The Modernity of Ancient Pigments: A Historical Approach
Abstract
1. Introduction
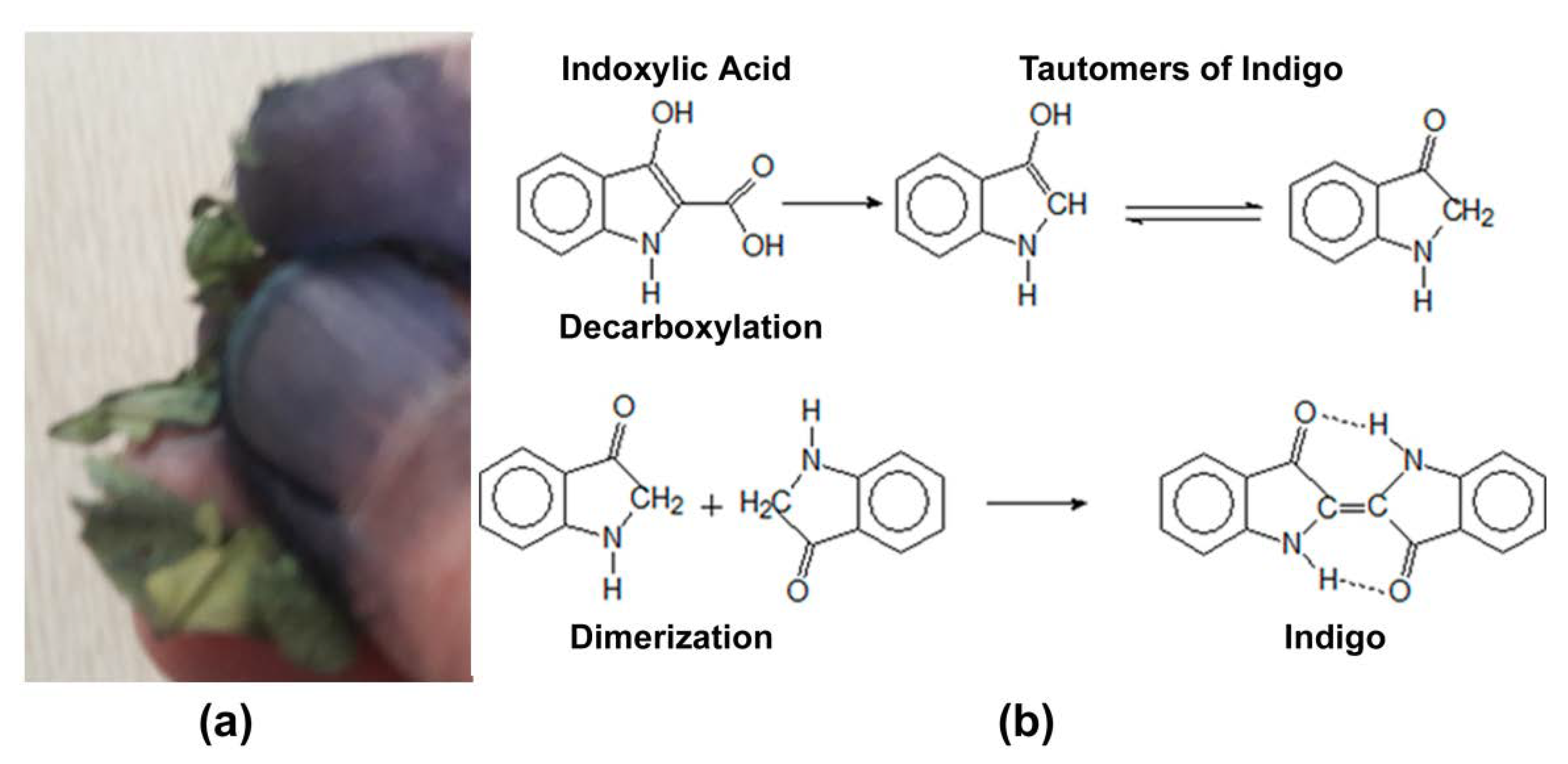
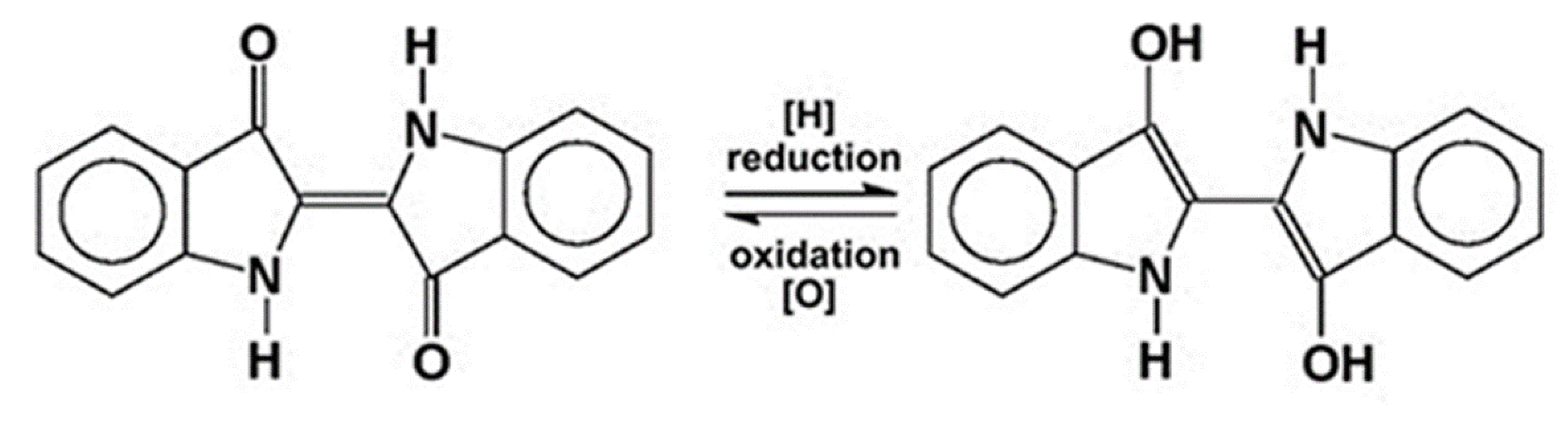
2. Indigo: From a Coveted Blue Dye to Genetic Engineering
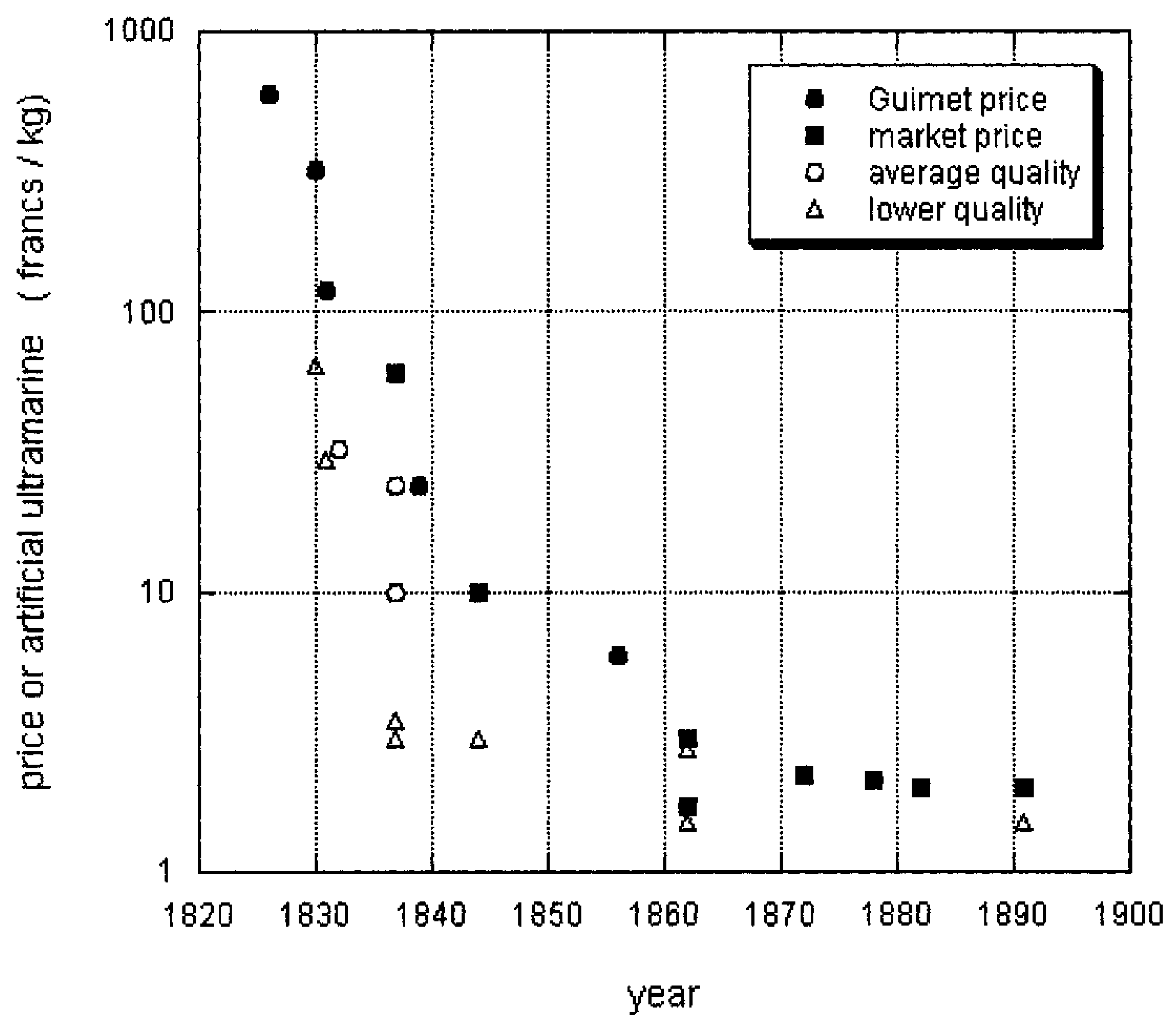
3. Ultramarine: From Priceless Gemstone to Workhorse Blue
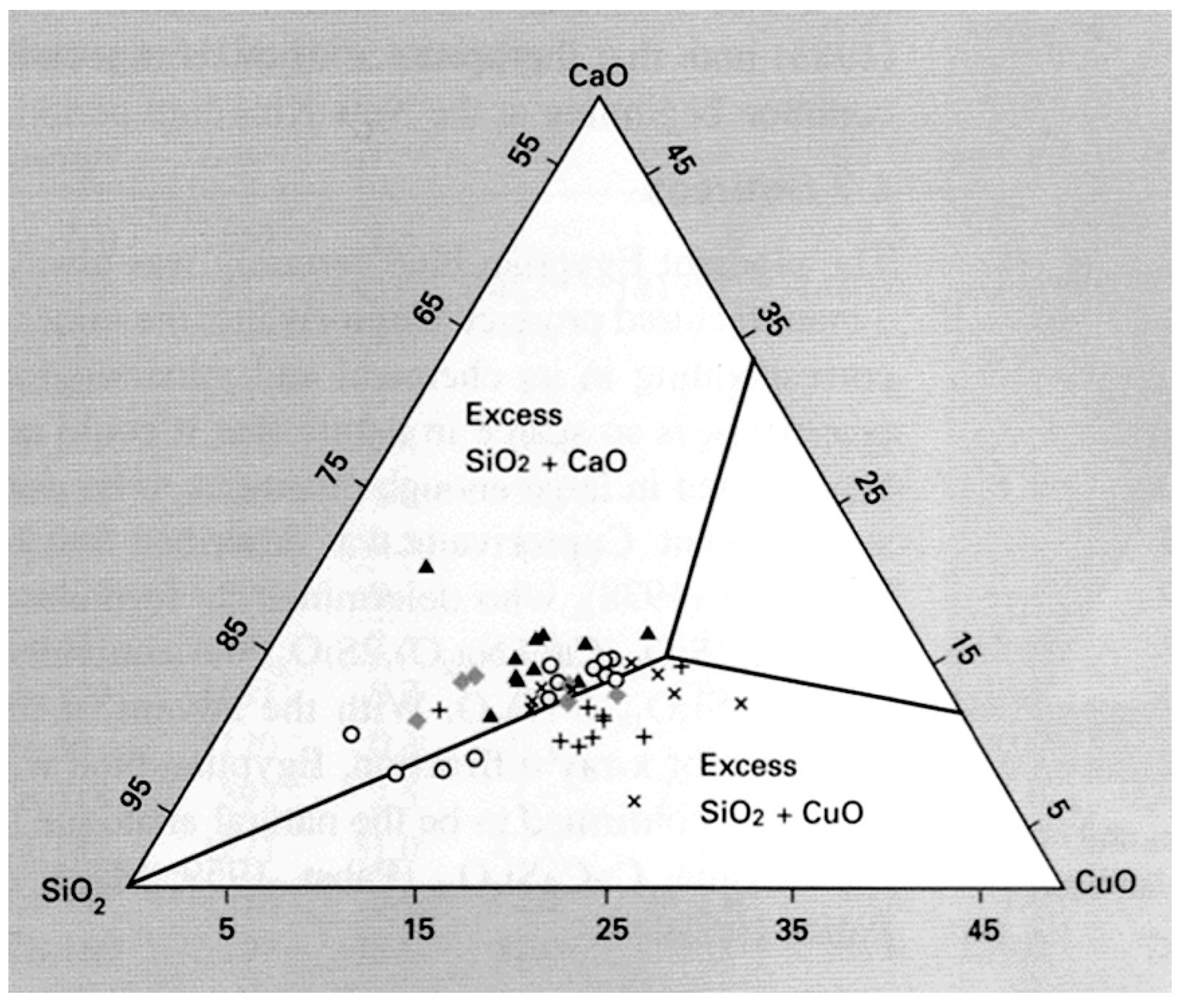
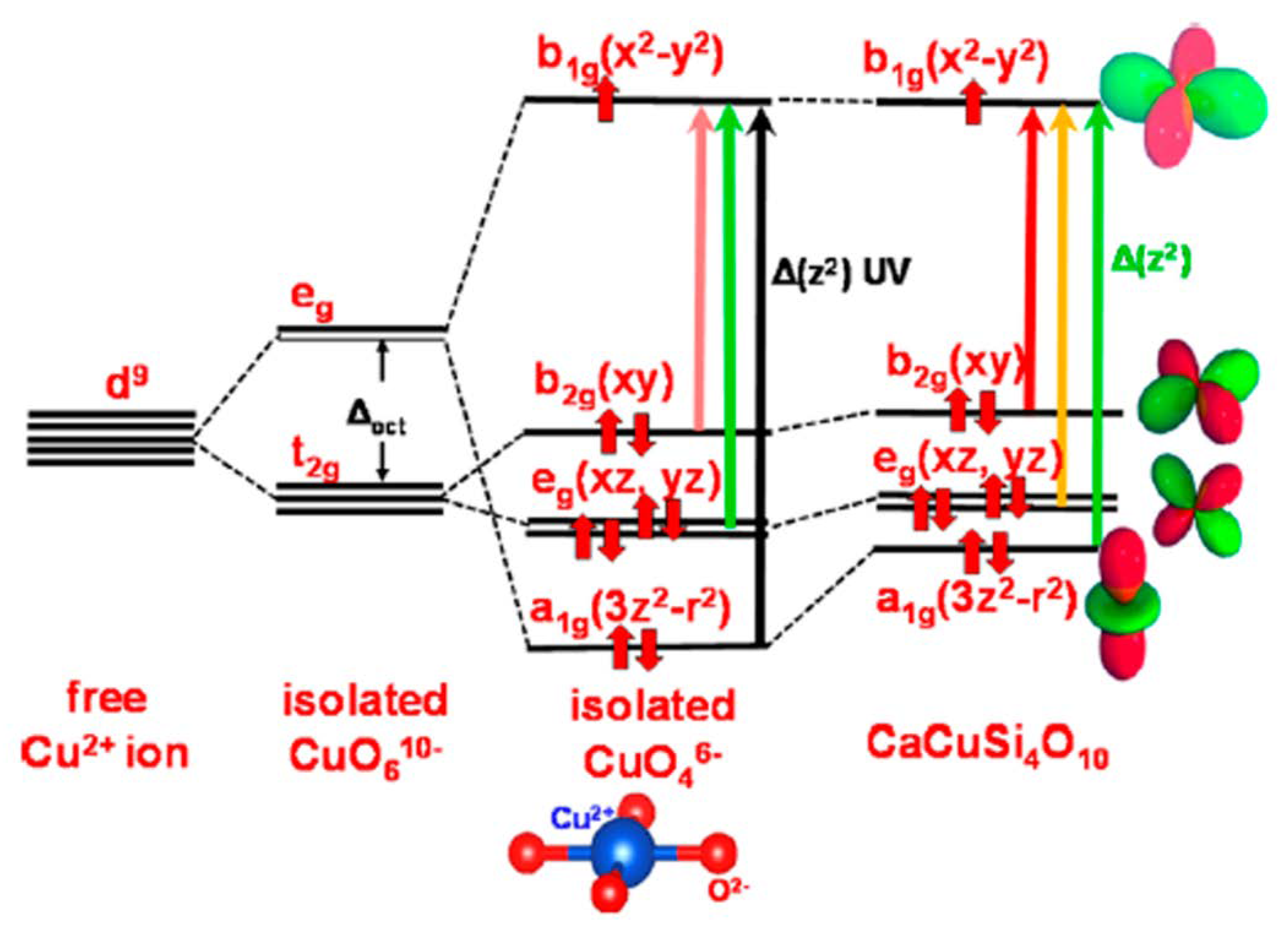
4. Egyptian Blue: From Pharaohs’ Tombs to Counterfeit Detection
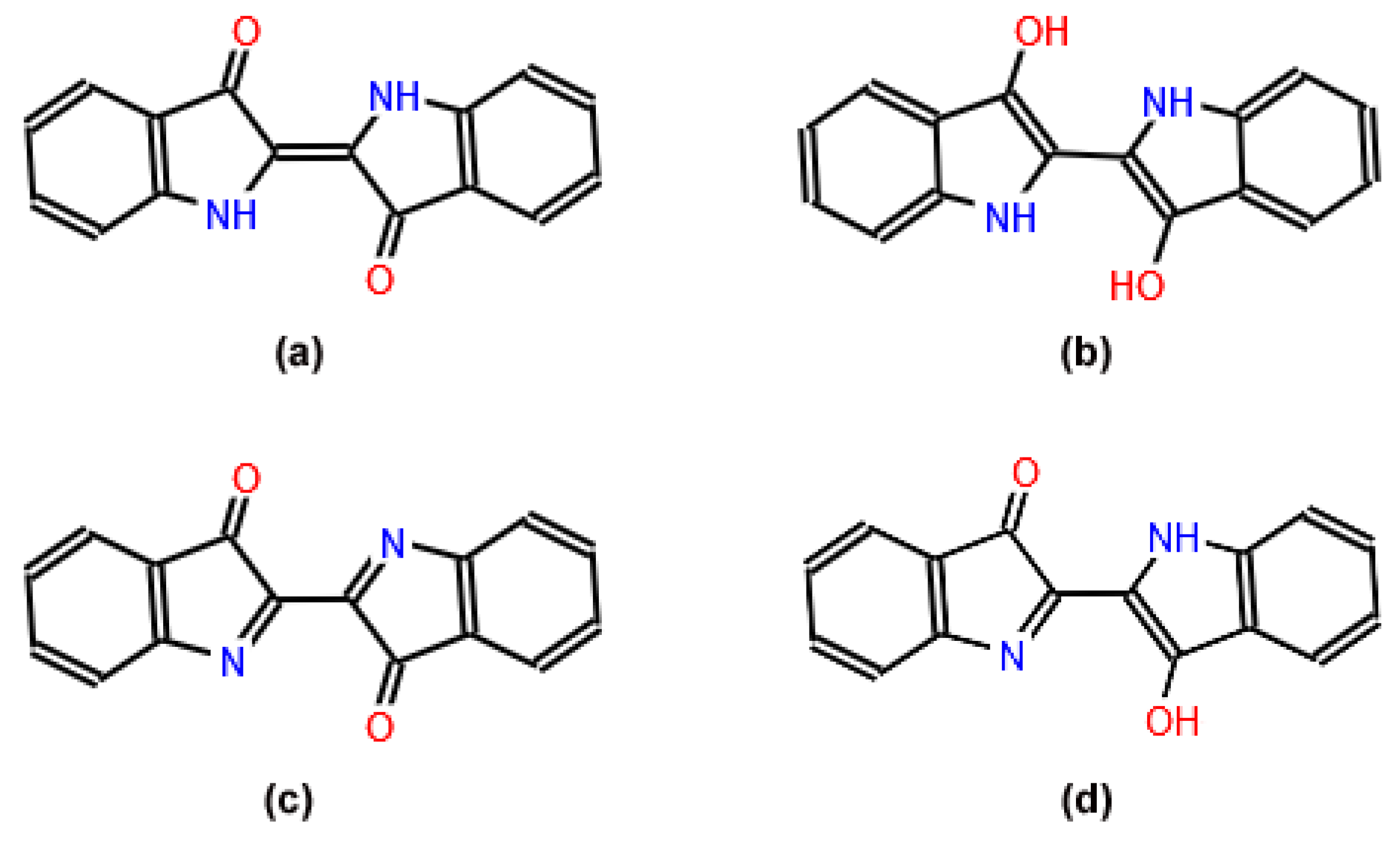
5. Maya Blue: From Indigenous Mystery to Nanotechnology
6. Purple of Cassius: From Ancient Assyria to the Nobel Prize—And beyond!
7. Prussian Blue: From Alchemical Accident to Semiconductor Technology
8. Structural Color: From Early Photography to Plasmonic Pigments
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Sanz de Sautuola, M. Breves Apuntes sobre Algunos Objetos Prehistóricos de la Provincia de Santander; Telesforo Martínez Blanca: Santander, Spain, 1880; pp. 11–23. [Google Scholar]
- Thurman, J. Letter from southern France. First impressions. What does the world’s oldest art say about us? The New Yorker, 23 June 2008; 58–67. [Google Scholar]
- Brunel, E.; Chauvet, J.-M.; Hillaire, C. The Discovery of the Chauvet-Pont d’Arc Cave; Éditions Équinox: Saint-Remy-de-Provence, France, 2014. [Google Scholar]
- The fact that most of the selected pigments are blue is largely coincidental. However, we note that many of the iconic blue pigments have received a great deal of attention in the recent literature.
- The term lake is derived from lac, the solid excrescence of the Indian wood insect, Laccifer lacca, from which the word lacquer is also derived. The general chemical reaction for lake formation is as follows: Dye(aq) + 2KAl(SO4)2·12H2O(aq) + 3K2CO3(aq) → 4K2SO4(aq) + 3CO2(g) + Dye·Al2O3(s) + 24H2O(l).
- Splitstoser, J.C.; Dillehay, T.D.; Wouters, J.; Claro, A. Early pre-Hispanic use of indigo blue in Peru. Sci. Adv. 2016, 2, e1501623. [Google Scholar] [CrossRef] [PubMed]
- Glen, C. The dyes of the Ancients. J. Soc. Dyers Colour. 1911, 27, 293–296. [Google Scholar] [CrossRef]
- Ruggli, P. Die Geschichte der Färberei. In Die Geschichte der Textil-Industrie; Johannsen, E.H.O., Fahrbach, R., Eds.; Süd-Verlag o.J.: Stuttgart, Germany; Zürich, Switzerland, 1932; p. 345. [Google Scholar]
- Struckmaier, S. Naturfarbstoffe: Farben mit Geschichte. Chem. Unserer Zeit 2003, 37, 402–409. [Google Scholar] [CrossRef]
- Maier, W.; Schumann, B.; Gröger, D. Biosynthesis of indoxyl derivatives in Isatis tinctoria and Polygonum tinctorium. Phytochemistry 1990, 29, 817–819. [Google Scholar] [CrossRef]
- Perkin, A.G. XCIII. Indoxylic acid. J. Chem. Soc. Trans. 1909, 95 Pt 2, 847–853. [Google Scholar] [CrossRef]
- Schweppe, H. Indigo and woad. In Artists’ Pigments: A Handbook of Their History and Characteristics; West FitzHugh, E., Ed.; National Gallery of Art: Washington, DC, USA, 1997; Volume 3, pp. 80–107. [Google Scholar]
- Travis, A.S. The Rainbow Makers: The Origins of the Synthetic Dyestuff Industry in Western Europe; Lehigh University Press: Bethlehem, PA, USA, 1993; p. 223. [Google Scholar]
- Schaefer, B. Natural Products in the Chemical Industry; Springer: New York, NY, USA; Heidelberg, Germany, 2014; pp. 23–28. [Google Scholar]
- Royal Economic Society. The rise and fall of the indigo industry in India. Asiat. Econ. J. 1912, 22, 237–247. [Google Scholar]
- Arnold, W.N. George III’s urine and indigo blue. Lancet 1996, 347, 1811–1813. [Google Scholar] [CrossRef]
- Rowe, M.; Blinman, E.; Jones, S. Everybody poops, even insects. Insect waste used to date ancient textile. New Mexico Archaeology, 4–5 November 2021. [Google Scholar]
- Peters, A. These Gorgeous Colors Come from Dye Made by Bacteria, not Chemicals. Available online: https://www.fastcompany.com/90257662/these-gorgeous-colors-come-from-dye-made-by-bacteria-not-chemicals (accessed on 8 January 2022).
- Hsu, T.M.; Welner, D.M.; Russ, Z.M.; Cervantes, B.; Prathuri, R.L.; Adams, P.D.; Dueber, J.E. Employing a biochemical protecting group for a sustainable indigo dyeing strategy. Nat. Chem. Biol. 2018, 14, 256–261. [Google Scholar] [CrossRef]
- Cho, S.; Shin, J.; Cho, B.-K. Applications of CRISPR/Cas system to bacterial metabolic engineering. Int. J. Mol. Sci. 2018, 19, 1089. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Glowacki, E.D.; Voss, G.; Leonat, L.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N.S. Indigo and Tyrian purple—From ancient natural dyes to modern organic semiconductors. Isr. J. Chem. 2012, 52, 540–551. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Liao, H.; Zhou, Y.; McCulloch, I.; Yue, W. The chemistry and applications of heteroisoindigo units as enabling links for semiconducting materials. Acc. Chem. Res. 2020, 53, 2855–2868. [Google Scholar] [CrossRef] [PubMed]
- Fallon, K.J.; Bronstein, H. Indolonaphthyridine: A versatile chromophore for organic electronics inspired by natural indigo dye. Acc. Chem. Res. 2021, 54, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Finlay, V. Color: A Natural History of the Palette; Random House: New York, NY, USA, 2004; p. 282. [Google Scholar]
- Plesters, J. Ultramarine blue, natural and artificial. In Artists’ Pigments: A Handbook of Their History and Characteristics; Roy, A., Ed.; National Gallery of Art: Washington, DC, USA, 1993; Volume 2, pp. 37–54. [Google Scholar]
- Cotton, F.A.; Harmon, J.B.; Hedges, R.M. Calculation of the ground state electronic structures and electronic spectra of di- and trisulfide radical anions by the scattered wave-SCF-X.alpha. method. J. Am. Chem. Soc. 1976, 98, 1417–1424. [Google Scholar] [CrossRef]
- Hamerton, I.; Tedaldi, L.; Eastaugh, N. A systematic examination of colour development in synthetic ultramarine according to historical methods. PLoS ONE 2013, 8, e50364. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0050364 (accessed on 3 June 2022). [CrossRef]
- Greeves, N.; University of Liverpool. Ultramarine—Na8[SiAlO4]6.(S3)2. Available online: https://www.chemtube3d.com/ss-ultramarine/ (accessed on 13 December 2021).
- Mertens, J. The history of artificial ultramarine (1787–1844): Science, industry and secrecy. Ambix 2004, 51, 219–244. [Google Scholar] [CrossRef]
- Kubersky-Piredda, S. The market for painters’ materials in Renaissance Florence. In Trade in Artists’ Materials: Markets and Commerce in Europe to 1700; Cannon, J., Kirby, J., Nash, S., Eds.; Archetype Publications: London, UK, 2010; pp. 223–243. [Google Scholar]
- UCoin.net Coin Catalog. Available online: https://en.ucoin.net/coin/france-1-franc-1825-1830/?tid=73762 (accessed on 18 December 2021).
- Delamare, F. Blue Pigments. 5000 Years of Art and Industry; Archetype Publications: London, UK, 2013; p. 250. [Google Scholar]
- Virtual Visit: Ultramarine Manufacture at Holliday Pigments, Hull. Available online: https://www.york.ac.uk/org/seg/salters/ChemistryArchive/V_VisitHP/applications.html. (accessed on 18 December 2021).
- Ables, K. Now Supply-Chain Woes Have Come to the Color Blue. Washington Post. 23 December 2021. Available online: https://www.washingtonpost.com/entertainment/museums/blue-paint-shortage-art/2021/12/21/486c667c-5df9-11ec-ae5b-5002292337c7_story.html (accessed on 27 December 2021).
- The File is Licensed under the Creative Commons Attribution-Share Alike 3.0 Unported (https://creativecommons.org/licenses/by-sa/3.0/deed.en) license. User: Jaredzimmerman. Available online: https://commons.wikimedia.org/w/index.php?title=File:L%27accord_bleu_(RE_10),_1960.jpg&oldid=493966780 (accessed on 18 December 2021).
- International Klein Blue. Available online: https://en.wikipedia.org/wiki/International_Klein_Blue (accessed on 18 December 2021).
- Jankowska, A.; Kowalak, S. New methods of ultramarine pigments synthesis from zeolites. Wiadomości Chemisczne 2008, 62, 659–682. (In Polish) [Google Scholar]
- Wiedemann, H.G.; Berke, H. Chemical and physical examinations of Egyptian blue and Chinese blue and purple. In Proceedings of the Conference: The Polychromy of Antique Sculptures and the Terra Cotta Army of the First Chinese Emperor, Xian, China, 22–28 March 1999; Yonggi, W., Tinghao, Z., Petzet, M., Emmerling, E., Blansdorf, C., Eds.; UNESCO International Council on Monuments and Sites: Paris, France, 2001; pp. 154–170. [Google Scholar]
- Riederer, J. Egyptian blue. In Artists’ Pigments: A Handbook of Their History and Characteristics; West FitzHugh, E., Ed.; National Gallery of Art: Washington, DC, USA, 1997; Volume 3, pp. 23–45. [Google Scholar]
- Johnson-McDaniel, D.; Barrett, C.A.; Sharafi, A.; Salguero, T.T. Nanoscience of an ancient pigment. J. Am. Chem. Soc. 2013, 135, 1677–1679. [Google Scholar] [CrossRef]
- Nadolny, J. The first century of published scientific analyses of the materials of historical painting and polychromy, circa 1780–1880. Rev. Conserv. 2003, 4, 39–51. [Google Scholar] [CrossRef]
- Gmelin, J.F. Experimenta Nonnulla cum Mumiis Instituta. Comment. Soc. Regiae Sci. Gott. Hist. Philol. Cl. 1781, 4, 3–25. [Google Scholar]
- Davey, C.J. Ancient Near Eastern pot bellows. Levant 1979, 11, 101–111. [Google Scholar] [CrossRef]
- Pabst, A. Structure of some tetragonal sheet silicates. Acta Crystallogr. 1959, 12, 733–739. [Google Scholar] [CrossRef]
- Mazzi, F.; Pabst, A. Reexamination of cuprorivaite. Am. Mineral. 1962, 47, 409–411. [Google Scholar]
- Minguzzi, C. Cuprorivaite: Un nuovo minerale. Period. Mineral. 1938, 9, 333–345. [Google Scholar]
- Tite, M.S.; Bimson, M.; Cowell, M.R. Technological examination of Egyptian blue. In Archaeological Chemistry III. Advances in Chemistry Series 205; Lambert, J.B., Ed.; American Chemical Society: Washington, DC, USA, 1984; pp. 215–242. [Google Scholar]
- Berke, H. The invention of blue and purple pigments in ancient times. Chem. Soc. Rev. 2007, 36, 15–30. [Google Scholar] [CrossRef]
- Kakoulli, I. Greek Painting Techniques and Materials from the Fourth to the First Century BC; Archetype Publications: London, UK, 2009; pp. 41–42, 138–140. [Google Scholar]
- García-Fernández, P.; Moreno, M.; Aramburu, J.A. Origin of the anomalous color of Egyptian and Han blue historical pigments: Going beyond the complex approximation in ligand field theory. J. Chem. Educ. 2016, 93, 111–117. [Google Scholar] [CrossRef]
- Pollio, M.V. De Architectura VII, Chaper 11, 1. Available online: https://penelope.uchicago.edu/Thayer/e/roman/texts/vitruvius/7*.html (accessed on 8 July 2022).
- Vezin, J.; Roger, P. Étude des matériaux de la couleur dans les manuscrits médiévaux emploi inédit de bleu égyptien dans trois manuscrits des VIIIe et Xe siècles. Comptes Rendus Séances Académie Inscr. Belles-Lettres 2007, 151, 67–87. [Google Scholar] [CrossRef]
- Gaetani, M.C.; Santamaria, U.; Seccaroni, C. The use of Egyptian blue and lapis lazuli in the Middle Ages: The wall paintings of the San Saba church in Rome. Stud. Conserv. 2004, 49, 13–22. [Google Scholar] [CrossRef]
- Bredal-Jørgensen, J.; Sanyova, J.; Rask, V.; Sargent, M.L.; Therkildsen, R.H. Striking presence of Egyptian blue identified in a painting of Giovanni Battista Benvenuto from 1524. Anal. Bioanal. Chem. 2011, 401, 1433–1439. [Google Scholar] [CrossRef]
- Anselmi, C.; Vagnini, M.; Seccaroni, C.; Azzarelli, M.; Frizzi, T.; Alberti, R.; Falcioni, M.; Sgamellotti, A. Imaging the antique: Unexpected Egyptian blue in Raphael’s Galatea by non-invasive mapping. Rendiconti Lincei. Scienze Fisiche e Naturali. Accademia Nazionale dei Lincei 2020, 31, 913–917. [Google Scholar] [CrossRef]
- Chaptal, J.A. Sur quelques couleurs trouvées à Pompeïa. Ann. Chim. 1809, 70, 22–31. [Google Scholar]
- Davy, H. Some experiments and observations on the colours used in painting by the Ancients. Phil. Trans. R. Soc. Lond. 1815, 105, 97–124. [Google Scholar]
- Spurrell, F.C.J. Notes on Egyptian colours. Archaeol. J. 1895, 52, 222–239. [Google Scholar] [CrossRef]
- Vauquelin, L.N. Lettre à M. Passalacqua, contenant l’analyse chimique des alliages des métaux qui composent la lame d’un poignard, deux miroirs et quelques instruments de sa collection, ainsi que l’analyse de las couleur bleue place sous le numéro 561. In Catalogue Raisonné et Historique des Antiquités découvertes en Égypte; Passalacqua, J., Ed.; Galerie D’antiquités Égyptiennes: Paris, France, 1826; pp. 238–239. [Google Scholar]
- Jaksch, H.; Seipel, W.; KWeiner, K.L.; El Goresy, A. Egyptian blue—Cuprorivaite a window to ancient Egyptian technology. Naturwissenschaften 1983, 70, 525–535. [Google Scholar] [CrossRef]
- Fouqué, F. Sur le bleu égyptien ou vestorien. Bull. Minéralogie 1889, 12, 36–38. [Google Scholar] [CrossRef]
- Laurie, A.P.; McLintock, W.F.P.; Miles, F.D. Egyptian blue. Proc. R. Soc. Lond. A 1914, 89, 418–429. [Google Scholar]
- Verri, G. The spatially resolved characterisation of Egyptian blue, Han blue and Han purple by photo-induced luminescence digital imaging. Anal. Bioanal. Chem. 2011, 401, 1011–1021. [Google Scholar] [CrossRef]
- Talbot, M. Color blind: Paint, politics, and Greek statues. The New Yorker, 29 October 2018; 44–51. [Google Scholar]
- Stokes, G.G. On the change of refrangibility of light. Phil. Trans. R.Soc. Lond. 1852, 142, 463–562. [Google Scholar]
- Bera, D.; Qian, L.; Holloway, P.H. Phosphor Quantum Dots. In Luminescent Materials and Applications; Kitai, A., Ed.; John Wiley and Sons: New York, NY, USA, 2008; pp. 19–37. [Google Scholar]
- Dyer, J.; Verri, G.; Cupitt, J. Multispectral Imaging in Reflectance and Photo-Induced Luminescence Modes: A User Manual; European CHARISMA Project, Version 1.0; The British Museum: London, UK, 2013; Available online: https://www.academia.edu/7276130/J_Dyer_G_Verri_and_J_Cupitt_Multispectral_Imaging_in_Reflectance_and_Photo_induced_Luminescence_modes_a_User_Manual_European_CHARISMA_Project (accessed on 14 January 2022).
- The Courtauld Institute of Art. Giovanni Verri. Available online: https://courtauld.pure.elsevier.com/en/persons/giovanni-verri (accessed on 14 January 2022).
- Accorsi, G.; Verri, G.; Bolognesi, M.; Armaroli, N.; Clementi, C.; Miliani, C.; Romani, A. The exceptional nearinfrared luminescence properties of cuprorivaite (Egyptian blue). Chem. Commun. 2009, 23, 3392–3394. [Google Scholar] [CrossRef]
- Linn, R.; Tepper, Y.; Bar-Oz, G. Visible induced luminescence reveals invisible rays shining from Christ in the early Christian wall painting of the Transfiguration in Shivta. PLoS ONE 2017, 12, e0185149. [Google Scholar] [CrossRef]
- Binet, L.; Lizion, J.; Bertaina, S.; Gourier, D. Magnetics and new optical properties in the UV-visible range of the Egyptian blue pigment cuprorivaite CaCuSi4O10. J. Phys. Chem. C 2021, 125, 25189–25196. [Google Scholar] [CrossRef]
- SciShow. Egyptian Blue: How an Ancient Pigment Could Save Lives. Available online: https://www.youtube.com/watch?v=K2Bwmdl61Sw&vl=en (accessed on 14 January 2022).
- Pogue, B.W.; Leblond, F.; Krishnaswamy, V.; Paulsen, K.D. Radiologic and near-infrared/optical spectroscopic imaging: Where is the synergy? Am. J. Roentgenol. 2010, 195, 321–332. [Google Scholar] [CrossRef][Green Version]
- Berdahl, P.; Boocock, S.K.; Chan, G.C.-Y.; Chen, S.S.; Levinson, R.M.; Zalich, M.A. High quantum yield of the Egyptian blue family of infrared phosphors (MCuSi4O10, M = Ca, Sr, Ba). J. Appl. Phys. 2018, 123, 193103. [Google Scholar] [CrossRef]
- Borisov, S.M.; Würth, C.; Resch-Genger, U.; Klimant, I. New life of ancient pigments: Application of high-performance optical sensing materials. Anal. Chem. 2013, 85, 9371–9377. [Google Scholar] [CrossRef] [PubMed]
- Canti, M.G.; Heathcote, J.L. Microscopic Egyptian blue (synthetic cuprorivaite) from sediments at two archaeological sites in West Central England. J. Archaeol. Sci. 2002, 29, 831–836. [Google Scholar] [CrossRef]
- West FitzHugh, E.; Zycherman, L.A. An early man-made blue pigment from China, barium copper silicate. Stud. Conserv. 1983, 28, 15–23. [Google Scholar] [CrossRef]
- West FitzHugh, E.; Zycherman, L.A. A purple barium copper silicate pigment from Early China. Stud. Conserv. 1992, 37, 145–154. [Google Scholar]
- Ma, Q.; Portmann, A.; Wild, F.; Berke, H. Raman and SEM studies of man-made barium copper silicate pigments in ancient Chinese artifacts. Stud. Conserv. 2006, 51, 81–98. [Google Scholar] [CrossRef]
- Thieme, C.; Emmerling, E.; Herm, C.; Wu, Y.; Zhou, T.; Zhang, Z. Research on paint materials, paint techniques and conservation experiments on the polychrome terracotta army of the First Emperor Qin Shi Huang. In The Ceramics Cultural Heritage: Proceedings of the International Symposium, the Ceramics Heritage, of the 8th CIMTEC-World Ceramics Congress and Forum on New Materials, Florence, Italy, 28 June–2 July 1994; Monographs in Material and Society 2 Series; Vincenzini, P., Ed.; Techna: Florence, Italy, 1994; pp. 591–601. [Google Scholar]
- Zhang, Z.; Ma, Q.; Berke, H. Man-made blue and purple barium copper silicate pigments and the pabstite (BaSnSi3O9) mystery of ancient Chinese wall paintings from Luoyang. Herit. Sci. 2019, 7, 97. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Feng, S. Hydrothermal synthesis and properties of pigments Chinese purple BaCuSi2O6 and dark blue BaCu2Si2O7. Dyes Pigm. 2014, 105, 167–173. [Google Scholar] [CrossRef]
- Powder samples of the Egyptian blue family of pigments prepared in the ancient manner of a solid state reaction held at very high temperatures are difficult to obtain in the pure phase of the compounds. There are always impurities present as by-products because of the complexity of the XO-CuO-SiO2 system. The hydrothemal method whereby stoichiometric ratios of reactants maintained in controlled pH aqueous solution at lower temperatures, typically about 250 °C, for about 48 hours yields crystalline products with few impurities.
- Lin, Y. Reconstruction of Ancient Production Technology of Chinese Blue Pigment and Synthesis of Chinese Blue Nanoscrolls as a Novel Optical Material. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2018; pp. 144–166. [Google Scholar]
- Orna, M.V. Archaeological blue pigments: Problem children from the get-go. In Archaeological Chemistry. A Multidisciplinary Analysis of the Past; Orna, M.V., Rasmussen, S.C., Eds.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2020; pp. 333–396. [Google Scholar]
- Chang, K. The grim story of Maya blue. The New York Times. 29 February 2008. Available online: https://www.nytimes.com/2008/02/29/science/29bluew.html (accessed on 19 January 2022).
- Morley, S.G. The Ancient Maya, 3rd ed; Stanford University Press: Redwood City, CA, USA, 1956; p. 171. [Google Scholar]
- Merwin, H.E. Chemical analysis of pigments. In Temple of the Warriors at Chitchen-Itzá; Yucatan; Morris, E.H., Charlot, J., Morris, A.A., Eds.; Carnegie Institution of Washington: Washington, DC, USA, 1931; pp. 355–356. [Google Scholar]
- Reyes Valerio, C. De Bonampak al Templo Mayor. El Azul Maya en Mesoamerica: Colección America Nuestra; Siglo XXI Editores: Mexico, DF, Mexico, 1993; Volume 40. [Google Scholar]
- Morley, S.G.; Brainerd, G.W.; Sharer, R.J. Bonampak, Chiapas, Mexico. Carnegie Institution of Washington Publication 602; Carnegie Institution of Washington: Washington, DC, USA, 1955. [Google Scholar]
- Gettens, R.J.; Stout, G.L. Painting Materials: A Short Encyclopaedia; Dover Publications: New York, NY, USA, 1966; p. 130. [Google Scholar]
- Gettens, R.J. Maya blue: An unsolved problem in ancient pigments. Am. Antiq. 1962, 27, 557–564. [Google Scholar] [CrossRef]
- Brindley, G.W. Index to the X-Ray Powder Data File (Minerals Section); American Society for Testing and Materials: Philadelphia, PA, USA, 1957. [Google Scholar]
- Sánchez del Río, M.; Doménech, A.; Doménech-Carbó, M.T.; Vásquez de Agredos Pascual, M.L.; Suárez, M.; García-Romero, E. The Maya blue pigment. In Developments in Palygorskite and Sepiolite Research: A New Outlook on These Nanomaterials; Developments in Clay Science; Galan, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 453–481. [Google Scholar] [CrossRef]
- Shepard, A.O.; Gottlieb, H.B. Maya Blue. Alternative Hypothesis. Notes from a Ceramic Laboratory 1; Carnegie Institute of Washington: Washington, DC, USA, 1962. [Google Scholar]
- Shepard, A.O. Maya blue: Alternative hypotheses. Am. Antiq. 1962, 27, 565–566. [Google Scholar] [CrossRef]
- Shepard, A.O.; Gottlieb, H.B.; Andrews, E.W.; Pollock, H.E.D. Notes from a Ceramic Laboratory; Carnegie Institution of Washington: Washington, DC, USA, 1977; p. 69. [Google Scholar]
- van Olphen, H. Maya blue: A clay-organic pigment? Science 1966, 154, 645–646. [Google Scholar] [CrossRef]
- Littmann, E.R. Maya blue. further perspectives and the possible use of indigo as the colorant. Am. Antiq. 1982, 47, 404–408. [Google Scholar] [CrossRef]
- Doménech, A.; Doménech-Carbó, M.T.; Sánchez del Río, M.; Vázquez de Agredos Pascual, M.L.; Lima, E. Maya blue as a nanostructured polyfunctional hybrid organic-inorganic material: The need to change paradigms. New J. Chem. 2009, 33, 2371–2379. [Google Scholar] [CrossRef]
- Chiari, G.; Giustetto, R.; Ricchiardi, G. Crystal structure refinement of palygorskite and Maya blue from molecular modelling and powder synchrotron diffraction. Eur. J. Mineral. 2003, 15, 21–33. [Google Scholar] [CrossRef]
- Ovarlez, S.; Chaze, A.-M.; Giulieri, F.; Delamare, F. Indigo chemisorption in sepiolite. application to Maya blue formation. C. R. Chim. 2006, 9, 1243–1248. [Google Scholar] [CrossRef]
- Structurally related clays, sepiolite and a form of montmorillonite, have been identified in a few samples of archaeological Maya blue.
- Tilocca, A.; Fois, E. The color and stability of Maya blue: TDDFT calculations. J. Phys. Chem. C 2009, 113, 8683–8687. [Google Scholar] [CrossRef]
- Giustetto, R.; Llabrés i Xamena, F.X.; Ricchiardi, G.; Bordiga, S.; Damin, A.; Gobetto, R.; Chierotti, M.R. Maya blue: A computational and spectroscopic study. J. Phys. Chem. B 2005, 109, 19360–19368. [Google Scholar] [CrossRef]
- Chiari, G. Measurement techniques to uncover mysteries in art. In Proceedings of the 3rd International Conference on Techniques, Measurements and Materials in Art and Archaeology, Jerusalem, Israel, 9–13 December 2018; Book of Abstracts. p. 21. [Google Scholar]
- Chiari, G.; Giustetto, R.; Druzik, J.; Doehne, E.; Ricchiardi, G. Pre-columbian nanotechnology: Reconciling the mysteries of the Maya blue pigment. Appl. Phys. A 2008, 90, 3–7. [Google Scholar] [CrossRef]
- Giustetto, R.; Seenivasan, K.; Bonino, F.; Ricchiardi, G.; Bordiga, S.; Chierotti, M.R.; Gobetto, R. Host/guest interactions in a sepiolite-based Maya blue pigment: A spectroscopic study. J. Phys. Chem. C 2011, 115, 16764–16776. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Bodé, S.; Alonso, A.; Moens, L. Raman spectroscopic analysis of the Maya wall paintings in Ek’Balam, Mexico. Spectrochim. Acta Part A 2005, 61, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Rondão, R.; Sérgio Seixas de Melo, J.; Bonifácio, V.D.B.; Melo, M.J. Dehydroindigo, the forgotten indigo and its contribution to the color of Maya blue. J. Phys. Chem. A 2010, 114, 1699–1708. [Google Scholar] [CrossRef]
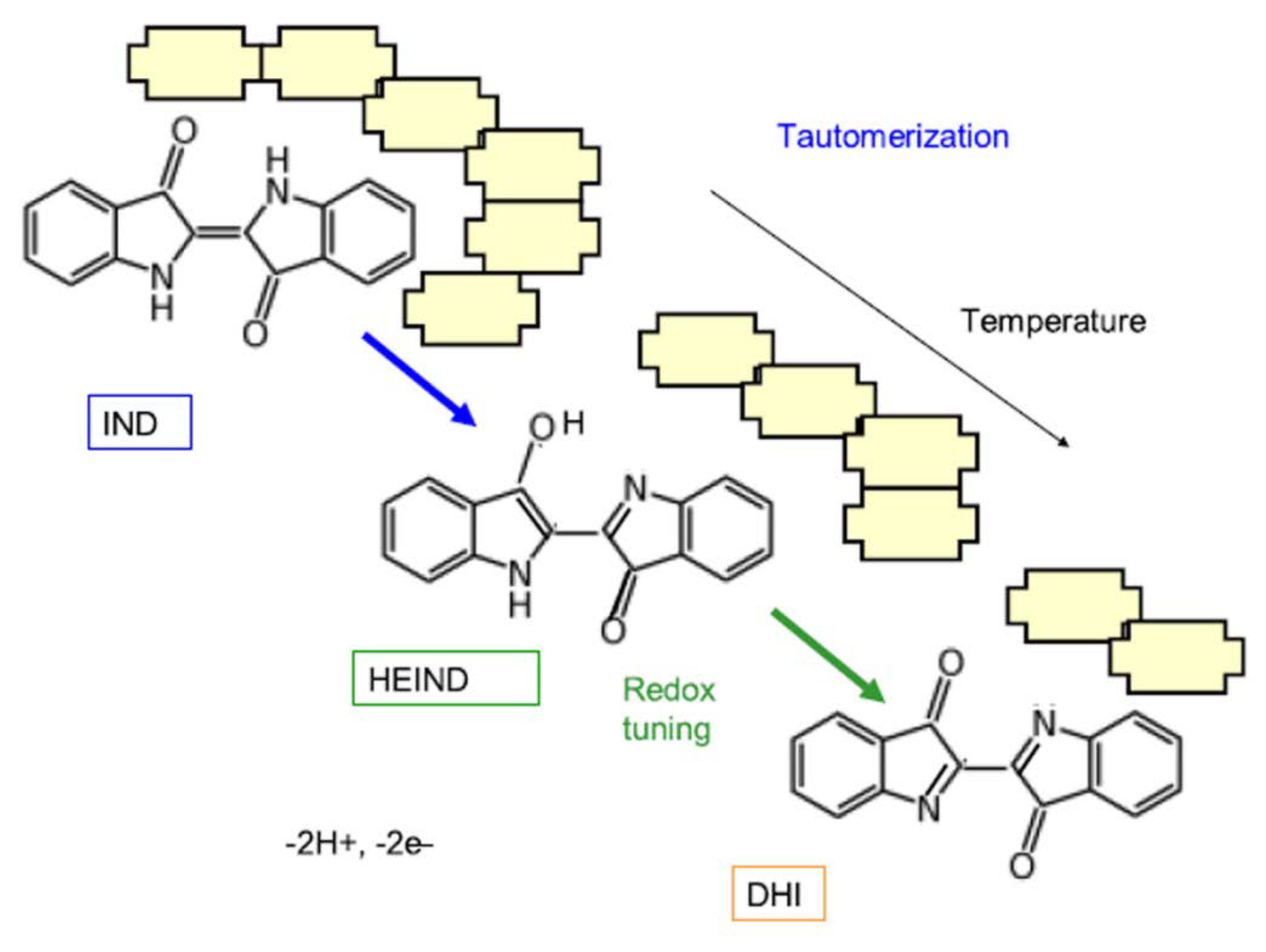
- Doménech-Carbó, A.; Costero, A.M.; Gil, S.; Montoya, N.; López-Carrasco, A.; Sáez, J.A.; Arroyo, P.; Doménech-Carbó, M.T. Isomerization and redox tuning: Reorganizing the Maya blue puzzle from synthetic, spectral, and electrochemical issues. J. Phys. Chem. C 2021, 125, 26188–26200. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Holmwood, S.; Di Turo, F.; Montoya, N.; Valle-Algarra, F.M.; Edwards, H.G.M.; Doménech-Carbó, M.T. Composition and color of Maya blue: Reexamination of literature data based on the dehydroindigo model. J. Phys. Chem. C 2019, 123, 770–782. [Google Scholar] [CrossRef]
- Dejoie, C.; Martinetto, P.; Tamura, N.; Kunz, M.; Porcher, F.; Bordat, P.; Brown, R.; Dooryhée, E.; Anne, M.; McCusker, L.B. Crystal structure of an indigo@Silicalite hybrid related to the ancient Maya blue pigment. J. Phys. Chem. C 2014, 118, 28032–28042. [Google Scholar] [CrossRef]
- Leitão, I.M.V.; Sérgio Seixas de Melo, J. Maya blue, an ancient guest-host pigment: Synthesis and models. J. Chem. Educ. 2013, 90, 1493–1497. [Google Scholar] [CrossRef]
- Winum, J.-Y.; Bernaud, L.; Filhol, J.-S. The hunt for Maya purple: Revisiting ancient pigments synthesis and properties. J. Chem. Educ. 2021, 98, 1389–1396. [Google Scholar] [CrossRef]
- Gómez-Romero, P.; Sanchez, C. Hybrid materials. functional properties. from Maya blue to 21st century materials. New J. Chem. 2005, 29, 57–58. [Google Scholar] [CrossRef]
- Giulieri, F.; Ovarlez, S.; Chaze, A.-M. Indigo/sepiolite nanohybrids: Stability of natural pigments inspired by Maya blue. Int. J. Nanotechnol. 2012, 9, 605–617. [Google Scholar]
- Wang, A.; Wang, W. (Eds.) Nanomaterials from Clay Minerals: A New Approach to Green Functional Materials; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Dong, J.; Zhang, J. Maya blue pigments derived from clay minerals. In Nanomaterials from Clay Minerals: A New Approach to Green Functional Materials; Wang, A., Wang, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 627–661. [Google Scholar]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic-inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, Z.; Li, Y.; Li, S.; Lu, G. Photosensitized reduction of water to hydrogen using novel Maya blue-like organic-inorganic hybrid material. J. Colloid Interface Sci. 2009, 333, 285–293. [Google Scholar] [CrossRef] [PubMed]
- McGovern, P.E. Alcoholic beverages as the universal medicine before synthetics. In Chemistry’s Role in Food Production and Sustainability: Past and Present; Orna, M.V., Eggleston, G., Bopp, A.F., Eds.; American Chemical Society: Washington, DC, USA, 2019; pp. 111–128. [Google Scholar]
- Liebman, A.J. History of distillation. J. Chem. Educ. 1956, 33, 166–173. [Google Scholar] [CrossRef]
- Cobb, C.; Goldwhite, H. Creations of Fire, Chemistry’s Lively History from Alchemy to the Atomic Age; Plenum Press: New York, NY, USA, 1995; p. 87. [Google Scholar]
- Cobb, C.; Fetterolf, M.L.; Goldwhite, H. The Chemistry of Alchemy: From Dragon’s Blood to Donkey Dung, How Chemistry was Forged; Prometheus Books: Amherst, MA, USA, 2014; p. 16. [Google Scholar]
- Forbes, R.J. Studies in Ancient Technology; E.J. Brill: Leiden, The Netherlands, 1955; Volume 3, p. 218. [Google Scholar]
- Barber, D.J.; Freestone, I.C.; Moulding, K.M. Ancient copper red glasses: Investigation and analysis by microbeam techniques. In From Mine to Microscope: Advances in the Study of Ancient Technology; Shortland, A.J., Freestone, I.C., Rehren, T., Eds.; Oxbow Books: Oxford, UK, 2009; pp. 115–128. [Google Scholar]
- Karpenko, V.; Norris, J.A. Vitriol in the history of chemistry. Chem. Listy 2002, 96, 997–1005. [Google Scholar]
- Cassius, A. De extremo Illo et Perfectissimo Naturae Opificio ac Principe Terrae Norum Sidere Auro; Georg Wolff: Hamburg, Germany, 1685. [Google Scholar]
- Richter, J.B. Über die neuern Gegenstände der Chymie. 11: Vorzüglich über die Glucine, Agust-Erde und Einige Besondres Eigenschaften des Goldes; Korn: München, Germany, 1802; p. 91. Available online: https://www.bavarikon.de/object/bav:BSB-MDZ-00000BSB10707959?cq=Thon&p=1&lang=de (accessed on 19 January 2022).
- Proust, J.L. Faits pour server à l’histoire de l’or. J. Phys. 1806, 62, 131–147. [Google Scholar]
- Faraday, M. Experimental relations of gold (and other metals) to light. Phil. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar]
- Freestone, I.; Meeks, N.; Sax, M.; Higgitt, C. The Lycurgus cup—A Roman nanotechnology. Gold Bull. 2007, 40, 270–277. [Google Scholar] [CrossRef]
- Thompson, D. Michael Faraday’s recognition of ruby gold: The birth of modern technology. Gold Bull. 2007, 40, 267–269. [Google Scholar] [CrossRef]
- Turkevich, J. Colloidal gold. Part I. Gold Bull. 1985, 18, 86–91. [Google Scholar] [CrossRef]
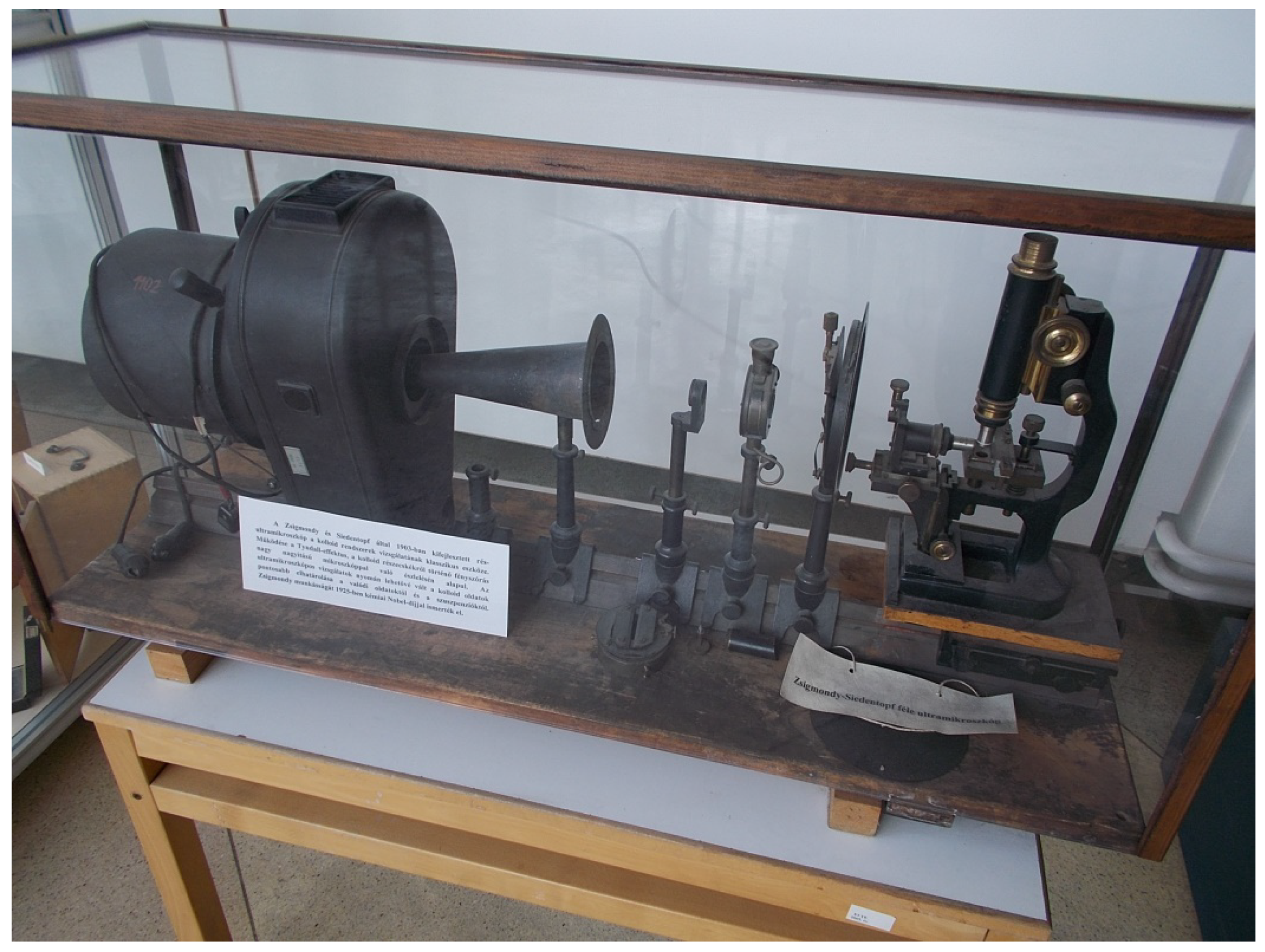
- Mappes, T.; Jahr, N.; Csaki, A.; Vogler, N.; Popp, J.; Fritzsche, W. The invention of immersion ultramicroscopy in 1912—The birth of nanotechnology. Angew. Chem. Int. Ed. 2012, 51, 11208–11212. [Google Scholar] [CrossRef]
- Hunt, L. Gold-based glass and enamel colours. Endeav. New Ser. 1981, 5, 61–67. [Google Scholar] [CrossRef]
- Zsigmondy, R.A. Neue Lüster und Farben auf Glas. Dingler’s Polytech. J. 1887, 266, 364–370. [Google Scholar]
- Zsigmondy, R.A. Die Löslichkeit der Sulfide im Glase (neue Farben). Dingler’s Polytech. J. 1889, 273, 29–37. [Google Scholar]
- Zsigmondy, R. Properties of Colloids. Nobel Lecture. 11 December 1926. Available online: https://www.nobelprize.org/prizes/chemistry/1925/zsigmondy/lecture/ (accessed on 20 April 2021).
- Siedentopf, H.; Zsigmondy, R. Ueber Sichtbarmachung und Größenbestimmung ultramikoskopischer† Teilchen, mit besonderer Anwendung auf Goldrubingläser. Ann. Der Phys. 1902, 315, 1–39. [Google Scholar] [CrossRef]
- Davis, D.J. A method of microscopic observation by means of lateral illumination. JAMA 1904, 42, 1075–1078. [Google Scholar] [CrossRef][Green Version]
- Siedentopf, H., IX. On the rendering visible of ultra-microscopic particles and of ultra-microscopic bacteria. J. R. Microsc. Soc. 1903, 23, 573–578. [Google Scholar]
- Fleck, G.M. Atomism in late nineteenth-century physical chemistry. J. Hist. Ideas 1963, 24, 106–114. [Google Scholar]
- Newburgh, R.; Peidle, J.; Rueckner, W. Einstein, Perrin, and the reality of atoms: 1905 revisited. Am. J. Phys. 2006, 74, 478–481. [Google Scholar]
- Einstein, A. Zur Theorie der Brownschen Bewegung. Ann. Phys. 1906, 19, 371–381. [Google Scholar]
- Fleck, G. Richard Zsigmondy (1865–1929). In Nobel Laureates in Chemistry 1901–1992; James, L.K., Ed.; American Chemical Society: Washington, DC, USA, 1993; pp. 151–157. [Google Scholar]
- Antonii, F. Panacea Aurea: Sive Tractatus Duo de Ipsius Auro Potabili; Bibliopolio Frobeniano: Hamburg, Germany, 1618; Available online: https://books.google.be/books?vid=GENT900000127717&printsec=frontcover&hl=nl#v=onepage&q&f=false (accessed on 7 January 2022).
- Huang, J.; Han, Y. Diverse near-infrared resonant gold nanostructures for biomedical applications. In Recent Progress in Colloid and Surface Chemistry with Biological Applications; ACS Symposium Series 1215; Wang, C., Leblanc, R.M., Eds.; American Chemical Society: Washington, DC, USA, 2015; pp. 213–243. [Google Scholar]
- Boisellier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar]
- Zuo, J.-Y.; Jiao, Y.-J.; Zhu, J.; Ding, S.-N. Rapid detection of severe fever with thrombocytopenia syndrome virus via colloidal gold immunochromatography assay. ACS Omega 2018, 3, 15399–15406. [Google Scholar] [CrossRef] [PubMed]
- Matus, M.F.; Malola, S.; Häkkinen, H. Ligand ratio plays a critical role in the design of optimal multifunctional gold nanoclusters for targeted gastric cancer therapy. ACS Nanosci. Au 2021, 1, 47–60. [Google Scholar] [CrossRef]
- Chen, J.; Saeki, F.; Wiley, B.J.; Cang, H.; Cobb, M.J.; Li, Z.-Y.; Au, L.; Zhang, H.; Kimmey, M.B.; Li, X.; et al. Gold nano cages: Bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2005, 5, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wu, X.; Cui, G.; Song, S.; Kuang, H.; Xu, C. Colloidal gold immunochromatographic assay for rapid detection of carbadox and cyadox in chicken breast. ACS Omega 2020, 5, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, A.; Mujawar, M.A.; Manickam, P.; Bhansali, S. Plasma-induced enhancement in electronic properties of gold nanoparticles: Application in electrochemical biosensing of cortisol. ACS Appl. Electron. Mater. 2021, 3, 230–237. [Google Scholar] [CrossRef]
- Edwards, P.P.; Thomas, J.M. Gold in a metallic divided state—From Faraday to present-day nanoscience. Angew. Chem. Int. Ed. 2007, 46, 5480–5486. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.; Spring, M.; Higgitt, C. The technology of red lake pigment manufacture: Study of the dyestuff substrate. Natl. Gallery Tech. Bull. 2005, 26, 71–87. [Google Scholar]
- Kraft, A. On the discovery and history of Prussian blue. Bull. Hist. Chem. 2008, 33, 61–67. [Google Scholar]
- Woodward, J. Preparation Coerulei Prussiaci ex Germania missa ad Johannem Woodward. Phil. Trans. 1724, 33, 15–17. [Google Scholar] [CrossRef]
- Method of Preparing Prussian Blue, Communicated from Germany to John Woodward, M. D. Gresham Professor of Physics and F. R. S., No 381, p. 15. Translated from the Latin. Phil. Trans. 1809. abridged series 7. pp. 4–6. Available online: https://archive.org/details/philosophicaltra07royarich/page/4/mode/2up?q=woodward&view=theater (accessed on 11 July 2022).
- Harley, R. Artists’ Pigments c 1600–1835; Butterworths: London, UK, 1970; p. 66. [Google Scholar]
- Ware, M. Prussian blue: Artists’ pigment and chemists’ sponge. J. Chem. Educ. 2008, 85, 612–621. [Google Scholar] [CrossRef]
- Ludi, A. Prussian blue, an inorganic evergreen. J. Chem. Educ. 1981, 58, 1013. [Google Scholar] [CrossRef]
- Berrie, B.H. Prussian blue. In Artists’ Pigments: A Handbook of Their History and Characteristics; West FitzHugh, E., Ed.; National Gallery of Art: Washington, DC, USA, 1997; Volume 3, pp. 191–217. [Google Scholar]
- Field, G. Chromatography; or, A Treatise on Colours and Pigments and of their Powers in Painting; Winsor & Newton: London, UK, 1885; p. 149. [Google Scholar]
- Kirby, J. Fading and colour change of prussian blue: Occurrences and early reports. Natl. Gallery Tech. Bull. 1993, 14, 62–71. [Google Scholar]
- Buser, H.J.; Schwarzenbach, D.; Petter, W.; Ludi, A. The crystal structure of Prussian blue: Fe4[Fe(CN)6]3·xH2O. Inorg. Chem. 1977, 16, 2704–2710. [Google Scholar] [CrossRef]
- Herschel, J.W.F. On the action of the rays of the solar spectrum on vegetable colours, and on some new photographic processes. Phil. Trans. R. Soc. Lond. 1842, 132, 181–215. [Google Scholar] [CrossRef]
- Neff, V.D. Electrochemical oxidation and reduction of thin films of Prussian blue. J. Electrochem. Soc. 1978, 125, 886–887. [Google Scholar] [CrossRef]
- Mortimer, R.J. Switching colors with electricity. Am. Sci. 2013, 101, 38–55. [Google Scholar] [CrossRef]
- Deb, S.K. A novel electrographic system. Appl. Opt. 1969, 8, 192–195. [Google Scholar] [CrossRef]
- Islam, S.M.; Barile, C.J. Dual tinting dynamic windows using reversible metal electrodeposition and Prussian blue. ACS Appl. Mater. Interfaces 2019, 11, 40043–40049. [Google Scholar] [CrossRef]
- Ide, M.; Sato, H.; Ozawa, H.; Haga, M. Regulation of ion transport in Prussian blue MOF films by a Ru-complex primer nanolayer on an ITO electrode and its energy storage appliction. ACS Appl. Electron. Mater. 2021, 3, 3962–3971. [Google Scholar] [CrossRef]
- Qin, M.; Ren, W.; Jiang, R.; Li, Q.; Yao, X.; Wang, S.; You, Y.; Mai, L. Highly crystallized Prussian blue with advanced kinetics for highly efficient sodium storage. ACS Appl. Mater. Interfaces 2021, 13, 3999–4007. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, B.; Mao, Y.; Shi, C.; Li, Y.; Huang, X.; Yang, F.; Gu, N. Achieving ultrasmall Prussian blue nanoparticles as high-performance biomedical agents with multifunctions. ACS Appl. Mater. Interfaces 2020, 12, 57382–57390. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Kotcherlakota, R.; Haque, S.; Das, S.; Nuthi, S.; Bhattacharya, D.; Madhusudana, K.; Chakravarty, S.S.; Sistla, R.; Patra, C.R. Silver Prussian blue analogue nanoparticles: Rationally designed advanced nanomedicine for multifunctional biomedical applications. ACS Biomater. Sci. Eng. 2020, 6, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.F.; Hoffman, H.A.; Yoon, P.R.S.; Conklin, L.S.; Saha, S.R.; Paglione, J.P.; Sze, R.W.; Fernandes, R. Biofunctionalized gadolinium-containing Prussian blue nanoparticles as multimodal molecular imaging agents. Bioconjugate Chem. 2014, 25, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.H.T.; Nossol, E. Self-recharging graphene oxide-Prussian blue electrodes for transparent batteries. ACS Appl. Nano Mater. 2019, 2, 2241–2249. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Yoon, J. Rocking chair desalination battery based on Prussian blue electrodes. ACS Omega 2017, 2, 1653–1659. [Google Scholar] [CrossRef]
- Katic, V.; dos Santos, P.L.; dos Santos, M.F.; Pires, B.M.; Loureiro, H.C.; Lima, A.P.; Queiroz, J.C.M.; Landers, R.; Muñoz, R.A.A.; Bonacin, J.A. 3D printed graphene electrodes modified with Prussian blue: Emerging electrochemical sensing platform for peroxide detection. ACS Appl. Mater. Interfaces 2019, 11, 35068–35078. [Google Scholar] [CrossRef]
- Chuang, C.H.; Hsiao, L.-Y.; Yeh, M.-H.; Wang, Y.-C.; Chang, S.-C.; Tsai, L.-D.; Ho, K.-C. Prussian blue analogue-derived metal oxides as electrocatalysts for oxygen evolution reaction: Tailoring the molar ratio of cobalt to iron. ACS Appl. Energy Mater. 2020, 3, 11752–11762. [Google Scholar] [CrossRef]
- Watson, M.-A. Natron energy. Chem. Eng. News 2020, 98, 40–41. [Google Scholar] [CrossRef]
- Hooke, R. Micrographia or Some Physiological Descriptions of Minute Bodies Made by Magnifying Glasses with Observations and Inquiries thereupon; Royal Society: London, UK, 1665; p. 168. [Google Scholar]
- Newton, I. Opticks, 4th ed.; William Innys at the West-End of St. Paul’s: London, UK, 1704; p. 251. [Google Scholar]
- McDougal, K.D.; Kang, S.; Yaqoob, Z.; So, P.T.C.; Kolle, M. In vivo visualization of butterfly scale cell morphogenesis in Vanessa cardui. Proc. Natl. Acad. Sci. USA 2021, 118, e2112009118. [Google Scholar] [CrossRef]
- Rayleigh, L. Studies of iridescent colour and the structure producing it. III. The colours of Labrador feldspar. Proc. Roy. Soc. Lond. 1923, 103, 34–45. [Google Scholar]
- Roeder, J. Manoharan Explains Structural Color; Teachers Clearinghouse for Science and Society Education Newsletter: Princeton, NJ, USA, 2022; Volume 41. [Google Scholar]
- Magkiriadou, S. Structural Color from Colloidal Glasses. Ph.D. Thesis, Harvard University, Graduate School of Arts & Sciences, Cambridge, MA, USA, 2015. [Google Scholar]
- Daguerre, L.J.M. Des procédés photogéniques considérés comme moyens de gravure. C. R. Hebd. Seances Acad. Sci. 1839, 9, 423–429. [Google Scholar]
- Forman, S.A. The dynamic interplay between photochemistry and photography. J. Chem. Educ. 1975, 52, 629–631. [Google Scholar] [CrossRef]
- Orna, M.V.; Goodstein, M.P. Chemistry and Artists’ Colors, 3rd ed.; ChemSource, Inc.: New Rochelle, NY, USA, 2017; pp. 334–349. [Google Scholar]
- Sheppard, S.E. The chemistry of photography. I. Historical considerations. J. Chem. Educ. 1927, 4, 298–312. [Google Scholar] [CrossRef]
- Schlather, A.E.; Gieri, P.; Robinson, M.; Centeno, S.A.; Manjavacas, A. Nineteenth century nanotechnology: The plasmonic properties of daguerreotypes. Proc. Natl. Acad. Sci. USA 2019, 116, 13791–13798. [Google Scholar] [CrossRef] [PubMed]
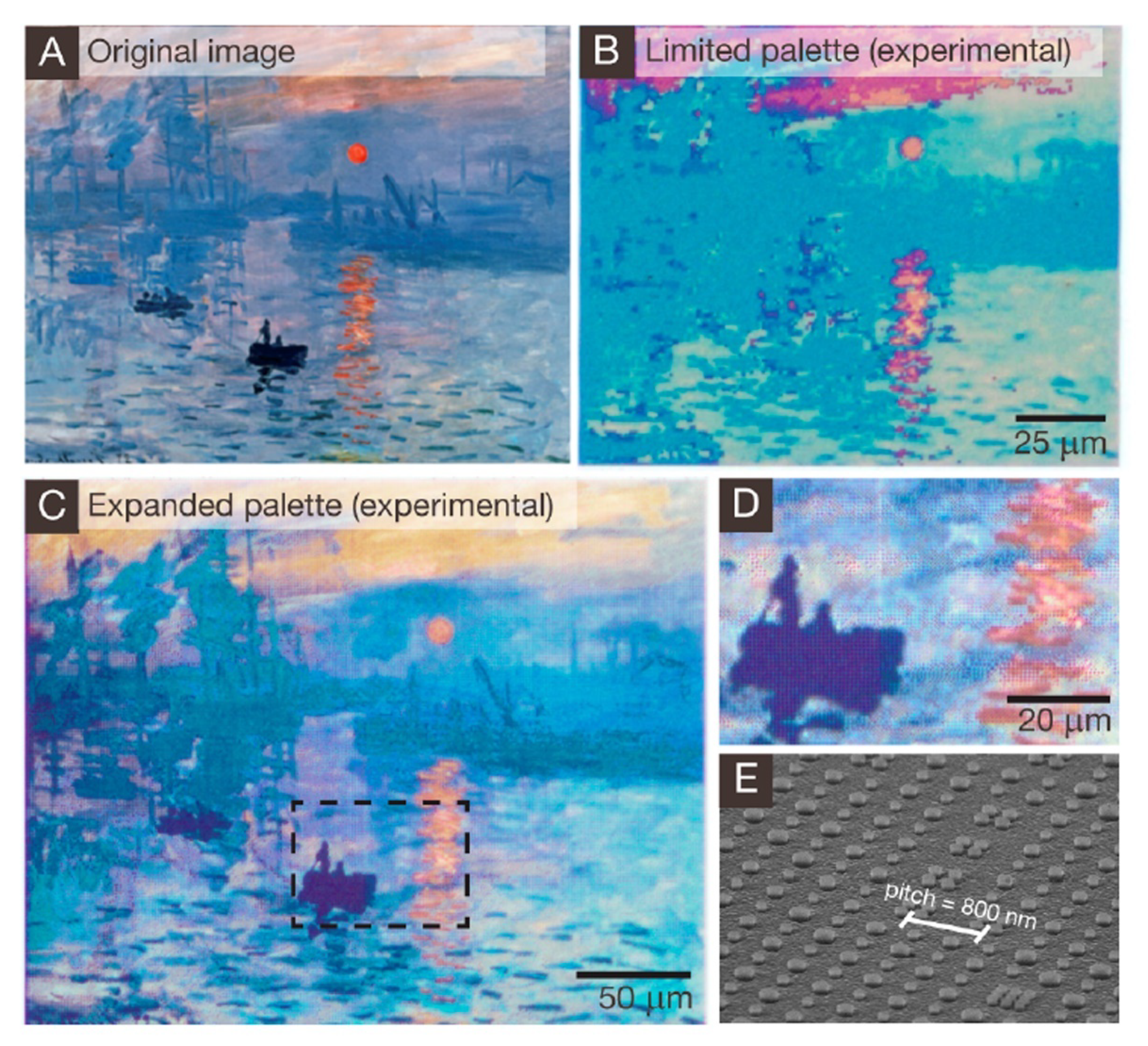
- Franklin, D.; He, Z.; Ortega, P.M.; Safaei, A.; Cencillo-Abad, P.; Wu, S.-T.; Chanda, D. Self-assembled plasmonics for angle-independent structural color displays with actively addressed black states. Proc. Natl. Acad. Sci. USA 2020, 117, 13350–13358. [Google Scholar] [CrossRef]
- Brazil, R. Plasmonic color makes a comeback. ACS Cent. Sci. 2020, 6, 332–335. [Google Scholar] [CrossRef]
- Tan, S.J.; Zhang, L.; Zhu, D.; Goh, X.M.; Wang, Y.M.; Kumar, K.; Qiu, C.-W.; Yang, J.K.W. Plasmonic color palettes for photorealistic printing with aluminum nanostructures. Nano Lett. 2014, 14, 4023–4029. [Google Scholar] [CrossRef]
- Müller, M.B.; Kuttner, C.; König, T.A.F.; Tsukruk, V.V.; Förster, S.; Karg, M.; Fery, A. Plasmonic library based on substrate-supportedgradiential plasmonicarrays. ACS Nano 2014, 8, 9410–9421. [Google Scholar] [CrossRef]
- Aristotle. De Sensu; The Clarendon Press: Oxford, UK, 1908; pp. 439–440. [Google Scholar]
- Newton, I. Opticks; Dover Publications: New York, NY, USA, 1952; p. 50. [Google Scholar]















| Pigment/Colorant | Type | Date |
|---|---|---|
| Indigo | Plant-derived organic compound | Antiquity |
| Ultramarine blue | Naturally occurring mineral | ~5000 BCE |
| Egyptian & Chinese blue | Synthetic pigment | ~3200 BCE |
| Maya blue | Composite material | 200–300 BCE |
| Purple of Cassius | Colloid | 16th Century |
| Prussian blue | Synthetic pigment | 1704–1707 |
| Structural colors | Microlamellar array | 1839 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orna, M.V.; Fontani, M. The Modernity of Ancient Pigments: A Historical Approach. Colorants 2022, 1, 307-346. https://doi.org/10.3390/colorants1030019
Orna MV, Fontani M. The Modernity of Ancient Pigments: A Historical Approach. Colorants. 2022; 1(3):307-346. https://doi.org/10.3390/colorants1030019
Chicago/Turabian StyleOrna, Mary Virginia, and Marco Fontani. 2022. "The Modernity of Ancient Pigments: A Historical Approach" Colorants 1, no. 3: 307-346. https://doi.org/10.3390/colorants1030019
APA StyleOrna, M. V., & Fontani, M. (2022). The Modernity of Ancient Pigments: A Historical Approach. Colorants, 1(3), 307-346. https://doi.org/10.3390/colorants1030019






