Abstract
This article compares spectroscopic properties of the series of dipyrromethene dyes, namely their complexes of boron (III), zinc(II) and cadmium(II) with the halogenated ligands of the same structure. Absorption and emission spectra, lifetimes of long-lived emission and quantum yields of luminescence were studied as the functions of molecular structure of dipyrromethene complexes. The role of the position and nature of a substituent in a ligand, polarity of a solvent and temperature of media were also investigated. The studies demonstrate that replacing the central atom boron(III) by zinc(II) decreases the fluorescence quantum yield, indicating the increased role of non-radiative processes in excitation energy deactivations such as intersystem crossings. In addition, according to the heavy atom effect, the efficiency of intersystem crossings in halogen-substituted zinc(II) and cadmium(II) dipyrromethene complexes is higher than in the corresponding boron fluoride dipyrromethenes (BODIPY), which leads to increase in phosphorescence at low temperatures (frozen solutions). The obtained results make it possible to carry out further investigations of potential sensory properties that are required for systematic use of halogenated dipyrromethene complexes for the creation of modern optical oxygen sensors and singlet oxygen photosensitizers for photodynamic therapy or photocatalytic oxidative reactions.
1. Introduction
The current stage in the development of world fundamental and material science is characterized by an increased interest in the creation of various materials capable of transforming the energy of light into a work function [1,2]. Such optically active materials are often based on hybrid organic compounds and their complexes. Dipyrromethene-based complexes of transition metals are of a particular interest among a variety of organic-based luminophores. The high interest in these compounds is due to their structural–optical property relationships and relative simplicity of synthesis. It is known that dipyrromethene-based complexes are the simplest chromophores, with an open chain oligopyrrole structure and a porphyrin-like fragment coordinated to the central atom which increases the structural rigidity of the entire system [3,4]. These complexes possess advanced optical characteristics (effective absorption and emission) in the visible range of the electromagnetic spectrum. Additionally, they possess high photostability, which is relevant in the design of optical devices. These properties define the unique role of dipyrromethene-based complexes of high demand in various areas of chemistry, physics and medicine [5,6].
Initially, the alkyl substituted complexes of BF2-dipyrromethenes with high fluorescence and stimulated emission yields in a wide spectral range were synthesized for use as active laser media [7,8,9]. It is also known that dipyrromethene complexes are used as active media for fluorescent markers and probes [10,11,12]. Moreover, derivatives of dipyrromethene complexes are potential sensors for detection of metal ions such as Pb(II), Hg(II), Pt(II) and gases in an air mixture (COCl2,CO2, COS, SO2 and SO3) [13,14,15,16,17,18]. Such sensors are used in the areas of biological and medical research.
Despite significant success in the design and application of dipyrromethene-based materials, the search for new compounds with improved spectroscopic characteristics is still a rapidly growing area of research. A particular focus is on dipyrromethene complexes of d-metals. At the moment, the photonics of dipyrromethene complexes of zinc(II) and cadmium(II) are not well investigated, especially the mechanisms of photorelaxation via long-lived excited states. The available studies demonstrate non-negligible phosphorescence even in the absence of heavy substituents in the ligand (halogenation) [19,20,21]. In this regard, in addition to their practical potential, halogen-substituted dipyrromethene systems are of a great fundamental interest due to the influence of the heavy atom effect on the photochemical and photophysical properties occurring in these compounds. The solution of the classical fundamental problem of establishing the “structure–property” relationship will evince the pathway towards practical use of dipyrromethene compounds in the creation of new materials for biosensorics and photodynamic therapy.
2. Materials and Methods
The object of study was a set of halogenated dipyrromethene complexes of BF2, Zn(II) and Cd(II) with substituents of various structures (Figure 1). There are three groups based on the different complexing ions B(III), Zn(II) and Cd(II). Halogen substituents also form three groups: two complexes with substitution of two bromines in the α- and β-positions relative to the central complexing metal and one with one atom of iodine in β-position. Full structural formulas and notations are contained in Supplementary Materials (Tables S1 and S2). It should be noted that the naming scheme adopted in this article is not generally accepted nomenclature but improves the clarity of this article. Analytical chemistry of the studied compounds has been performed by means of mass, IR and NMR spectroscopy at the G.A. Krestov Institute of Solution Chemistry at the Russian Academy of Sciences [22,23].
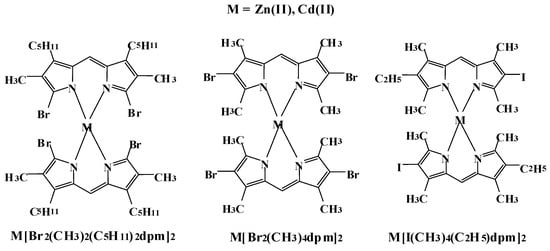
Figure 1.
Structural formulas and designations of the studied dipyrromethene complexes.
Analytically pure ethanol, propanol-2, chloroform and cyclohexane were used as solvents (all HPLC grade). This set of solvents is basic and makes it possible to study the spectroscopic properties of compounds under diametrically opposite conditions. These solvents were used to test the impact of differences in polarity, protonic properties and ability to form hydrogen bonds on the spectral properties of target compounds.
The spectral–luminescent properties (the stationary absorption, fluorescence, phosphorescence, and luminescence excitation spectra) of liquid and frozen solutions of the studied dipyrromethene complexes were recorded with the accuracy of resolution of 0.5 nm and of 3% in intensity using Cary5000 (Agilent) and Cary Eclipse (Varian) spectrometers with Optistat DN cryostat (Oxford Instruments). The Cary Eclipse spectrometer also allows measuring the lifetime of long-lived emissions (for τ > 100 µs) of compounds in frozen solutions. Luminescence quantum yields were measured by the comparative method using the fluorescence data from the previously studied compound as reference: (CH3)4BODIPY (λfl = 514 x. nm, γfl = 0.8) [21]. An ethanolic solution of zinc tetraphenylporphyrin (ZnTPP) (λphos = 780 nm, γphos = 0.015) was used as standard for phosphorescence measurements [24]. The error in determining the luminescence quantum yields does not exceed 10%.
3. Results
3.1. Spectral–Luminescent Characteristics at Room Temperature
The spectroscopic characteristics of zinc(II) and cadmium(II) dipyrromethene complexes are given in Table 1. They are compared to the previously studied properties of boron fluoride dipyrromethenes with similar substituents [21,25]. Comparative analysis of the spectral features of the studied complexes indicates a common shape of the spectra, which is characteristic of compounds of the dipyrromethene dyes family. There are two bands in the electronic absorption spectra. In the region of 330–400 nm, there is a band with a lower molar absorption coefficient corresponding to S0-S2 absorption. The long wavelength band corresponding to S0-S1 absorption is in the region of 450–550 nm and is the most sensitive to ligand substitution (Figure 2a,c). The typical fluorescence spectra appear as “mirror reflections” of the absorption spectra which is evidence that it is the same state that absorbs and emits (Figure 2b,d).

Table 1.
Spectral and luminescent characteristics of BF2, Zn(II) and Cd(II) dipyrromethene complexes at room temperature.
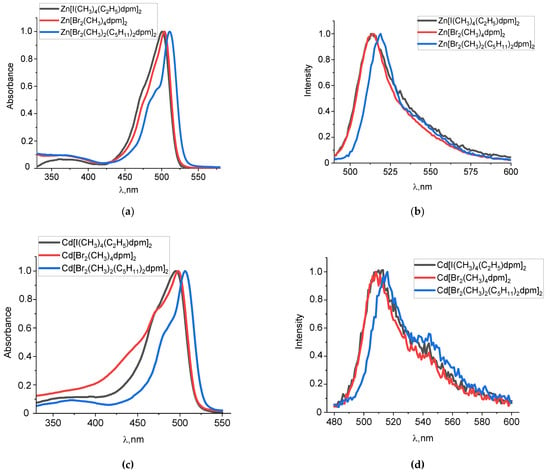
Figure 2.
Normalized absorption (a,c) and fluorescence (λex = 470 nm) (b,d) spectra of zinc(II) (a,b) and cadmium(II) (c,d) dipyrromethene complexes in ethanol solutions at 298 K.
Substitution of boron with either zinc(II) or cadmium(II) leads to the hypsochromic shift of the absorption and fluorescence maxima by 24–33 nm in a series of complexes of the same type in terms of the ligand. For the dipyrromethene complexes of zinc(II) and cadmium(II), an increase in extinction coefficients is observed, which can be explained by a twofold increase in a number of chromophore ligands when compared to BODIPY complexes containing only one dipyrromethene ligand.
The monoiodine-substituted complex absorbs and emits at the shortest wavelength among the complexes of the same central atom (Table 1, Figure 2). In turn, the introduction of two heavy bromine atoms into α-positions to the pyrrole ring leads to a bathochromic shift of the main absorption and emission maxima by 5–9 nm relative to the β-dibromo-substituted analog. It should be noted that for boron fluoride and zinc(II) complexes, the α-bromination leads to a slight decrease in the extinction coefficients, which can be explained by the different inductive effect of alkyl substituents (CH3/C5H11) and the additional influence on the polarization of intramolecular interactions of bromine atoms in α-positions to the central atom.
An increase in the polarity of the medium causes a general hypsochromic shift of the absorption and fluorescence maxima by 7–10 nm for BODIPY complexes and by 3–7 nm for zinc(II) and cadmium(II) complexes. At the same time, a slight decrease in intensity is observed, i.e., the molar extinction coefficient. The value of the Stokes shift is generally not large and is in the range of 6–19 nm for BODIPY complexes, 8–14 nm for zinc(II) complexes and 10–20 nm for cadmium(II) complexes. For BODIPY complexes, the Stokes shift increases in more polar solvents, which indicates an increase in the solvation interactions of excited molecules with a polar solvent compared to a non-polar one.
The nature of the solvent also has a significant effect on the luminescence efficiency of zinc(II) and cadmium(II) dipyrromethene complexes. Fluorescence quantum yield decreases in polar solvents such as ethanol and propanol-2 (Table 1). This is explained by the additional specific interaction of the solvent molecules with the central atom, which increases the yield of nonradiative relaxation in the deactivation of the excitation energy due to the internal reorganization of the first solvation sphere in the excited state in comparison with the main one.
3.2. Phosphorescence Properties in Frozen Solutions
According to the heavy atom effect, the halogen-substituted complexes of dipyrromethenes exhibit a decrease in the fluorescence efficiency and an increase in the intersystem crossing yield. Due to this, non-zero phosphorescence has been observed for these complexes in frozen ethanol and propanol-2 solutions. Unfortunately, attempts to register reliable phosphorescence spectra in chloroform and cyclohexane have not been successful. The solutions of these solvents formed frost on the cuvette walls, hindering the path for theoptical beam. Polar solvents, such as ethanol and propanol-2, form optically transparent ice floes upon freezing and are suitable for recording phosphorescence at 77 K.
Previously, we studied the phosphorescence of halogen-substituted complexes of BODIPY [20,21,25]. The results of the phosphorescence of the zinc(II) and cadmium(II) complexes are shown in Table 2. The maxima of the long-lived emission spectra of the zinc(II) and cadmium(II) complexes are hypsochromically shifted relative to the analogous BODIPY complexes and are in the region of 735–745 nm. Phosphorescence spectra exhibit only single peak. For α-dibromo-substituted complexes of zinc(II) and cadmium(II), and a clearer resolution of the vibrational structure is observed at the right edge of the phosphorescence spectrum (Figure 3). The coincidence of the phosphorescence excitation spectra with the absorption spectrum of halogen dipyrromethenates confirms that phosphorescence belongs to complexes Zn[Br2(CH3)2(C5H11)2dpm]2 and Cd[Br2(CH3)2(C5H11)2dpm]2 (Figure 4).

Table 2.
Spectral and luminescent characteristics of BF2, Zn(II) and Cd(III) dipyrromethene complexes at temperature 77 K.
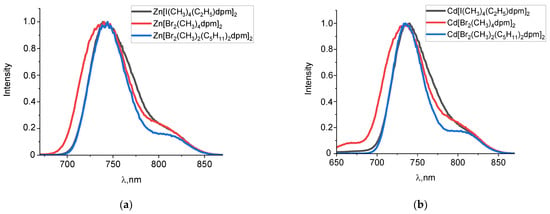
Figure 3.
Normalized phosphorescence (λex = 470 nm) spectra of zinc(II) (a) and cadmium(II) (b) dipyrromethene complexes in ethanol solutions at 77 K. Phosphorescence spectra of zinc(II) and cadmium(II) dipyrromethene complexes in propanol-2 solutions at 77 K are contained in Supplementary Materials (Tables S1 and S2).
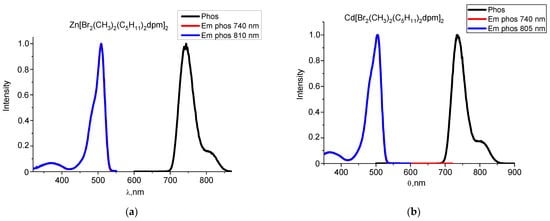
Figure 4.
Normalized phosphorescence (λex = 470 nm) and extinction of phosphorescence spectra of zinc(II) (a) and cadmium(II) (b) dipyrromethene complexes in ethanol solutions at 77 K.
The lifetimes of long-lived emissions were determined for the studied compounds (Figure 5). The complexes with β-dibromo substitution in the ligand possess an increased phosphorescence lifetime. This is the key value our study, as the long lifetime of triplet states indicates a higher probability of the formation of singlet oxygen via intersystem crossing [26,27,28]. This fact requires further studies on the probability of the formation of singlet oxygen.
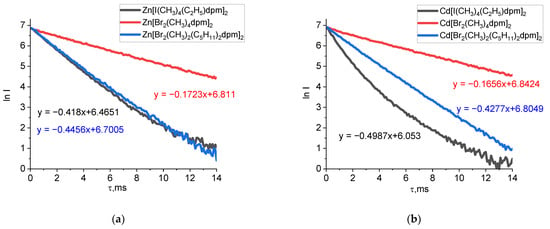
Figure 5.
Dependence of the logarithm of phosphorescence attenuation on the lifetime (λex = 470 nm, λem = 740 nm) of zinc(II) (a) and cadmium(II) (b) dipyrromethene complexes in ethanol solutions at 77 K. Dependence of the logarithm of phosphorescence attenuation on the lifetime of zinc(II) and cadmium(II) dipyrromethene complexes in propanol-2 solutions at 77 K are contained in Supplementary Materials (Tables S1 and S2).
The phosphorescence quantum yields for halogen-substituted BODIPY complexes decrease from bromine to iodine complexes according to the heavy atom effect. However, for zinc(II) and cadmium(II) complexes, this regularity is violated. The highest values of phosphorescence quantum yields were obtained for α-dibromo-substituted zinc(II) complexes. Similar values were obtained for cadmium(II) complexes with the same ligand. These results indicate the additional influence of both structural factors (namely the ability of metal complexes to coordinate several chromophore dipyrromethene groups) and a special electronic activity of bromination in the α-position in the ongoing photophysical processes.
4. Conclusions
The spectral properties of halogen-substituted zinc(II) and cadmium(II) complexes of dipyrromethenes were studied and compared with similar BODIPY complexes. The spectral characteristics of absorption and emission of the studied compounds were studied as the function of the ligand structure, polarity of solvents and temperature (298 K and 77 K). Results show that the photonics of dipyrromethenates is determined not only by the type of complexing metal but also by the substituents introduced into the ligand. Specifically, the nature and location of the substituents in the dipyrromethene core significantly affect the efficiency of the radiative T–S deactivation of the excitation energy. This work provides the basis for further studies on the phosphorescent properties of the dipyrromethene-based compounds, especially on the interactions with molecular oxygen in order to create diverse, active media for optical sensors and photodynamic therapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/colorants1030018/s1, Table S1: Structural formulas, absorption and luminescence spectra of zinc(II) dipyrromethene complexes; Table S2: Structural formulas, absorption and luminescence spectra of cadmium(II) dipyrromethene complexes.
Author Contributions
Conceptualization, I.A.; formal analysis, E.B.; funding acquisition, I.A.; investigation, E.B. and M.A.; methodology, I.A.; validation, I.A. and E.B.; visualization, E.B. and M.A.; writing—original draft, I.A. and E.B.; writing—review and editing, I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (project No 21-73-00073).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We kindly thank our colleagues from the G.A. Krestov Institute of Solution Chemistry of the RAS—M.B. Berezin and E.V. Antina—for synthesis and determination of dipyrromethene complexes structure by mass spectrometry and IR spectroscopy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhuang, Y.; Ren, X.; Che, X.; Liu, S.; Huang, W.; Zhao, Q. Organic photoresponsive materials for information storage: A review. Adv. Photonics 2021, 3, 014001. [Google Scholar] [CrossRef]
- Ha, J.M.; Hur, S.H.; Pathak, A.; Jeong, J.; Woo, H.Y. Recent advances in organic luminescent materials with narrowband emission. NPG Asia Mater. 2021, 53, 1–36. [Google Scholar] [CrossRef]
- Radunz, S.; Kraus, W.; Bischoff, F.A.; Emmerling, F.; Tschiche, H.R.; Resch-Genger, U. Temperature-and structural-dependent optical properties and photophysics of BODIPY dyes. J. Chem. A 2020, 124, 1787–1797. [Google Scholar] [CrossRef]
- Li, W.; Gong, Q.; Guo, X.; Wu, Q.; Chang, F.; Wang, H.; Zhang, F.; Hao, E.; Jiao, L. Synthesis, reactivity, and properties of a class of π-extended BODIPY derivatives. J. Org. Chem. 2021, 86, 17110–17118. [Google Scholar] [CrossRef] [PubMed]
- Ziessel, R.; Ulrich, G.; Harriman, A. The chemistry of Bodipy: A new El Dorado for fluorescence tools. New J. Chem. 2007, 31, 496–501. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY dye, the most versatile fluorophore ever? Chem. Record. 2016, 16, 335–348. [Google Scholar] [CrossRef]
- Pérez-Ojeda, M.E.; Thivierge, C.; Martín, V.; Costela, A.; Burgess, K.; García-Moreno, I. Highly efficient and photostable photonic materials from diiodinated BODIPY laser dyes. Opt. Mater. Express 2011, 1, 243–251. [Google Scholar] [CrossRef]
- Duran-Sampedro, G.; Agarrabeitia, A.R.; Garcia-Moreno, I.; Costela, A.; Bañuelos, J.; Arbeloa, T.; López Arbeloa, I.; Chiara, J.L.; Ortiz, M.J. Chlorinated BODIPYs: Surprisingly efficient and highly photostable laser dyes. Eur. J. Org. Chem. 2012, 32, 6335–6350. [Google Scholar] [CrossRef]
- Esnal, I.; Valois-Escamilla, I.; Gomez-Duran, C.F.A.; Urias-Benavides, A.; Betancourt-Mendiola, M.L.; Lopez-Arbeloa, I.; Banuelos, J.; Garcia-Moreno, I.; Costela, A.; Pena-Cabrera, E. Blue-to-orange color-tunable laser emission from tailored borondipyrromethene dyes. ChemPhysChem 2013, 14, 4134–4142. [Google Scholar] [CrossRef]
- Sevinç, G.; Özgür, M.; Küçüköz, B.; Karatay, A.; Aslan, H.; Yılmaz, H. Synthesis and spectroscopic properties of a novel “turn off” fluorescent probe: Thienyl-pyridine substituted BODIPY. J. Lumin. 2019, 211, 334–340. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Sun, Y.; Liu, M.; Guo, W. Carbon-dipyrromethenes: Bright cationic fluorescent dyes and potential application in revealing cellular trafficking of mitochondrial glutathione conjugates. J. Am. Chem. Soc. 2020, 142, 17069–17078. [Google Scholar] [CrossRef] [PubMed]
- Antina, E.V.; Berezin, M.B.; V’yugin, A.I.; Guseva, G.B.; Bumagina, N.A.; Antina, L.A.; Ksenofontov, A.A.; Nuraneeva, E.N.; Kalyagin, A.A.; Bocharov, P.S.; et al. Chemistry and Practical Application of Dipyrromethene Ligands, Salts, and Coordination Compounds as Optical Sensors for Analytes of Various Nature (A Review). Russ. J. Inorg. Chem. 2022, 67, 321–337. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Wu, J.; Qin, Y.; Jiang, D. Rational design of piperidine functionalized boron–dipyrromethene as fluorescent chromoionophore for ion-selective optodes. Sens. Actuators B: Chem. 2016, 232, 37–42. [Google Scholar] [CrossRef]
- Xia, H.C.; Xu, X.H.; Song, Q.H. BODIPY-based fluorescent sensor for the recognization of phosgene in solutions and in gas phase. Anal. Chem. 2017, 89, 4192–4197. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.J.; Liu, J.Y.; Lo, P.C.; Ng, D.K.P. Selective detection of Hg 2+ ions with boron dipyrromethene-based fluorescent probes appended with a bis(1,2,3-triazole)amino receptor. Chem. Asian J. 2019, 14, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cheng, W.; Ji, C.; Zhang, J.; Yin, M. Detection of metal ions in biological systems: A review. Rev. Anal. Chem. 2020, 39, 231–246. [Google Scholar] [CrossRef]
- Tang, F.K.; Zhu, J.; Kong, F.K.W.; Ng, M.; Bian, Q.; Yam, V.W.W.; Tse, A.K.W.; Tse, Y.C.; Leung, K.C.F. A BODIPY-based fluorescent sensor for the detection of Pt2+ and Pt drugs. Chem. Commun. 2020, 56, 2695–2698. [Google Scholar] [CrossRef]
- Wang, H. Fluoride ion-induced gas sensor based on the dipyrromethene boron difluoride derivative: A theoretical investigation. J. Phys. Org. Chem. 2021, 34, e4265. [Google Scholar] [CrossRef]
- Kuznetsova, R.T.; Aksenova, Y.V.; Bashkirtsev, D.E.; Prokopenko, A.A.; Tel’minov, E.N.; Mayer, G.V.; Dudina, N.A.; Antina, E.V.; Nikonova, A.Y.; Berezin, M.B.; et al. Photonics of zinc(II) and boron(III) chelates with methyl- and phenyl-substituted dipyrromethenes and azadipyrromethenes. High Energy Chem. 2015, 49, 16–23. [Google Scholar] [CrossRef]
- Kuznetsova, R.T.; Aksenova, I.V.; Bashkirtsev, D.E.; Prokopenko, A.A.; Pomogaev, V.A.; Antina, E.V.; Berezin, M.B.; Bumagina, N.A. Photonics of coordination complexes of dipyrrins with p- and d-block elements for application in optical devices. J. Photochem. Photobiol. A: Chem. 2018, 354, 147–154. [Google Scholar] [CrossRef]
- Kuznetsova, R.T.; Aksenova, I.V.; Prokopenko, A.A.; Pomogaev, V.A.; Antina, E.V.; Berezin, M.B.; Antina, L.A.; Bumagina, N.A. Photonics of boron(III) and zinc(II) dipyrromethenates as active media for modern optical devices. J. Mol. Liq. 2019, 278, 5–11. [Google Scholar] [CrossRef]
- Nuraneeva, E.N.; Guseva, G.B.; Antina, E.V.; Berezin, M.B.; V’yugin, A.I. Synthesis and luminescent properties of zinc(II) complexes with iodo- and bromosubstituted 2,2′ -dipyrrines. J. Lumin. 2016, 170, 248–254. [Google Scholar] [CrossRef]
- Nuraneeva, E.N.; Guseva, G.B.; Antina, E.V.; Berezin, M.B.; Ksenofontov, A.A. Cadmium(II) complexes with monoiodo- and dibromodipyrromethenes: Synthesis, molecular structure, spectral-luminescent properties, and stability in solutions. Russ. Chem. Bull. 2018, 67, 1231–1240. [Google Scholar] [CrossRef]
- Chrysochoos, J.; Beyene, K. Oxidative fluorescence quenching of zinc tetraphenylporphyrin (ZnTPP) by trivalent lanthanide ions in several solvents: Role of lanthanide-induced singlet–triplet crossing. J. Lumin. 1999, 81, 209–218. [Google Scholar] [CrossRef]
- Nikonova, A.Y.; Kuznetsova, R.T.; Aksenova, I.V.; Tel’minov, E.N.; Mayer, G.V.; Dudina, N.A.; Nuraneeva, E.N.; Antina, E.V. Optical properties of zinc(II) and boron(III) dipyrrinates with different structures. Opt. Spectrosc. 2016, 120, 395–402. [Google Scholar] [CrossRef]
- Chen, K.; Dong, Y.; Zhao, X.; Imran, M.; Tang, G.; Zhao, J.; Liu, Q. Bodipy derivatives as triplet photosensitizers and the related intersystem crossing mechanisms. Front. Chem. 2019, 7, 821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filatov, M.A. Heavy-atom-free BODIPY photosensitizers with intersystem crossing mediated by intramolecular photoinduced electron transfer. Org. Biomol. Chem. 2020, 18, 10–27. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Huang, L.; Yan, Y.; El-Zohry, A.M.; Toffoletti, A.; Zhao, J.; Barbon, A.; Dick, B.; Mohammed, O.F.; Han, G. Elucidation of the intersystem crossing mechanism in a helical bodipy for low-dose photodynamic therapy. Angew. Chem. Int. Ed. 2020, 59, 16114–16121. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).