Commercial, Non-Commercial and Experimental Wound Dressings Based on Bacterial Cellulose: An In-Depth Comparative Study of Physicochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Samples of Bacterial Cellulose Wound Dressings
2.2. Characterization of Samples of Bacterial Cellulose Wound Dressings
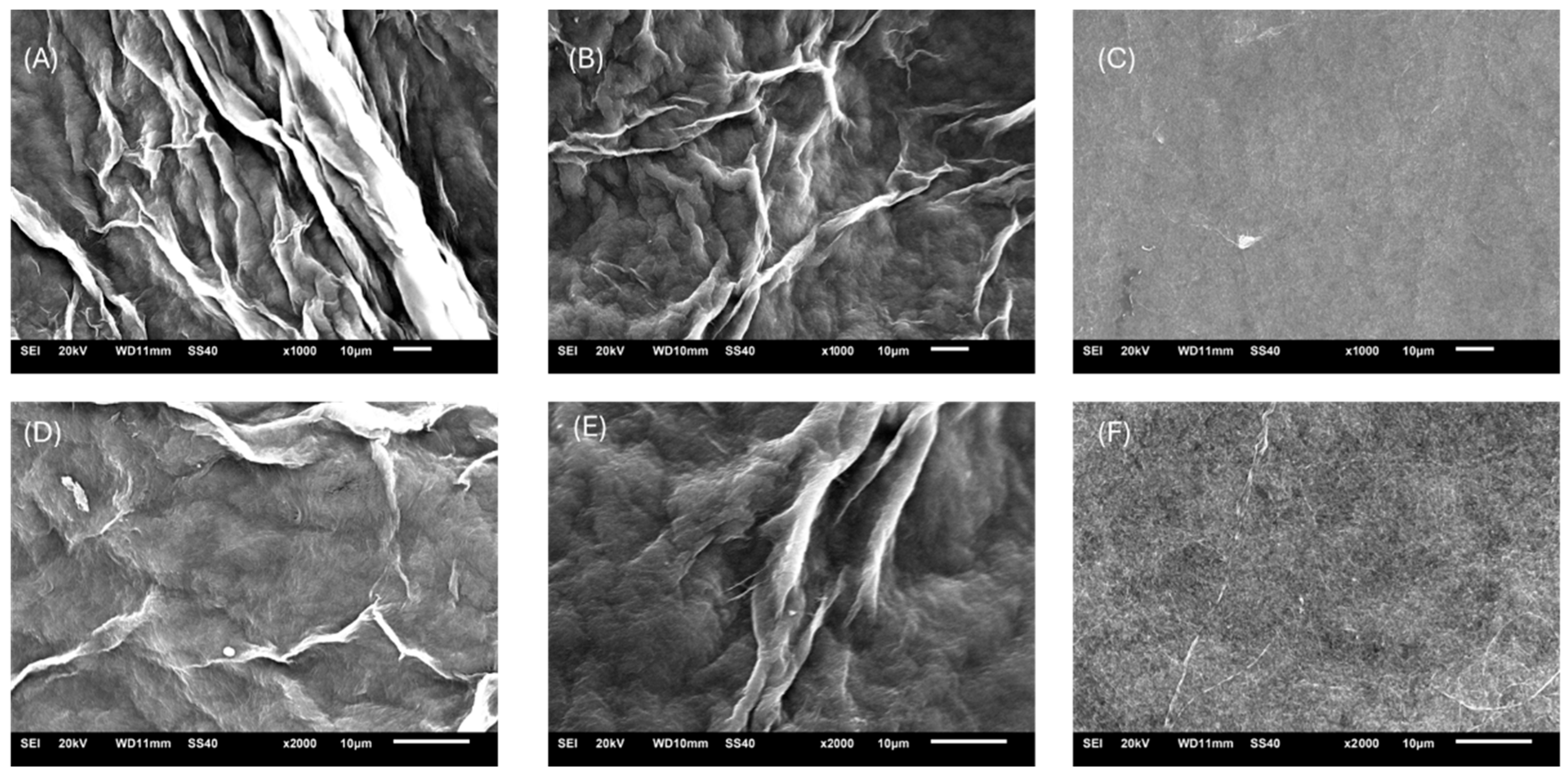
2.2.1. Scanning Electron Microscopy (SEM)
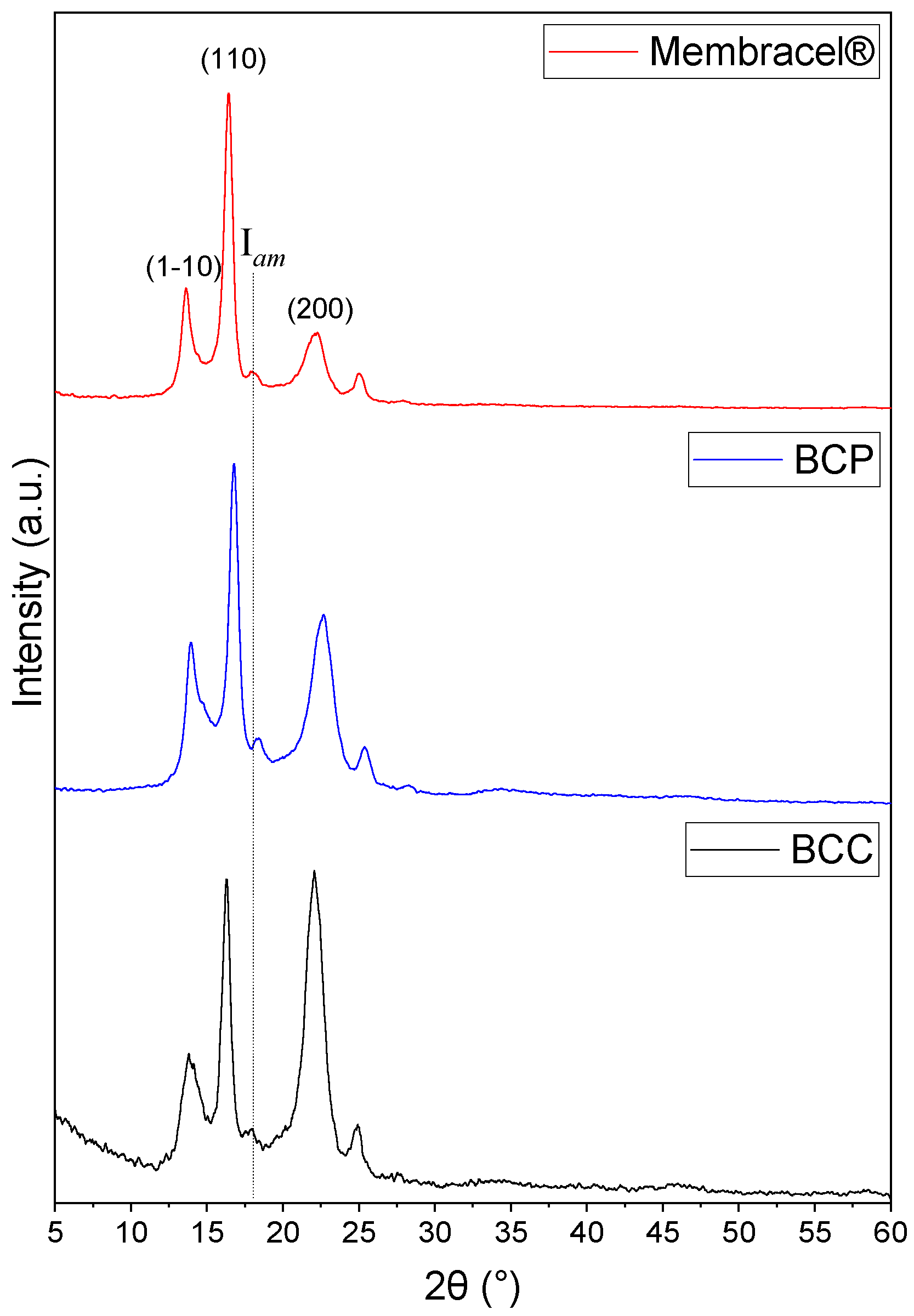
2.2.2. X-Ray Diffraction (XRD) and Fourier Transformed InfraRed Spectroscopy (FTIR)
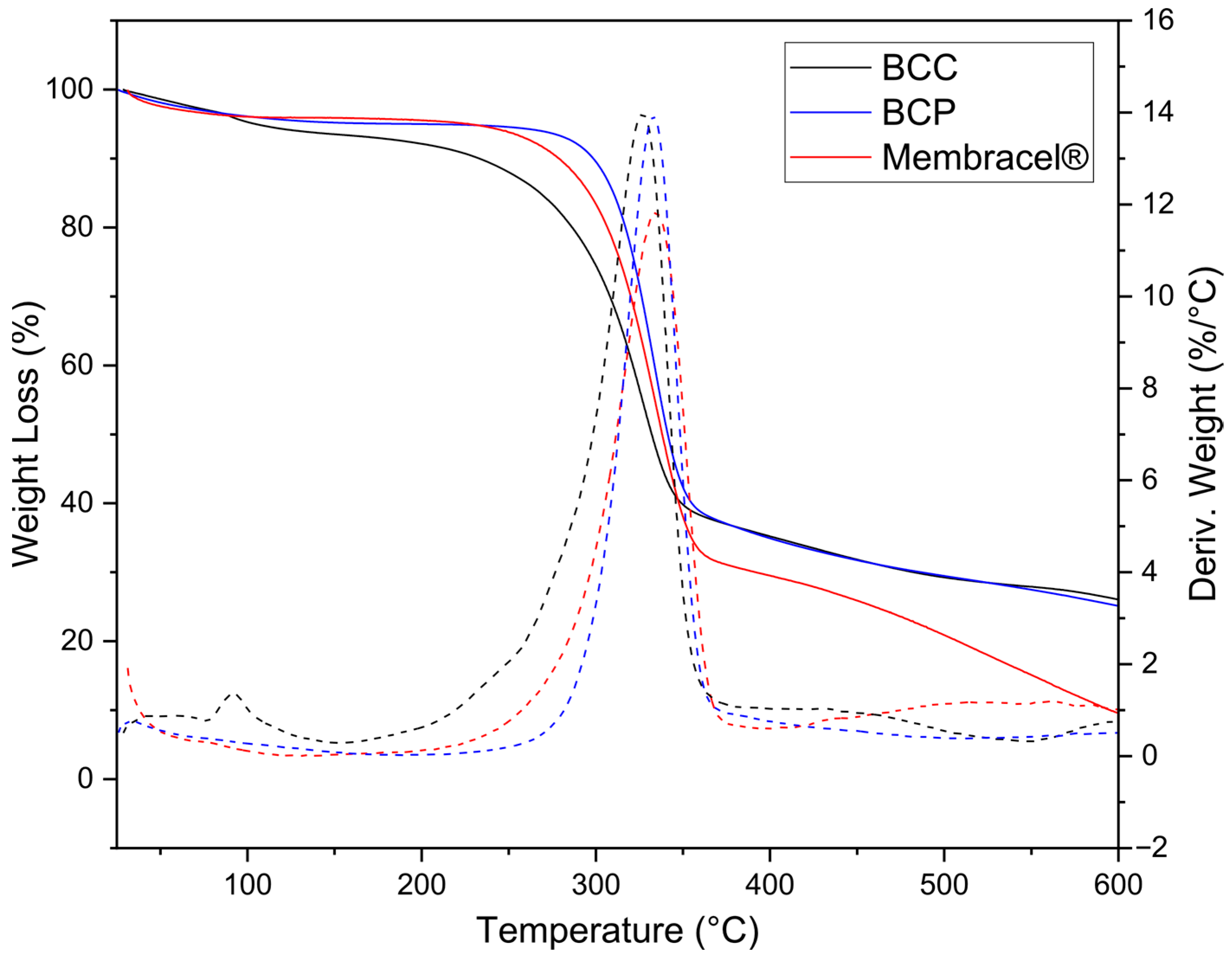
2.2.3. Thermogravimetric Analysis
2.2.4. Solid-State 13C Nuclear Magnetic Resonance (NMR) Analysis
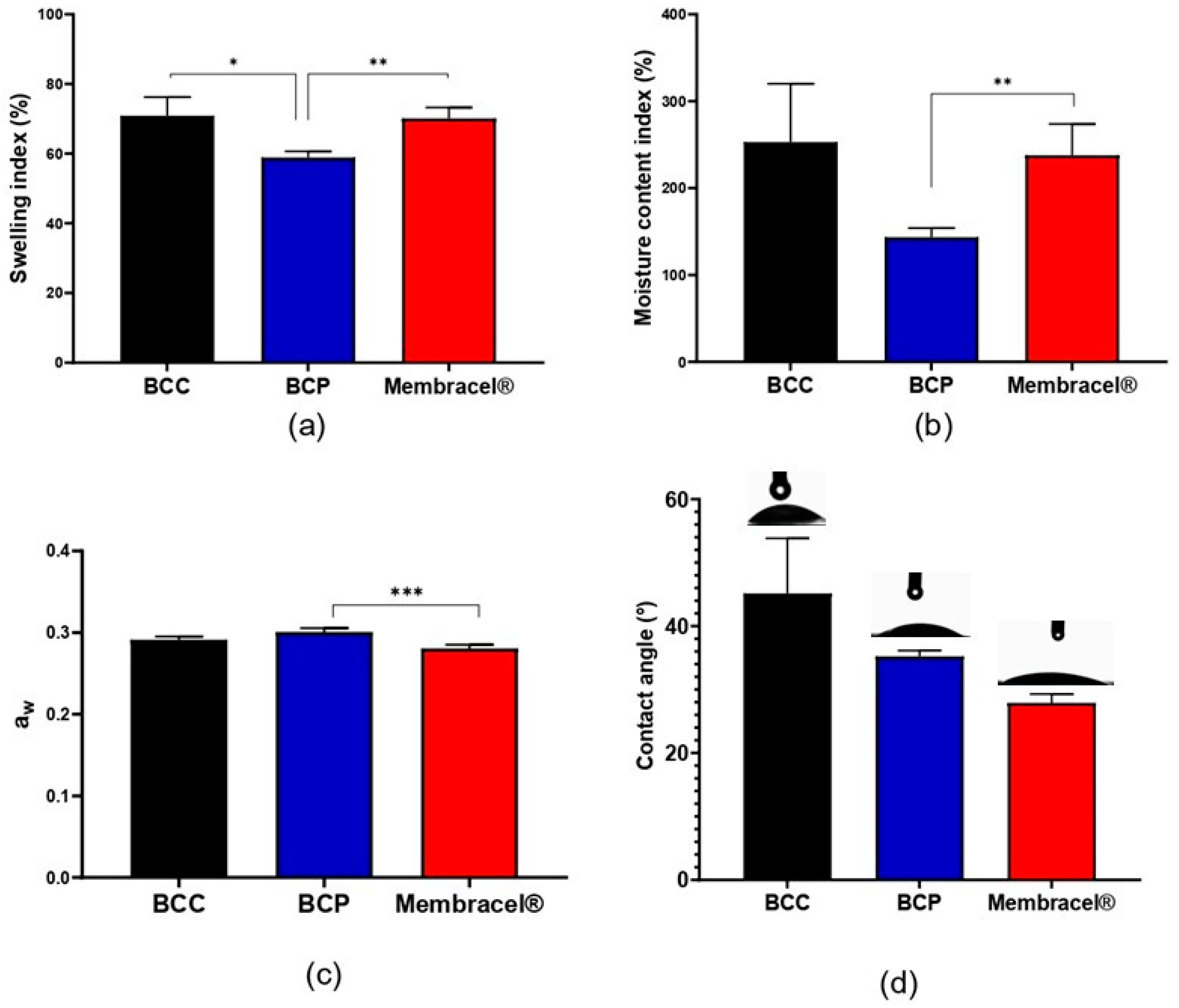
2.2.5. Water Interaction: Swelling Index, Moisture Content Index, Water Activity (aw) and Contact Angle
2.2.6. Porosity and Water Vapor Transmission Rate (WVTR) Analysis
2.2.7. Optical Properties
2.2.8. Tensile Mechanical Properties
2.2.9. In Vitro Stability and Fluid Uptake in Simulated Wound Fluid
2.3. Data Analysis
3. Results and Discussion
3.1. Morphological Properties
3.2. Physicochemical Characterization by FTIR and XRD
3.3. Thermal Characterization by TGA/DTG
3.4. Solid-State 13C NMR Analysis
3.5. Water Interaction Properties
3.6. Porosity and Water Vapor Permeability Characterization
3.7. Optical Properties
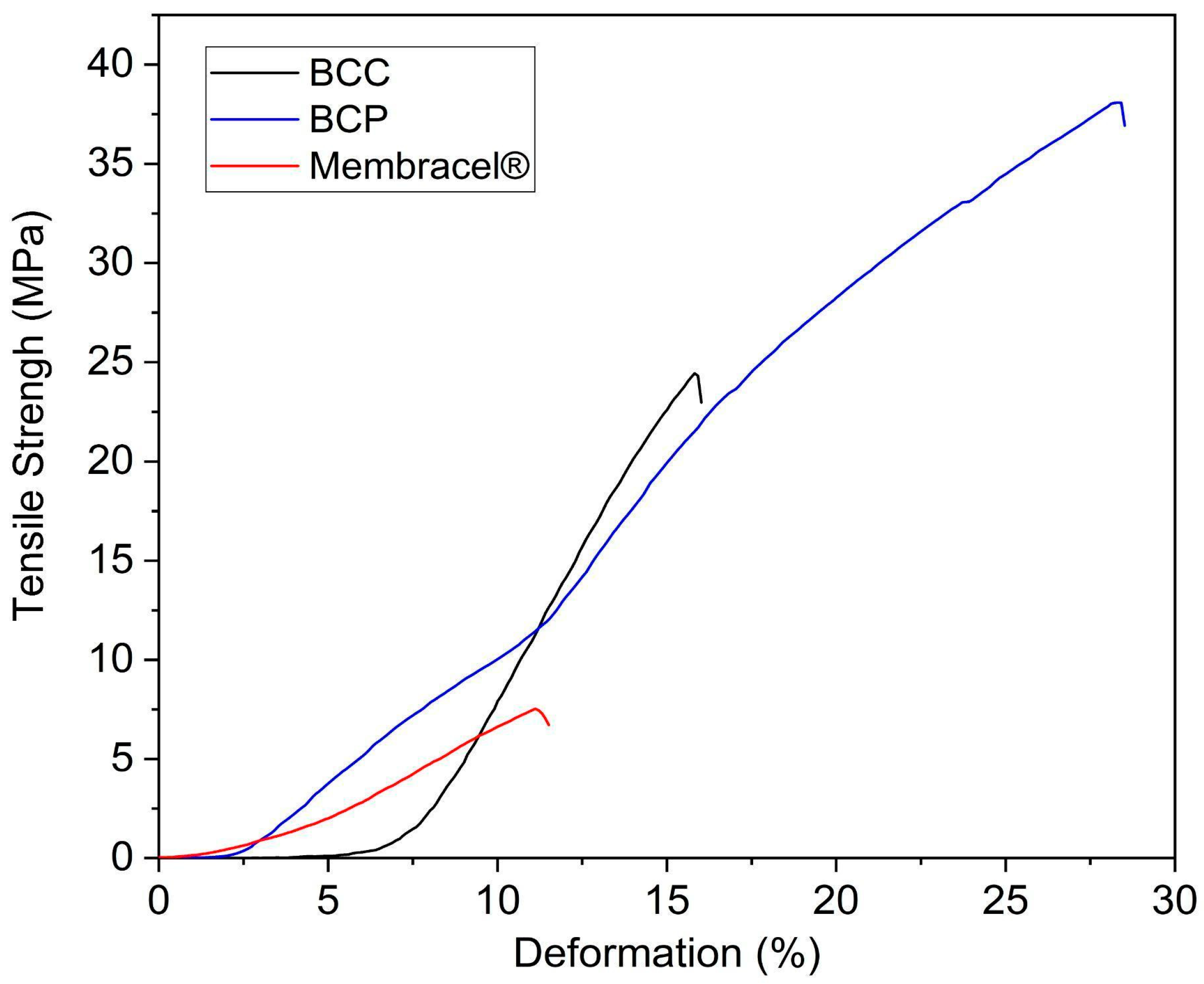
3.8. Mechanical Properties
3.9. In Vitro Stability and Fluid Uptake in Simulated Wound Fluid
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Bacterial cellulose |
| BCP | Bacterial cellulose from POLISA® |
| BCC | Bacterial cellulose from SENAI CIMATEC |
| SEM | Scanning electron microscopy |
| XRD | X-ray Diffraction |
| FTIR | Fourier Transformed Infrared Spectroscopy |
| TGA | Thermogravimetric analysis |
| DTG | Derivative Thermogravimetry |
| NMR | Nuclear Magnetic Resonance |
| SWF | Simulated wound fluid |
| ANOVA | Analysis of variance |
| CrI | Crystallinity index |
| WVTR | Water vapor transmission rate |
References
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic Wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2022 Compendium of Estimates. Adv. Wound Care 2023, 12, 657–670. [Google Scholar] [CrossRef]
- Díaz-Herrera, M.Á.; González-Durán, M.; Rodríguez-Martínez, F.J.; Tujillo-Flores, G.; Tuset-Mateu, N.; Verdú-Soriano, J.; Gea-Caballero, V.; Sanllorente-Melenchón, A.; Almeda-Ortega, J.; Cunillera-Puértolas, O.; et al. The Financial Burden of Chronic Wounds in Primary Care: A Real-World Data Analysis on Cost and Prevalence. Int. J. Nurs. Stud. Adv. 2025, 8, 100313. [Google Scholar] [CrossRef]
- Sharma, A.; Shankar, R.; Yadav, A.K.; Pratap, A.; Ansari, M.A.; Srivastava, V. Burden of Chronic Nonhealing Wounds: An Overview of the Worldwide Humanistic and Economic Burden to the Healthcare System. Int. J. Low Extrem. Wounds 2024, 15347346241246339. [Google Scholar] [CrossRef]
- Galvão Duarte, J.; Piedade, A.P.; Sarmento, B.; Mascarenhas-Melo, F. The Printed Path to Healing: Advancing Wound Dressings through Additive Manufacturing. Adv. Healthc. Mater. 2025, 14, 2402711. [Google Scholar] [CrossRef]
- Hodge, J.G.; Zamierowski, D.S.; Robinson, J.L.; Mellott, A.J. Evaluating Polymeric Biomaterials to Improve next Generation Wound Dressing Design. Biomater. Res. 2022, 26, 50. [Google Scholar] [CrossRef]
- Monami, M.; Ragghianti, B.; Scatena, A.; Miranda, C.; Monge, L.; Uccioli, L.; Stefanon, L.; Cappella, C.; Silverii, A.; Vermigli, C. Effectiveness of Different Advanced Wound Dressings versus Standard of Care for the Management of Diabetic Foot Ulcers: A Meta-Analysis of Randomized Controlled Trials for the Development of the Italian Guidelines for the Treatment of Diabetic Foot Syndrome. Acta Diabetol. 2024, 61, 1517–1526. [Google Scholar] [CrossRef]
- Pang, Q.; Yang, F.; Jiang, Z.; Wu, K.; Hou, R.; Zhu, Y. Smart Wound Dressing for Advanced Wound Management: Real-Time Monitoring and on-Demand Treatment. Mater. Des. 2023, 229, 111917. [Google Scholar] [CrossRef]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.d.S.; Castro, G.R. Bacterial Cellulose-Based Materials as Dressings for Wound Healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef]
- Utoiu, E.; Manoiu, V.S.; Oprita, E.I.; Craciunescu, O. Bacterial Cellulose: A Sustainable Source for Hydrogels and 3D-Printed Scaffolds for Tissue Engineering. Gels 2024, 10, 387. [Google Scholar] [CrossRef]
- Rumon, M.M.H. Advances in Cellulose-Based Hydrogels: Tunable Swelling Dynamics and Their Versatile Real-Time Applications. RSC Adv. 2025, 15, 11688–11729. [Google Scholar] [CrossRef]
- Hou, S.; Xia, Z.; Pan, J.; Wang, N.; Gao, H.; Ren, J.; Xia, X. Bacterial Cellulose Applied in Wound Dressing Materials: Production and Functional Modification—A Review. Macromol. Biosci. 2024, 24, 2300333. [Google Scholar] [CrossRef]
- Aditya, T.; Allain, J.P.; Jaramillo, C.; Restrepo, A.M. Surface Modification of Bacterial Cellulose for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 610. [Google Scholar] [CrossRef]
- Castilhos, C.; Rubira, L.T.; Nogeuira, M.L.S.; Santos, T.S. Estudo de Caso: Tratamento de Amputação de Antepé Com Membrana Regeneradora Membracel. Feridas 2020, 8, 1652–1657. [Google Scholar] [CrossRef]
- Barbosa Da Silva, S.; Salani, R.; De Cássia Ferreira, R.; Gazote Eloy Geraldo, Y.; Pavani, C.; Setúbal Destro Rodrigues, M.F.; Motta, L.J.; Fátima Teixeira Silva, D. Can Photons Pass through Primary Coatings Used to Treat Cutaneous Wounds? Adv. Ski. Wound Care 2021, 34, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.A.; Magalhães, M.A.B.; Matioski, A.; Ribas-Filho, J.M.; Magalhães, W.L.E.; Claro, F.C.; Ramos, R.K.; de Camargo, T.M.S.; Malafaia, O. Pine Nanocellulose and Bacterial Nanocellulose Dressings are Similar in the Treatment of Second-Degree Burn? Experimental Study in Rats. Arq. Bras. Cir. Dig. 2020, 33, e1533. [Google Scholar] [CrossRef]
- Nunes, C.A.d.B.; Melo, P.G.; Malaquias, S.G.; Amaral, K.V.Á.; Alves, G.R.; Meira, A.A.; Cardoso, A.L.; Pereira, L.V.; Bachion, M.M. Effectiveness of Two Bundles in Venous Leg Ulcer Healing: A Randomized Controlled Trial. J. Vasc. Nurs. 2019, 37, 232–245. [Google Scholar] [CrossRef]
- de Carvalho, R.S.F.; Mahnke, L.C.; Palácio, S.B.; Barbosa, W.T.; Hodel, K.V.S.; Barbosa, J.D.V.; Melo, F.d.A.D.; Chorilli, M.; Meneguin, A.B.; Pinto, F.C.M.; et al. Bacterial Cellulose Hydrogel Produced by Gluconacetobacter Hansenii Using Sugarcane Molasses as Medium: Physicochemical Characterization for Wound Healing Applications. Carbohydr. Polym. Technol. Appl. 2025, 9, 100632. [Google Scholar] [CrossRef]
- Hodel, K.V.S.; Fonseca, L.M.d.S.; Santos, I.M.d.S.; Cerqueira, J.C.; Santos-Júnior, R.E.d.; Nunes, S.B.; Barbosa, J.D.V.; Machado, B.A.S. Evaluation of Different Methods for Cultivating Gluconacetobacter Hansenii for Bacterial Cellulose and Montmorillonite Biocomposite Production: Wound-Dressing Applications. Polymers 2020, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-Bacterial Cellulose Patch of Ciprofloxacin for Wound Dressing: Preparation and Characterization Studies. Int. J. Biol. Macromol. 2020, 147, 1136–1145. [Google Scholar] [CrossRef]
- Maria, L.C.S.; Santos, A.L.C.; Oliveira, P.C.; Valle, A.S.S.; Barud, H.S.; Messaddeq, Y.; Ribeiro, S.J.L. Preparation and Antibacterial Activity of Silver Nanoparticles Impregnated in Bacterial Cellulose. Polímeros 2010, 20, 72–77. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Jędrzejczak-Krzepkowska, M.; Ludwicka, K. Comparative Analysis of Bacterial Cellulose Membranes Synthesized by Chosen Komagataeibacter Strains and Their Application Potential. Int. J. Mol. Sci. 2022, 23, 3391. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Du, R.; Zhao, F.; Peng, Q.; Zhou, Z.; Han, Y. Production and Characterization of Bacterial Cellulose Produced by Gluconacetobacter Xylinus Isolated from Chinese Persimmon Vinegar. Carbohydr. Polym. 2018, 194, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Leal, I.L.; Rosa, Y.C.d.S.; Penha, J.d.S.; Correia, P.R.C.; Melo, P.d.S.; Guimarães, D.H.; Barbosa, J.D.V.; Druzian, J.I.; Machado, B.A.S. Development and Application Starch Films:PBAT with Additives for Evaluating the Shelf Life of Tommy Atkins Mango in the Fresh-Cut State. J. Appl. Polym. Sci. 2019, 136, 48150–48169. [Google Scholar] [CrossRef]
- Ao, H.; Jiang, W.; Nie, Y.; Zhou, C.; Zong, J.; Liu, M.; Liu, X.; Wan, Y. Engineering Quaternized Chitosan in the 3D Bacterial Cellulose Structure for Antibacterial Wound Dressings. Polym. Test. 2020, 86, 106490. [Google Scholar] [CrossRef]
- ASTM E96-00; Standard Test Methods for Water Vapor Transmission of Materials. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2000. [CrossRef]
- Almeida, D.M.; Prestes, R.A.; Pinheiro, L.A.; Woicechowski, A.L.; Wosiacki, G. Propriedades Físicas, Químicas e de Barreira Em Filme Formados Por Blenda de Celulose Bacteriana e Fécula de Batata. Polímeros Ciência Tecnol. 2013, 23, 538–546. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M.; Velazquez, G. Environmentally Friendly Films Combining Bacterial Cellulose, Chitosan, and Polyvinyl Alcohol: Effect of Water Activity on Barrier, Mechanical, and Optical Properties. Biomacromolecules 2020, 21, 753–760. [Google Scholar] [CrossRef]
- Atila, D.; Karataş, A.; Keskin, D.; Tezcaner, A. Pullulan Hydrogel-Immobilized Bacterial Cellulose Membranes with Dual-Release of Vitamin C and E for Wound Dressing Applications. Int. J. Biol. Macromol. 2022, 218, 760–774. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Abdelraof, M.; Hashem, A.H.; El Saied, H. Sustainable Bacterial Cellulose Production by Achromobacter Using Mango Peel Waste. Microb. Cell Factories 2023, 22, 24. [Google Scholar] [CrossRef]
- Santos, S.M.; Carbajo, J.M.; Gómez, N.; Quintana, E.; Ladero, M.; Sánchez, A.; Chinga-Carrasco, G.; Villar, J.C. Use of Bacterial Cellulose in Degraded Paper Restoration. Part II: Application on Real Samples. J. Mater. Sci. 2016, 51, 1553–1561. [Google Scholar] [CrossRef]
- Das, M.; Zandraa, O.; Mudenur, C.; Saha, N.; Sáha, P.; Mandal, B.; Katiyar, V. Composite Scaffolds Based on Bacterial Cellulose for Wound Dressing Application. ACS Appl. Bio Mater. 2022, 5, 3722–3733. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Kiselev, E.G.; Nemtsev, I.V.; Vasiliev, A.D.; Kuzmin, A.P.; Shishatskaya, E.I. Bacterial Cellulose (BC) and BC Composites: Production and Properties. Nanomaterials 2022, 12, 192. [Google Scholar] [CrossRef]
- Wang, X.; Guo, C.; Hao, W.; Ullah, N.; Chen, L.; Li, Z.; Feng, X. Development and Characterization of Agar-Based Edible Films Reinforced with Nano-Bacterial Cellulose. Int. J. Biol. Macromol. 2018, 118, 722–730. [Google Scholar] [CrossRef]
- Ahmed, E.F.; Shawkat Ali, W.; Hasan Heider, N. Description and determination of the nanocellulose components produced from acetic acid bacteria. Rev. Bionatura 2023, 8, 112. [Google Scholar] [CrossRef]
- Dawwan, G.E.; El-Sayed, N.S.; Al-Shemy, M.T. Bacterial cellulose doped with ZnO as a multifunctional bioactive plataform for Curcumin and propolis Immobilization: Synthesis, characterization, and wound healing potential. Microb Cell Fact. 2025, 24, 196. [Google Scholar] [CrossRef] [PubMed]
- Don, T.-M.; Lee, K.-T.; Chen, B.-Y.; Tang, S.; Huang, Y.-C.; Chuang, A.E.-Y. Physicochemical Properties of Bacterial Cellulose/Phototherapeutic Polypyrrole/Antibacterial Chitosan Composite Membranes and Their Evaluation as Chronic Wound Dressings. Int. J. Biol. Macromol. 2025, 308, 142183. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Ul-Islam, M.; Kamal, T.; Ahmad, M.d.W.; Abbas, Y.; Manan, S.; Ullah, M.W.; Yang, G. Ex Situ Development and Characterization of Green Antibacterial Bacterial Cellulose-Based Composites for Potential Biomedical Applications. Adv. Compos. Hybrid Mater. 2022, 5, 307–321. [Google Scholar] [CrossRef]
- Pasaribu, K.M.; Ilyas, S.; Tamrin, T.; Radecka, I.; Swingler, S.; Gupta, A.; Stamboulis, A.G.; Gea, S. Bioactive Bacterial Cellulose Wound Dressings for Burns with Collagen In-Situ and Chitosan Ex-Situ Impregnation. Int. J. Biol. Macromol. 2023, 230, 123118. [Google Scholar] [CrossRef]
- Zulkarnain, N.N.; Abd Rahman, N.; Othman, A.R.; Md Saleh, N.; Mohd Ali, J.; Shukor, H.; Mohd Said, M.; Wan Zaki, W.R. Characterization and Antibacterial Activity of Bacterial Cellulose Impregnated with Moringa Oleifera Leaf Extract and Silver Nanoparticles. Cellulose 2024, 31, 5213–5227. [Google Scholar] [CrossRef]
- Mensah, A.; Chen, Y.; Christopher, N.; Wei, Q. Membrane Technological Pathways and Inherent Structure of Bacterial Cellulose Composites for Drug Delivery. Bioengineering 2022, 9, 3. [Google Scholar] [CrossRef]
- Claro, A.M.; Dias, I.K.R.; Fontes, M.d.L.; Colturato, V.M.M.; Lima, L.R.; Sávio, L.B.; Berto, G.L.; Arantes, V.; Barud, H.d.S. Bacterial Cellulose Nanocrystals Obtained through Enzymatic and Acidic Routes: A Comparative Study of Their Main Properties and in Vitro Biological Responses. Carbohydr. Res. 2024, 539, 109104. [Google Scholar] [CrossRef]
- Uğurel, C.; Öğüt, H. Optimization of Bacterial Cellulose Production by Komagataeibacter Rhaeticus K23. Fibers 2024, 12, 29. [Google Scholar] [CrossRef]
- Mamouri, K.S.; Rahaiee, S.; Zare, M.; Kenari, M.N.; Mirzakhani, N. Physicochemical and Thermal Characterization, and Evaluation of a Bacterial Cellulose/Barhang Gum-Based Dressing for Wound Healing. Int. J. Biol. Macromol. 2023, 242, 124660. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, M.; Won, S.-Y.; Zo, S.M.; Han, S.S. Fungal Carboxymethyl Chitosan-Impregnated Bacterial Cellulose Hydrogel as Wound-Dressing Agent. Gels 2023, 9, 184. [Google Scholar] [CrossRef]
- Pasaribu, K.M.; Gea, S.; Ilyas, S.; Tamrin, T.; Radecka, I. Characterization of Bacterial Cellulose-Based Wound Dressing in Different Order Impregnation of Chitosan and Collagen. Biomolecules 2020, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Inoue, B.S.; Streit, S.; dos Santos Schneider, A.L.; Meier, M.M. Bioactive Bacterial Cellulose Membrane with Prolonged Release of Chlorhexidine for Dental Medical Application. Int. J. Biol. Macromol. 2020, 148, 1098–1108. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. Physical, Structural, Mechanical and Thermal Characterization of Bacterial Cellulose by G. Hansenii NCIM 2529. Carbohydr. Polym. 2014, 106, 132–141. [Google Scholar] [CrossRef]
- Deng, L.; Huang, Y.; Chen, S.; Han, Z.; Han, Z.; Jin, M.; Qu, X.; Wang, B.; Wang, H.; Gu, S. Bacterial Cellulose-Based Hydrogel with Antibacterial Activity and Vascularization for Wound Healing. Carbohydr. Polym. 2023, 308, 120647. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Wu, C.; Chen, Y. Bacterial Cellulose-Based Superabsorbent Hydrogel for Wet Wound Dressing. Molecules 2025, 30, 737. [Google Scholar] [CrossRef]
- Teshima, R.; Osawa, S.; Yoshikawa, M.; Kawano, Y.; Otsuka, H.; Hanawa, T. Low-Adhesion and Low-Swelling Hydrogel Based on Alginate and Carbonated Water to Prevent Temporary Dilation of Wound Sites. Int. J. Biol. Macromol. 2024, 254, 127928. [Google Scholar] [CrossRef]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef]
- Wong, C.C.Q.; Tomura, K.; Yamamoto, O. Characterization of Sucrose-Impregnated Crystalline Glucose/Mannose Films as Moisturizing Wound Dressings and Their Significant Healing Effect on Deep Wounds in a Rat Model. Bioengineering 2025, 12, 327. [Google Scholar] [CrossRef]
- Hodel, K.V.S.; Machado, B.A.S.; Sacramento, G.d.C.; Maciel, C.A.d.O.; Oliveira-Junior, G.S.; Matos, B.N.; Gelfuso, G.M.; Nunes, S.B.; Barbosa, J.D.V.; Godoy, A.L.P.C. Active Potential of Bacterial Cellulose-Based Wound Dressing: Analysis of Its Potential for Dermal Lesion Treatment. Pharmaceutics 2022, 14, 1222. [Google Scholar] [CrossRef]
- Milne, S.D.; Seoudi, I.; Al Hamad, H.; Talal, T.K.; Anoop, A.A.; Allahverdi, N.; Zakaria, Z.; Menzies, R.; Connolly, P. A Wearable Wound Moisture Sensor as an Indicator for Wound Dressing Change: An Observational Study of Wound Moisture and Status. Int. Wound J. 2015, 13, 1309. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velázquez, G.; Vázquez, M. Bacterial Cellulose Films: Evaluation of the Water Interaction. Food Packag. Shelf Life 2020, 25, 100526. [Google Scholar] [CrossRef]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; Trujillo de Santiago, G. Structural and Biological Engineering of 3D Hydrogels for Wound Healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685. [Google Scholar] [CrossRef]
- Schmitz, M.; Eberlein, T.; Andriessen, A. Wound Treatment Costs Comparing a Bio-Cellulose Dressing with Moist Wound Healing Dressings and Conventional Dressings. Wound Med. 2014, 6, 11–14. [Google Scholar] [CrossRef]
- Rauscher, M.; Rauscher, A.; Hu, L.Y.; Schlitt, H.J.; Krauß, S.; Illg, C.; Wolfertstetter, P.R.; Hofmann, A.; Knorr, C.; Denzinger, M. Influence of Accumulation of Humidity under Wound Dressings and Effects on Transepidermal Water Loss (TEWL) and Skin Hydration. Appl. Sci. 2024, 14, 7739. [Google Scholar] [CrossRef]
- Yue, M.; Lei, M.; Liu, Y.; Gui, N. The Application of Moist Dressings in Wound Care for Tracheostomy Patients: A Meta-analysis. J. Clin. Nurs. 2019, 28, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Zhao, Z.; Li, J.; Huang, Y.; Chen, W. Multifunctional Dressings for Wound Exudate Management. Prog Mater Sci 2024, 146, 101328. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial Cellulose: A Versatile Biopolymer for Wound Dressing Applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Z.; Guo, T.; Jing, J.; Dang, Y. Accelerating Infectious Wound Healing through Bacterial Cellulose/Ag Composite Film Enriched with GM-CSF. Sci. Rep. 2025, 15, 22142. [Google Scholar] [CrossRef]
- Ciecholewska-Juśko, D.; Żywicka, A.; Junka, A.; Drozd, R.; Sobolewski, P.; Migdał, P.; Kowalska, U.; Toporkiewicz, M.; Fijałkowski, K. Superabsorbent Crosslinked Bacterial Cellulose Biomaterials for Chronic Wound Dressings. Carbohydr. Polym. 2021, 253, 117247. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; Cavalcanti, Y.d.F.; de Medeiros, A.D.M.; Silva Junior, C.J.G.d.; Durval, I.J.B.; Costa, A.F.d.S.; Sarubbo, L.A. Synthesis of Transparent Bacterial Cellulose Films as a Platform for Targeted Drug Delivery in Wound Care. Processes 2024, 12, 1282. [Google Scholar] [CrossRef]
- Kuddushi, M.; Shah, A.A.; Ayranci, C.; Zhang, X. Recent Advances in Novel Materials and Techniques for Developing Transparent Wound Dressings. J. Mater. Chem. B 2023, 11, 6201–6224. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Winarto, N.; Dosan, R.; Aisyah, P.B. The Benefits Of Occlusive Dressings In Wound Healing. Open Dermatol. J. 2019, 13, 27–33. [Google Scholar] [CrossRef]
- Chen, S.Q.; Lopez-Sanchez, P.; Wang, D.; Mikkelsen, D.; Gidley, M.J. Mechanical Properties of Bacterial Cellulose Synthesised by Diverse Strains of the Genus Komagataeibacter. Food Hydrocoll. 2018, 81, 87–95. [Google Scholar] [CrossRef]
- Dayal, M.S.; Catchmark, J.M. Mechanical and Structural Property Analysis of Bacterial Cellulose Composites. Carbohydr. Polym. 2016, 144, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Wang, L.; Zhao, J.C.; Zhu, P. Effect of Drying Methods on Structure and Mechanical Properties of Bacterial Cellulose Films. Adv. Mater. Res. 2011, 239, 2667–2670. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhao, X.Q.; Li, D.M.; Wu, Y.M.; Wahid, F.; Xie, Y.Y.; Zhong, C. Review on the Strategies for Enhancing Mechanical Properties of Bacterial Cellulose. J. Mater. Sci. 2023, 58, 15265–15293. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Biodegradation and Thermal Stability of Bacterial Cellulose as Biomaterial: The Relevance in Biomedical Applications. Polym. Degrad. Stab. 2020, 179, 109232. [Google Scholar] [CrossRef]
- Liu, G.; Zou, F.; He, W.; Li, J.; Xie, Y.; Ma, M.; Zheng, Y. The Controlled Degradation of Bacterial Cellulose in Simulated Physiological Environment by Immobilization and Release of Cellulase. Carbohydr. Polym. 2023, 314, 120906. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palácio, S.B.; Penello, S.O.; Hodel, K.V.S.; Barbosa, W.T.; Reis, G.A.; Machado, B.A.S.; Godoy, A.L.P.C.; Tavares, M.I.B.; Mahnke, L.C.; Viana Barbosa, J.D.; et al. Commercial, Non-Commercial and Experimental Wound Dressings Based on Bacterial Cellulose: An In-Depth Comparative Study of Physicochemical Properties. Fibers 2025, 13, 127. https://doi.org/10.3390/fib13090127
Palácio SB, Penello SO, Hodel KVS, Barbosa WT, Reis GA, Machado BAS, Godoy ALPC, Tavares MIB, Mahnke LC, Viana Barbosa JD, et al. Commercial, Non-Commercial and Experimental Wound Dressings Based on Bacterial Cellulose: An In-Depth Comparative Study of Physicochemical Properties. Fibers. 2025; 13(9):127. https://doi.org/10.3390/fib13090127
Chicago/Turabian StylePalácio, Sarah Brandão, Simone Oliveira Penello, Katharine Valéria Saraiva Hodel, Willams Teles Barbosa, Gisele Assunção Reis, Bruna Aparecida Souza Machado, Ana Leonor Pardo Campos Godoy, Maria Inês Bruno Tavares, Layla Carvalho Mahnke, Josiane Dantas Viana Barbosa, and et al. 2025. "Commercial, Non-Commercial and Experimental Wound Dressings Based on Bacterial Cellulose: An In-Depth Comparative Study of Physicochemical Properties" Fibers 13, no. 9: 127. https://doi.org/10.3390/fib13090127
APA StylePalácio, S. B., Penello, S. O., Hodel, K. V. S., Barbosa, W. T., Reis, G. A., Machado, B. A. S., Godoy, A. L. P. C., Tavares, M. I. B., Mahnke, L. C., Viana Barbosa, J. D., & Aguiar, J. L. d. A. (2025). Commercial, Non-Commercial and Experimental Wound Dressings Based on Bacterial Cellulose: An In-Depth Comparative Study of Physicochemical Properties. Fibers, 13(9), 127. https://doi.org/10.3390/fib13090127





