Fibrillated Nanocellulose Obtained by Mechanochemical Processes from Coconut Fiber Residue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Treatment to Obtain Cellulose
2.3. Obtaining Nanocellulose by the Grinding Method
2.4. Characterization Techniques
2.4.1. Fourier Transform Infrared Spectroscopy—FTIR
2.4.2. Elemental Analysis (CHNS)
2.4.3. X-Ray Diffraction (XRD) Analysis
2.4.4. Thermogravimetric Analysis and Differential Thermal Analysis
2.4.5. Morphological Surfaces (SEM, AFM)
- Scanning Electron Spectroscopy (SEM)
- Atomic Force Microscopy (AFM)
2.4.6. Rheology
3. Results
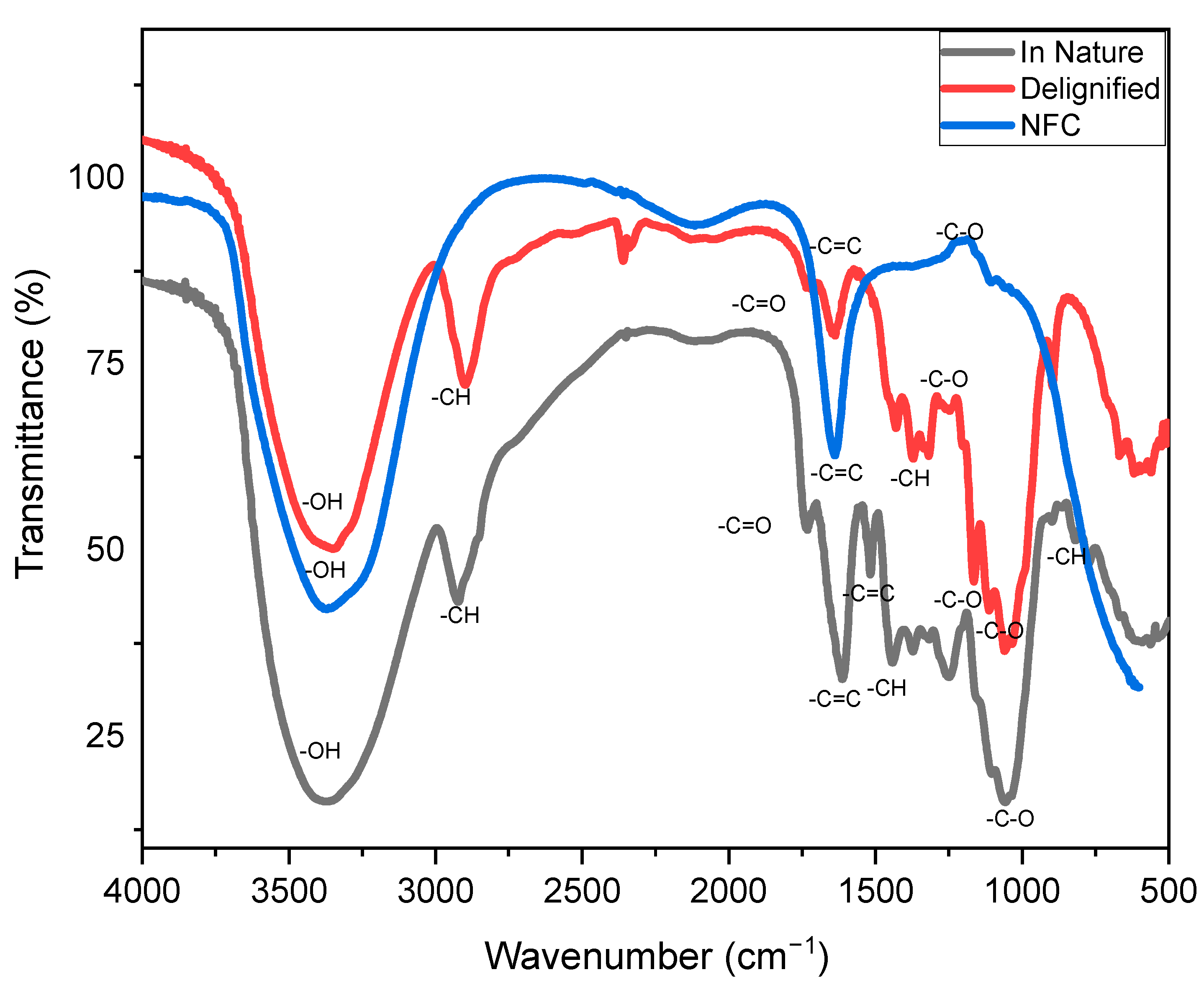
3.1. Fourier Transform Infrared Spectroscopy—FTIR
3.2. Elemental Analysis (CHNS)
3.3. Analysis of the Crystallinity of Green Coconut Fibers and Nanocellulose (XRD)
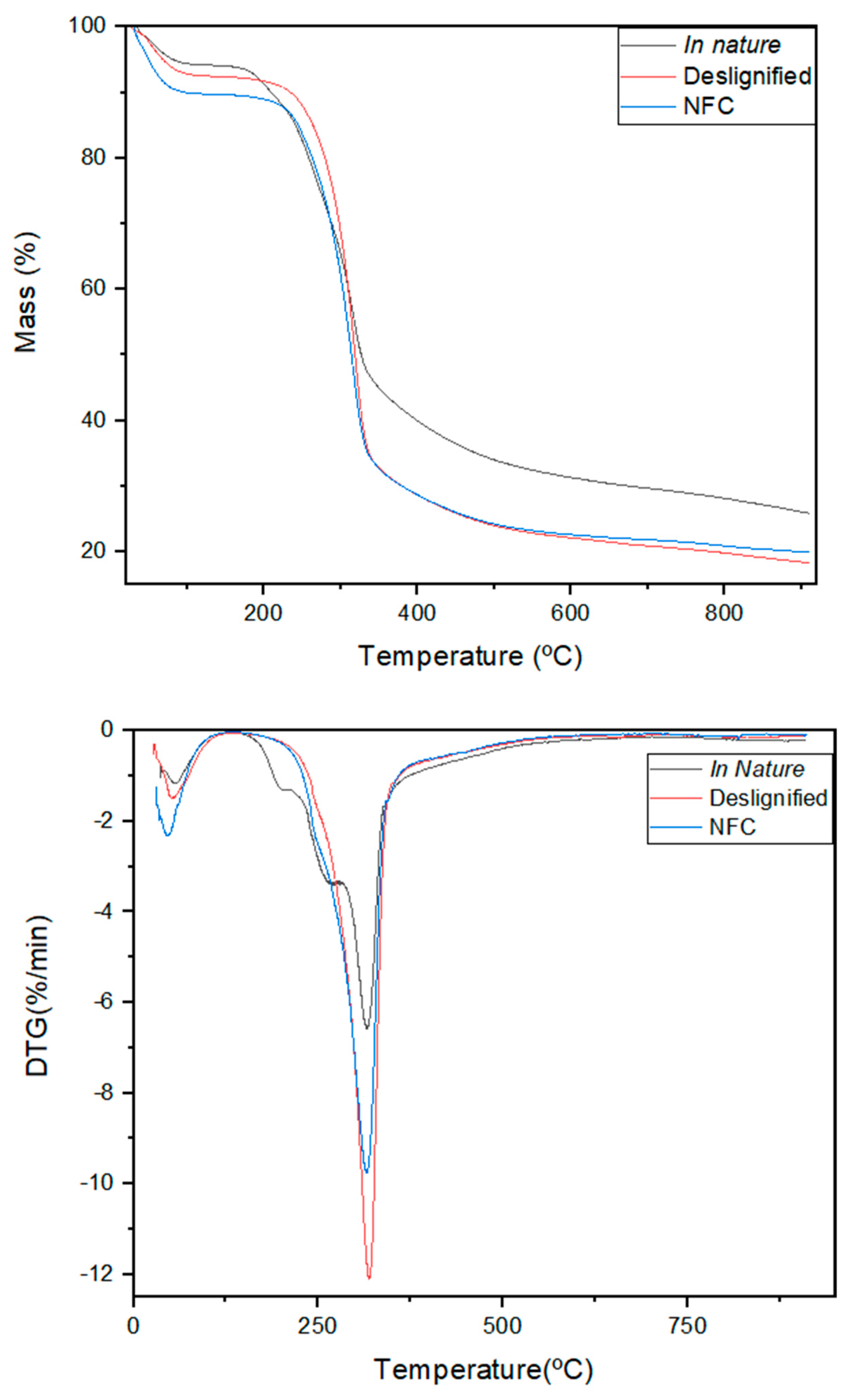
3.4. Thermogravimetric Analysis and Differential Thermal Analysis (TG/DTG)
3.5. Morphological Surfaces (SEM and AFM)
3.6. Rheology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNF | Cellulose Nanofibril |
| CNC | Cellulose Nanocrystal |
| FTIR | Fourier transform infrared spectroscopy |
| TG | Thermogravimetric Analysis |
| DTG | Thermogravimetric Derivative Analysis |
| DRX | X-ray diffraction |
| SEM | Scanning Electron Spectroscopy |
| AFM | Atomic Force Microscopy |
References
- Nagarajan, K.J. A Comprehensive Review on Cellulose Nanocrystals and Cellulose Nanofibers: Pretreatment, Preparation, and Characterization; Wiley: Hoboken, NJ, USA, 2021; ISBN 4860771060964. [Google Scholar]
- TAPPI International Nanotecnology Division. Roadmap for the Development of International Standards for Nanocellulose; TAPPI: Peachtree Corners, GA, USA, 2011; Volume 33. [Google Scholar]
- Nargotra, P.; Sharma, V.; Tsai, M.L.; Hsieh, S.L.; Dong, C.D.; Wang, H.M.D.; Kuo, C.H. Recent Advancements in the Valorization of Agro-Industrial Food Waste for the Production of Nanocellulose. Appl. Sci. 2023, 13, 6159. [Google Scholar] [CrossRef]
- Jose, J.; Vinod, T.P. Nanocellulose from Coconut Midrib Used for Antibacterial and Electromagnetic Interference Shielding Applications. New J. Chem. 2025, 49, 9475–9483. [Google Scholar] [CrossRef]
- Vincent, S.; Kandasubramanian, B. Cellulose Nanocrystals from Agricultural Resources: Extraction and Functionalisation. Eur Polym. J. 2021, 160, 110789. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Leow, Y.; Boo, Y.J.; Lin, M.; Tan, Y.C.; Goh, R.Z.R.; Zhu, Q.; Loh, X.J.; Xue, K.; Kai, D. Coconut Husk-Derived Nanocellulose as Reinforcing Additives in Thermal-Responsive Hydrogels. Carbohydr. Polym. 2024, 323, 121453. [Google Scholar] [CrossRef]
- Arun, R.; Shruthy, R.; Preetha, R.; Sreejit, V. Biodegradable Nano Composite Reinforced with Cellulose Nano Fiber from Coconut Industry Waste for Replacing Synthetic Plastic Food Packaging. Chemosphere 2022, 291, 132786. [Google Scholar] [CrossRef]
- Marakana, P.G.; Dey, A.; Saini, B. Isolation of Nanocellulose from Lignocellulosic Biomass: Synthesis, Characterization, Modification, and Potential Applications. J. Environ. Chem. Eng. 2021, 9, 106606. [Google Scholar] [CrossRef]
- Rosa, M.d.F.; Abreu, F.A.P.d.; Furtado, Â.A.L.; Brígido, A.K.L.; Norões, E.R.d.V. Processo Agroindustrial: Obtenção de Pó de Casca de Coco Verde; Embrapa Comunicado Técnico: Brasília, DF, Brazil, 2001; Volume 61, pp. 1–4. [Google Scholar]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose Nanowhiskers from Coconut Husk Fibers: Effect of Preparation Conditions on Their Thermal and Morphological Behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Mahrouz, M.; Malainine, M.E.; Dufresne, A.; Vuong, R.; Vignon, M.R. Structure and Morphology of Cladodes and Spines of Opuntia Ficus-Indica. Cellul. Extr. Characterisation 2003, 51, 77–83. [Google Scholar]
- Zenni, M.R.; Santos, C.P.d.; Bianchi, R.M.d.C. Celulose Nanofibrilada: Estudo Da Obtenção E Aplicação Na Indústria Papeleira. Eng. Mod. Soluções Probl. Soc. Indústria 2021, 2, 107–119. [Google Scholar] [CrossRef]
- Petersohn Junior, E.; Pires, C.; de Freitas, R.A.; Magalhães, W.L.E. Effect of the Addition of Hydroxypropyl Methylcellulose and Hydroxyethyl Cellulose on the Rheological Properties and Thermogravimetric Kinetics of Dried and Redispersed Microfibrillated Cellulose. Fibers Polym. 2025, 26, 1465–1478. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different Preparation Methods and Properties of Nanostructured Cellulose from Various Natural Resources and Residues: A Review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of Cellulose Nanofibrils: A Review of Recent Advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Fonseca, A.S.; Panthapulakkal, S.; Konar, S.K.; Sain, M.; Bufalino, L.; Raabe, J.; Miranda, I.P.A.; Martins, M.A.; Tonoli, G.H.D. Improving Cellulose Nanofibrillation of Non-Wood Fiber Using Alkaline and Bleaching Pre-Treatments. Ind. Crops Prod. 2019, 131, 203–212. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Abd Hamid, S.B. Facile Production of Nanostructured Cellulose from Elaeis Guineensis Empty Fruit Bunch via One Pot Oxidative-Hydrolysis Isolation Approach. Carbohydr. Polym. 2017, 157, 1511–1524. [Google Scholar] [CrossRef]
- Clecius, A.; Lima, A.D.; Nascimento, R.F.; Sousa, F.F.D.; Filho, J.M.; Oliveira, A.C. Modified Coconut Shell Fibers: A Green and Economical Sorbent for the Removal of Anions from Aqueous Solutions. Chem. Eng. J. 2012, 185–186, 274–284. [Google Scholar] [CrossRef]
- Ferreira, H.S.; Pires, C.A.D.M. Ag/TiO2 Photocatalyst Immobilized onto Modified Natural Fibers for Photodegradation of Anthracene. Chem. Eng. Sci. 2020, 227, 115939. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, Preparation and Characterization of Cellulose Fibres and Nanocrystals from Rice Husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Ruiz, A.; Silvino, E.; Avelino, F. Bioethanol Production by Saccharomyces Cerevisiae, Pichia Stipitis and Zymomonas Mobilis from Deligni Fi Ed Coconut Fi Bre Mature and Lignin Extraction According to Biore Fi Nery Concept. Renew. Energy 2016, 94, 353–365. [Google Scholar] [CrossRef]
- Soriano, J.A.; García-Contreras, R.; Carpio de Los Pinos, A.J. Study of the Thermochemical Properties of Lignocellulosic Biomass from Energy Crops. Energies 2021, 14, 3780. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Nizamuddin, S.; Xu, J.; Vancov, T.; Chen, C. Cellulose Nanocrystals from Agriculture and Forestry Biomass: Synthesis Methods, Characterization and Industrial Applications. Environ. Sci. Pollut. Res. 2024, 31, 58745–58778. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley: Hoboken, NJ, USA, 2017; pp. 1–49. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A Review of Nanocellulose as a New Material towards Environmental Sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Hernandez, C.C.; Ferreira, F.F.; Rosa, D.S. X-Ray Powder Diffraction and Other Analyses of Cellulose Nanocrystals Obtained from Corn Straw by Chemical Treatments. Carbohydr. Polym. 2018, 193, 39–44. [Google Scholar] [CrossRef]
- Wu, J.; Du, X.; Yin, Z.; Xu, S.; Xu, S.; Zhang, Y. Preparation and Characterization of Cellulose Nanofibrils from Coconut Coir Fibers and Their Reinforcements in Biodegradable Composite Films. Carbohydr. Polym. 2019, 211, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chirayil, C.J.; Joy, J.; Mathew, L.; Mozetic, M.; Koetz, J.; Thomas, S. Isolation and Characterization of Cellulose Nanofibrils from Helicteres Isora Plant. Ind. Crops Prod. 2014, 59, 27–34. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Dhlamini, M.S.; Mothudi, B.M.; Kommula, V.P.; Zhang, J.; Zhang, J.; Rajulu, A.V. Extraction and Characterization of Cellulose Single Fibers from Native African Napier Grass. Carbohydr. Polym. 2018, 188, 85–91. [Google Scholar] [CrossRef]
- Rosa, S.M.L.; Rehman, N.; De Miranda, M.I.G.; Nachtigall, S.M.B.; Bica, C.I.D. Chlorine-Free Extraction of Cellulose from Rice Husk and Whisker Isolation. Carbohydr. Polym. 2012, 87, 1131–1138. [Google Scholar] [CrossRef]
- Mishra, L.; Basu, G. Coconut Fibre: Its Structure, Properties and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 1, ISBN 9780128206669. [Google Scholar]
- Nagarajan, K.J.; Balaji, A.N.; Ramanujam, N.R. Extraction of Cellulose Nano Fi Bers from Cocos Nucifera Var Aurantiaca Peduncle by Ball Milling Combined with Chemical Treatment. Carbohydr. Polym. 2019, 212, 312–322. [Google Scholar] [CrossRef]
- Moura, H.O.M.A.; Câmara, A.B.F.; Silva, B.R.; Albuquerque, I.M.; Oliveira, K.G.; Campos, L.M.A.; Carvalho, L.S. Nova Metodologia De Caracterização Composicional Da Lignocelulose Aplicando a Deconvolução De Dados Termogravimétricos. Open Sci. Res. IV 2022, 4, 604–619. [Google Scholar] [CrossRef]
- Iotti, M.; Gregersen, Ø.W.; Moe, S.; Lenes, M. Rheological Studies of Microfibrillar Cellulose Water Dispersions. J. Polym. Environ. 2011, 19, 137–145. [Google Scholar] [CrossRef]
- Moberg, T.; Sahlin, K.; Yao, K.; Geng, S.; Westman, G.; Zhou, Q.; Oksman, K.; Rigdahl, M. Rheological Properties of Nanocellulose Suspensions: Effects of Fibril/Particle Dimensions and Surface Characteristics. Cellulose 2017, 24, 2499–2510. [Google Scholar] [CrossRef]
- Pääkko, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic Hydrolysis Combined with Mechanical Shearing and High-Pressure Homogenization for Nanoscale Cellulose Fibrils and Strong Gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Schramm, G. A Practical Approach to Rheology and Rheometry, 2nd ed.; Rheology; Gebrueder HAAKE GmbH: Karlsruhe, Germany, 1994. [Google Scholar]
- Zhang, H.; Wang, H.; Dai, H.; Chen, H.; Ma, L.; Zhang, Y. Regulating Emulsion Properties through Tannin Acid-Mediated Gelatin/Cellulose Nanocrystal Complexes: From Low-Oil Emulsion to High Internal Phase Emulsion Gel for 3D Printing. Food Hydrocoll. 2025, 159, 110647. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, R.; Chen, Y.; Wang, T.; Wu, J.; Wang, S. The Preparation and Characterization of Pineapple Peel Cellulose Nanofibers and Its Application in Oil-Water Emulsions. Carbohydr. Polym. 2025, 353, 123245. [Google Scholar] [CrossRef] [PubMed]







| Sample | Crystallinity Index (C.I.), % |
|---|---|
| In natura | 30 |
| Delignified NFC | 40 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.I.d.S.; Pires, C.; Petersohn Junior, E.; Cordeiro, A.M.T.d.M.; Freitas, R.A.d.; Santos, N.A.d. Fibrillated Nanocellulose Obtained by Mechanochemical Processes from Coconut Fiber Residue. Fibers 2025, 13, 123. https://doi.org/10.3390/fib13090123
Silva SIdS, Pires C, Petersohn Junior E, Cordeiro AMTdM, Freitas RAd, Santos NAd. Fibrillated Nanocellulose Obtained by Mechanochemical Processes from Coconut Fiber Residue. Fibers. 2025; 13(9):123. https://doi.org/10.3390/fib13090123
Chicago/Turabian StyleSilva, Sarah Inglid dos Santos, Cassiano Pires, Egon Petersohn Junior, Angela Maria Tribuzy de Magalhães Cordeiro, Rilton Alves de Freitas, and Nataly Albuquerque dos Santos. 2025. "Fibrillated Nanocellulose Obtained by Mechanochemical Processes from Coconut Fiber Residue" Fibers 13, no. 9: 123. https://doi.org/10.3390/fib13090123
APA StyleSilva, S. I. d. S., Pires, C., Petersohn Junior, E., Cordeiro, A. M. T. d. M., Freitas, R. A. d., & Santos, N. A. d. (2025). Fibrillated Nanocellulose Obtained by Mechanochemical Processes from Coconut Fiber Residue. Fibers, 13(9), 123. https://doi.org/10.3390/fib13090123







