Comparative Study of the Synthesis of a Red Ceramic Pigment Using Microwave Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis by Traditional Route
2.2. Synthesis by Coprecipitation Route
2.3. Conventional Heat Treatment
2.4. Microwave Assisted Heat Treatment
2.5. Characterization of Samples
3. Results
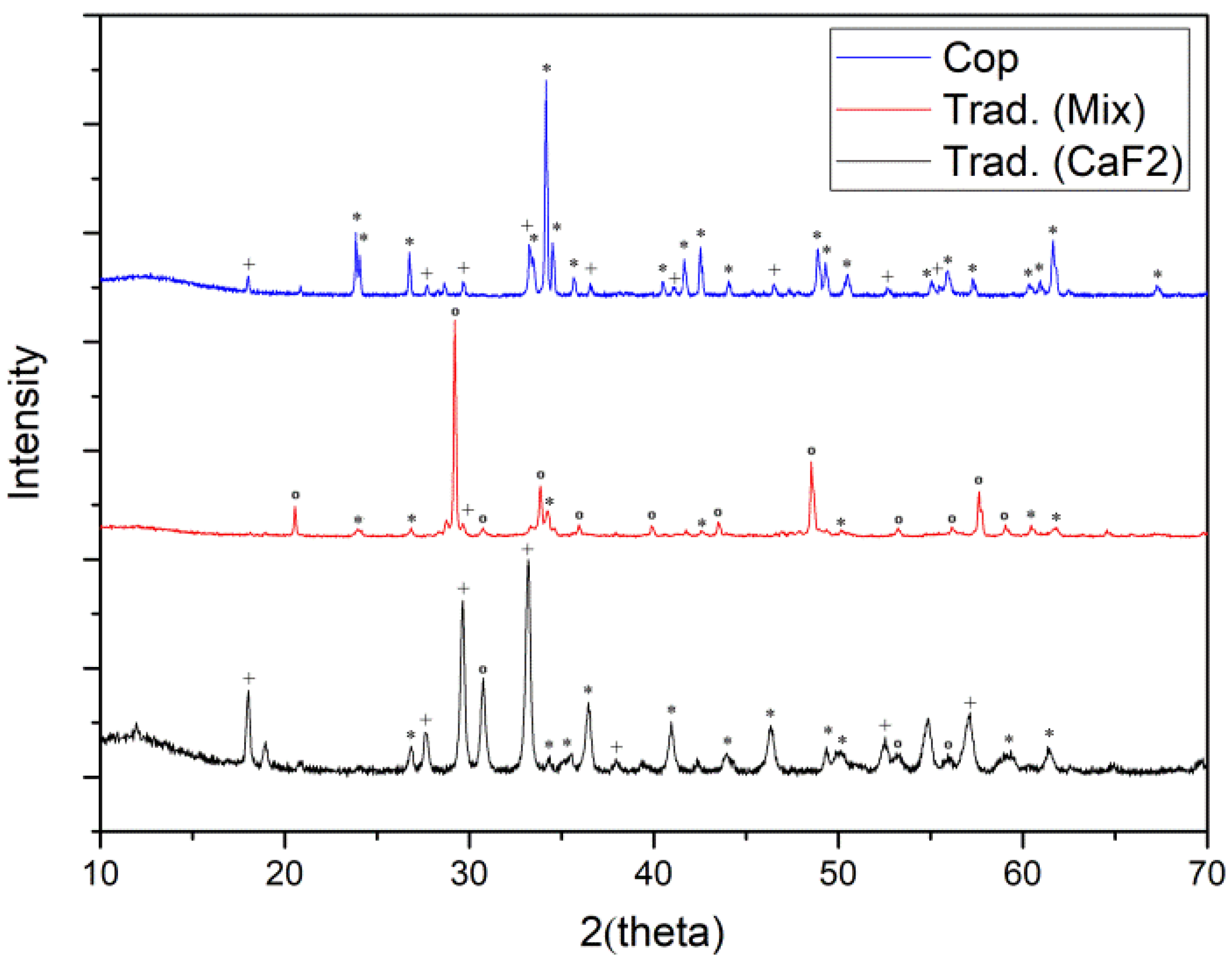
3.1. Synthesis by Traditional Route
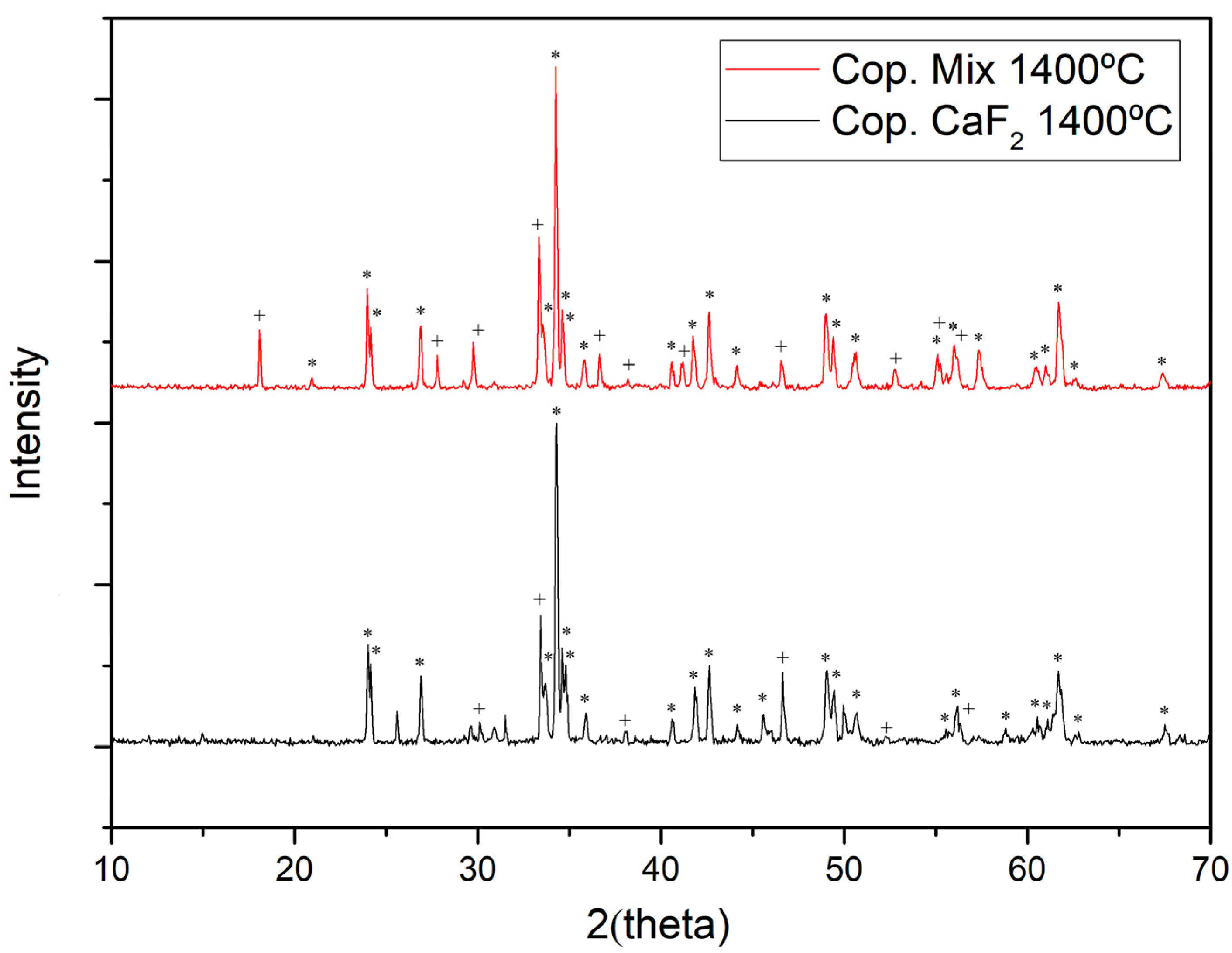
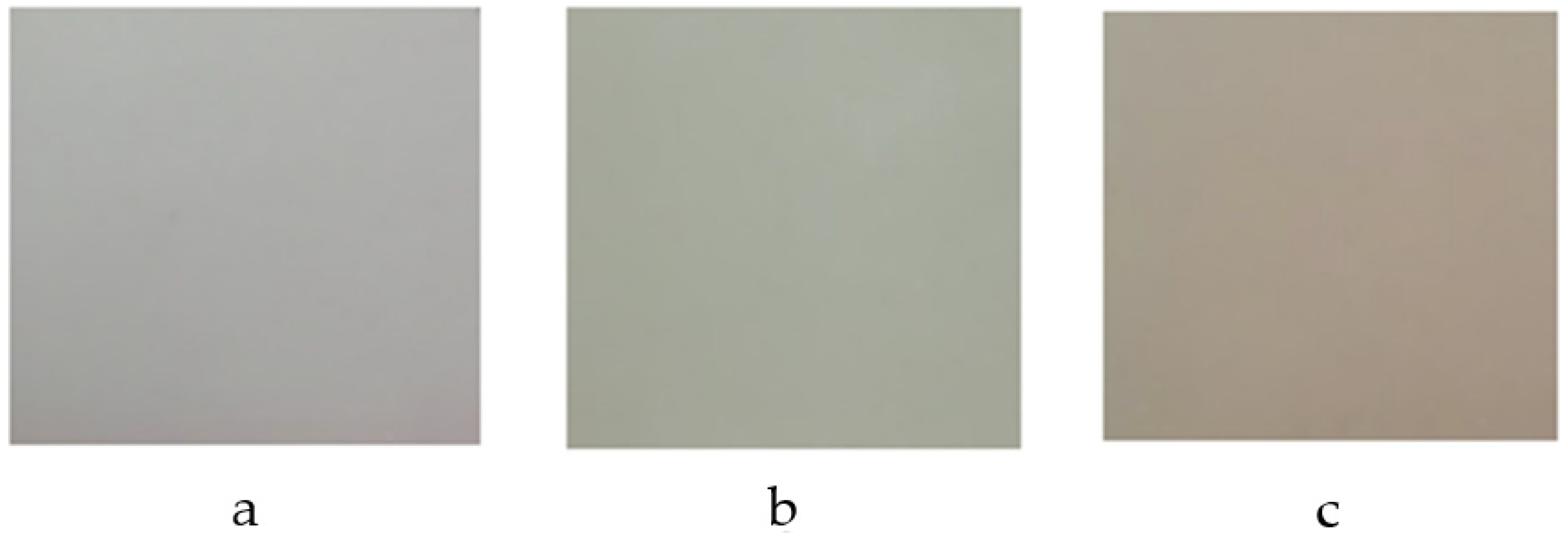
3.2. Coprecipitation Synthesis
3.3. Microwave-Assisted Synthesis
3.4. Comparative Results with Other Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miguel, E.; Carda, J.B.; Nebot-Díaz, I. Development of Red Ceramic Pigments with Perovskite Structure Prepared through a Traditional Route. Eng 2023, 4, 159–173. [Google Scholar] [CrossRef]
- Monrós, G.; Badenes, J.; García, A.; Tena, M. El Color de la Cerámica: Nuevos Mecanismos en Pigmentos para los Nuevos Procesados de la Industria Cerámica, 1st ed.; Jaume I University: Castellón, Spain, 2003. [Google Scholar]
- Chen, Y.; Zou, J. Cr and Mg co-doped YAlO3 red cool pigments with high NIR reflectance and infrared emissivity for sustainable energy-saving applications. Cer. Int. 2023, 49, 13717–13727. [Google Scholar] [CrossRef]
- Lyubenova, T.S.; Carda, J.B.; Ocaña, M. Synthesis by pyrolysis of aerosols and ceramic application of Cr-doped CaYAlO4 red-orange pigments. J. Eur. Ceram. Soc. 2009, 29, 2193–2198. [Google Scholar] [CrossRef]
- Ahmadi, S.; Aghaei, A.; Eftekhari Yerta, B.E. Synthesis of Y(Al,Cr)O3 red pigments by co-precipitation method and their interactions with glazes. Ceram. Int. 2009, 35, 3485–3488. [Google Scholar] [CrossRef]
- Glasser, F. Vidriados y Pigmentos. Nuevos Productos y Tecnologías de Esmaltes y Pigmentos Cerámicos, 1st ed.; Carda Castelló, J.B., Ed.; Faenza Editrice Iberica, S.L.: Castellón, Spain, 1992. [Google Scholar]
- Marinova, Y.; Hohemberger, J.M.; Cordoncillo, E.; Escribano, P.; Carda, J. Study of solid solutions, with perovskite structure, for application in the field of the ceramic pigments. J. Eur. Ceram. Soc. 2003, 23, 213–220. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G. Química Inorgánica Avanzada, 4th ed.; Limusa: Balderas, México, 1993; pp. 47–69. [Google Scholar]
- Casabó-Gispert, J. Estructura Atómica y Enlace Químico, 1st ed.; Editorial Reverté, S.A.: Barcelona, Spain, 1999; pp. 29–31. [Google Scholar]
- López-Navarrete, E.; Orera, V.M.; Lázaro, F.J.; Carda, J.B.; Ocaña, M. Preparation through Aerosols of Cr-Doped Y2Sn2O7. J. Am. Ceram. Soc. 2005, 87, 2108–2113. [Google Scholar] [CrossRef]
- Pavlov, R.; Blasco, V.; Cordoncillo, E.; Carda, P.E.J.B. Síntesis de nuevos pigmentos cerámicos de color rojo mediante el método de Pechini. Boletín De La Soc. Española De Cerámica Y Vidrio 2000, 39, 609–616. [Google Scholar] [CrossRef]
- Bucko, M.M.; Stobierska, E.; Lis, J.; Molasy, B. Pigments in the Y2O3-Al2O3-Cr2O3 system. Ceram. Mater. 2010, 4, 62. [Google Scholar]
- Prado-Gonjal, J.; Morán, E. Síntesis asistida por microondas de sólidos inorgánicos. An. Quím. 2011, 107, 129–136. [Google Scholar]
- Gargori, C.; Galindo, R.; Cerro, S.; Llusar, M.; García, A.; Badenes, J.; Monrós, G. Obtención de pigmentos cerámicos de perovskita CaTiO3 dopada con cromo y vanadio por descomposición metal-orgánica (MOD). Boletín De La Soc. Española De Cerámica Y Vidrio 2012, 6, 343–352. [Google Scholar] [CrossRef]
- Norkus, M.; Skaudzius, R. Enhanced NIR region emission of chromium by changing the chromium concentration in yttrium aluminum garnet (YAG) host matrix. J. Alloys Compd. 2022, 908, 164601. [Google Scholar] [CrossRef]
- Chaika, M.A.; Dulina, N.A.; Doroshenko, A.G.; Parkhomenko, S.V.; Gayduk, O.V.; Tomala, R.; Strek, W.; Hreniak, D.; Mancardi, G.; Vovk, O.M. Influence of calcium concentration on formation of tetravalent chromium doped Y3Al5O12 ceramics. Cer. Int. 2018, 44, 13513–13519. [Google Scholar] [CrossRef]
- Nebot-Díaz, I.; Dal Corso, P. (Eds.) Digital Ceramic Decoration, an Introduction; ATC: Castellón, Spain, 2017. [Google Scholar]
- Oset, M.; Moya, A.; Paulo-Redondo, G.; Nebot-Díaz, I. Nanoparticle black ceramic pigment obtained by hydrotalcite-like compound microwave treatment. Chemengineerig 2022, 6, 54. [Google Scholar] [CrossRef]
- Rives, V.; Pérez-Bernal, M.E.; Ruano-Casero, R.J.; Nebot-Diaz, I. Development of a black piment from non-stoichiometric hydrotalcites. J. Eur. Cer. Soc. 2012, 32, 975–987. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O.; Deac, I.G.; Lazar, M.; Borodi, G.; Petean, I. Effect of amorphous SiO2 matrix on structural and magnetic properties of Cu0.6Co0.4Fe2O4/SiO2 nanocomposites. J. Alloys Compd. 2020, 849, 156695. [Google Scholar] [CrossRef]
- Enríquez, E.; Reinosa, J.J.; Fuertes, V.; Fernández, J.F. Advances and challenges of ceramic pigments for inkjet printing. Cer. Int. 2022, 48, 31080–31101. [Google Scholar] [CrossRef]
- Abima, S.; Sumathi, S. Influence of method of synthesis on the colour and NIR reflective properties of bismuth and vanadium doped cerium phosphate. J. Solid State Chem. 2023, 324, 124140. [Google Scholar] [CrossRef]
- Veronesi, P.; Colombini, E.; Canarslan, O.; Baldi, G.; Leonelli, C. Procedure to generate a selection chart for microwave sol-gel synthesis of nanoparticles. Chem. Eng. Proc.-Process Intensif. 2023, 189, 109383. [Google Scholar] [CrossRef]
- Trujillano, R.; Nieto, D.; Rives, V. Microwave-assisted synthesis of Ni, Zn layered double hydroxy salts. Microporous Mesoporous Mater. 2017, 253, 129–136. [Google Scholar] [CrossRef]

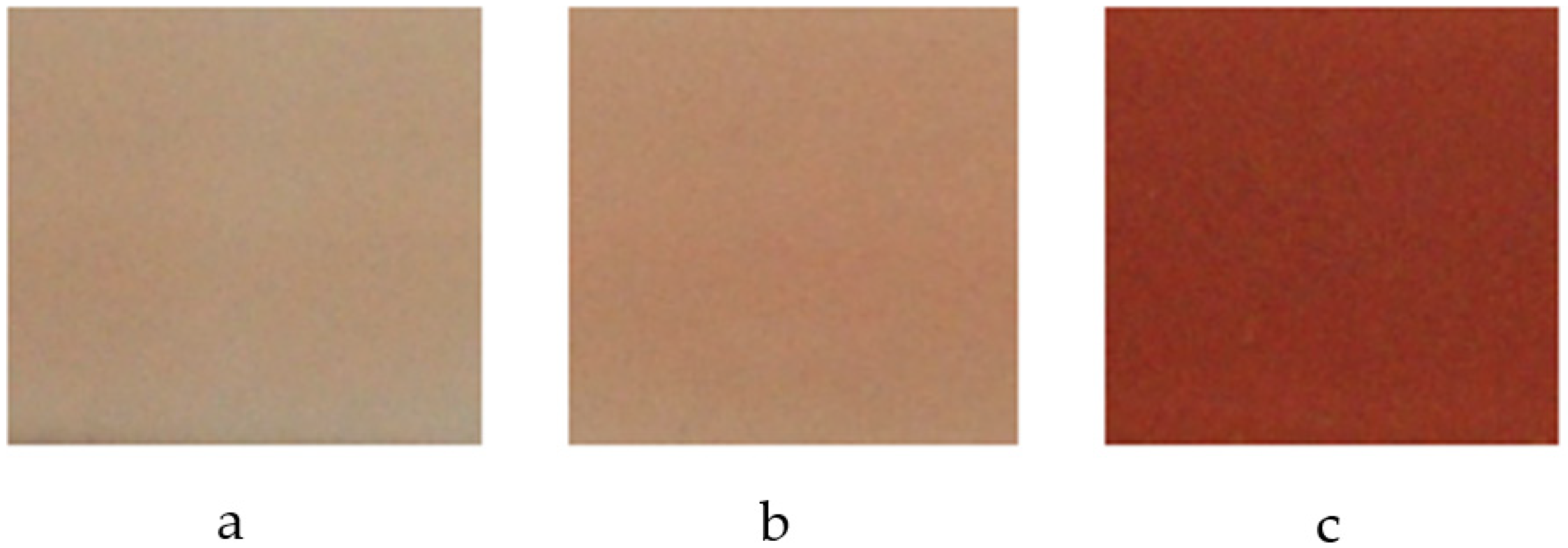

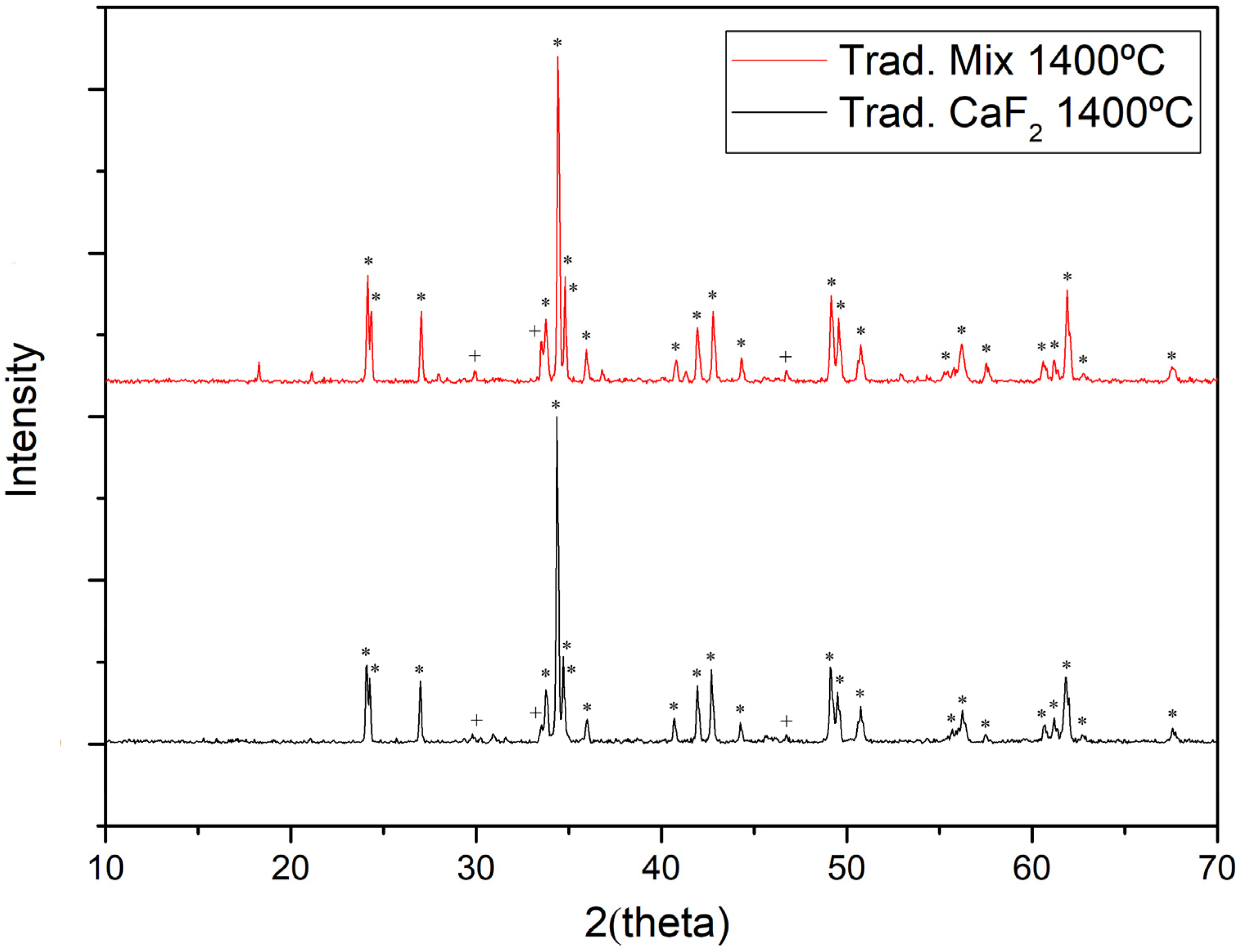
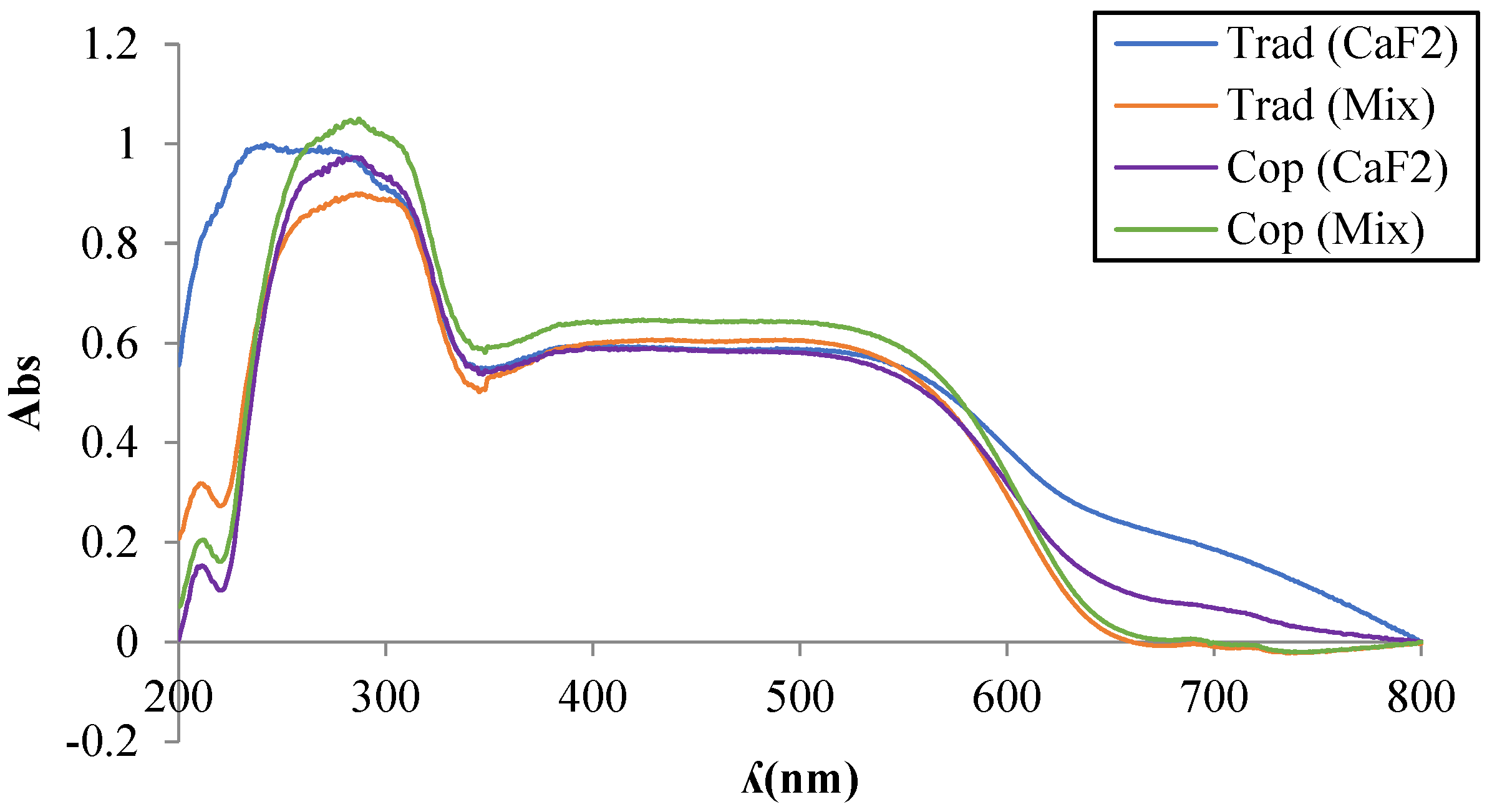
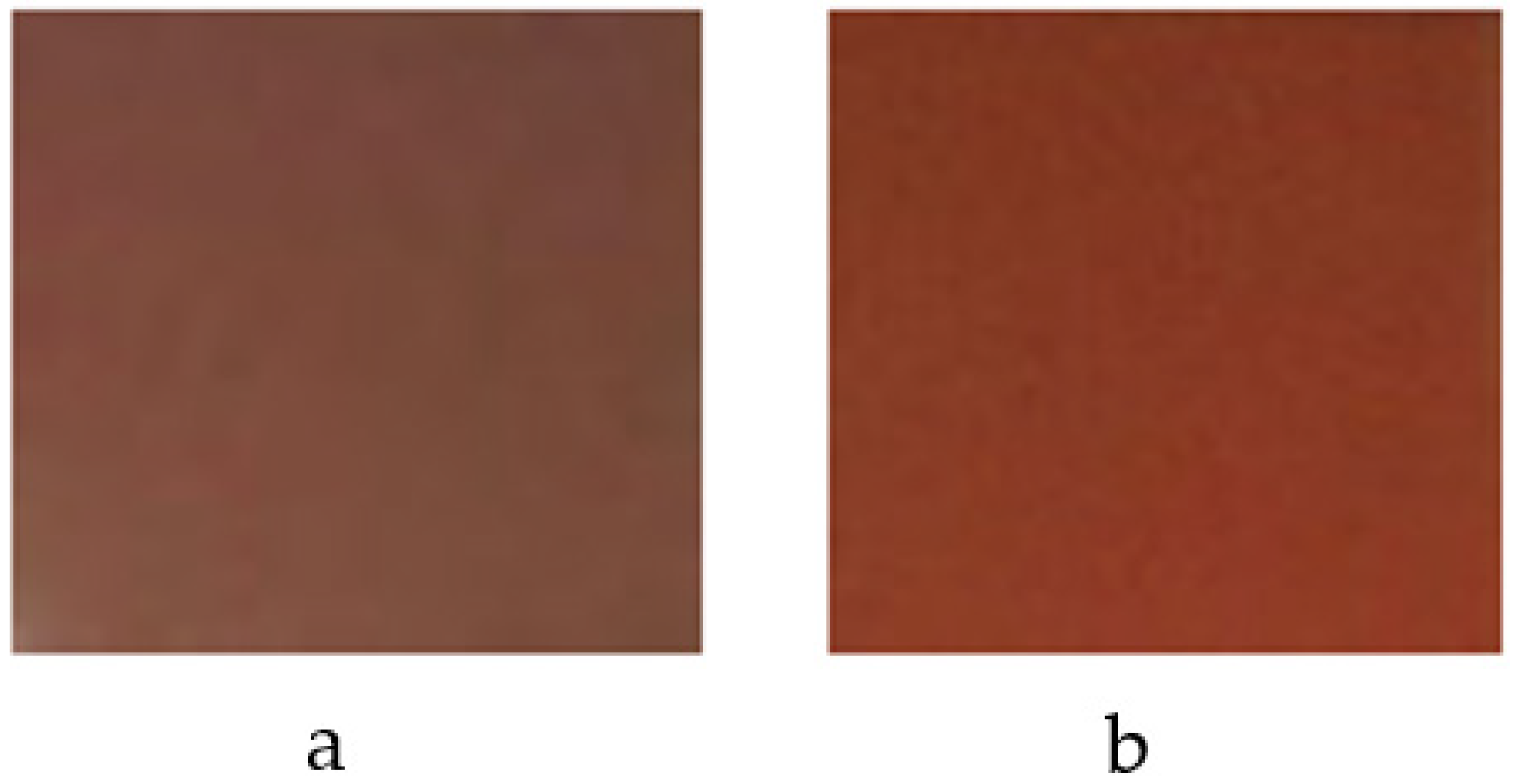




| Flux | T (°C) | Color | L* | a* | b* |
|---|---|---|---|---|---|
| CaF2 | 1300 | Pink | 71.36 | 15.82 | 13.04 |
| 1400 | Pink | 57.74 | 21.34 | 12.69 | |
| 1500 | Red | 60.50 | 36.06 | 23.24 | |
| NaF + MgF2 + Li2CO3 | 1200 | Red | 58.50 | 34.81 | 21.49 |
| 1300 | Red | 54.82 | 23.68 | 11.41 | |
| 1400 | Red | 60.50 | 36.06 | 23.24 |
| Flux | T (°C) | Color | L* | a* | b* |
|---|---|---|---|---|---|
| CaF2 | 1400 | Red | 68.34 | 19.53 | 8.24 |
| NaF + MgF2 + Li2CO3 | 1200 | Red | 63.00 | 27.62 | 18.97 |
| 1300 | Red | 59.57 | 31.67 | 19.20 | |
| 1400 | Red | 60.98 | 28.33 | 15.00 |
| Synthesis Route | Flux | T (°C) | Color | L* | a* | b* |
|---|---|---|---|---|---|---|
| Ceramic | CaF2 | 1017 | Light green | 78.96 | 0.31 | 7.13 |
| NaF + MgF2 + Li2CO3 | 1017 | Light green | 75.85 | −1.96 | 13.00 | |
| Coprecipitation | NaF + MgF2 + Li2CO3 | 1017 | Light Pink | 71.97 | 2.83 | 13.05 |
| Stoichiometry | Synthesis Route | T (°C)/dwell (h) | Flux | Crystalline Phase [Ref] |
|---|---|---|---|---|
| Y0.98Al0.98Cr0.04O3 | Ceramic | 1400/6 | CaF2 | P >>> G |
| NaF + MgF2 + Li2CO3 | P >>>> G | |||
| Y0.98Al0.98Cr0.04O3 | Coprecipitation | CaF2 | P >> G | |
| NaF + MgF2 + Li2CO3 | P >>> G | |||
| Y0.98Al0.98Cr0.04O3 | Ceramic + microwave heating | 1017 | CaF2 | G >>> P >> Y |
| NaF + MgF2 + Li2CO3 | Y >>> P | |||
| Coprecipitation + microwave heating | - | P >> G | ||
| Ceramic + microwave heating | 1185 | CaF2 | P >> G > Y | |
| NaF + MgF2 + Li2CO3 | P >>> G | |||
| Coprecipitation + microwave heating | - | P >>> G | ||
| Y0.99AlCr0.01O3 | Ceramic | 1500/6 | CaF2 | P >> G [1] |
| Y0.97AlCr0.03O3 | P >>> G [1] | |||
| Y0.95AlCr0.05O3 | P >>> G [1] | |||
| Y0.90AlCr0.10O3 | P >> G [1] | |||
| Y0.98Al0.98Cr0.04O3 | Ceramic | 1300/6 | CaF2 | P >> G [1] |
| 1400/6 | P >>> G [1] | |||
| 1500/6 | P >>> G [1] | |||
| Y0.97AlCr0.03O3 | Ceramic | 1300/6 | CaF2 | P >> G [12] |
| 1400/6 | P >>> G [12] | |||
| 1500/6 | P [12] | |||
| YAl0.97Cr0.03O3 | Ceramic | 1300/6 | CaF2 | P >> G [12] |
| 1400/6 | P >>> G [12] | |||
| 1500/6 | P >>> G [12] | |||
| YAlO3 | Coprecipitation | 1400/4 | - | P > G [5] |
| NaF + MgF2 + Li2CO3 | P [5] | |||
| YAl0.97Cr0.03O3 | Coprecipitation | 1400/4 | NaF + MgF2 + Li2CO3 | P [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguel, E.; Paulo-Redondo, G.; Carda Castelló, J.B.; Nebot-Díaz, I. Comparative Study of the Synthesis of a Red Ceramic Pigment Using Microwave Heat Treatment. Colorants 2023, 2, 518-532. https://doi.org/10.3390/colorants2030025
Miguel E, Paulo-Redondo G, Carda Castelló JB, Nebot-Díaz I. Comparative Study of the Synthesis of a Red Ceramic Pigment Using Microwave Heat Treatment. Colorants. 2023; 2(3):518-532. https://doi.org/10.3390/colorants2030025
Chicago/Turabian StyleMiguel, Eva, Guillermo Paulo-Redondo, Juan Bautista Carda Castelló, and Isaac Nebot-Díaz. 2023. "Comparative Study of the Synthesis of a Red Ceramic Pigment Using Microwave Heat Treatment" Colorants 2, no. 3: 518-532. https://doi.org/10.3390/colorants2030025
APA StyleMiguel, E., Paulo-Redondo, G., Carda Castelló, J. B., & Nebot-Díaz, I. (2023). Comparative Study of the Synthesis of a Red Ceramic Pigment Using Microwave Heat Treatment. Colorants, 2(3), 518-532. https://doi.org/10.3390/colorants2030025






