Biodegradable Nanofiber for Delivery of Immunomodulating Agent in the Treatment of Basal Cell Carcinoma
Abstract
:1. Introduction
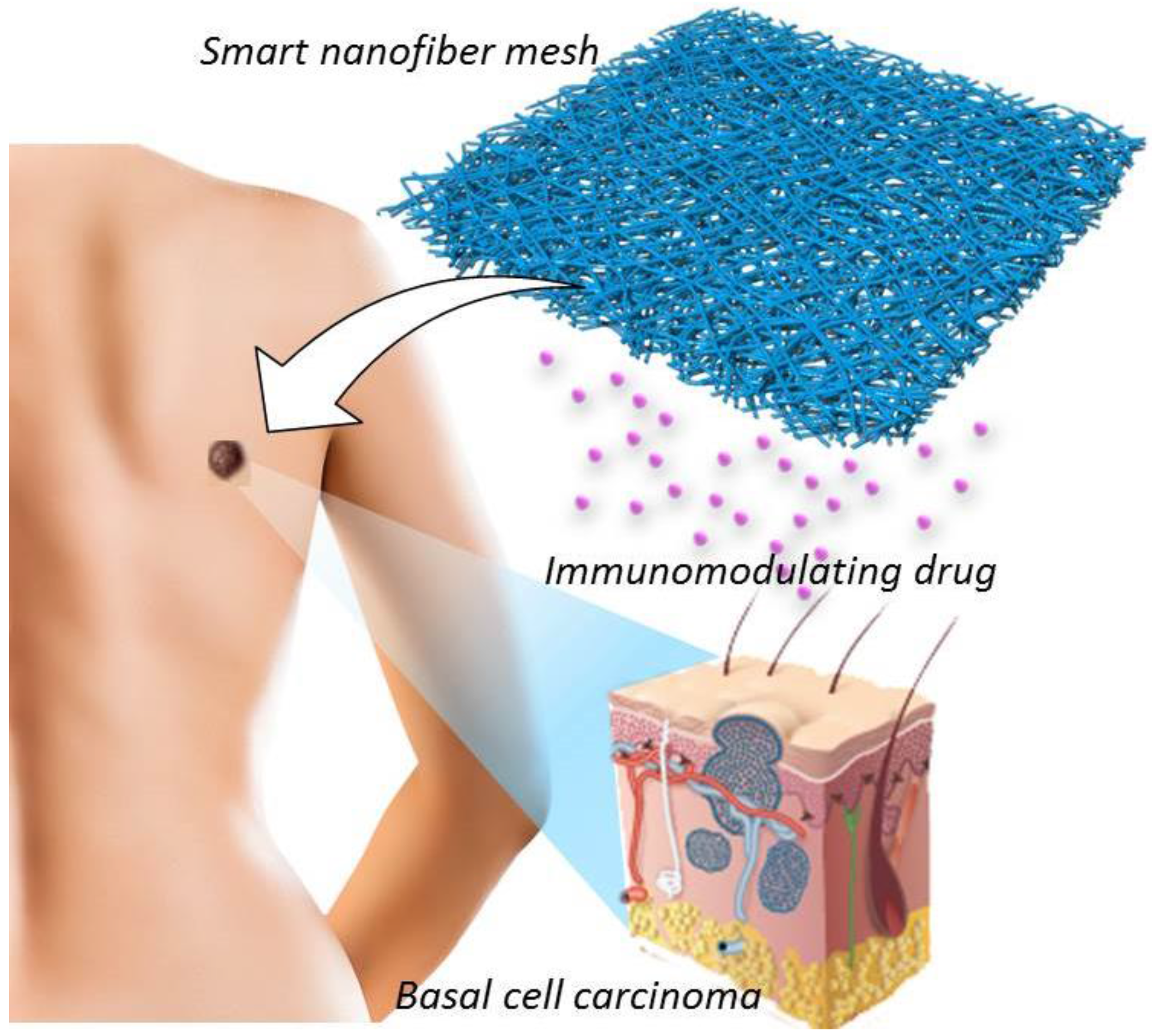
2. Experimental Section
2.1. Preparation of Poly(ε-caprolactone) (PCL)
2.2. Fiber Production
2.3. Characterization
2.4. Drug Release Profile
3. Results and Discussion
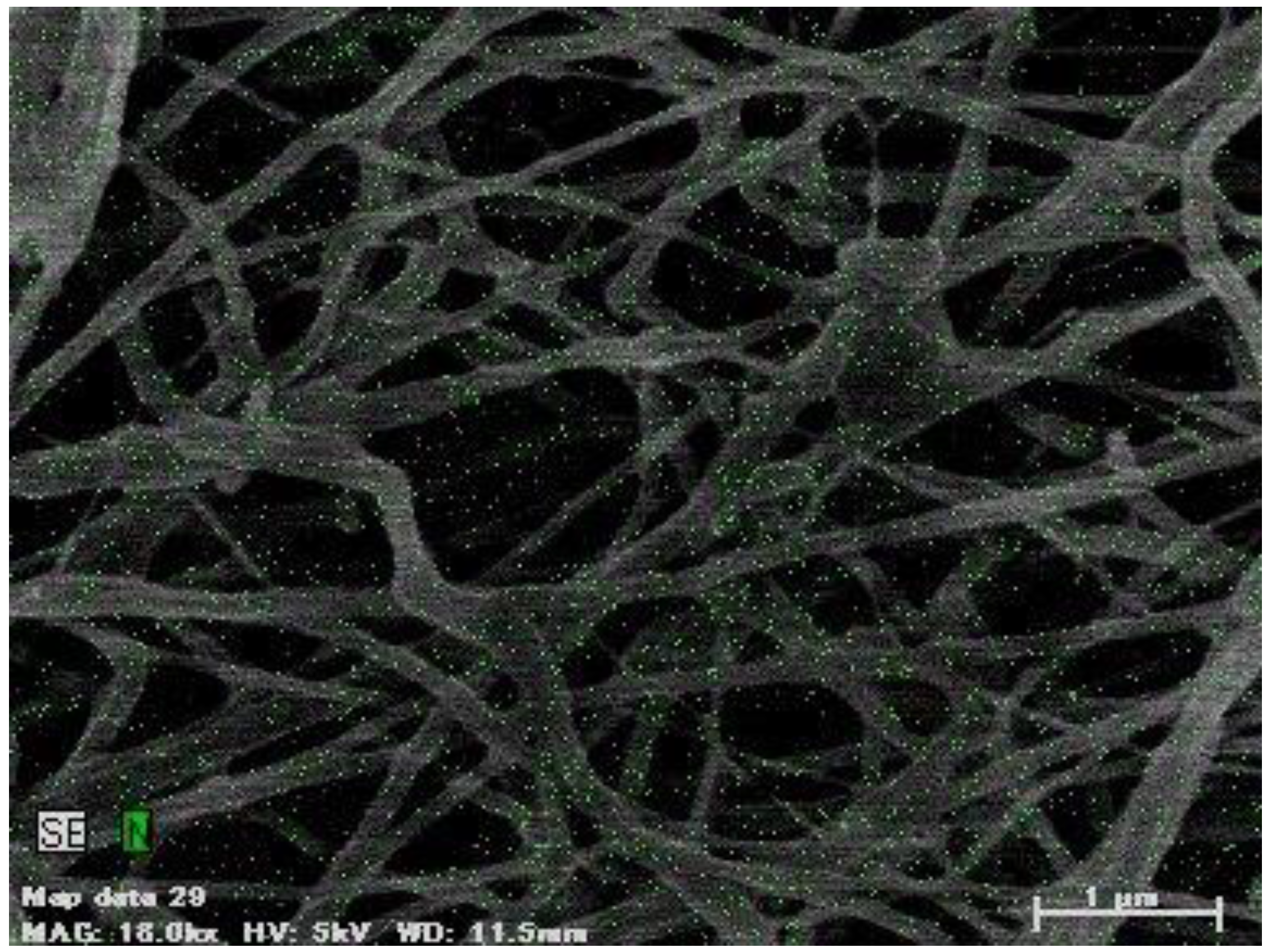
3.1. SEM of PCL/Imiquimod Nanofibers
| PCL | Mn,NMR | Mn,GPC | Mw/Mn |
| (g/mol) | (g/mol) | (-) | |
| 27,600 | 27,000 | 1.35 |
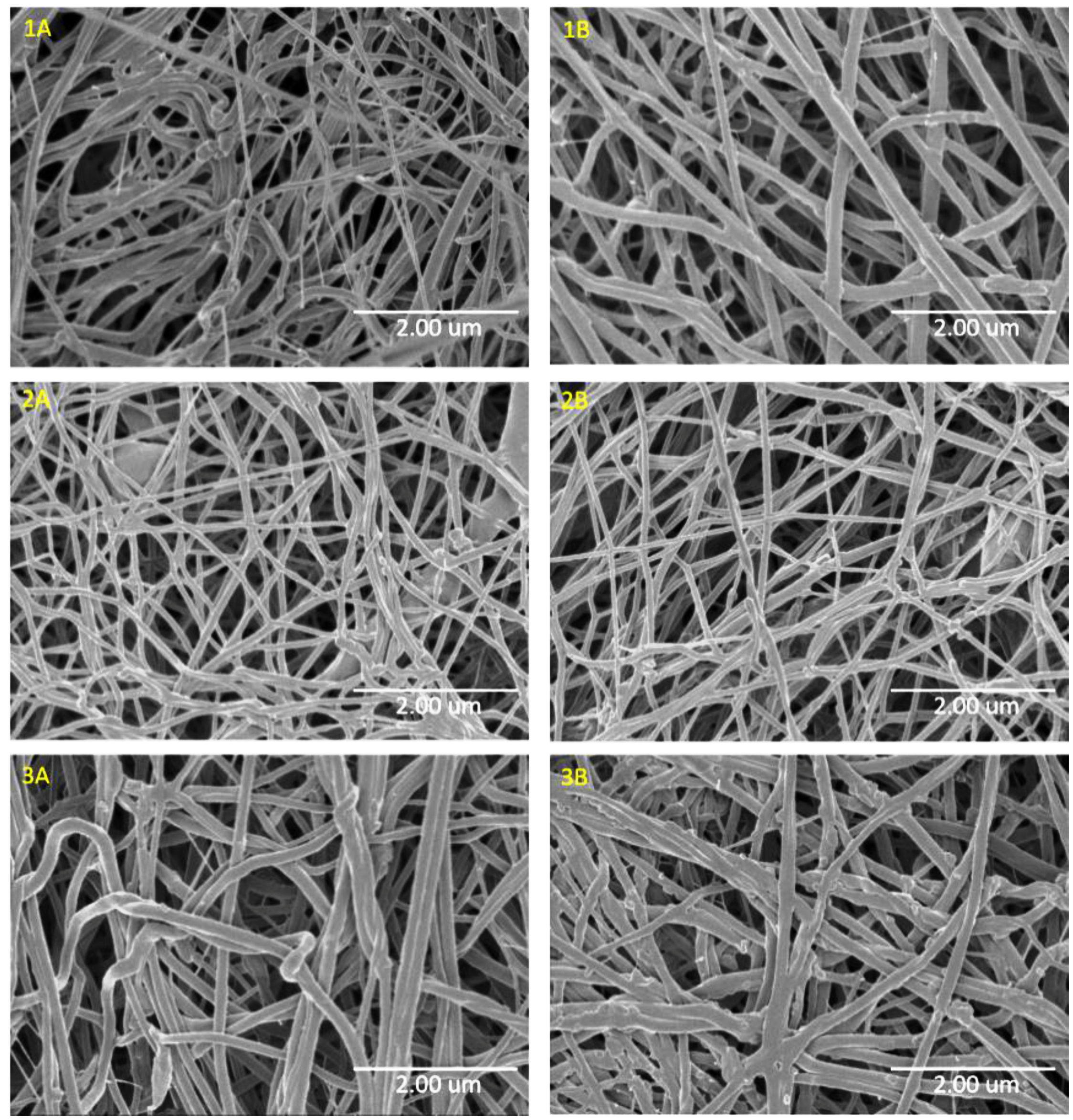
| Sample | PCL w/v% | Flow rate (mL/h) | Imiquimod wt% | Average fiber size (nm) ± standard deviation |
|---|---|---|---|---|
| 1 | 15 | 1 | 0 | 540 ± 281 |
| 1A | 15 | 1 | 5 | 72 ± 22 |
| 1B | 15 | 1 | 10 | 120 ± 56 |
| 2 | 15 | 2 | 0 | 1028 ± 382 |
| 2A | 15 | 2 | 5 | 94 ± 20 |
| 2B | 15 | 2 | 10 | 95 ± 92 |
| 3 | 20 | 1 | 0 | 1320 ± 504 |
| 3A | 20 | 1 | 5 | 132 ± 33 |
| 3B | 20 | 1 | 10 | 150 ± 36 |
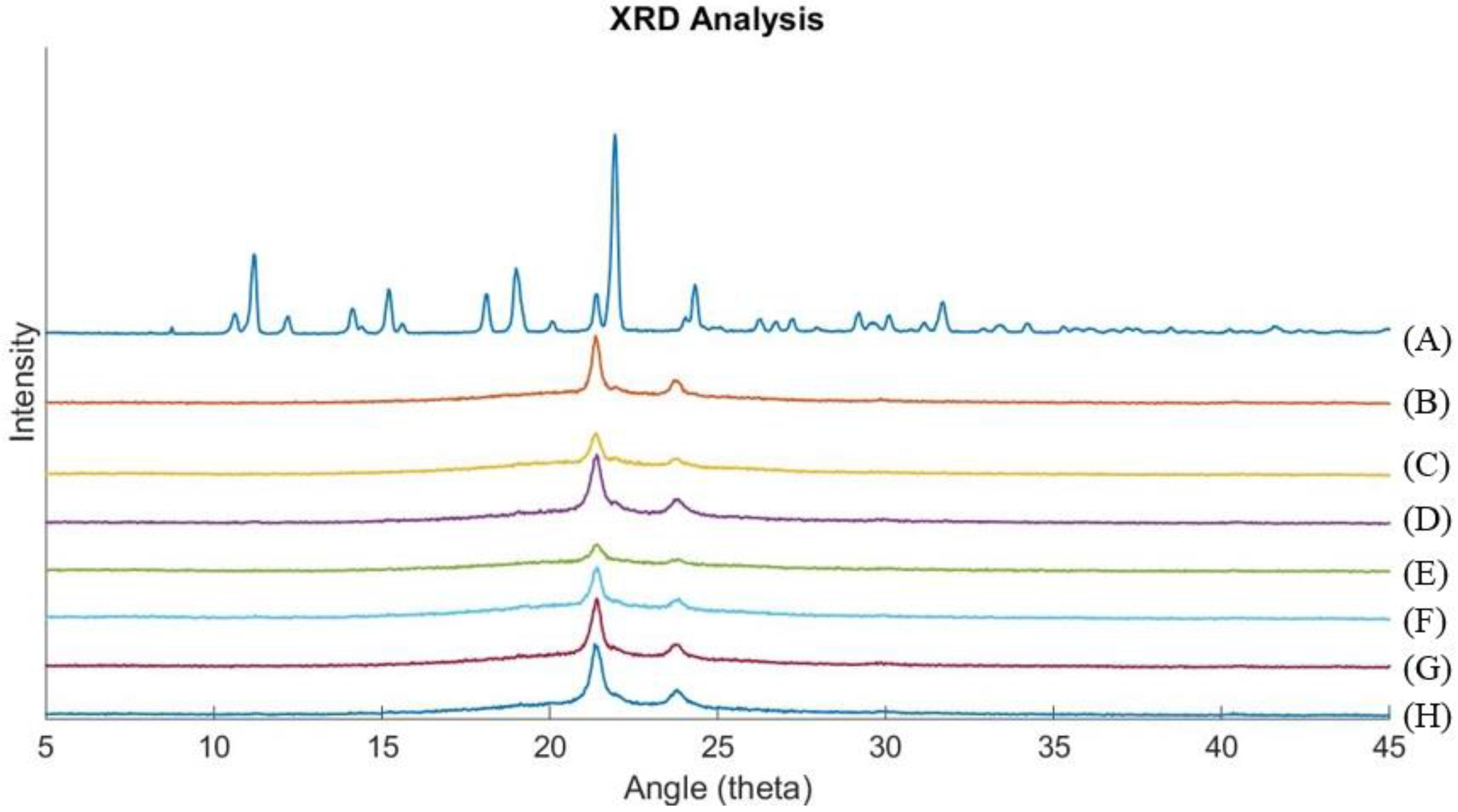
3.2. X-Ray Diffraction
3.3. Drug Release Profile
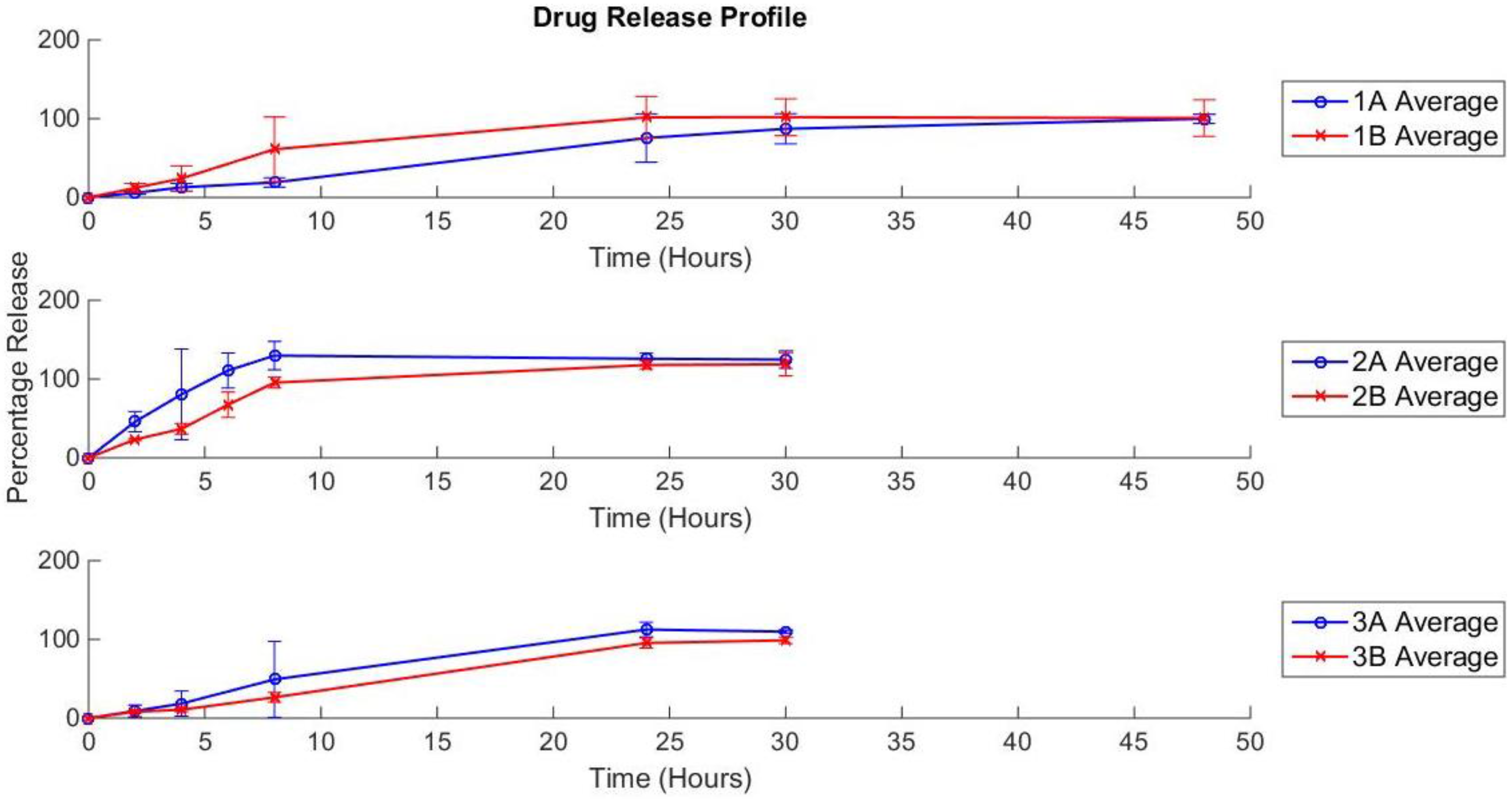
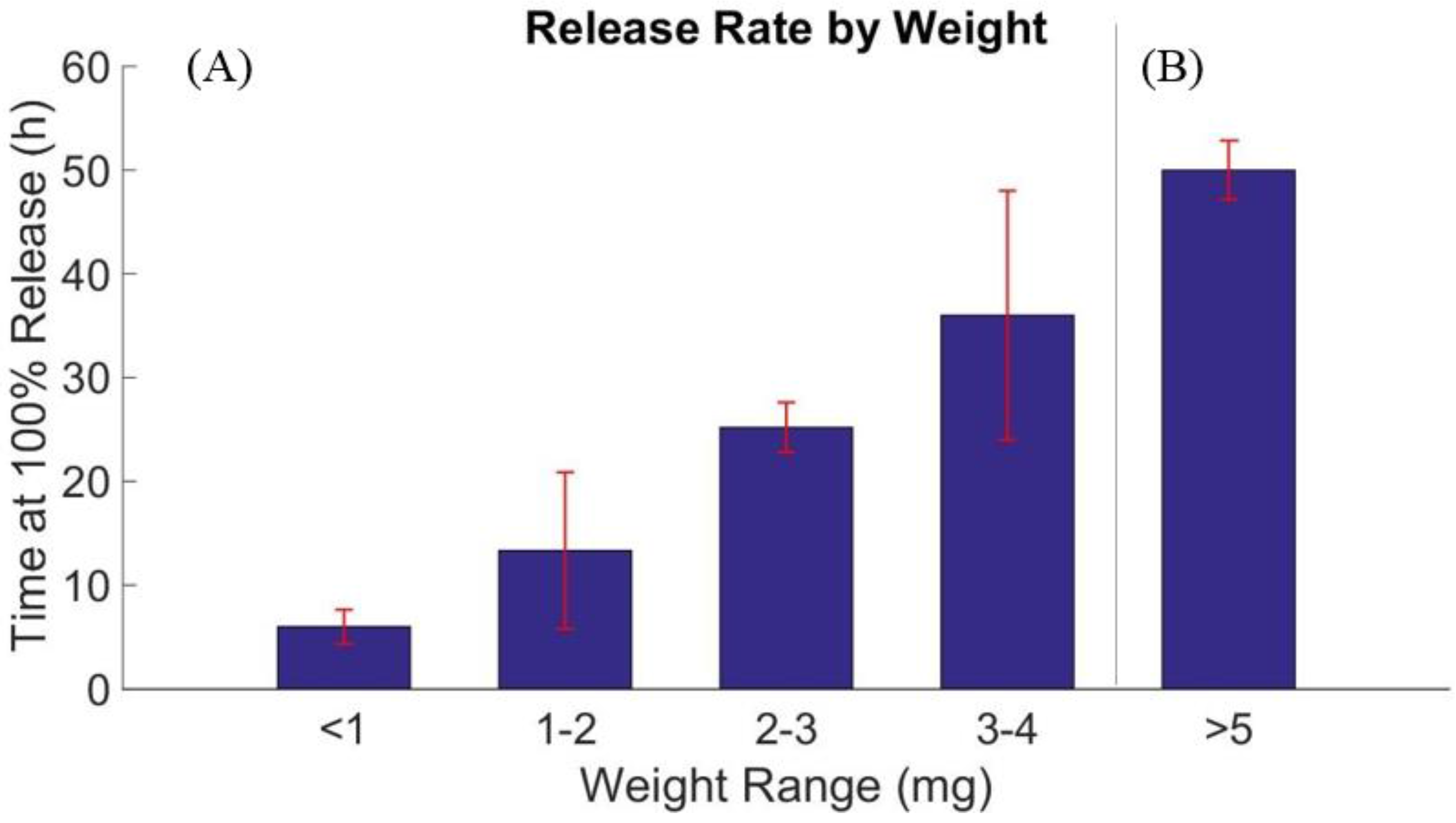
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Staples, M.P.; Elwood, M.; Burton, R.C.; Williams, J.L.; Marks, R.; Giles, G.G. Non-melanoma skin cancer in australia: The 2002 national survey and trends since 1985. Med. J. Aust. 2006, 184, 6–10. [Google Scholar] [PubMed]
- Katalinic, A.; Kunze, U.; Schäfer, T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in schleswig-holstein, germany: Incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer). Br. J. Dermatol. 2003, 149, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Hoey, S.E.H.; Devereux, C.E.J.; Murray, L.; Catney, D.; Gavin, A.; Kumar, S.; Donnelly, D.; Dolan, O.M. Skin cancer trends in northern ireland and consequences for provision of dermatology services. Br. J. Dermatol. 2007, 156, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.W.; Metelitsa, A.I.; Dover, D.C.; Salopek, T.G. Trends in incidence of nonmelanoma skin cancers in alberta, canada, 1988–2007. Br. J. Dermatol. 2010, 163, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Cancer Council Australia. Skin Cancer. Available online: http://www.cancer.org.au/about-cancer/types-of-cancer/skin-cancer.html (accessed on 1 September 2015).
- Madan, V.; Lear, J.T.; Szeimies, R.-M. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef]
- Sinclair, R. Nonmelanoma skin cancer in australia. Br. J. Dermatol. 2013, 168, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Bahner, J.D.; Bordeaux, J.S. Non-melanoma skin cancers: Photodynamic therapy, cryotherapy, 5-fluorouracil, imiquimod, diclofenac, or what? Facts and controversies. Clin. Dermatol. 2013, 31, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Santiago, F.; Serra, D.; Vieira, R.; Figueiredo, A. Incidence and factors associated with recurrence after incomplete excision of basal cell carcinomas: A study of 90 cases. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.J.; Zitelli, J.A. Surgical margins for basal cell carcinoma. Arch. Dermatol. 1987, 123, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Smeets, N.W.J.; Kuijpers, D.I.M.; Nelemans, P.; Ostertag, J.U.; Verhaegh, M.E.J.M.; Krekels, G.A.M.; Neumann, H.A.M. Mohs’ micrographic surgery for treatment of basal cell carcinoma of the face—Results of a retrospective study and review of the literature. Br. J. Dermatol. 2004, 151, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.A.; Breen, D.; Culleton, S.; Zhang, L.; Kamra, J.; Tsao, M.; Balogh, J. Palliative radiotherapy for non-melanoma skin cancer. Clin. Oncol. 2010, 22, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Hamdoon, Z.; Jerjes, W.; Upile, T.; Hopper, C. Optical coherence tomography-guided photodynamic therapy for skin cancer: Case study. Photodiagn. Photodyn. Ther. 2011, 8, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Kokoszka, A.; Scheinfeld, N. Evidence-based review of the use of cryosurgery intreatment of basal cell carcinoma. Dermatol. Surg. 2003, 29, 566–571. [Google Scholar] [PubMed]
- Sauder, D.N. Imiquimod: Modes of action. Br. J. Dermatol. 2003, 149, 5–8. [Google Scholar] [CrossRef]
- Stanley, M.A. Imiquimod and the imidazoquinolones: Mechanism of action and therapeutic potential. Clin. Exp. Dermatol. 2002, 27, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Madkan, V.; Cook-Norris, R.; Sra, K.; Tyring, S. Current and potential uses of imiquimod. South. Med. J. 2005, 98, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.V. Imiquimod: An immune response modifier. J. Am. Acad. Dermatol. 2000, 43, 1–5. [Google Scholar] [CrossRef]
- Turan, A.; Saricaoglu, H.; Baskan, E.B.; Toker, S.C.; Tunali, S. Treatment of infiltrating basal cell carcinoma with the combination of intralesional ifnα-2b and topical imiquimod 5% cream. Int. J. Dermatol. 2009, 48, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Beutner, K.R.; Geisse, J.K.; Helman, D.; Fox, T.L.; Ginkel, A.; Owens, M.L. Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream. J. Am. Acad. Dermatol. 1999, 41, 1002–1007. [Google Scholar] [CrossRef]
- Geisse, J.; Caro, I.; Lindholm, J.; Golitz, L.; Stampone, P.; Owens, M. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: Results from two phase iii, randomized, vehicle-controlled studies. J. Am. Acad. Dermatol. 2004, 50, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Imiquimod Prescribing Information; Food and Drug Administration: Silver Spring, MD, USA, 2010. [Google Scholar]
- Taveira, S.F.; Lopez, R.F.V. Topical Administration of Anticancer Drugs for Skin Cancer Treatment. In Skin Cancers—Risk, Factors, Prevention and Therapy; InTech: Rijeka, Croatia, 2011; Chapter 11. [Google Scholar]
- Chen, W.; Wu, Z.; Yang, H.; Guo, S.; Li, D.; Cheng, L. In vitro and in vivo evaluation of injectable implants for intratumoral delivery of 5-fluorouracil. Pharm. Dev. Technol. 2014, 19, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wei, H.; Zhang, X.-Z.; Cheng, S.-X.; Zhuo, R.-X. Thermo-triggered and biotinylated biotin-p(nipaam-co-hmaam)-b-pmma micelles for controlled drug release. J. Biomed. Mater. Res. A 2009, 88, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafie, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, C.; Cheng, L.; Guo, S.; Zhang, Y.; Tang, T. Study on hydrophilic 5-fluorouracil release from hydrophobic poly(ε-caprolactone) cylindrical implants. Drug Dev. Ind. Pharm. 2011, 37, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Ischakov, R.; Adler-Abramovich, L.; Buzhansky, L.; Shekhter, T.; Gazit, E. Peptide-based hydrogel nanoparticles as effective drug delivery agents. Bioorg. Med. Chem. 2013, 21, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. A smart hyperthermia nanofiber with switchable drug release for inducing cancer apoptosis. Adv. Funct. Mater. 2013, 23, 5753–5761. [Google Scholar] [CrossRef]
- Kranz, H.; Bodmeier, R. A novel in situ forming drug delivery system for controlled parenteral drug delivery. Int. J. Pharm. 2007, 332, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Tu, M.; Liu, H.-W.; Zhao, J.-H.; Zha, Z.-G.; Zhou, C.-R. Preparation, structure and drug release behaviour of chitosan-based nanofibre. IET Nanobiotechnol. 2009, 3, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Morrow, D.I.J.; Woolfson, A.D. Fast-drying multi-laminate bioadhesive films for transdermal and topical drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Zawislak, A.A.; Woolfson, A.D. Design and physicochemical characterisation of a bioadhesive patch for dose-controlled topical delivery of imiquimod. Int. J. Pharm. 2006, 307, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Alves da Silva, M.L.; Martins, A.; Gomes, M.; Costa-Pinto, A.R.; Reis, R.L.; Costa, P.; Neves, N.M. Cartilage tissue engineering using electrospun PCL nanofiber meshes and MSCs. Biomacromolecules 2010, 11, 3228–3236. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Namekawa, K.; Schreiber, M.T.; Aoyagi, T.; Ebara, M. Fabrication of zeolite-polymer composite nanofibers for removal of uremic toxins from kidney failure patients. Biomater. Sci. 2014, 2, 674–679. [Google Scholar] [CrossRef]
- Bordes, C.; Fréville, V.; Ruffin, E.; Marote, P.; Gauvrit, J.Y.; Briancon, S.; Lantéri, P. Determination of polycaprolactone solubility parameters: Application to solvent substitution in a microencapsulation process. Int. J. Pharm. 2010, 383, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Gholipour Kanani, A.; Hajir Bahrami, S. Effect of changing solvents on poly(ε-caprolactone) nanofibrous webs morphology. J. Nanomater. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Qin, X.; Wu, D. Effect of different solvents on poly(caprolactone) (PCL) electrospun nonwoven membranes. J. Therm. Anal. Calorim. 2012, 107, 1007–1013. [Google Scholar] [CrossRef]
- Dias, J.; Bártolo, P. Morphological characteristics of electrospun PCL meshes—The influence of solvent type and concentration. Procedia CIRP 2013, 5, 216–221. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. Temperature-responsive electrospun nanofibers for “on–off” switchable release of dextran. Sci. Technol. Adv. Mater. 2012, 12, 064203:1–064203:9. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. A smart nanofiber web that captures and releases cells. Angew. Chem. Int. Ed. 2012, 51, 10537–10541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kotsuchibashi, Y.; Uto, K.; Ebara, E.; Aoyagi, T.; Liu, Y.; Narain, R. pH and glucose responsive nanofibers for the reversible capture and release of lectins. Biomater. Sci. 2015, 3, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kotsuchibashi, Y.; Liu, Y.; Narain, R. Study of bacterial adhesion on biomimetic temperature responsive glycopolymer surfaces. ACS Appl. Mater. Interf. 2015, 7, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Uto, K.; Idota, N.; Hoffman, J.M.; Aoyagi, T. Shape-memory surface with dynamically tunable nanogeometry activated by body heat. Adv. Mater. 2012, 24, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Chmura, A.J.; Davidson, M.G.; Jones, M.D.; Lunn, M.D.; Mahon, M.F.; Johnson, A.F.; Khunkamchoo, P.; Roberts, S.L.; Wong, S.S.F. Group 4 complexes with aminebisphenolate ligands and their application for the ring opening polymerization of cyclic esters. Macromolecules 2006, 39, 7250–7257. [Google Scholar] [CrossRef]
- Sinnwell, S.; Inglis, A.J.; Davis, T.P.; Stenzel, M.H.; Barner-Kowollik, C. An atom-efficient conjugation approach to well-defined block copolymers using raft chemistry and hetero diels-alder cycloaddition. Chem. Commun. 2008, 2052–2054. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Gerster, J.F.; Owens, M.L.; Slade, H.B.; Tomai, M.A. Imiquimod applied topically a novel immune response modifier and new class of drug. Int. J. Immunopharmacol. 1999, 21, 1–14. [Google Scholar] [CrossRef]
- Lee, I.-H.; An, S.; Yu, M.K.; Kwon, H.-K.; Im, S.-H.; Jon, S. Targeted chemoimmunotherapy using drug-loaded aptamer-dendrimer bioconjugates. J. Control. Release 2011, 155, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, O.; Pasut, G.; Moro, S.; Orsolini, P.; Guiotto, A.; Veronese, F.M. PEG-Ara-C conjugates for controlled release. Eur. J. Med. Chem. 2004, 39, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Veeren, A.; Bhaw-Luximon, A. Polymer-drug encapsulation using various PEG- and polypeptide-based block copolymer micelles. Macromol. Symp. 2012, 313, 59–68. [Google Scholar] [CrossRef]
- Lee, P.; Zhang, R.; Li, V.; Liu, X.; Sun, R.W.; Che, C.-M.; Wong, K.K. Enhancement of anticancer efficacy using modified lipophilic nanoparticle drug encapsulation. Int. J. Nanomed. 2012, 7, 731–737. [Google Scholar]
- Choi, S.-H.; Youn, D.-Y.; Jo, S.M.; Oh, S.-G.; Kim, I.-D. Micelle-mediated synthesis of single-crystalline β(3c)-sic fibers via emulsion electrospinning. ACS Appl. Mater. Interf. 2011, 3, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrett, R.; Niiyama, E.; Kotsuchibashi, Y.; Uto, K.; Ebara, M. Biodegradable Nanofiber for Delivery of Immunomodulating Agent in the Treatment of Basal Cell Carcinoma. Fibers 2015, 3, 478-490. https://doi.org/10.3390/fib3040478
Garrett R, Niiyama E, Kotsuchibashi Y, Uto K, Ebara M. Biodegradable Nanofiber for Delivery of Immunomodulating Agent in the Treatment of Basal Cell Carcinoma. Fibers. 2015; 3(4):478-490. https://doi.org/10.3390/fib3040478
Chicago/Turabian StyleGarrett, Richard, Eri Niiyama, Yohei Kotsuchibashi, Koichiro Uto, and Mitsuhiro Ebara. 2015. "Biodegradable Nanofiber for Delivery of Immunomodulating Agent in the Treatment of Basal Cell Carcinoma" Fibers 3, no. 4: 478-490. https://doi.org/10.3390/fib3040478
APA StyleGarrett, R., Niiyama, E., Kotsuchibashi, Y., Uto, K., & Ebara, M. (2015). Biodegradable Nanofiber for Delivery of Immunomodulating Agent in the Treatment of Basal Cell Carcinoma. Fibers, 3(4), 478-490. https://doi.org/10.3390/fib3040478







