Chemoinformatics Analysis of the Colour Fastness Properties of Acid and Direct Dyes in Textile Coloration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database for Colour Fastness of Wool, Polyamide, and Cotton Fibres Dyed with Acid and Direct Dyes
2.2. Chemoinformatics Tools
3. Results and Discussion
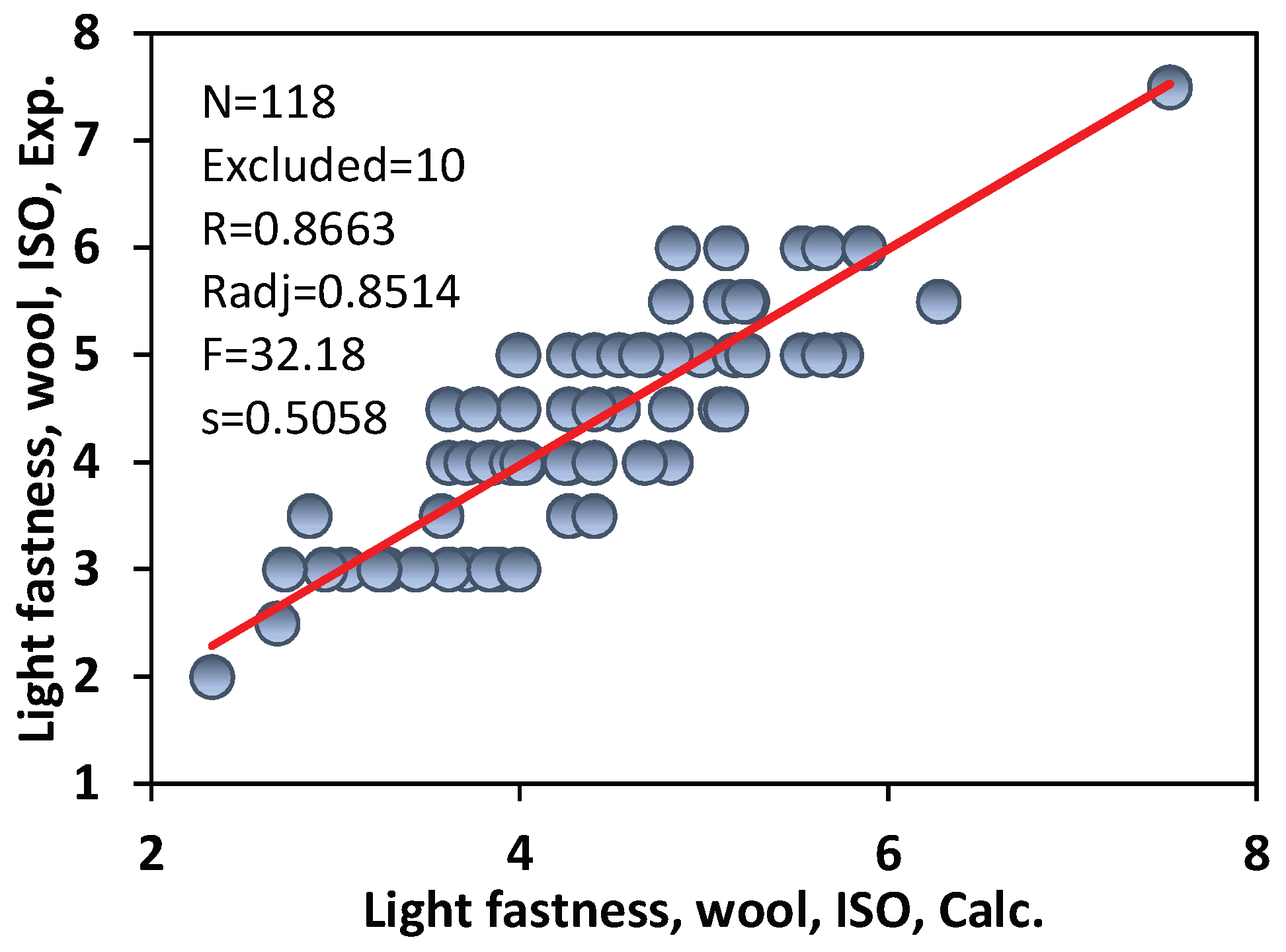
3.1. Light Fastness of Commercial Acid Azo Dyes on Wool
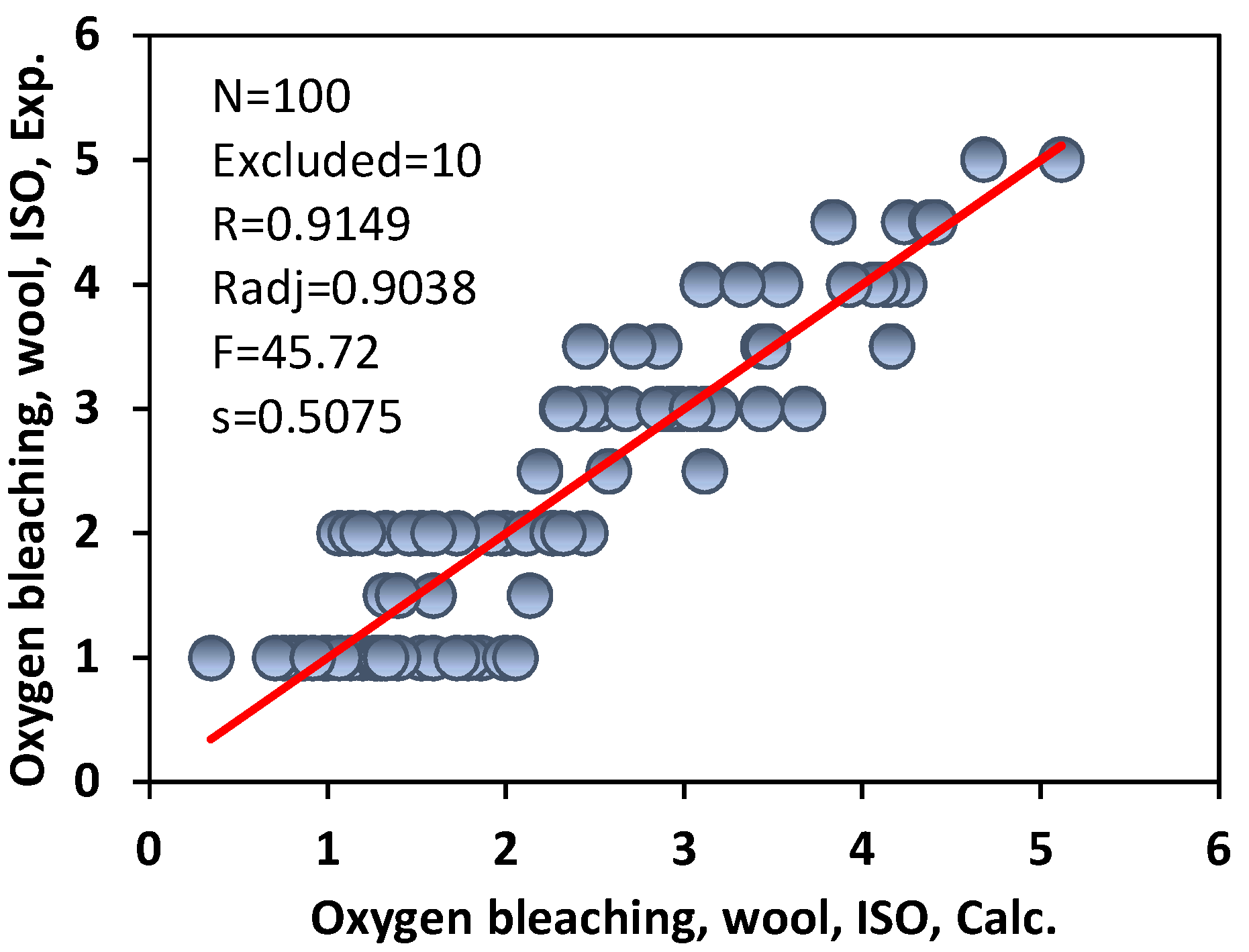
3.2. Colour Fastness to Oxygen Bleaching of Commercial Acid Azo Dyes on Wool
3.3. Wash Fastness of Commercial Acid Azo Dyes on Wool
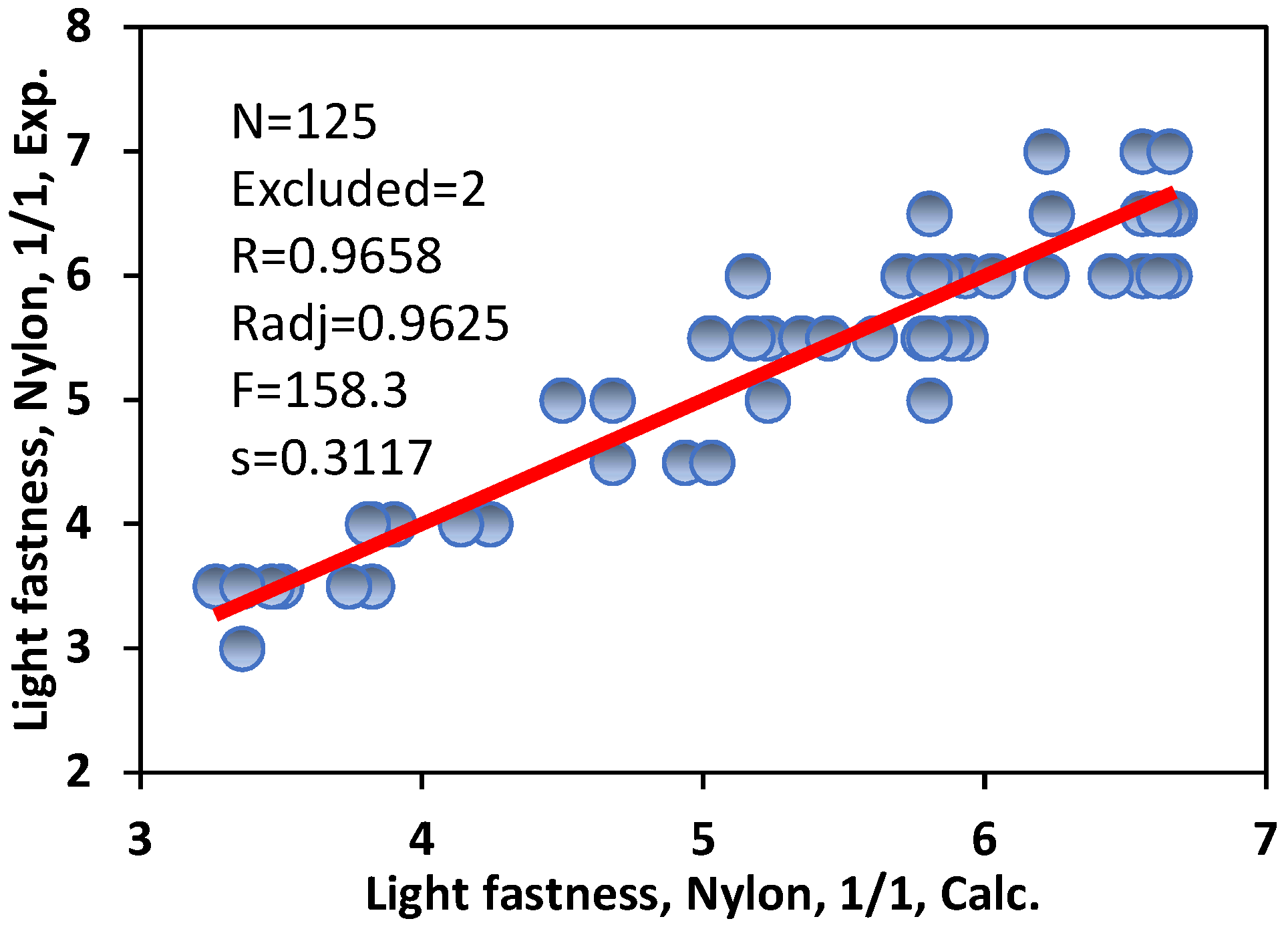
3.4. Light Fastness of Acid Dyes on Polyamide (Nylon) Fibres
3.5. Adsorption Properties of Direct Dyes on Cotton Fibres
3.6. Photodegradation of Azo Dyes in Solution
3.7. Comparative Analysis of Fragment Descriptors of Regression Models for Different Kinds of Fibres and Colour Fastness Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burkinshaw, S.M. Physico-Chemical Aspects of Textile Coloration; SDC, John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; ISBN 978-1-118-72569-6. [Google Scholar]
- Burkinshaw, S.M. The role of inorganic electrolyte (salt) in cellulosic fibre dyeing: Part 1 fundamental aspects. Coloration Technol. 2021, 137, 421–444. [Google Scholar] [CrossRef]
- Burkinshaw, S.M. The role of inorganic electrolyte (salt) in cellulosic fibre dyeing: Part 2 theories of how inorganic electrolyte promotes dye uptake. Coloration Technol. 2021, 137, 547–586. [Google Scholar] [CrossRef]
- Vickerstaff, T. The Physical Chemistry of Dyeing, 2nd ed.; Oliver & Boyd: Edinburgh, UK, 1954. [Google Scholar]
- Peters, R.H. Textile Chemistry: The Physical Chemistry of Dyeing; Elsevier Sci. Publ. Co.: Amsterdam, The Netherlands; Oxford, UK; New York, NY, USA, 1975. [Google Scholar]
- Giles, C.H.; Duff, D.G.; Sinclair, R.S. Relation between the molecular structure of dyes and their technical properties: Chapter VII. In The Chemistry of Synthetic Dyes; Venkataraman, K., Ed.; Academic Press: Cambridge, MA, USA, 1978; pp. 279–329. [Google Scholar]
- The Theory of Coloration of Textiles, 2nd ed.; Johnson, A. (Ed.) Society of Dyers and Colourists: Bradford, UK, 1989. [Google Scholar]
- Colorants and Auxiliaries. Organic Chemistry and Application Properties; Colorants; Shore, J., Ed.; Society of Dyers & Colourists: Bradford, UK, 2002; Volume 1, ISBN 9780901956774. [Google Scholar]
- Telegin, F.; Shushina, I.; Ran, J.; Biba, Y.; Mikhaylov, A.; Priazhnikova, V. Structure—Property relationships for dyes of different nature. Adv. Mater. Res. 2013, 821–822, 488–492. [Google Scholar] [CrossRef]
- SDC; AATCC. Colour Index™ Online. Available online: https://colour-index.com/about (accessed on 29 April 2022).
- World Dye Variety. Available online: http://www.worlddyevariety.com/ (accessed on 29 April 2022).
- Kuenemann, M.A.; Szymczyk, M.; Chen, Y.; Sultana, N.; Hinks, D.; Freeman, H.S.; Williams, A.J.; Fourches, D.; Vinueza, N.R. Weaver’s historic accessible collection of synthetic dyes: A cheminformatics analysis. Chem. Sci. 2017, 8, 4334–4339. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.N.; Kuenemann, M.A.; Van Den Driessche, G.A.; Williams, A.J.; Fourches, D.; Freeman, H.S. Toward the rational design of sustainable hair dyes Using cheminformatics approaches: Step 1. Database development and analysis. ACS Sustain. Chem. Eng. 2018, 6, 2344–2352. [Google Scholar] [CrossRef]
- Williams, T.N.; Van Den Driessche, G.A.; Valery, A.R.B.; Fourches, D.; Freeman, H.S. Toward the rational design of sustainable hair dyes using cheminformatics approaches: Step 2. Identification of hair dye substance database analogs in the Max Weaver dye library. ACS Sustain. Chem. Eng. 2018, 6, 14248–14256. [Google Scholar] [CrossRef]
- Churchley, J.; Greaves, A.; Hutchings, M.; Phillips, D.A.S.; Taylor, J.A. A chemometric approach to understanding the bioelimination of anionic, water-soluble dyes by a biomass—Part 2: Acid dyes. Coloration Technol. 2000, 116, 222–228. [Google Scholar] [CrossRef]
- Churchley, J.H.; Greaves, A.J.; Hutchings, M.G.; Phillips, D.A.S.; Taylor, J.A. A chemometric approach to understanding the bioelimination of anionic, water-soluble dyes by a biomass—Part 3: Direct dyes. Coloration Technol. 2000, 116, 279–284. [Google Scholar] [CrossRef]
- Churchley, J.H.; Greaves, A.J.; Hutchings, M.G.; Phillips, D.A.S.; Taylor, J.A. A chemometric approach to understanding the bioelimination of anionic, water-soluble dyes by a biomass—Part 4: Reactive dyes. Coloration Technol. 2000, 116, 323–329. [Google Scholar] [CrossRef]
- De Giorgi, M.R.; Cerniani, A.; Carpignano, R.; Savarino, P. Design of high fastness acid dyes for silk: A chemometric approach. J. Soc. Dye. Colour. 1993, 109, 405–410. [Google Scholar] [CrossRef]
- De Giorgi, M.R.; Carpignano, R.; Scano, P. Structure optimization in a series of dyes for wool and cotton. A chemometric approach. Dyes Pigments 1994, 26, 175–189. [Google Scholar] [CrossRef]
- De Giorgi, M.; Carpignano, R. Design of dyes of high technical properties for silk by a chemometric approach. Dyes Pigments 1996, 30, 79–88. [Google Scholar] [CrossRef]
- De Giorgi, M.R.; Carpignano, R.; Crisponi, G. Structure optimization in a series of acid dyes for wool and nylon. Dyes Pigments 1997, 34, 1–12. [Google Scholar] [CrossRef]
- De Giorgi, M.; Carpignano, R.; Cerniani, A. Structure optimization in a series of thiadiazole disperse dyes using a chemometric approach. Dyes Pigments 1998, 37, 187–196. [Google Scholar] [CrossRef]
- Kats, M.D.; Krichevskii, G.E. Mathematical model for the relationship between lightfastness and chemical structure of monoazo disperse dyes. Izv. Vuzov. Techn. Text. Prom 1979, 60–63. [Google Scholar]
- Kats, M.D.; Lysun, N.V.; Mostoslavskaya, E.I.; Krichevskii, G.E. Studies of the relationships between chemical structure of diperse monoazo dyes and their light protecting properties on polyamide fibres. Zh. Prikl. Khim. 1988, 1196–1199. [Google Scholar]
- Hilal, S.H.; Carreira, L.A.; Baughman, G.L.; Karickhoff, S.W.; Melton, C.M. Estimation of ionization constants of azo dyes and related aromatic amines: Environmental implication. J. Phys. Org. Chem. 1994, 7, 122–141. [Google Scholar] [CrossRef]
- Timofei, S.; Schmidt, W.; Kurunczi, L.; Simon, Z. A review of QSAR for dye affinity for cellulose fibres. Dyes Pigments 2000, 47, 5–16. [Google Scholar] [CrossRef]
- Funar-Timofei, S.; Fabian, W.M.; Kurunczi, L.; Goodarzi, M.; Ali, S.T.; Heyden, Y.V. Modelling heterocyclic azo dye affinities for cellulose fibres by computational approaches. Dyes Pigments 2012, 94, 278–289. [Google Scholar] [CrossRef]
- Polanski, J.; Gieleciak, R.; Wyszomirski, M. Comparative molecular surface analysis (CoMSA) for modeling dye-fiber affinities of the azo and anthraquinone dyes. J. Chem. Inf. Comput. Sci. 2003, 43, 1754–1762. [Google Scholar] [CrossRef]
- Polanski, J.; Gieleciak, R.; Wyszomirski, M. Mapping dye pharmacophores by the Comparative Molecular Surface Analysis (CoMSA): Application to heterocyclic monoazo dyes. Dyes Pigments 2004, 62, 61–76. [Google Scholar] [CrossRef]
- Zhokhova, N.I.; Baskin, I.I.; Palyulin, V.A.; Zefirov, A.N.; Zefirov, N.S. A study of the affinity of dyes for cellulose fiber within the framework of a fragment approach in QSPR. Russ. J. Appl. Chem. 2005, 78, 1013–1017. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Wu, L.; Gu, S.; Liu, R.; Liu, L.; Liu, X.; Xu, J. Quantitative structure–affinity relationship study of azo dyes for cellulose fibers by multiple linear regression and artificial neural network. Chemom. Intell. Lab. Syst. 2014, 134, 1–9. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, Q.; Zhang, X.; Jia, S.; Gan, Y.; Zhang, Y.; Shi, J.; Yuan, J. Hologram quantitative structure–activity relationship and topomer comparative molecular-field analysis to predict the affinities of azo dyes for cellulose fibers. Dyes Pigments 2018, 153, 35–43. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A. In silico enhancement of azo dye adsorption affinity for cellulose fibre through mechanistic interpretation under guidance of QSPR models using Monte Carlo method with index of ideality correlation. SAR QSAR Environ. Res. 2020, 31, 697–715. [Google Scholar] [CrossRef]
- Oakes, J.; Dixon, S. Adsorption of dyes to cotton and inhibition by polymers. Coloration Technol. 2003, 119, 140–149. [Google Scholar]
- Zhang, G.; Zhang, S. Quantitative structure-activity relationship in the photodegradation of azo dyes. J. Environ. Sci. 2020, 90, 41–50. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Li, Y.; Ge, F.; Zhu, R. Quantitative structure–property relationship (QSPR) study for the degradation of dye wastewater by Mo–Zn–Al–O catalyst. J. Mol. Liq. 2016, 215, 461–466. [Google Scholar] [CrossRef]
- Li, B.; Dong, Y.; Ding, Z. Heterogeneous Fenton degradation of azo dyes catalyzed by modified polyacrylonitrile fiber Fe complexes: QSPR (quantitative structure property relationship) study. J. Environ. Sci. 2013, 25, 1469–1476. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Mendoza, D.; Yoshimura, C. Application of Quantitative Structure–Property Relationship Predictive Models to Water Treatment: A Critical Review. ACS EST Water 2021, 1, 498–517. [Google Scholar] [CrossRef]
- Umbuzeiro, G.d.A.; Albuquerque, A.F.; Vacchi, F.I.; Szymczyk, M.; Sui, X.; Aalizadeh, R.; von der Ohe, P.C.; Thomaidis, N.S.; Vinueza, N.R.; Freeman, H.S. Towards a reliable prediction of the aquatic toxicity of dyes. Environ. Sci. Eur. 2019, 31, 76. [Google Scholar] [CrossRef]
- Funar-Timofei, S.; Ilia Gheorghe. QSAR Modeling of Dye Ecotoxicity. In Ecotoxicological QSARs; Roy, K., Ed.; Springer: New York, NY, USA, 2020; pp. 405–436. ISBN 978-1-0716-0149-5. [Google Scholar]
- Ecotoxicological QSARs; Roy, K. (Ed.) Springer: New York, NY, USA, 2020; ISBN 978-1-0716-0149-5. [Google Scholar]
- Oakes, J.; Gratton, P.; Clark, R.; Wilkes, I. Kinetic investigation of the oxidation of substituted arylazonaphthol dyes by hydrogen peroxide in alkaline solution. J. Chem. Soc. Perkin Trans. 1998, 2, 2569–2576. [Google Scholar] [CrossRef]
- Oakes, J. Principles of colour loss. Part 1: Mechanisms of oxidation of model azo dyes by detergent bleaches. Rev. Prog. Color. Relat. Top. 2002, 32, 63–79. [Google Scholar] [CrossRef]
- Oakes, J. Principles of colour loss. Part 2: Degradation of azo dyes by electron transfer, catalysis and radical routes. Rev. Prog. Color. Relat. Top. 2003, 33, 72–84. [Google Scholar] [CrossRef]
- Chudgar, R.J.; Oakes, J. Dyes, Azo. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; ISBN 9780471238966. [Google Scholar]
- Oakes, J.; Dixon, S. Adsorption of dyes to cotton and inhibition by surfactants, polymers and surfactant–polymer mixtures. Coloration Technol. 2003, 119, 315–323. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Salihu, G. The role of auxiliaries in the immersion dyeing of textile fibres part 2: Analysis of conventional models that describe the manner by which inorganic electrolytes promote direct dye uptake on cellulosic fibres. Dyes Pigments 2019, 161, 531–545. [Google Scholar] [CrossRef] [Green Version]
- Burkinshaw, S.M.; Salihu, G. The role of auxiliaries in the immersion dyeing of textile fibres: Part 3 theoretical model to describe the role of inorganic electrolytes used in dyeing cellulosic fibres with direct dyes. Dyes Pigments 2019, 161, 546–564. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Salihu, G. The role of auxiliaries in the immersion dyeing of textile fibres: Part 4 theoretical model to describe the role of liquor ratio in dyeing cellulosic fibres with direct dyes in the absence and presence of inorganic electrolyte. Dyes Pigments 2019, 161, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Burkinshaw, S.M.; Salihu, G. The role of auxiliaries in the immersion dyeing of textile fibres: Part 5 practical aspects of the role of inorganic electrolytes in dyeing cellulosic fibres with direct dyes. Dyes Pigments 2019, 161, 581–594. [Google Scholar] [CrossRef] [Green Version]
- Ferus-Comelo, M. An analysis of the substantivity of hydrolysed reactive dyes and its implication for rinsing processes. Coloration Technol. 2013, 129, 24–31. [Google Scholar] [CrossRef]
- Bredereck, K.; Schumacher, C. Structure reactivity correlations of azo reactive dyes based on H-acid. III. Dye degradation by peroxide. Dyes Pigments 1993, 23, 121–133. [Google Scholar] [CrossRef]
- Bredereck, K.; Schumacher, C. Structure reactivity correlations of azo reactive dyes based on H-acid. IV. Investigations into the light fastness in the dry state, in the wet state, and in presence of perspiration. Dyes Pigments 1993, 23, 135–147. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Karelson, M.; Lobanov, V.S. QSPR as a means of predicting and understanding chemical and physical properties in terms of structure. Pure Appl. Chem. 1997, 69, 245–248. [Google Scholar] [CrossRef]
- Cherkasov, A.; Muratov, E.N.; Fourches, D.; Varnek, A.; Baskin, I.I.; Cronin, M.; Dearden, J.; Gramatica, P.; Martin, Y.C.; Todeschini, R.; et al. QSAR modeling: Where have you been? Where are you going to? J. Med. Chem. 2014, 57, 4977–5010. [Google Scholar] [CrossRef] [Green Version]
- Tetko, I.V.; Engkvist, O.; Koch, U.; Reymond, J.-L.; Chen, H. BIGCHEM: Challenges and opportunities for big data analysis in chemistry. Mol. Inform. 2016, 35, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.V.; Filimonov, D.; Poroikov, V.; Oprea, T.I.; Baskin, I.I.; Varnek, A.; Roitberg, A.; et al. QSAR without borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Xu, X.; Liu, H.; Cordeiro, M.N.D.S. Review of quantitative structure-activity/property relationship studies of dyes: Recent advances and perspectives. Coloration Technol. 2013, 129, 173–186. [Google Scholar] [CrossRef]
- Yu, X.; Wang, H. Support vector machine classification model for color fastness to ironing of vat dyes. Text. Res. J. 2021, 91, 1889–1899. [Google Scholar] [CrossRef]
- Hilal, S.H.; Karichhoff, S.W.; Careira, L.A. Prediction of Chemical Reactivity Parameters and Physical Properties of Organic Compounds from Molecular Structure Using; SPARC: Research Triangle Park, NC, USA, 2003. [Google Scholar]
- CODESSA PRO: COmprehensive DEscriptors for Structural and Statistical Analysis. Available online: www.codessa-pro.com (accessed on 29 April 2022).
- DRAGON. Available online: https://chm.kode-solutions.net/products_dragon.php (accessed on 29 April 2022).
- Baskin, I.I.; Palyulin, V.A.; Zefirov, N.S. A Neural Device for Searching Direct Correlations between Structures and Properties of Chemical Compounds. J. Chem. Inf. Comput. Sci. 1997, 37, 715–721. [Google Scholar] [CrossRef]
- Baskin, I.I.; Ait, A.O.; Halberstam, N.M.; Palyulin, V.A.; Alfimov, M.V.; Zefirov, N.S. Application of methodology of artificial neural networks for predicting the properties of sophisticated molecular systems: Prediction of the long-wave absorption band position for symmetric cyanine dyes. In Doklady Physical Chemistry; Consultants Bureau: New York, NY, USA, 1997; Volume 357, pp. 353–355. [Google Scholar]
- Baskin, I.I.; Keschtova, S.V.; Palyulin, V.A.; Zefirov, N.S. Combining Molecular Modelling with the Use of Artificial Neural Networks as an Approach to Predicting Substituent Constants and Bioactivity. In Molecular Modeling and Prediction of Bioactivity; Gundertofte, K., Jørgensen, F.S., Eds.; Springer: Boston, MA, USA, 1999. [Google Scholar]
- Baskin, I.; Varnek, A. Fragment Descriptors in SAR/QSAR/QSPR Studies, Molecular Similarity Analysis and in Virtual Screening. In Chemoinformatics Approaches to Virtual Screening; Varnek, A., Tropsha, A., Eds.; RSC Publishing: Cambridge, UK, 2008; pp. 1–43. ISBN 9780854041442. [Google Scholar]
- CORAL Software/Databases. Available online: www.insilico.eu/coral/ (accessed on 29 April 2022).
- Sushko, I.; Novotarskyi, S.; Körner, R.; Pandey, A.K.; Rupp, M.; Teetz, W.; Brandmaier, S.; Abdelaziz, A.; Prokopenko, V.V.; Tanchuk, V.Y.; et al. Online chemical modeling environment (OCHEM): Web platform for data storage, model development and publishing of chemical information. J. Comput. Aided Mol. Des. 2011, 25, 533–554. [Google Scholar] [CrossRef] [Green Version]
- ChemAxon: Free Academic License for JChem. Available online: www.chemaxon.com (accessed on 12 January 2017).
- Telegin, F.Y.; Ran, J.H.; Morshed, M.; Pervez, M.N.; Sun, L.; Zhang, C.; Priazhinikova, V.G. Structure and Properties of Dyes in Coloration of Textiles. Application of Fragment Approach. KEM 2016, 703, 261–266. [Google Scholar] [CrossRef]
- Telegin, F.Y.; Ran, J.; Pryazhnikova, V.G. Colour, colour fastness and chemical constitution of anionic azo dyes: Chemoinformatics analysis. In The Scientific Notes of Color Society of Russia. First Russian Congress on Color; Schindler, V., Griber, M., Yulia, A., Eds.; Smolensk State University: Smolensk, Russia, 2019; Volume 1, pp. 97–102. [Google Scholar]
- Telegin, F.Y.; Marfin, Y.S. Polarity of borondipyrrins and their structure: Semiempirical and chemoinformatics analysis. Zh. Neorg. Khimii/J. Inorg. Chem. Russ. 2022, 67, 384–396. [Google Scholar] [CrossRef]
- Grecu, R.; Pieroni, M. Quantitative relationships between chemical structure and technical properties of arylazoindole sulphonic acid dyes. Dyes Pigments 1981, 2, 305–318. [Google Scholar] [CrossRef]
- Carpignano, R.; Barni, E.; Di Modica, G.; Grecu, R.; Bottaccio, G. Quantitative relationships between chemical structure and technical properties of 4-aminoazobenzene sulphonic acid dyes. Dyes Pigments 1983, 4, 195–211. [Google Scholar] [CrossRef]
- Carpignano, R.; Savarino, P.; Barni, E.; Clementi, S.; Giulietti, G. Quantitative Structure–Property relationships study of azo dyes using partial least squares analysis in latent variables (PLS). Dyes Pigments 1985, 6, 189–212. [Google Scholar] [CrossRef]
- Barni, E.; Savarino, P.; di Modica, G.; Carpignano, R. Monoazo Dyes for Polyamide Derived from 4-Alkylamido-Shydroxybenzoic Acids. Dyes Pigments 1984, 5, 15–36. [Google Scholar] [CrossRef]
- Kraska, J.; Blus, K. Synthesis and properties of acid dye derivatives of arylsulphonanilides. Dyes Pigments 1984, 5, 415–430. [Google Scholar] [CrossRef]
- Blus, K. Synthesis and Properties of Acid Dyes Derived from 3-Hydroxy-2-naphthanilides. Dyes Pigments 1992, 18, 163–177. [Google Scholar] [CrossRef]
- Blus, K.; Kraska, J. The influence of arylamide groups on the properties of acid dyes. Dyes Pigments 1993, 22, 163–172. [Google Scholar] [CrossRef]
- Blus, K. Synthesis and properties of acid dyes derived from 1-phenyl-3-methyl-5-pyrazolone. Dyes Pigments 1992, 20, 53–65. [Google Scholar] [CrossRef]
- Blus, K. Photo-stability of Acid Dyes, Derivatives of 1-phenyl-3-methylpyrazol-5-one in Polyamide Fibers. Fibres Text. East. Eur. 2005, 13, 70–74. [Google Scholar]
- Kraska, J.; Blus, K. Synthesis and properties of disazo dyes derived from 4-amino-2′-nitrodiphenylamine. Dyes Pigments 1996, 31, 97–109. [Google Scholar] [CrossRef]
- Chao, Y.C.; Yang, S.S. Disazo direct dyes derived from 4,4′-diamino derivatives of benzanilide, diphenylamine-2-sulfonic acid and stilbene-2,2′-disulfonic acid. Dyes Pigments 1995, 29, 131–138. [Google Scholar] [CrossRef]
- Chao, Y.C.; Pan, Y.I. Trisazo dyes derived from 4,4′-diaminodiphenylsulphide: Substitutes for C.I. Direct black 38 and C.I. Direct Green 1. Dyes Pigments 1996, 31, 253–262. [Google Scholar] [CrossRef]
- Practical Applications of Quantitative Structure-Activity Relationships (QSAR) in Environmental Chemistry and Toxicology; Karcher, W.; Devillers, J. (Eds.) Springer: Dordrecht, The Netherlands, 1990; ISBN 0792308271. [Google Scholar]
- Telegin, F.Y.; Khaylenko, E.S.; Telegin, P.F. Quantitative relationships for design of disperse dyes of high technical properties. In Proceedings of the 21th IFATCC Congress, New Horizons in Textile Finishing, Barcelona, Spain, 6–9 May 2008. [Google Scholar]
- Telegin, F.Y. Structure and properties of dyes in theory and practice of coloration. Des. Mater. Technol. 2009, 11, 163–167. [Google Scholar]


| Software | Number of Descriptors |
|---|---|
| SPARC, by L.A. Carreira et al., ARChem, USA, 1994 [60] | not specified |
| CODESSA, by A.R. Katritzky, M. Karelson, R. Petrukhin, University of Florida USA, 2001-2005 [61] | about 1500 |
| DRAGON, by Kode Chemoinformatics, R. Todecini et al., Pisa, Italy, 1994 [62] | 5270 |
| NASAWIN, by I.I. Baskin et al., Moscow State University, Russia, 1995 [63,64,65,66] | unlimited |
| CORAL, Mario Negri Institute, E. Benfenati, A.A. Toropov, A.P. Toropova, Italy, 2010 [67] | unlimited |
| OCHEM, I.I. Tetko, et al., International project, 2011 [68] | unlimited |
| Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment |
|---|---|---|
| Coeff0 = 3.161944, T-stat = 18.3618 | Coeff1 = 0.414375, T-stat = 8.8966 LF-W-1 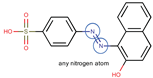 | Coeff2 = −0.604511, T-stat = −4.3458 LF-W-2 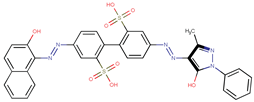 |
| Coeff3 = −1.037494, T-stat = −6.3766 LF-W-3 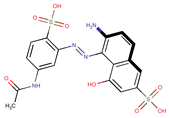 | Coeff4 = −0.287592, T-stat = −3.3646 LF-W-4 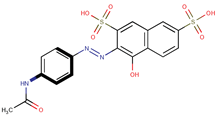 | Coeff5 = 0.685249, T-stat = 4.1154 LF-W-5 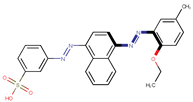 |
| Coeff6 = 0.668728, T-stat = 7.1565 LF-W-6 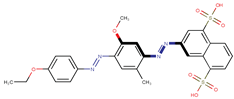 | Coeff7 = 0.288798, T-stat = 4.1107 LF-W-7 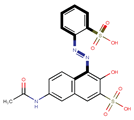 | Coeff8 = −0.492495, T-stat = −3.5045 LF-W-8 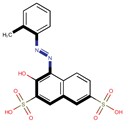 |
| Coeff9 = −0.376856, T-stat = −3.9398 LF-W-9 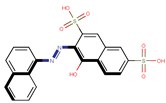 | Coeff10 = −0.55531, T-stat = −6.5707 LF-W-10 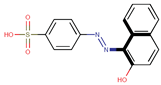 |
| Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment |
|---|---|---|
| Coeff0 = −0.714316, T-stat = −2.9669 | Coeff1 = 0.065831, T-stat = 10.1430 OB-W-1  | Coeff2 = −0.938693, T-stat = −4.1047 OB-W-2  |
| Coeff3 = 1.477873, T-stat = 4.8356 OB-W-3 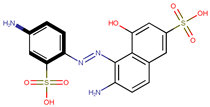 | Coeff4 = 2.706476, T-stat = 7.3958 OB-W-4 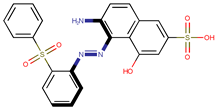 | Coeff5 = −1.376763, T-stat = −5.2326 OB-W-5 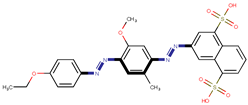 |
| Coeff6 = 0.101659, T-stat = 4.6905 OB-W-6 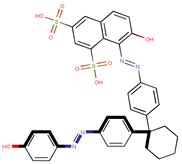 | Coeff7 = 1.20228, T-stat = 6.8330 OB-W-7 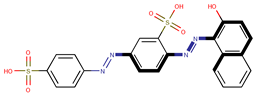 | Coeff8 = 0.492824, T-stat = 5.1165 OB-W-8 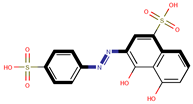 |
| Coeff9 =−1.134224, T-stat = −5.6996 OB-W-9 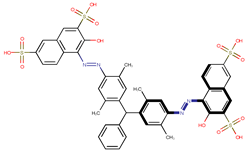 | Coeff10 = 2.800875, T-stat = 7.3944 OB-W-10 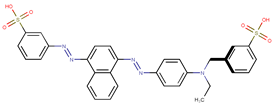 |
| Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment |
|---|---|---|
| Coeff0 = 0.963902, T-stat = 4.9380 | Coeff1 = 0.058948, T-stat = 12.5825 WF-W-1 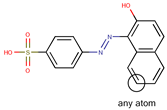 | Coeff2 = −0.309573, T-stat = −3.9703 WF-W-2 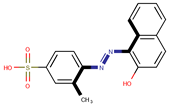 |
| Coeff3 = −0.335361, T-stat = −3.8736 WF-W-3  | Coeff4 = −0.256796, T-stat = −3.8610 WF-W-4 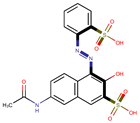 | Coeff5 = 0.199498, T-stat3 = 3.3687 WF-W-5 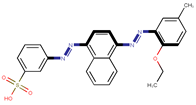 |
| Coeff6 = 0.511187, T-stat = 4.1279 WF-W-6  | Coeff7 = −0.532824, T-stat = −3.5236 WF-W-7 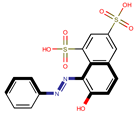 | Coeff8 = −0.938293, T-stat = −3.4897 WF-W-8  |
| Coeff9 = 0.407356, T-stat = 6.1331 WF-W-9 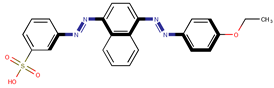 | Coeff10 = −0.0876, T-stat = −4.8110 WF-W-10 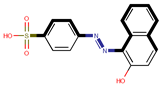 |
| Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment |
|---|---|---|
| Coeff0 = 4.930911, T-stat = 30.6418 | Coeff1 = 0.43608, T-stat = 7.3894 LF-PA-1 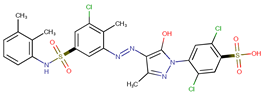 | Coeff2 = −2.4247, T-stat =−17.7498 LF-PA-2 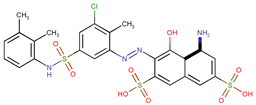 |
| Coeff3 = 0.85488, T-stat = 10.6573 LF-PA-3 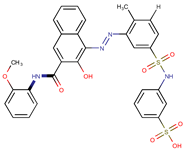 | Coeff4 = −0.31445, T-stat = −7.2016 LF-PA-4 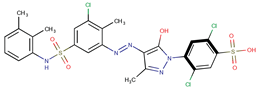 | Coeff5 = 0.758169, T-stat = 8.1588 LF-PA-5 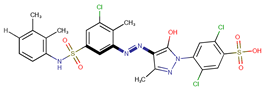 |
| Coeff6 = −1.66501, T-stat = −19.3869 LF-PA-6 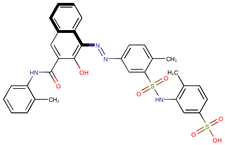 | Coeff7 = −0.77779, T-stat = −9.5075 LF-PA-7 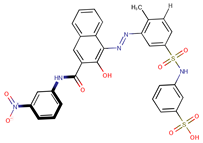 | Coeff8 = −0.42405, T-stat = −5.5203 LF-PA-8 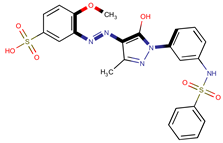 |
| Coeff9 = 0.416039, T-stat = 7.6620 LF-PA-9 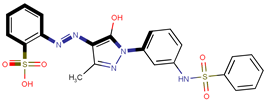 | Coeff10 = 0.552282, T-stat = 4.6392 LF-PA-10 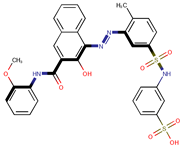 |
| Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment |
|---|---|---|
| N = 225, R = 0.9979, R_adj = 0.9978, F = 5095, s = 0.0338, RMSE_t = 0.00330, MAE_t = 0.0259 Coeff0 = 0.824114, T-stat = 70.9156 Coeff1(C=1%) = 0.421999, T-stat = 53.6620 Coeff2 (C=0.5%) = −0.21474, T-stat = −27.3063 Coeff3 (C=0.1%) = −0.88427, T-stat = −112.4455 | ||
| Coeff4 = −0.13195, T-stat = −11.8450 A-C-4  | Coeff5 = −0.01797, T-stat = −5.4787 A-C-5 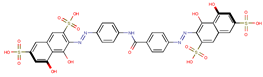 | Coeff6 = −0.00622, T-stat = −6.4462 A-C-6  |
| Coeff7 = −0.01134, T-stat = −3.4668 A-C-7  | Coeff8 = 0.002434, T-stat = 5.8381 A-C-8 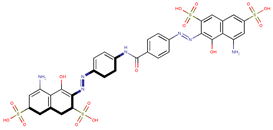 | Coeff9 = −0.06541, T-stat = −4.0627 A-C-9 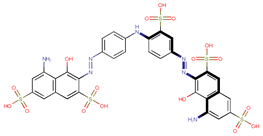 |
| Coeff10 = −0.01152, T-stat = −6.5432 A-C-10 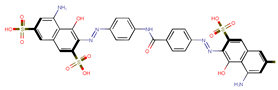 | ||
| Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment | Regression Coefficient, Molecular Fragment |
|---|---|---|
| N = 22, R = 0.9814, R_adj = 0.9726, F = 65.43, s = 0.111, RMSE_t = 0.0917, MAE_t = 0.0774 Coeff0 = −2.2095; T-stat = −30.6200 | ||
Coeff1 = −0.0509; T-stat = −3.4380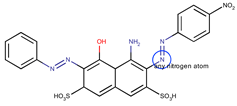 | Coeff2 = −0.0672; T-stat = −5.7746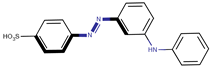 | Coeff3 = −0.1204; T-stat = −4.6107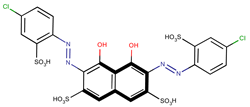 |
Coeff4 = −0.2012; T-stat = −5.9598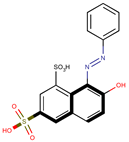 | Coeff5 = −0.1825; T-stat = −3.3489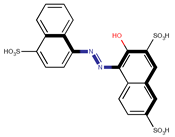 | Coeff6 = 0.5413; T-stat = 137513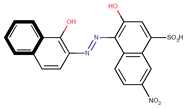 |
| The Primary or Substituted Amino Group | Azo-Bond and the Primary or Substituted Amino Group | Aromatic Chain and Nitrogen of Azo Group |
|---|---|---|
| Coeff3 = −1.037494, T-stat = −6.3766 LF-W-3, Table 2 | Coeff10 = −0.55531, T-stat = −6.5707 LF-W-10, Table 2 | |
| Coeff3 = 1.477873, T-stat = 4.8356 OB-W-3, Table 3 | Coeff4 = 2.706476, T-stat = 7.3958 OB-W-4, Table 3 | |
| Coeff2 = −2.4247, T-stat = −17.7498 LF-PA-2, Table 5 | Coeff8 = −0.42405, T-stat = −5.5203 LF-PA-8, Table 5 | Coeff6 = −1.66501, T-stat = −19.3869 LF-PA-6, Table 5 |
| Azo Group in a Chain of Conjugated Double Bonds | Azo Group in a Chain of Conjugated Double Bonds and Sulphonic Group | Azo-Bond in a Chain of Conjugated Double Bonds and Carbamide Group |
|---|---|---|
| Coeff7 = 0.288798, T-stat = 4.1107 LF-W-7, Table 2 | ||
| Coeff9 = −1.134224, T-stat = −5.6996 OB-W-9, Table 3 | ||
| Coeff5 = 0.758169, T-stat = 8.1588 LF-PA-5, Table 5 | Coeff9 = 0.416039, T-stat = 7.6620 LF-PA-9, Table 5 | Coeff10 = 0.552282, T-stat = 4.6392 LF-PA-10, Table 5 |
| Two Azo-Bonds in a Chain of Conjugated Double Bonds | Azo-Bond in a Chain of Conjugated Double Bonds and Terminal Hydrophobic Terminal Group |
|---|---|
| Coeff5 = 0.199498, T-stat = 3.3687 WF-W-5, Table 4 | Coeff6 = 0.511187, T-stat = 4.1279 WF-W-6, Table 4 |
| Coeff8 = 0.002434, T-stat = 5.8381 A-C-8, Table 6 |
| Sulphonic Group | Azo-Bond and Hydrophilic Terminal Group |
|---|---|
| Coeff3 = −0.335361, T-stat = −3.8736 WF-W-3, Table 4 | Coeff7 = −0.532824, T-stat = −3.5236 WF-W-7, Table 4 |
| Coeff10 = −0.01152, T-stat = −6.5432 A-C-10, Table 6 | Coeff9 = −0.06541, T-stat = −4.0627 A-C-9, Table 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, J.; Pryazhnikova, V.G.; Telegin, F.Y. Chemoinformatics Analysis of the Colour Fastness Properties of Acid and Direct Dyes in Textile Coloration. Colorants 2022, 1, 280-297. https://doi.org/10.3390/colorants1030017
Ran J, Pryazhnikova VG, Telegin FY. Chemoinformatics Analysis of the Colour Fastness Properties of Acid and Direct Dyes in Textile Coloration. Colorants. 2022; 1(3):280-297. https://doi.org/10.3390/colorants1030017
Chicago/Turabian StyleRan, Jianhua, Victoria G. Pryazhnikova, and Felix Y. Telegin. 2022. "Chemoinformatics Analysis of the Colour Fastness Properties of Acid and Direct Dyes in Textile Coloration" Colorants 1, no. 3: 280-297. https://doi.org/10.3390/colorants1030017
APA StyleRan, J., Pryazhnikova, V. G., & Telegin, F. Y. (2022). Chemoinformatics Analysis of the Colour Fastness Properties of Acid and Direct Dyes in Textile Coloration. Colorants, 1(3), 280-297. https://doi.org/10.3390/colorants1030017






