Environmentally Benign Phytic Acid-Based Nanocoating for Multifunctional Flame-Retardant/Antibacterial Cotton
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Textile Exchange Organization. Preferred Fiber & Materials Market Report. 2020. Available online: https://textileexchange.org/about-us/#annualreports (accessed on 7 October 2021).
- Cellulose-Based Graft Copolymers; CRC Press: Boca Raton, FL, USA, 2015; pp. 235–270. [CrossRef]
- Magovac, E.; Bischof, S. Non-Halogen FR Treatment of Cellulosic Textiles. Tekstil 2015, 64, 298–309. [Google Scholar]
- Hull, T.; Law, R.; Bergman, Å. Environmental Drivers for Replacement of Halogenated Flame Retardants. In Polymer Green Flame Retardants; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 119–179. [Google Scholar] [CrossRef]
- Gao, Y.; Cranston, R. Recent Advances in Antimicrobial Treatments of Textiles. Text. Res. J. 2008, 78, 60–72. [Google Scholar] [CrossRef]
- Choudhury, A.K.R. Finishes for protection against microbial, insect and UV radiation. In Principles of Textile Finishing; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 319–382. [Google Scholar] [CrossRef]
- Choudhury, A.K.R. Flame- and fire-retardant finishes. In Principles of Textile Finishing; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 195–244. [Google Scholar] [CrossRef]
- Vukušić, S.B.; Grgac, S.F.; Budimir, A.; Kalenić, S. Cotton textiles modified with citric acid as efficient anti-bacterial agent for prevention of nosocomial infections. Croat. Med. J. 2011, 52, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Schramm, C.; Vukusic, S.B.; Katovic, D. Non-formaldehyde durable press finishing of dyed fabrics: Evaluation of cotton-bound polycarboxylic acids. Color. Technol. 2002, 118, 244–249. [Google Scholar] [CrossRef]
- Jordanov, I.; Magovac, E.; Fahami, A.; Lazar, S.; Kolibaba, T.; Smith, R.J.; Bischof, S.; Grunlan, J.C. Flame retardant polyester fabric from nitrogen-rich low molecular weight additives within intumescent nanocoating. Polym. Degrad. Stab. 2019, 170, 108998. [Google Scholar] [CrossRef]
- Jordanov, I.; Kolibaba, T.J.; Lazar, S.; Magovac, E.; Bischof, S.; Grunlan, J.C. Flame suppression of polyamide through combined enzymatic modification and addition of urea to multilayer nanocoating. J. Mater. Sci. 2020, 55, 15056–15067. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Frache, A.; Malucelli, G. Layer by Layer coatings assembled through dipping, vertical or horizontal spray for cotton flame retardancy. Carbohydr. Polym. 2013, 92, 114–119. [Google Scholar] [CrossRef]
- Holder, K.M.; Smith, R.J.; Grunlan, J.C. A review of flame retardant nanocoatings prepared using layer-by-layer assembly of polyelectrolytes. J. Mater. Sci. 2017, 52, 12923–12959. [Google Scholar] [CrossRef]
- Shirvan, A.R.; Nejad, N.H.; Bashari, A. Antibacterial finishing of cotton fabric via the chitosan/TPP self-assembled nano layers. Fibers Polym. 2014, 15, 1908–1914. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Liu, Y.-Y.; Xu, Y.-J.; Jiang, Z.-M.; Dong, C.-H.; Zhang, L.; Liu, Y.; Zhu, P. Fully bio-based coating from chitosan and phytate for fire-safety and antibacterial cotton fabrics. Carbohydr. Polym. 2020, 237, 116173. [Google Scholar] [CrossRef]
- Xue, C.-H.; Wu, Y.; Guo, X.-J.; Liu, B.-Y.; Wang, H.-D.; Jia, S.-T. Superhydrophobic, flame-retardant and conductive cotton fabrics via layer-by-layer assembly of carbon nanotubes for flexible sensing electronics. Cellulose 2020, 27, 3455–3468. [Google Scholar] [CrossRef]
- Lin, D.; Zeng, X.; Li, H.; Lai, X. Facile fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via layer-by-layer assembly. Cellulose 2018, 25, 3135–3149. [Google Scholar] [CrossRef]
- Magovac, E.; Jordanov, I.; Grunlan, J.C.; Bischof, S. Environmentally-Benign Phytic Acid-Based Multilayer Coating for Flame Retardant Cotton. Materials 2020, 13, 5492. [Google Scholar] [CrossRef] [PubMed]
- Magovac, E.; Budimir, A.; Jordanov, I.; Bischof, S.; Grunlan, J.C. Antibacterial cotton from novel phytic acid-based multilayer nanocoating. Green Mater. 2021, 0, 1–6. [Google Scholar] [CrossRef]
- ISO 4589-2:2017(En). Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test. Available online: https://www.iso.org/obp/ui/#iso:std:iso:24444:ed-1:v1:en (accessed on 25 October 2021).
- ASTM D6413/D6413M-15. Standard Test Method for Flame Resistance of Textiles (Vertical Test). Available online: https://www.astm.org/Standards/D6413.htm (accessed on 25 October 2021).
- ASTM D7309-21a. Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry. Available online: https://www.astm.org/Standards/D7309.htm (accessed on 25 October 2021).
- AATCC TM100-2019. Test Method for Antibacterial Finishes on Textile Materials: Assessment of Antibacterial Activity Fin-ishes on Textile Material. Available online: https://members.aatcc.org/store/tm100/513/ (accessed on 25 October 2021).
- Weil, E.D.; Levchik, S.V. Flame Retardants in Commercial Use or Development for Textiles. In Flame Retardants for Plastics and Textiles; Carl Hanser Verlag GmbH & Co. KG: München, Germany, 2009; pp. 197–225. [Google Scholar] [CrossRef]
- Flecknoe-Brown, K.W.; Van Hees, P. Sensitivity analysis on the microscale combustion calorimeter for polyurethane foam using a full factorial design methodology. J. Fire Sci. 2018, 36, 453–471. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Fu, Y. Pyrolysis of cellulose. Carbohydr. Res. 1973, 29, 113–122. [Google Scholar] [CrossRef]
- Lengyel, J.; Ončák, M.; Herburger, A.; Van Der Linde, C.; Beyer, M.K. Infrared spectroscopy of O− and OH− in water clusters: Evidence for fast interconversion between O− and OH˙OH−. Phys. Chem. Chem. Phys. 2017, 19, 25346–25351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarano, D.; Bertarione, S.; Spoto, G.; Zecchina, A.; Areán, C.O. FTIR spectroscopy of hydrogen, carbon monoxide, and methane adsorbed and co-adsorbed on zinc oxide. Thin Solid Film. 2001, 400, 50–55. [Google Scholar] [CrossRef]
- Falk, M.; Miller, A.G. Infrared spectrum of carbon dioxide in aqueous solution. Vib. Spectrosc. 1992, 4, 105–108. [Google Scholar] [CrossRef]
- Horrocks, A.; Price, D.; Akalin, M. FTIR analysis of gases evolved from cotton and flame retarded cotton fabrics pyrolysed in air. Polym. Degrad. Stab. 1996, 52, 205–213. [Google Scholar] [CrossRef]
- Gilbert, A.S. IR Spectral Group Frequencies of Organic Compounds. In Encyclopedia of Spectroscopy and Spectrometry; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 408–418. [Google Scholar] [CrossRef]
- Franklin, W.E.; Rowland, S.P. Mechanistic Aspects of Flame Retardancy in Cotton Cellulose. Text. Res. J. 1979, 49, 170–175. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, W.; Yan, M.; Liu, J.; Wang, B.; Xia, Y. Pyrolysis products and thermal degradation mechanism of intrinsically flame-retardant carrageenan fiber. RSC Adv. 2017, 7, 25253–25264. [Google Scholar] [CrossRef] [Green Version]
- Camino, G. Flame retardants: Intumescent systems. In Polymer Science and Technology Series; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1998; pp. 297–306. [Google Scholar] [CrossRef]
- Ishida, S.T. Bacteriolyses of Bacterial Cell Walls by Cu(II) and Zn(II) Ions Based on Antibacterial Results of Dilution Medium Method and Halo Antibacterial Test. J. Adv. Res. Biotechnol. 2017, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
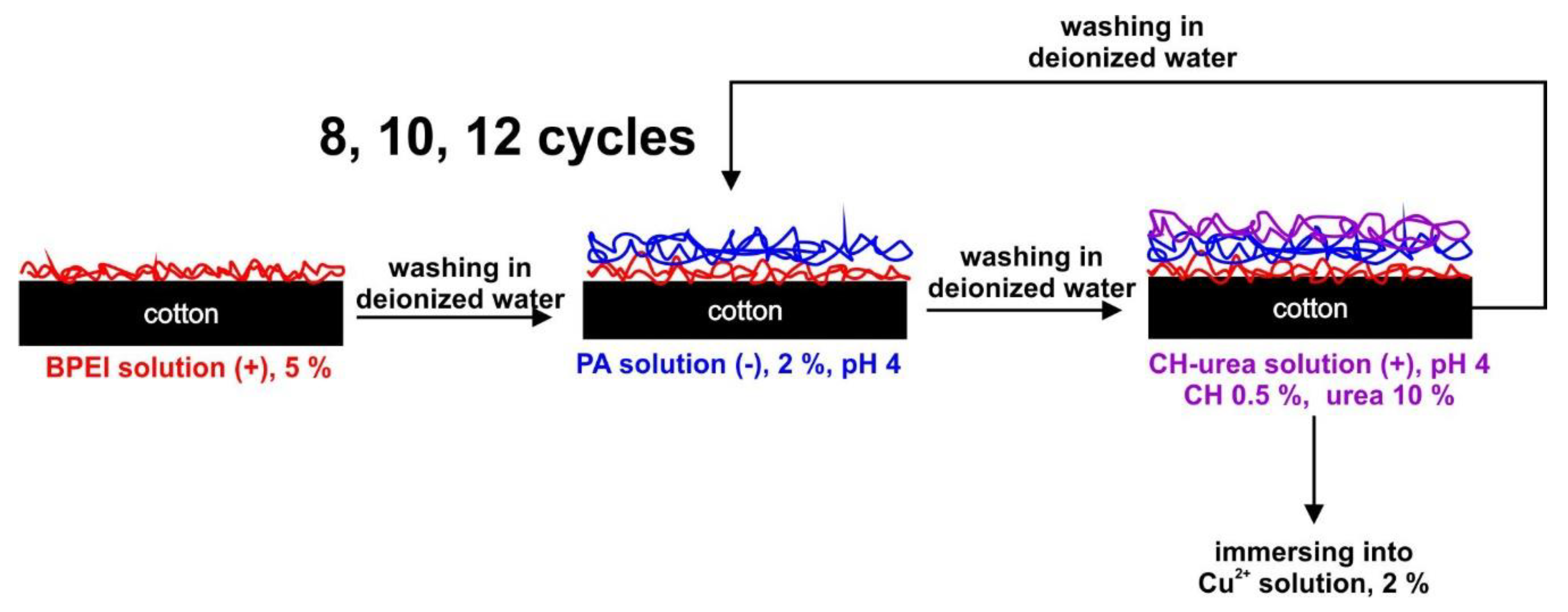
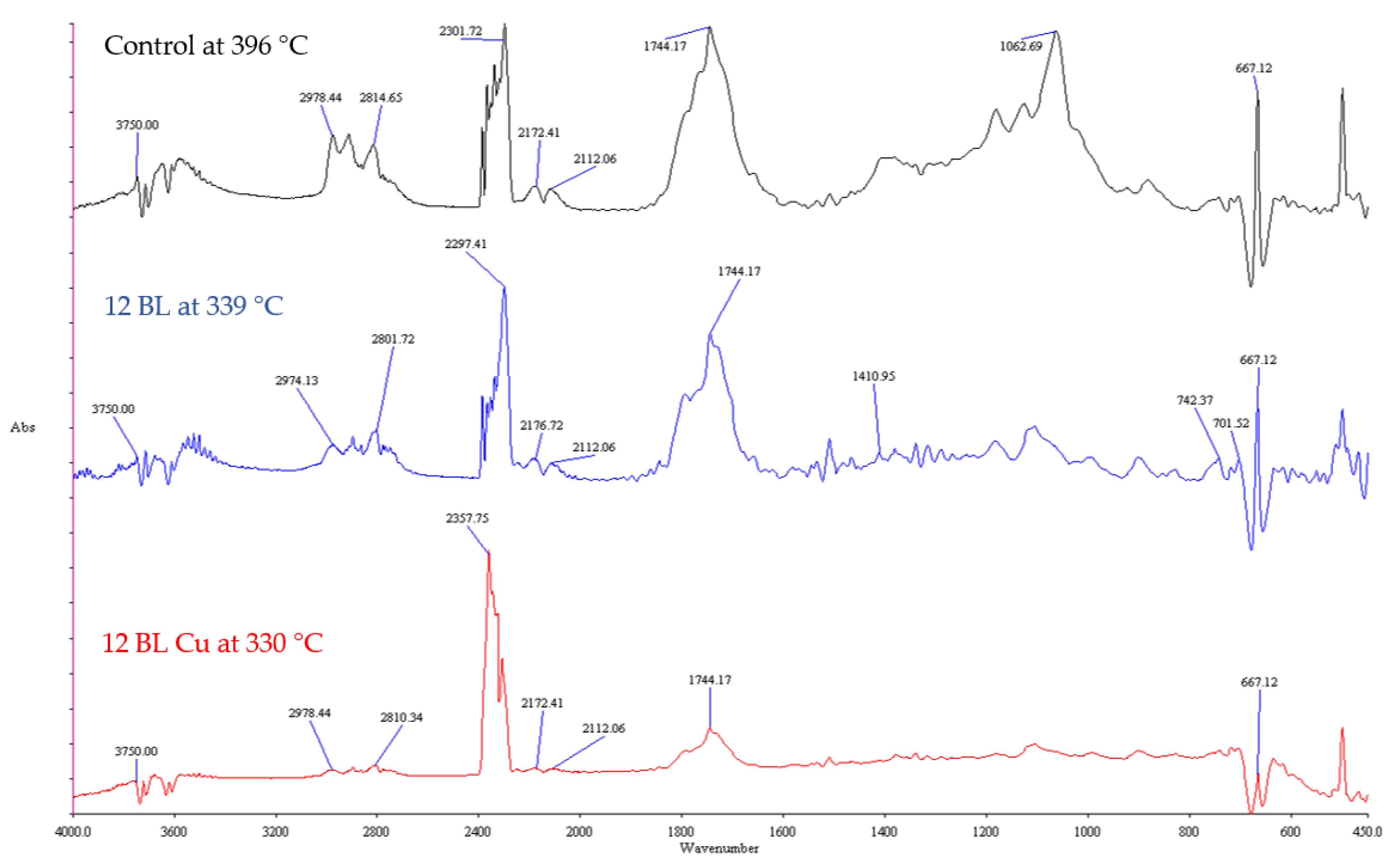
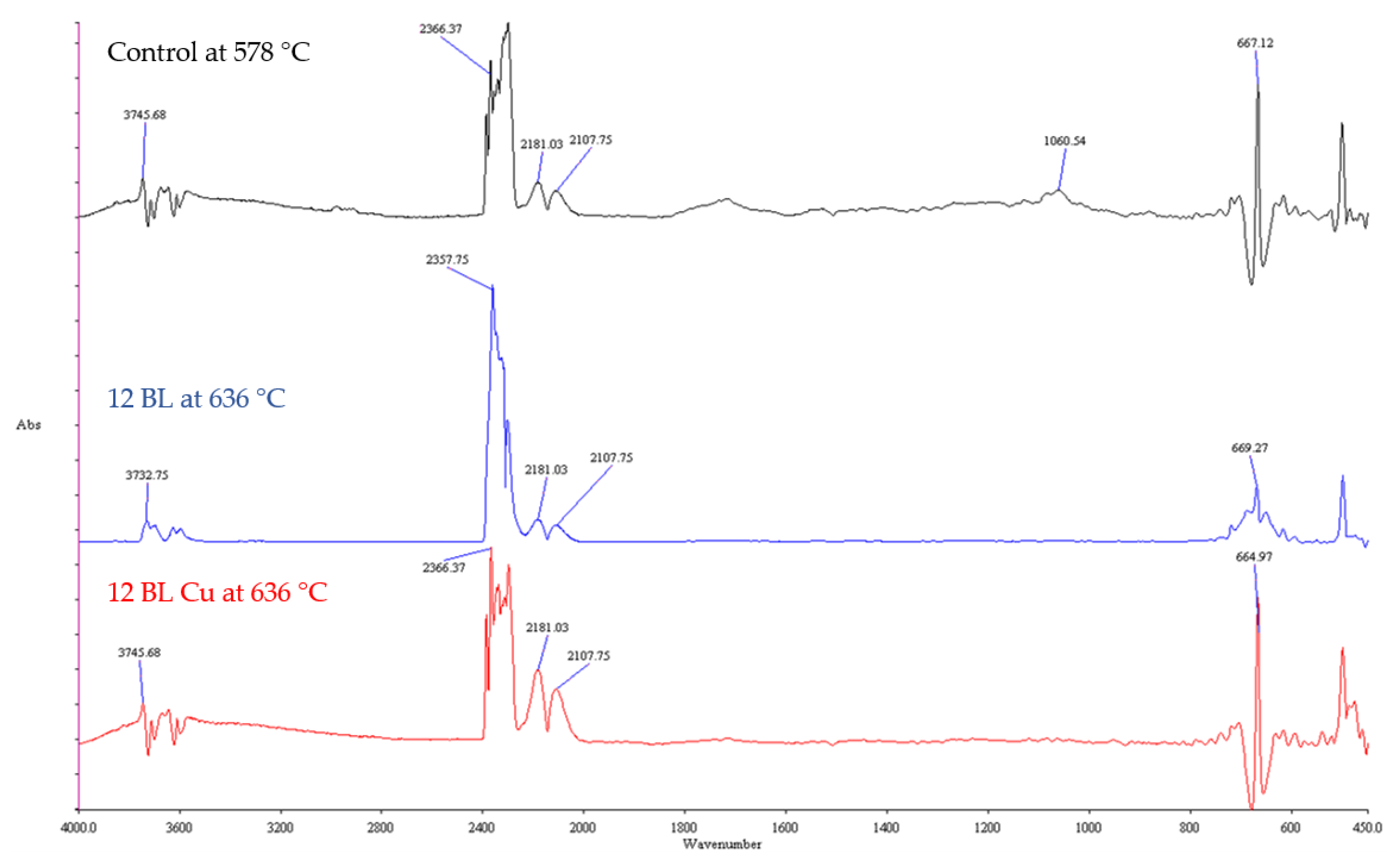
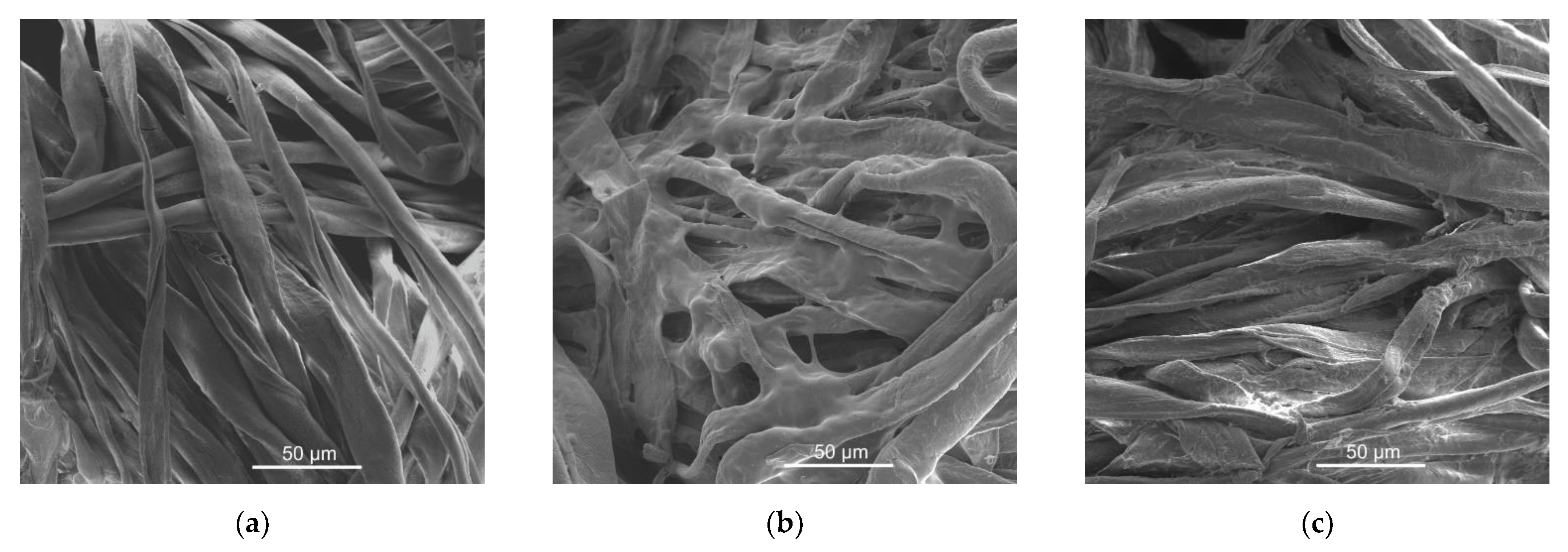

| Batch | Number of BLs | Weight Gain (%) | LOI (%) |
|---|---|---|---|
| Control | n/a | n/a | 18.0 |
| PA/CH-urea | 8 | 12.34 | 21.5 |
| 10 | 17.58 | 24.0 | |
| 12 | 18.54 | 24.5 | |
| PA/CH-urea + Cu2+ | 8 | 12.96 | 23.5 |
| 10 | 18.05 | 25.5 | |
| 12 | 18.97 | 26.0 |
| Control | PA/CH-Urea | PA/CH-Urea + Cu2+ | |||||
|---|---|---|---|---|---|---|---|
| Number of BL | n/a | 8 | 10 | 12 | 8 | 10 | 12 |
| Image |  |  |  |  |  |  |  |
| Char length (cm) | n/a | n/a | n/a | 6.7 | n/a | n/a | 6.5 |
| After flame time (s) | n/a | n/a | n/a | 0 | n/a | n/a | 0 |
| After glow time (s) | n/a | n/a | n/a | 0 | n/a | n/a | 0 |
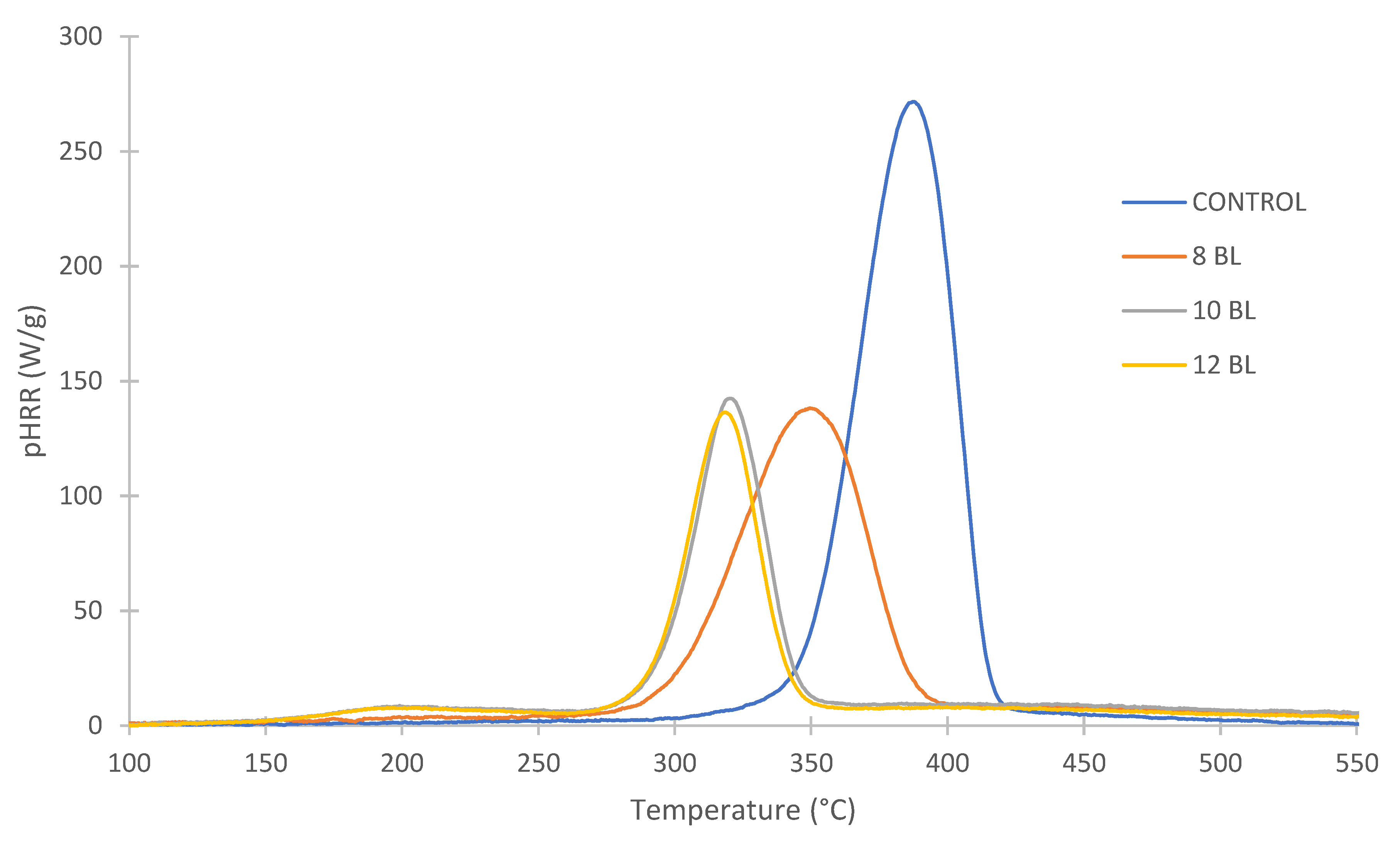
| Sample | pHRR (W/g) | ΔHRR (%) | THR (kJ/g) | ΔTHR (%) | TpHRR (°C) |
|---|---|---|---|---|---|
| control | 269.4 ± 4.8 | 0.0 | 11.6 ± 0.9 | 0.0 | 395 ± 1.4 |
| 8 BL | 133.2 ± 5.7 | 50.6 | 7.7 ± 0.8 | 33.6 | 360 ± 2.4 |
| 10 BL | 136.7 ± 5.5 | 49.3 | 5.0 ± 0.6 | 56.9 | 322 ± 2.7 |
| 12 BL | 132.2 ± 6.4 | 50.9 | 5.0 ± 1.1 | 56.9 | 318 ± 3.0 |
| 8 BL Cu | 110.1 ± 6.1 | 59.1 | 7.9 ± 1.0 | 31.9 | 320 ± 2.9 |
| 10 BL Cu | 108.8 ± 4.2 | 59.6 | 5.1 ± 0.7 | 56.0 | 310 ± 1.8 |
| 12 BL Cu | 103.0 ± 4.1 | 61.8 | 5.3 ± 0.6 | 54.3 | 311 ± 1.7 |
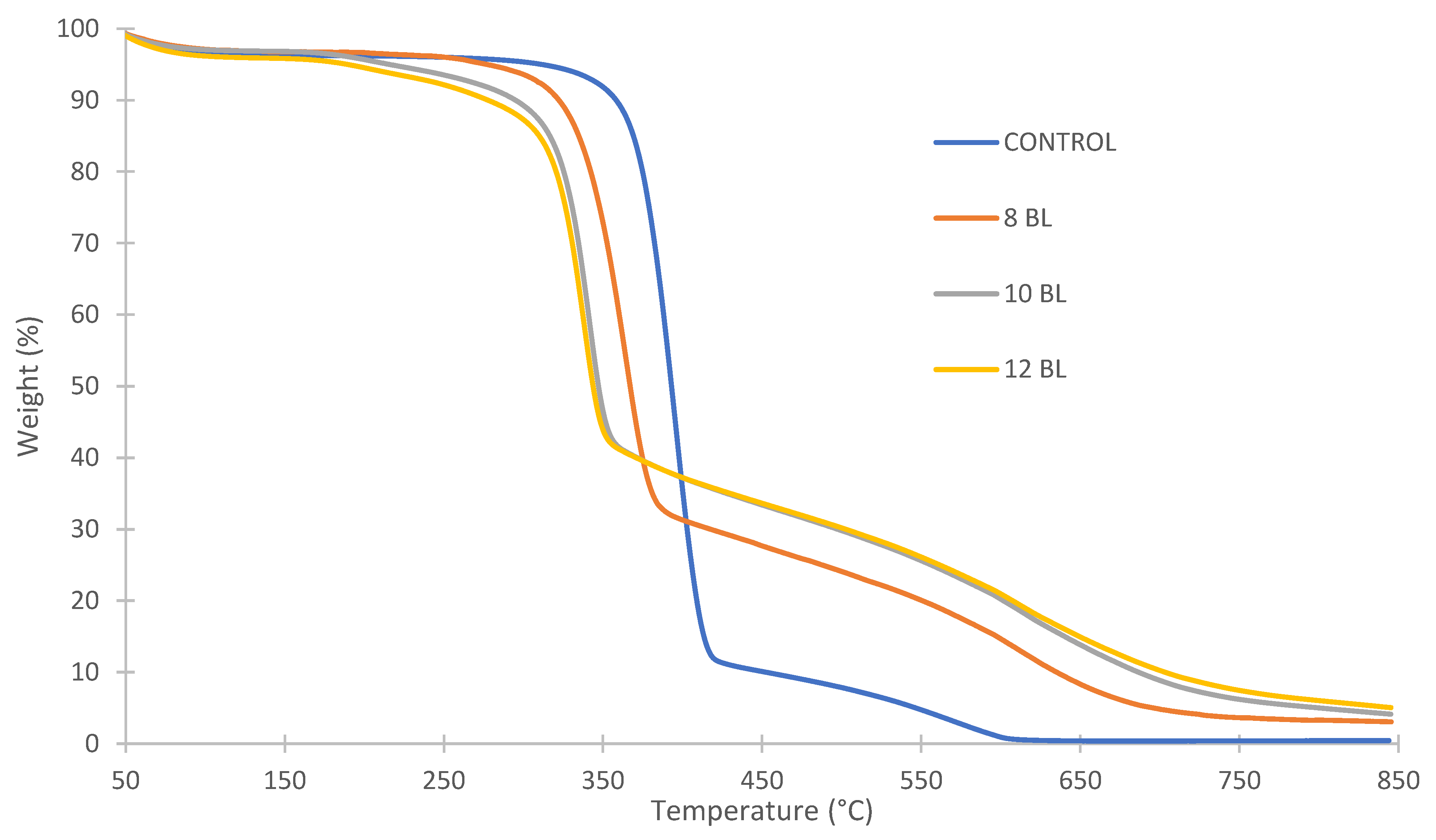
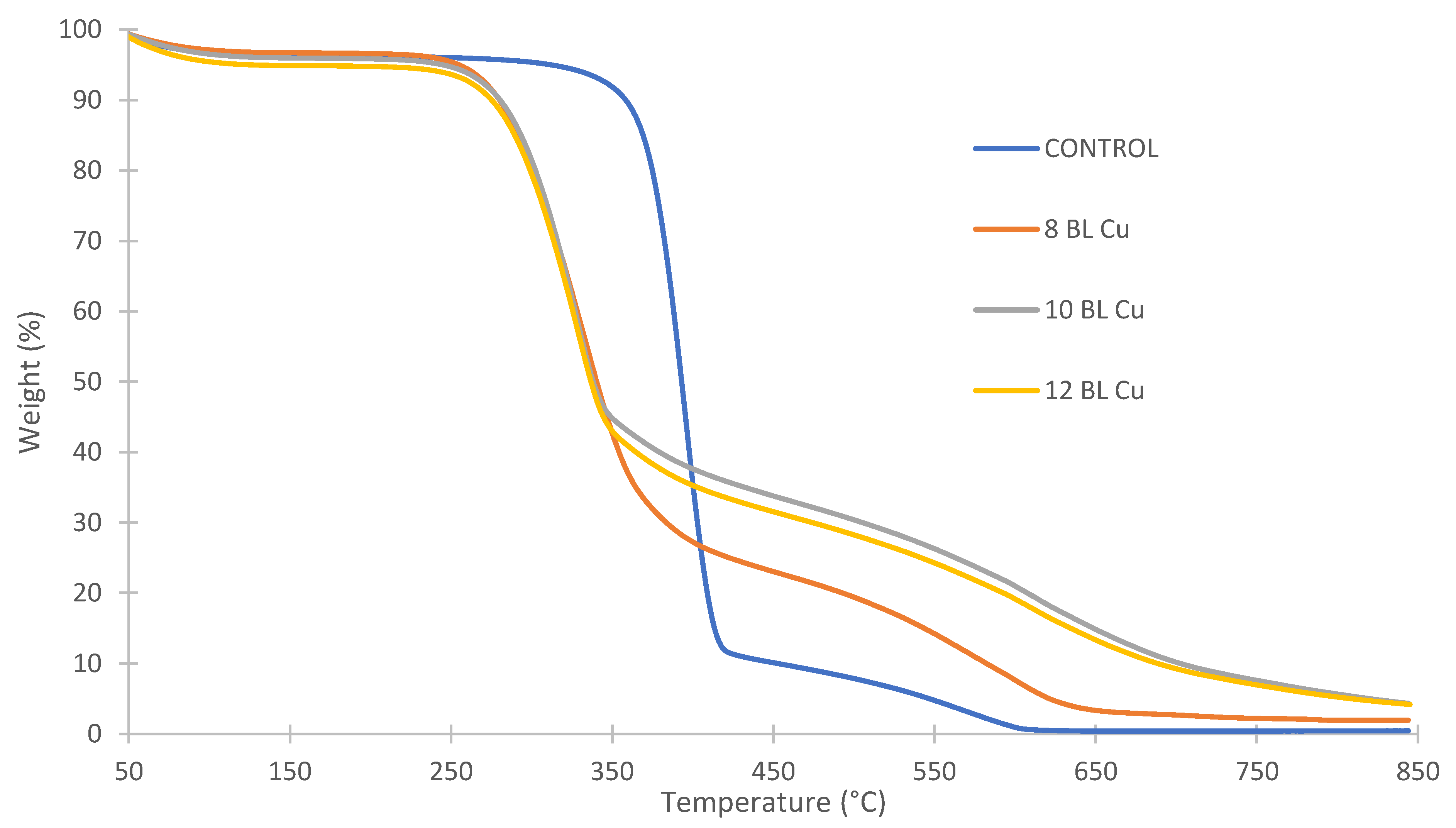
| Sample | Onset 1 (°C) | T1 (°C) | Time (s) | Weight at T1 (%) | End 1 (°C) | Onset 2 (°C) | T2 (°C) | Time (s) | End 2 (°C) | Weight at 650 °C (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| control | 360 | 396 | 727 | 43.6 | 420 | 496 | 578 | 1027 | 607 | 0.4 |
| 8 BL | 324 | 364 | 646 | 54.4 | 388 | 604 | 623 | 1158 | 813 | 8.3 |
| 10 BL | 316 | 342 | 602 | 58.7 | 357 | 527 | 640 | 1142 | 745 | 13.8 |
| 12 BL | 312 | 339 | 609 | 57.8 | 354 | 512 | 636 | 1161 | 711 | 14.9 |
| 8 BL + Cu | 258 | 334 | 588 | 54.9 | 376 | 488 | 578 | 1066 | 648 | 3.3 |
| 10 BL + Cu | 267 | 328 | 576 | 58.5 | 350 | 502 | 634 | 1182 | 705 | 14.8 |
| 12 BL + Cu | 261 | 330 | 597 | 56.2 | 354 | 464 | 636 | 1130 | 700 | 13.3 |
| Element | Phosphorus | Nitrogen | Copper |
|---|---|---|---|
| Atomic number | 15 | 7 | 29 |
| Series | K-series | K-series | L-series |
| Sample | Average wt % | ||
| 8 BL | 12.1 | 5.2 | n/a |
| 10 BL | 1.7 | 3.3 | n/a |
| 12 BL | 11.7 | 6.8 | n/a |
| 8 BL Cu | 5.8 | 2.6 | 17.0 |
| 10 BL Cu | 8.3 | 2.3 | 6.6 |
| 12 BL Cu | 14.0 | 4.6 | 8.2 |
| Bacterium Reduction (%) | ||
|---|---|---|
| Sample | Klebsiella pneumoniae | Staphylococcus aureus |
| 8 BL Cu | 99.9 | 100 |
| 10 BL Cu | 99.7 | 99.9 |
| 12 BL Cu | 100.0 | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magovac, E.; Vončina, B.; Budimir, A.; Jordanov, I.; Grunlan, J.C.; Bischof, S. Environmentally Benign Phytic Acid-Based Nanocoating for Multifunctional Flame-Retardant/Antibacterial Cotton. Fibers 2021, 9, 69. https://doi.org/10.3390/fib9110069
Magovac E, Vončina B, Budimir A, Jordanov I, Grunlan JC, Bischof S. Environmentally Benign Phytic Acid-Based Nanocoating for Multifunctional Flame-Retardant/Antibacterial Cotton. Fibers. 2021; 9(11):69. https://doi.org/10.3390/fib9110069
Chicago/Turabian StyleMagovac, Eva, Bojana Vončina, Ana Budimir, Igor Jordanov, Jaime C. Grunlan, and Sandra Bischof. 2021. "Environmentally Benign Phytic Acid-Based Nanocoating for Multifunctional Flame-Retardant/Antibacterial Cotton" Fibers 9, no. 11: 69. https://doi.org/10.3390/fib9110069
APA StyleMagovac, E., Vončina, B., Budimir, A., Jordanov, I., Grunlan, J. C., & Bischof, S. (2021). Environmentally Benign Phytic Acid-Based Nanocoating for Multifunctional Flame-Retardant/Antibacterial Cotton. Fibers, 9(11), 69. https://doi.org/10.3390/fib9110069







