Effect of GNPs on the Piezoresistive, Electrical and Mechanical Properties of PHA and PLA Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Production
2.3. Sample Testing
3. Results
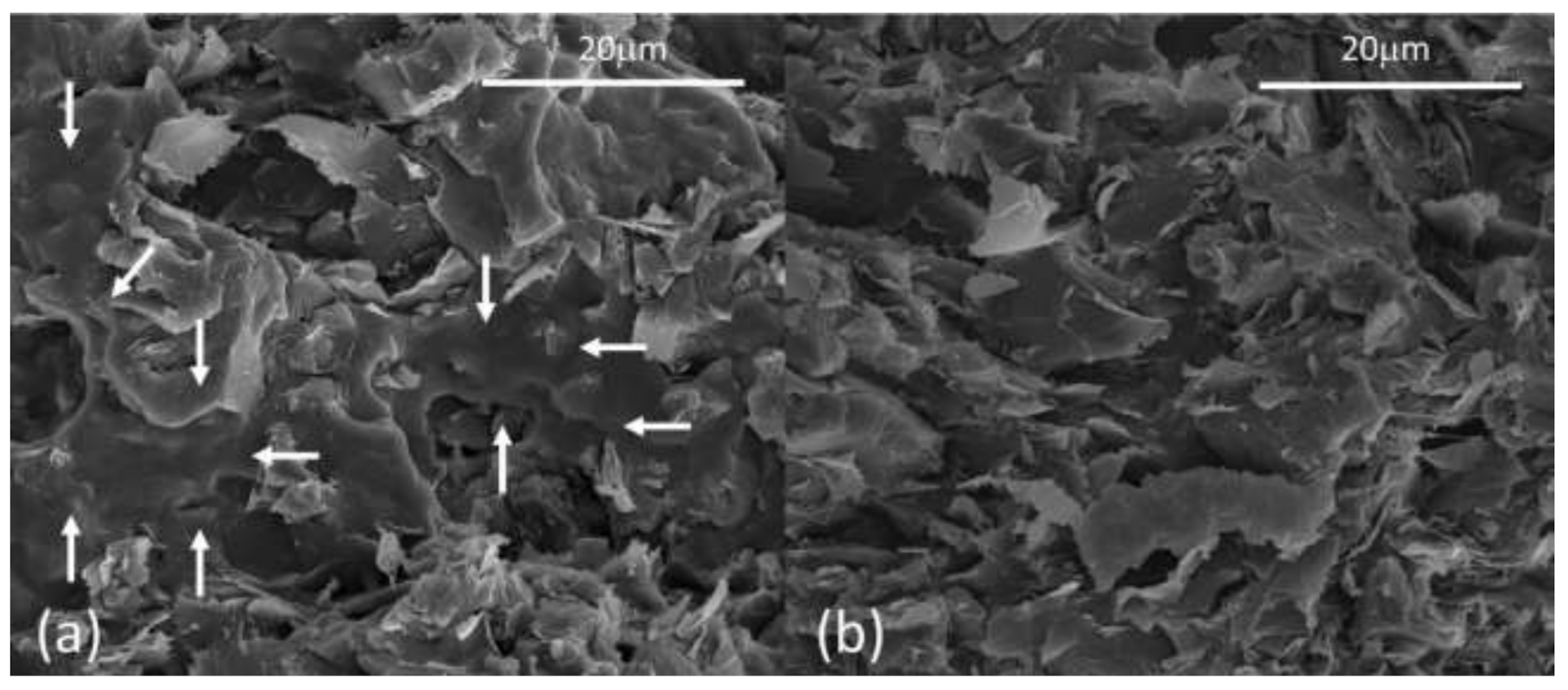
3.1. GNPs Dispersion
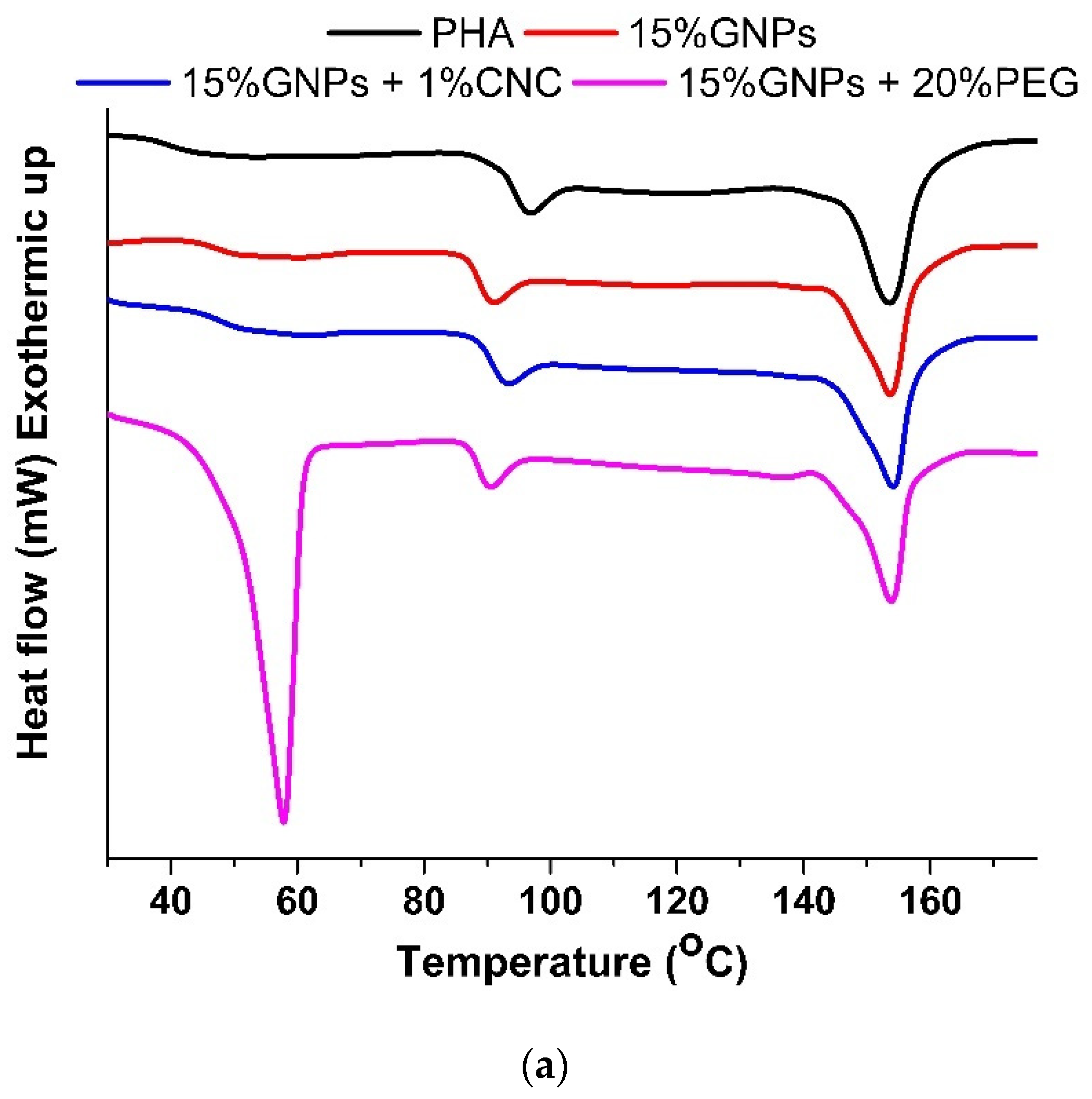
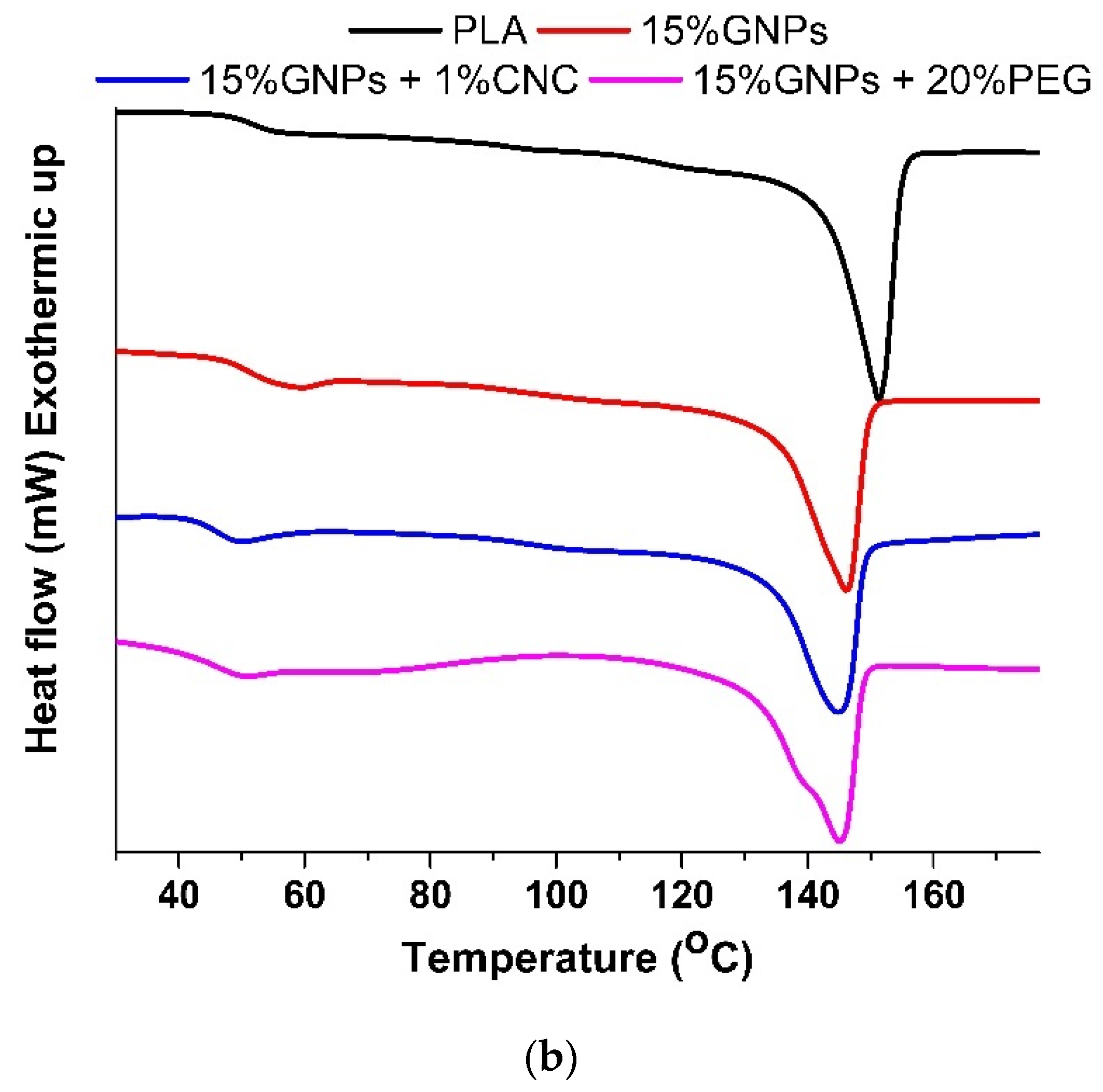
3.2. Differential Scanning Calorimetry
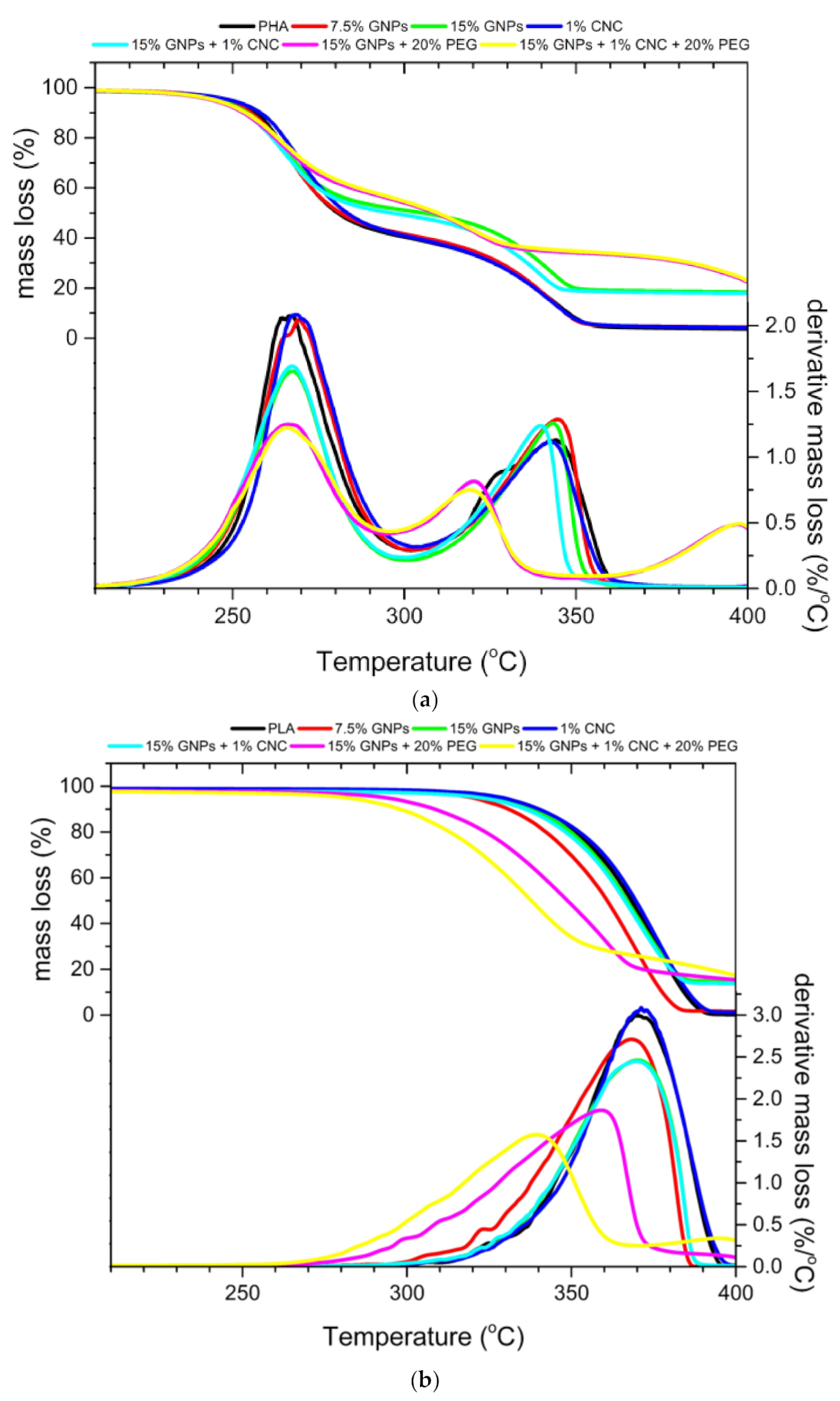
3.3. Thermogravimetric Analysis
3.4. Tensile Tests
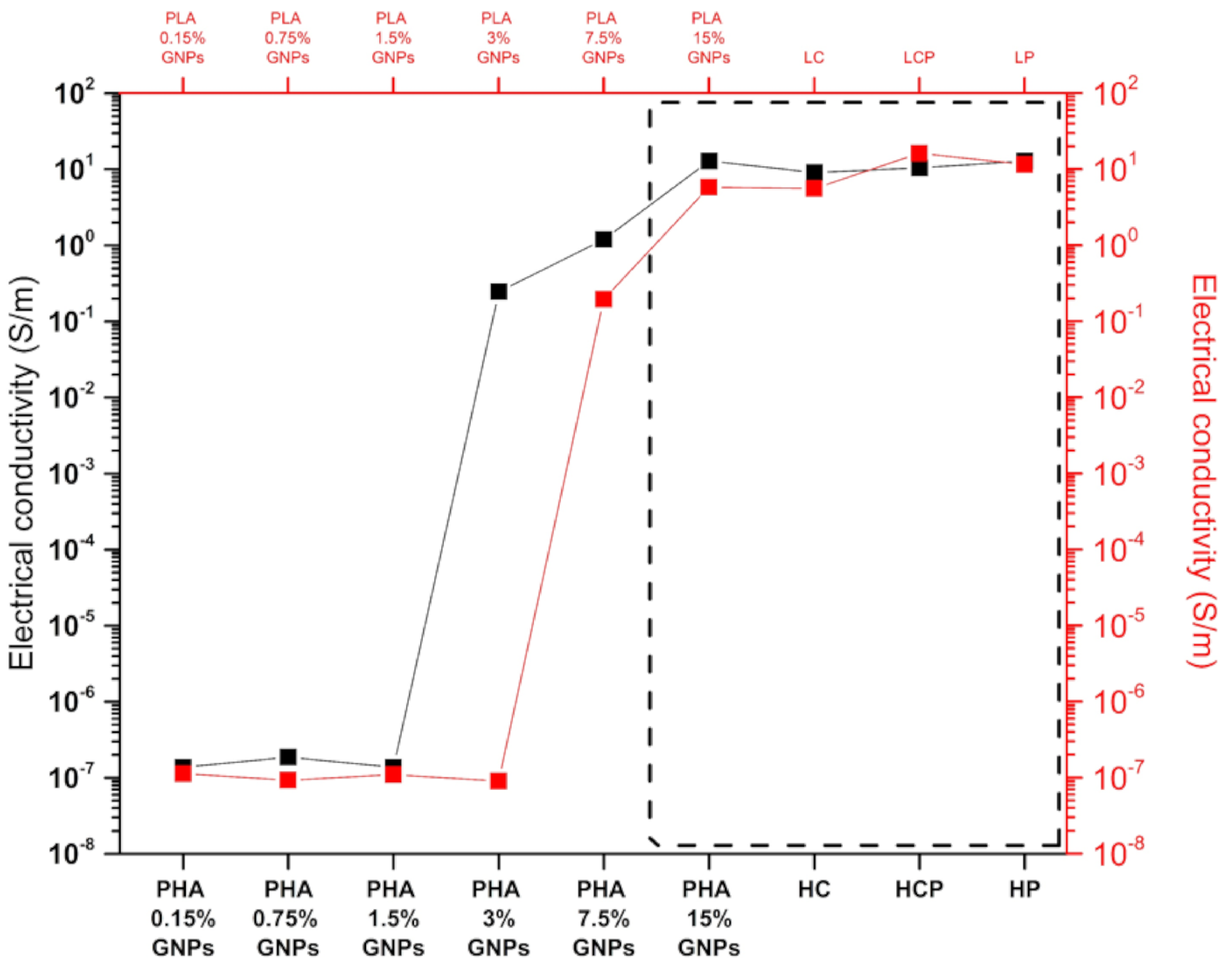
3.5. Electrical Conductivity
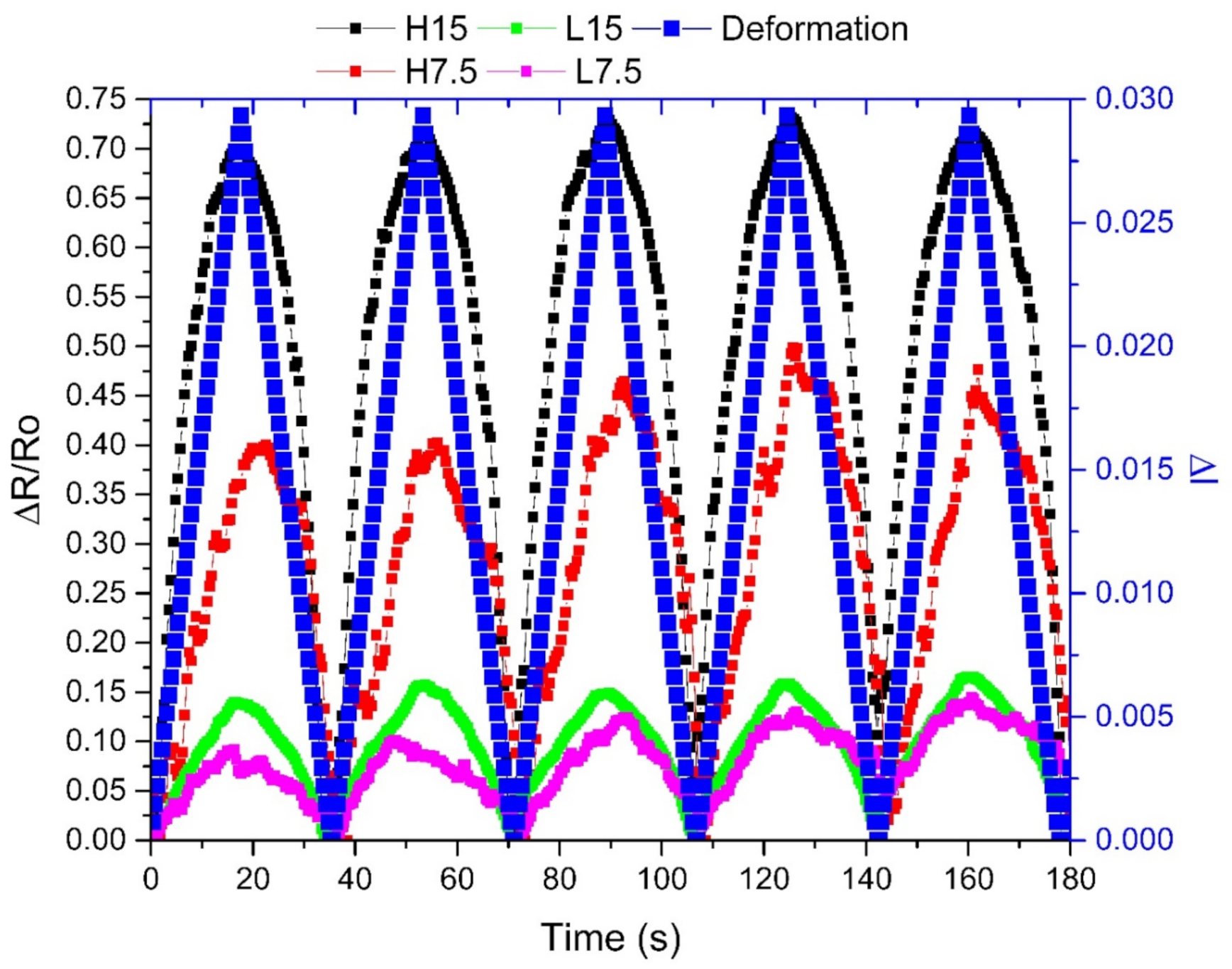
3.6. Piezoresistive Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Díez-Pascual, A.M. Synthesis and Applications of Biopolymer Composites. Int. J. Mol. Sci. 2019, 20, 2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambabu, G.; Bhat, S.D.; Figueiredo, F.M.L. Carbon Nanocomposite Membrane Electrolytes for Direct Methanol Fuel Cells—A Concise Review. Nanomaterials 2019, 9, 1292. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhang, Y.; Zhou, R.; Meng, L.; Chen, T.; Mai, W.; Pan, C. Recent Advances of Wearable and Flexible Piezoresistivity Pressure Sensor Devices and Its Future Prospects. J. Mater. 2020, 6, 86–101. [Google Scholar] [CrossRef]
- Jang, D.; Yoon, H.N.; Nam, I.W.; Lee, H.K. Effect of Carbonyl Iron Powder Incorporation on the Piezoresistive Sensing Characteristics of CNT-Based Polymeric Sensor. Compos. Struct. 2020, 244, 112260. [Google Scholar] [CrossRef]
- Chang, X.; Sun, S.; Sun, S.; Liu, T.; Xiong, X.; Lei, Y.; Dong, L.; Yin, Y. ZnO Nanorods/Carbon Black-Based Flexible Strain Sensor for Detecting Human Motions. J. Alloys Compd. 2018, 738, 111–117. [Google Scholar] [CrossRef]
- Morteza, A.; Aekachan, P.; Sangjun, L.; Seunghwa, R.; Inkyu, P. Highly Stretchable and Sensitive Strain Sensor Based on Silver Nanowire-Elastomer Nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar]
- Park, J.J.; Hyun, W.J.; Mun, S.C.; Park, Y.T.; Park, O.O. Highly Stretchable and Wearable Graphene Strain Sensors with Controllable Sensitivity for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2015, 7, 6317–6324. [Google Scholar] [CrossRef] [PubMed]
- Salski, B.; Gwarek, W.; Korpas, P.; Reszewicz, S.; Chong, A.Y.B.; Theodorakeas, P.; Hatziioannidis, I.; Kappatos, V.; Selcuk, C.; Gan, T.H.; et al. Non-Destructive Testing of Carbon-Fibre-Reinforced Polymer Materials with a Radio-Frequency Inductive Sensor. Compos. Struct. 2015, 122, 104–112. [Google Scholar] [CrossRef]
- Sun, Y.W.; Liu, W.; Hernandez, I.; Gonzalez, J.; Rodriguez, F.; Dunstan, D.J.; Humphreys, C.J. 3D Strain in 2D Materials: To What Extent Is Monolayer Graphene Graphite? Phys. Rev. Lett. 2019, 123, 135501. [Google Scholar] [CrossRef] [Green Version]
- Rafiei, Z.; Zahedi-Dizaji, S.M.; Kang, A.K. Two-Dimensional Nanomaterials. In Nanostructures; Intech Open: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Peres, N.M.R. Colloquium: The Transport Properties of Graphene: An Introduction. Rev. Mod. Phys. 2010, 82, 2673–2700. [Google Scholar] [CrossRef] [Green Version]
- Trikkaliotis, D.G.; Mitropoulos, A.C.; Kyzas, G.Z. Low-Cost Route for Top-down Synthesis of over- and Low-Oxidized Graphene Oxide. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 600, 124928. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Ceriotti, G.; Metzger, A.; Kim, N.D.; Tour, J.M. Chemical Mass Production of Graphene Nanoplatelets in ∼100% Yield. ACS Nano 2016, 10, 274–279. [Google Scholar] [CrossRef]
- Cataldi, P.; Athanassiou, A.; Bayer, I.S. Graphene Nanoplatelets-Based Advanced Materials and Recent Progress in Sustainable Applications. Appl. Sci. 2018, 8, 1438. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.D.L. A Review of Exfoliated Graphite. J. Mater. Sci. 2015, 51, 554–568. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Li, C.; Qin, C.; Ye, M. Synthesis of Amphiphilic Graphene Nanoplatelets. Small 2009, 5, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.P.; Sanivada, U.K.; Bessa, J.; Cunha, F.; Fangueiro, R. A Review of Multiple Scale Fibrous and Composite Systems for Heating Applications. Molecules 2021, 26, 3686. [Google Scholar] [CrossRef] [PubMed]
- Sanivada, U.K.; MarMol, G.; Fangueiro, R. Hierarchical Vegetal Fiber Reinforced Composites. In Vegetable Fiber Composites and their Technological Applications; Jawaid, M., Khan, A., Eds.; Springer Nature: Singapore, 2021; pp. 379–412. [Google Scholar] [CrossRef]
- Shekar, H.S.S.; Ramachandra, M. Green Composites: A Review. Mater. Today Proc. 2018, 5, 2518–2526. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant Fibre Based Bio-Composites: Sustainable and Renewable Green Materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Christian, S.J.; Billington, S.L. Mechanical Response of PHB- and Cellulose Acetate Natural Fiber-Reinforced Composites for Construction Applications. Compos. Part B Eng. 2011, 42, 1920–1928. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, L.; Nie, J.; Wang, H.; Yang, D. Study of Poly(Lactic Acid)/Natural Fiber Composites. J. Appl. Polym. Sci. 2012, 125, E526–E533. [Google Scholar] [CrossRef]
- Sanivada, U.K.; MarMol, G.; Brito, F.P.; Fangueiro, R. PLA Composites Reinforced with Flax and Jute Fibers—A Review of Recent Trends, Processing Parameters and Mechanical Properties. Polymers 2020, 12, 2373. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Jawaid, M.; Al-Othman, O. An Overview on Polylactic Acid, Its Cellulosic Composites and Applications. Curr. Org. Synth. 2017, 14, 156–170. [Google Scholar] [CrossRef] [Green Version]
- Mathuriya, A.S.; Yakhmi, J.V. Polyhydroxyalkanoates: Biodegradable Plastics and Their Applications. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer: Cham, Switzerland, 2019; Volume 4, pp. 2873–2900. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of Synthesis, Characteristics, Processing and Potential Applications in Packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.-Q.; Chen, X.-Y.; Wu, F.-Q.; Chen, J.-C. Polyhydroxyalkanoates (PHA) toward Cost Competitiveness and Functionality. Adv. Ind. Eng. Polym. Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P.; Torre, L.; Fortunati, E.; Puglia, D. Effect of Cellulose Nanocrystals and Bacterial Cellulose on Disintegrability in Composting Conditions of Plasticized PHB Nanocomposites. Polymers 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigotti, D.; Checchetto, R.; Tarter, S.; Caretti, D.; Rizzuto, M.; Fambri, L.; Pegoretti, A. Polylactic Acid-Lauryl Functionalized Nanocellulose Nanocomposites: Microstructural, Thermo-Mechanical and Gas Transport Properties. Express Polym. Lett. 2019, 13, 858–876. [Google Scholar] [CrossRef]
- Xu, P.; Yang, W.; Niu, D.; Yu, M.; Du, M.; Dong, W.; Chen, M.; Jan Lemstra, P.; Ma, P. Multifunctional and Robust Polyhydroxyalkanoate Nanocomposites with Superior Gas Barrier, Heat Resistant and Inherent Antibacterial Performances. Chem. Eng. J. 2020, 382, 122864. [Google Scholar] [CrossRef]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; McWilliams, J.L.; Hamon, M.A.; Haddon, R.C. Solution Properties of Graphite and Graphene. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Kim, K.T.; Lee, J.U.; Jo, W.H. Facile Method to Functionalize Graphene Oxide and Its Application to Poly(Ethylene Terephthalate)/Graphene Composite. ACS Appl. Mater. Interfaces 2012, 4, 4184–4191. [Google Scholar] [CrossRef]
- Mukheem, A.; Muthoosamy, K.; Manickam, S.; Sudesh, K.; Shahabuddin, S.; Saidur, R.; Akbar, N.; Sridewi, N. Fabrication and Characterization of an Electrospun PHA/Graphene Silver Nanocomposite Scaffold for Antibacterial Applications. Materials 2018, 11, 1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Wu, L.P.; Chen, G.Q. Synthesis and Characterization of Electroconductive PHA- Graft-Graphene Nanocomposites. Biomacromolecules 2019, 20, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Dikin, D.A.; Piner, R.D.; Ruoff, R.S. Tunable Electrical Conductivity of Individual Graphene Oxide Sheets Reduced at “Low” Temperatures. Nano Lett. 2008, 8, 4283–4287. [Google Scholar] [CrossRef] [PubMed]
- Kashi, S.; Gupta, R.K.; Kao, N.; Hadigheh, S.A.; Bhattacharya, S.N. Influence of Graphene Nanoplatelet Incorporation and Dispersion State on Thermal, Mechanical and Electrical Properties of Biodegradable Matrices. J. Mater. Sci. Technol. 2018, 34, 1026–1034. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Dhar, P.; Kumar, A.; Katiyar, V. Polyhydroxyalkanoates (PHA)-Cellulose Based Nanobiocomposites for Food Packaging Applications. In Food Additives and Packaging; Komolprasert, V., Turowski, P., Eds.; ACS Publications: Washington, DC, USA, 2014; Volume 1162, pp. 275–314. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mawatari, Y.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y.C. Polyhydroxyalkanoates (PHA) Production from Synthetic Waste Using Pseudomonas Pseudoflava: PHA Synthase Enzyme Activity Analysis from P. Pseudoflava and P. Palleronii. Bioresour. Technol. 2017, 234, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundar, N.; Kumar, S.A.; Pavithra, A.; Ghosh, S. Studies on Semi-Crystalline Poly Lactic Acid (PLA) as a Hydrophobic Coating Material on Kraft Paper for Imparting Barrier Properties in Coated Abrasive Applications. Prog. Org. Coat. 2020, 145. [Google Scholar] [CrossRef]
- Adesina, O.T.; Sadiku, E.R.; Jamiru, T.; Ogunbiyi, O.F.; Adesina, O.S. Thermal Properties of Spark Plasma -Sintered Polylactide/Graphene Composites. Mater. Chem. Phys. 2020, 242, 122545. [Google Scholar] [CrossRef]
- Gao, Y.; Picot, O.T.; Bilotti, E.; Peijs, T. Influence of Filler Size on the Properties of Poly(Lactic Acid) (PLA)/Graphene Nanoplatelet (GNP) Nanocomposites. Eur. Polym. J. 2017, 86, 117–131. [Google Scholar] [CrossRef]
- Szuman, K.; Krucińska, I.; Boguń, M.; Draczyński, Z. PLA/PHA- Biodegradable Blends for Pneumothermic Fabrication of Nonwovens. Autex Res. J. 2016, 16, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Reddy, G.V.S.; Reddy, A.B.; Sudhakar, K.; Sadiku, E.R. Preparation and Characterization of Poly(Ethylene Glycol) Stabilized Nano Silver Particles by a Mechanochemical Assisted Ball Mill Process. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Gupta, A.; Simmons, W.; Schueneman, G.T.; Hylton, D.; Mintz, E.A. Rheological and Thermo-Mechanical Properties of Poly(Lactic Acid)/Lignin-Coated Cellulose Nanocrystal Composites. ACS Sustain. Chem. Eng. 2017, 5, 1711–1720. [Google Scholar] [CrossRef]
- Ojha, N.; Das, N. A Statistical Approach to Optimize the Production of Polyhydroxyalkanoates from Wickerhamomyces Anomalus VIT-NN01 Using Response Surface Methodology. Int. J. Biol. Macromol. 2018, 107, 2157–2170. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Yi, C.; Wang, L.; Liu, H.; Xu, W. Thermal Degradation of Poly(Lactic Acid) Measured by Thermogravimetry Coupled to Fourier Transform Infrared Spectroscopy. J. Therm. Anal. Calorim. 2009, 97, 929–935. [Google Scholar] [CrossRef]
- Achaby, M.E.; Arrakhiz, F.; Vaudreuil, S.; Qaiss, A.K.; Bousmina, M.; Fassi-Fehri, O. Mechanical, Thermal, and Rheological Properties of Graphene-Based Polypropylene Mechanical, Thermal, and Rheological Properties of Graphene-Based Polypropylene Nanocomposites Prepared by Melt of Graphene-Based Polypropylene Nanocomposites Prepared by Melt. Polym. Compos. 2012, 33, 733–744. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Petrova-Doycheva, I.; Menseidov, D.; Ivanov, E.; Paddubskaya, A.; Kuzhir, P. Exploring Thermal Annealing and Graphene-Carbon Nanotube Additives to Enhance Crystallinity, Thermal, Electrical and Tensile Properties of Aged Poly(Lactic) Acid-Based Filament for 3D Printing. Compos. Sci. Technol. 2019, 181, 107712. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Ni, X.; Leong, K.W. Synthesis and Characterization of New Biodegradable Amphiphilic Poly(Ethylene Oxide)-b-Poly [(R)-3-Hydroxy Butyrate]-b-Poly(Ethylene Oxide) Triblock Copolymers. Macromolecules 2003, 36, 2661–2667. [Google Scholar] [CrossRef]
- Pan, J.; Li, G.; Chen, Z.; Chen, X.; Zhu, W.; Xu, K. Alternative Block Polyurethanes Based on Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) and Poly(Ethylene Glycol). Biomaterials 2009, 30, 2975–2984. [Google Scholar] [CrossRef]
- Ozdemir, E.; Hacaloglu, J. Characterizations of PLA-PEG Blends Involving Organically Modified Montmorillonite. J. Anal. Appl. Pyrolysis 2017, 127, 343–349. [Google Scholar] [CrossRef]
- Ghalia, M.A.; Dahman, Y. Investigating the Effect of Multi-Functional Chain Extenders on PLA/PEG Copolymer Properties; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 95. [Google Scholar] [CrossRef]
- Graphene, L.; Rafiee, M.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.; Koratkar, N. Enhanced Mechanical Properties of Nanocomposites at Low Graphene Content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef]
- Ladani, R.B.; Bhasin, M.; Wu, S.; Ravindran, A.R.; Ghorbani, K.; Zhang, J.; Kinloch, A.J.; Mouritz, A.P.; Wang, C.H. Fracture and Fatigue Behaviour of Epoxy Nanocomposites Containing 1-D and 2-D Nanoscale Carbon Fillers. Eng. Fract. Mech. 2018, 203, 102–114. [Google Scholar] [CrossRef]
- Li, L.; Bao, R.Y.; Gao, T.; Liu, Z.Y.; Xie, B.H.; Yang, M.B.; Yang, W. Dopamine-Induced Functionalization of Cellulose Nanocrystals with Polyethylene Glycol towards Poly(L-Lactic Acid) Bionanocomposites for Green Packaging. Carbohydr. Polym. 2019, 203, 275–284. [Google Scholar] [CrossRef]
- Kashi, S.; Gupta, R.K.; Baum, T.; Kao, N.; Bhattacharya, S.N. Morphology, Electromagnetic Properties and Electromagnetic Interference Shielding Performance of Poly Lactide/Graphene Nanoplatelet Nanocomposites. Mater. Des. 2016, 95, 119–126. [Google Scholar] [CrossRef]
- Kim, I.H.; Jeong, Y.G. Polylactide/Exfoliated Graphite Nanocomposites with Enhanced Thermal Stability, Mechanical Modulus, and Electrical Conductivity. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 850–858. [Google Scholar] [CrossRef]
- Papadopoulou, E.L.; Basnett, P.; Paul, U.C.; Marras, S.; Ceseracciu, L.; Roy, I.; Athanassiou, A. Green Composites of Poly(3-Hydroxybutyrate) Containing Graphene Nanoplatelets with Desirable Electrical Conductivity and Oxygen Barrier Properties. ACS Omega 2019, 4, 19746–19755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis, 6th ed.; Skoog, D.A., Holler, F.J., Crouch, S.R., Eds.; Thomson Brooks/Cole: Belmont, CA, USA, 2007. [Google Scholar]



| Material | Technical Specifications | Company Details |
|---|---|---|
| PHA | Melt Flow Rate = 3 g/10 min at 170 °C | Goodfellow Cambridge, Huntingdon, UK |
| Peak Melt Temperature = 140–160 °C | ||
| Specific Gravity = 1.24 g/cm3 | ||
| PLA | Melt Flow Rate = 3 g/10 min at 170 °C | Nature Works®, Blair, WI, USA |
| Peak Melt Temperature = 140–160 °C | ||
| Specific Gravity = 1.24 g/cm3 | ||
| GNPs | 8–30 layers | Graphenest, Aveiro, Portugal |
| Thickness = 3 and 10 nm | ||
| Planar size = 0.5 to 0.2 μm | ||
| Surface Area = 150 m2/g | ||
| CNCs | Average Particle Size = 75 nm | Celluforce, Windsor, QC, Canada |
| Polydispersity Index = 0.181 | ||
| Spray dried form | ||
| Chloroform | Density = 1.48 g/cm3 (20 °C) | Honeywell, Seelze, Germany |
| Vapor pressure = 210 hPa (25 °C) | ||
| 99% purity | ||
| PEG 4000 | Solidification point = 54.1 °C | Acofarma, Barcelona, Spain |
| OHCH2-(CH2OCH2)n-CH2OH n: 68–84 | ||
| Viscosity = 121.0 mPas |
| ΔHm | Xc | Tm | Mass Loss (%) | ||||
|---|---|---|---|---|---|---|---|
| (mJ) | (J/g) | (%) | (°C) | 300 °C | 350 °C | 400 °C | |
| PHA | 98.9 | 11.8 | 8.1 | 153.8 | 59.6 | 91.8 | 96.5 |
| PHA + 1% CNC | 88.4 | 10.4 | 7.2 | 152.6 | 59.7 | 92.4 | 95.8 |
| PHA + 7.5% GNPs | ** | ** | ** | ** | 58.3 | 92.8 | 95.6 |
| PHA + 15% GNPs | 75.8 | 8.9 | 7.2 | 153.8 | 48.7 | 79.9 | 81.4 |
| PHA + 15% GNPs + 1%CNC | 80.9 | 9.1 | 7.4 | 154.3 | 50.6 | 81.1 | 82.3 |
| PHA + 15% GNPs + 20%PEG | 81.5 | 9.5 | 7.6 | 153.9 | 45.8 | 65.8 | 77.6 |
| PHA + 15% GNPs + 1%CNC + 20%PEG | ** | ** | ** | ** | 54.8 | 65.2 | 76.9 |
| PLA | 323.8 | 40.0 | 42.7 | 151.2 | 2.1 | 19.1 | 99.9 |
| PLA + 1% CNC | 254.5 | 30.7 | 33.1 | 150.7 | 1.6 | 17.4 | 98.9 |
| PLA + 7.5% GNPs | ** | ** | ** | ** | 1.8 | 30.1 | 98.1 |
| PLA + 15% GNPs | 245.4 | 27.6 | 22.2 | 146.4 | 1.5 | 19.9 | 85.2 |
| PLA + 15% GNPs + 1%CNC | 192.8 | 22.2 | 18.1 | 145.0 | 2.9 | 22.2 | 86.2 |
| PLA + 15% GNPs + 20%PEG | 223.0 | 24.5 | 19.8 | 145.2 | 6.7 | 51.6 | 84.4 |
| PLA + 15% GNPs + 1%CNC + 20%PEG | ** | ** | ** | ** | 10.9 | 65.7 | 82.3 |
| GNPS (wt.%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.15 | 0.75 | 1.5 | 3 | 7.5 | 15 | ||||||||
| Additives (wt.%) | ||||||||||||||
| *** | 1% CNC | *** | *** | *** | *** | *** | *** | 1% CNC | 20% PEG | 20% PEG + 1% CNC | ||||
| Tensile properties | Tensile strength (MPa) | PLA | ℜ | 59.65 | 72.91 | 61.45 | 62.19 | 49.49 | *** | 38.13 | 28.33 | 24.20 | 12.35 | 8.42 |
| σ | 3.17 | 3.98 | 3.40 | 1.67 | 1.28 | *** | 2.43 | 2.40 | 3.17 | 0.73 | 23.69 | |||
| CV | 5.31% | 5.46% | 5.53% | 2.68% | 2.59% | *** | 6.36% | 8.48% | 13.08% | 5.90% | 2.82% | |||
| PHA | ℜ | 18.67 | 22.48 | 19.06 | 19.77 | 16.85 | *** | 11.73 | 11.14 | 12.37 | 7.72 | 6.36 | ||
| σ | 0.79 | 0.52 | 1.09 | 0.58 | 1.04 | *** | 1.22 | 2.00 | 1.08 | 0.97 | 0.30 | |||
| CV | 4.22% | 2.30% | 5.74% | 2.92% | 6.20% | *** | 10.38% | 17.93% | 8.71% | 12.51% | 4.74% | |||
| Strain at break(%) | PLA | ℜ | 3.94 | 3.94 | 4.56 | 5.80 | 3.25 | *** | 2.70 | 2.49 | 2.07 | 10.24 | 17.31 | |
| σ | 0.44 | 0.49 | 0.64 | 0.45 | 0.50 | *** | 0.32 | 0.12 | 0.27 | 2.96 | 0.94 | |||
| CV | 11.27% | 12.42% | 14.08% | 7.68% | 15.50% | *** | 11.75% | 4.97% | 13.04% | 28.91% | 5.40% | |||
| PHA | ℜ | 4.75 | 10.70 | 4.38 | 3.99 | 3.68 | *** | 2.19 | 2.35 | 2.29 | 2.45 | 1.77 | ||
| σ | 0.21 | 0.06 | 0.16 | 0.35 | 0.18 | *** | 0.21 | 0.02 | 0.29 | 0.48 | 0.07 | |||
| CV | 4.42% | 0.59% | 3.67% | 8.80% | 4.92% | *** | 9.65% | 1.06% | 12.57% | 19.42% | 3.81% | |||
| Elastic Modulus(GPa) | PLA | ℜ | 1.95 | 2.12 | 2.11 | 2.10 | 1.98 | *** | 1.75 | 1.30 | 1.84 | 0.56 | 0.30 | |
| σ | 0.07 | 0.14 | 0.11 | 0.10 | 0.15 | *** | 0.14 | 0.24 | 0.14 | 0.12 | 0.03 | |||
| CV | 3.40% | 6.66% | 5.44% | 4.94% | 7.56% | *** | 8.16% | 18.15% | 7.73% | 20.95% | 9.24% | |||
| PHA | ℜ | 0.54 | 0.72 | 0.72 | 0.95 | 0.96 | *** | 0.80 | 0.56 | 0.93 | 0.48 | 0.35 | ||
| σ | 0.08 | 0.02 | 0.08 | 0.06 | 0.01 | *** | 0.03 | 0.13 | 0.11 | 0.01 | 0.03 | |||
| CV | 14.97% | 2.42% | 11.38% | 6.27% | 1.40% | *** | 3.59% | 23.68% | 11.30% | 2.27% | 8.28% | |||
| Electrical properties | Piezoresistiveanalysis(GF) | PLA | ℜ | *** | *** | *** | *** | *** | *** | 1.02 | 1.12 | 1.38 | 4.31 | 3.24 |
| σ | *** | *** | *** | *** | *** | *** | 0.41 | 0.26 | 0.38 | 1.51 | 0.87 | |||
| CV | *** | *** | *** | *** | *** | *** | 39.7 | 23.2 | 27.8 | 35.0 | 26.9 | |||
| PHA | ℜ | *** | *** | *** | *** | *** | *** | 3.77 | 6.30 | 1.91 | 1.02 | 2.00 | ||
| σ | *** | *** | *** | *** | *** | *** | 1.67 | 1.75 | 1.1 | 0.50 | 0.85 | |||
| CV | *** | *** | *** | *** | *** | *** | 44.38% | 27.74% | 60.03% | 49.20% | 42.64% | |||
| Electricalconductivity(S/m) | PLA | ℜ | *** | *** | 1.1 × 10−7 | 9.2 × 10−8 | 1.1 × 10−7 | 9.0 × 10−8 | 0.20 | 5.81 | 5.59 | 16.11 | 11.54 | |
| σ | *** | *** | 5.24 × 10−9 | 1.31 × 10−8 | 9.17 × 10−9 | 1.02 × 10−8 | 0.02 | 0.25 | 0.78 | 1.91 | 0.60 | |||
| CV | *** | *** | 4.65% | 14.20% | 8.39% | *** | 11.57% | 4.36% | 13.93% | 11.88% | 5.20% | |||
| PHA | ℜ | *** | *** | 1.37 × 10−7 | 1.86 × 10−7 | 1.38 × 10−7 | 0.25 | 1.20 | 12.94 | 9.07 | 10.52 | 12.87 | ||
| σ | *** | *** | 8.21 × 10−9 | 1.54 × 10−8 | 9.20 × 10−9 | 0.02 | 0.18 | 1.51 | 1.28 | 2.29 | 2.39 | |||
| CV | *** | *** | 5.98% | 8.28% | 6.68% | *** | 15.24% | 11.68% | 14.08% | 21.78% | 18.56% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mármol, G.; Sanivada, U.K.; Fangueiro, R. Effect of GNPs on the Piezoresistive, Electrical and Mechanical Properties of PHA and PLA Films. Fibers 2021, 9, 86. https://doi.org/10.3390/fib9120086
Mármol G, Sanivada UK, Fangueiro R. Effect of GNPs on the Piezoresistive, Electrical and Mechanical Properties of PHA and PLA Films. Fibers. 2021; 9(12):86. https://doi.org/10.3390/fib9120086
Chicago/Turabian StyleMármol, Gonzalo, Usha Kiran Sanivada, and Raul Fangueiro. 2021. "Effect of GNPs on the Piezoresistive, Electrical and Mechanical Properties of PHA and PLA Films" Fibers 9, no. 12: 86. https://doi.org/10.3390/fib9120086
APA StyleMármol, G., Sanivada, U. K., & Fangueiro, R. (2021). Effect of GNPs on the Piezoresistive, Electrical and Mechanical Properties of PHA and PLA Films. Fibers, 9(12), 86. https://doi.org/10.3390/fib9120086