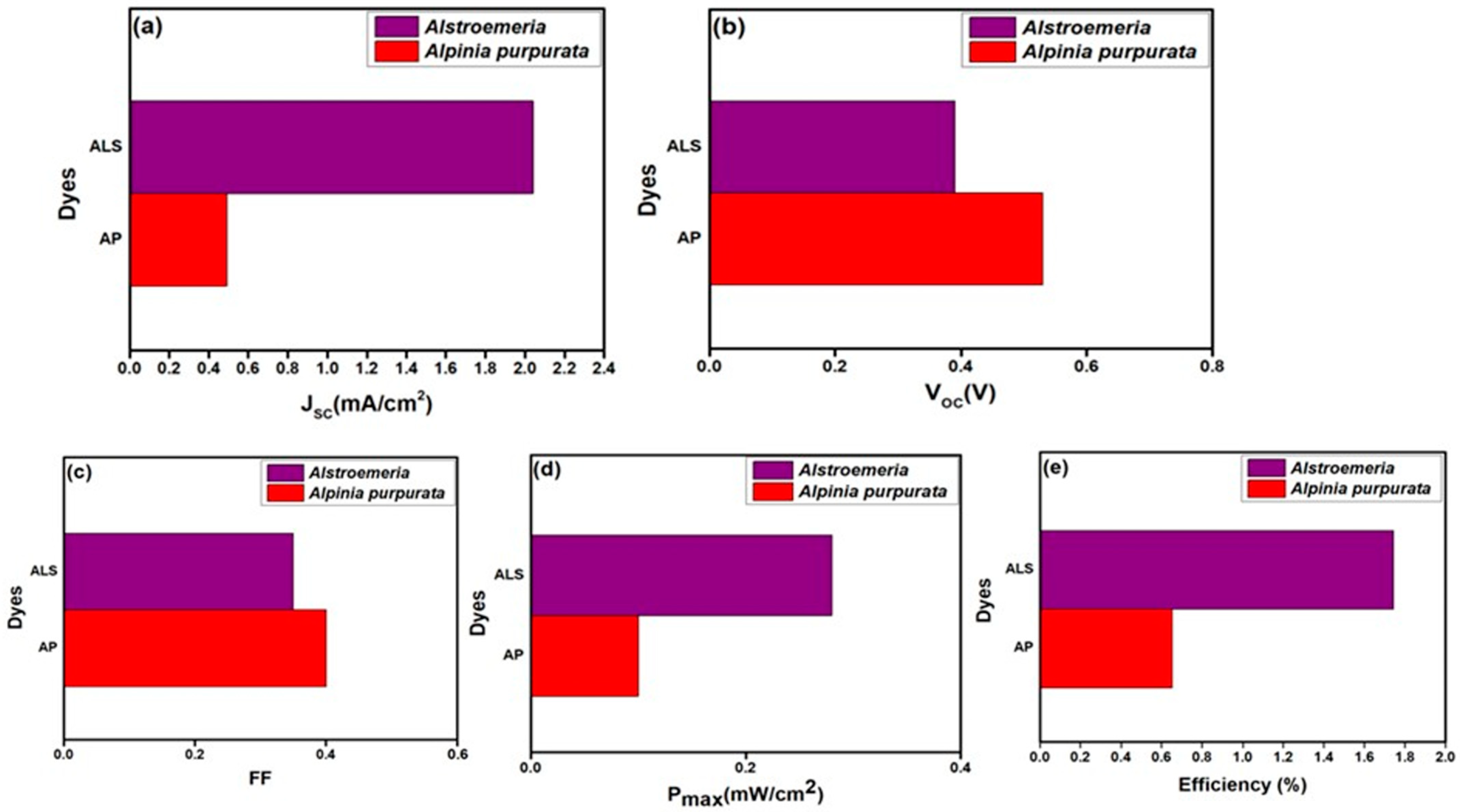

| Dye | JSC (mA/cm2) | VOC (V) | FF | η (%) | Ref. |
|---|---|---|---|---|---|
| Jabuticaba | 0.38 | 0.41 | 0.29 | 0.13 | [34] |
| Thubergia Erecta | 0.27 | 0.54 | 0.08 | 0.38 | [35] |
| Achiote seeds | 1.1 | 0.57 | 0.59 | 0.37 | [54] |
| Henna | 0.42 | 0.54 | 0.38 | 0.09 | [55] |
| Sorghum Stem | 1.69 | 0.34 | 0.31 | 0.18 | [56] |
| Leucanthemum | 0.42 | 0.54 | 0.27 | 0.88 | [37] |
| Prickly Pear | 1.17 | 0.56 | 0.85 | 0.56 | [57] |
| Carica papaya | 1.77 | 0.40 | 0.42 | 0.29 | [58] |
| Chrysanthemum | 0.85 | 0.58 | 0.48 | 1.35 | [59] |
| Tropaeolum majus | 0.72 | 0.55 | 0.70 | 0.28 | [60] |
| Gerbera | 1.22 | 0.59 | 0.53 | 1.54 | [41] |
| Amaranthus cruentus | 5.81 | 0.49 | 0.28 | 0.81 | [61] |
| Areca catechu | 0.9 | 0.51 | 0.63 | 0.38 | [62] |
| Lantana repens | 0.45 | 0.69 | 0.34 | 0.12 | [63] |
| Solidago canadensis | 0.93 | 0.79 | 0.42 | 0.31 | [63] |
| Alstroemeria | 2.04 | 0.39 | 0.35 | 1.74 | this work |
| Alpinia purpurata | 0.49 | 0.53 | 0.40 | 0.65 | this work |
| Dye | Rs (Ω) | R1 (Ω) | R2 (Ω) | R3 (Ω) | C1 (μF) | C2 (μF) | C3 (μF) |
|---|---|---|---|---|---|---|---|
| Alpinia purpurata | 28.34 | 163.6 | 24.50 | 8.097 | 64.36 | 71.75 | 93.45 |
| ±0.53 | ±1.50 | ±9.85 | ±8.26 | ±3.63 | ±4.15 | ±5.29 | |
| Alstroemeria | 38.56 | 73.16 | 15.75 | 6.017 | 44.29 | 144.28 | 87.43 |
| ±0.78 | ±1.27 | ±0.21 | ±1.21 | ±2.28 | ±3.01 | ±3.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Conceição, L.R.B.; da Cunha, H.O.; Leite, A.M.B.; Suresh Babu, R.; Raja, S.; Ribeiro, C.; de Barros, A.L.F. Evaluation of Solar Conversion Efficiency in Dye-sensitized Solar Cells Using Natural Dyes Extracted from Alpinia purpurata and Alstroemeria Flower Petals as Novel Photosensitizers. Colorants 2023, 2, 618-631. https://doi.org/10.3390/colorants2040032
da Conceição LRB, da Cunha HO, Leite AMB, Suresh Babu R, Raja S, Ribeiro C, de Barros ALF. Evaluation of Solar Conversion Efficiency in Dye-sensitized Solar Cells Using Natural Dyes Extracted from Alpinia purpurata and Alstroemeria Flower Petals as Novel Photosensitizers. Colorants. 2023; 2(4):618-631. https://doi.org/10.3390/colorants2040032
Chicago/Turabian Styleda Conceição, Leonardo Ricardo Bernardes, Higor Oliveira da Cunha, Arcano Matheus Bragança Leite, Rajendran Suresh Babu, Sebastian Raja, Caue Ribeiro, and Ana Lucia Ferreira de Barros. 2023. "Evaluation of Solar Conversion Efficiency in Dye-sensitized Solar Cells Using Natural Dyes Extracted from Alpinia purpurata and Alstroemeria Flower Petals as Novel Photosensitizers" Colorants 2, no. 4: 618-631. https://doi.org/10.3390/colorants2040032
APA Styleda Conceição, L. R. B., da Cunha, H. O., Leite, A. M. B., Suresh Babu, R., Raja, S., Ribeiro, C., & de Barros, A. L. F. (2023). Evaluation of Solar Conversion Efficiency in Dye-sensitized Solar Cells Using Natural Dyes Extracted from Alpinia purpurata and Alstroemeria Flower Petals as Novel Photosensitizers. Colorants, 2(4), 618-631. https://doi.org/10.3390/colorants2040032









