Poly-D,L-Lactic Acid as a Compatibilizer for Nootkatone-Embedded Nylon 12 Fabric Manufacturing
Abstract
Highlights
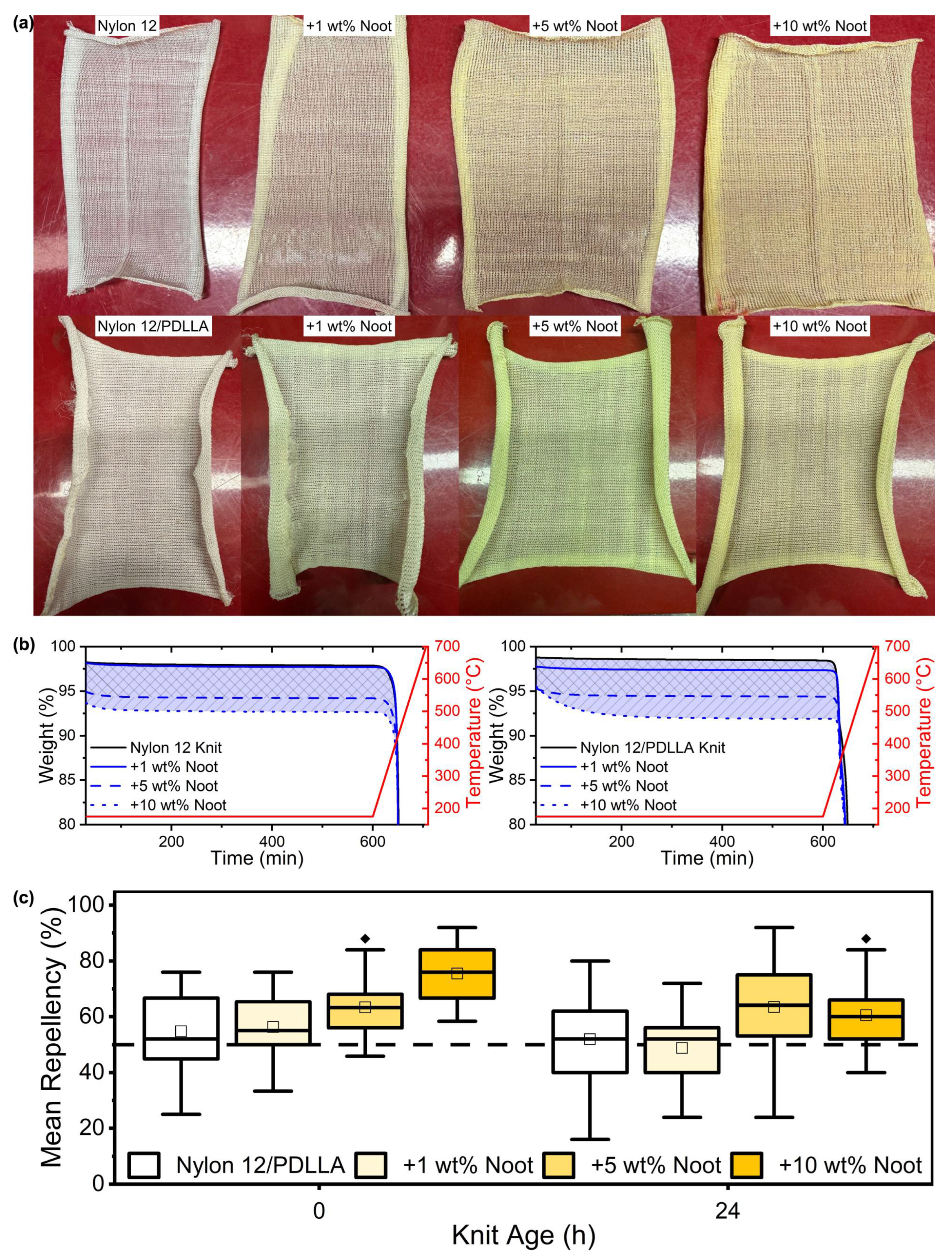
- Nootkatone retention throughout Nylon 12 fabric manufacturing was increased through the addition of poly-D,L-lactic acid (PDLLA) as a compatibilizer.
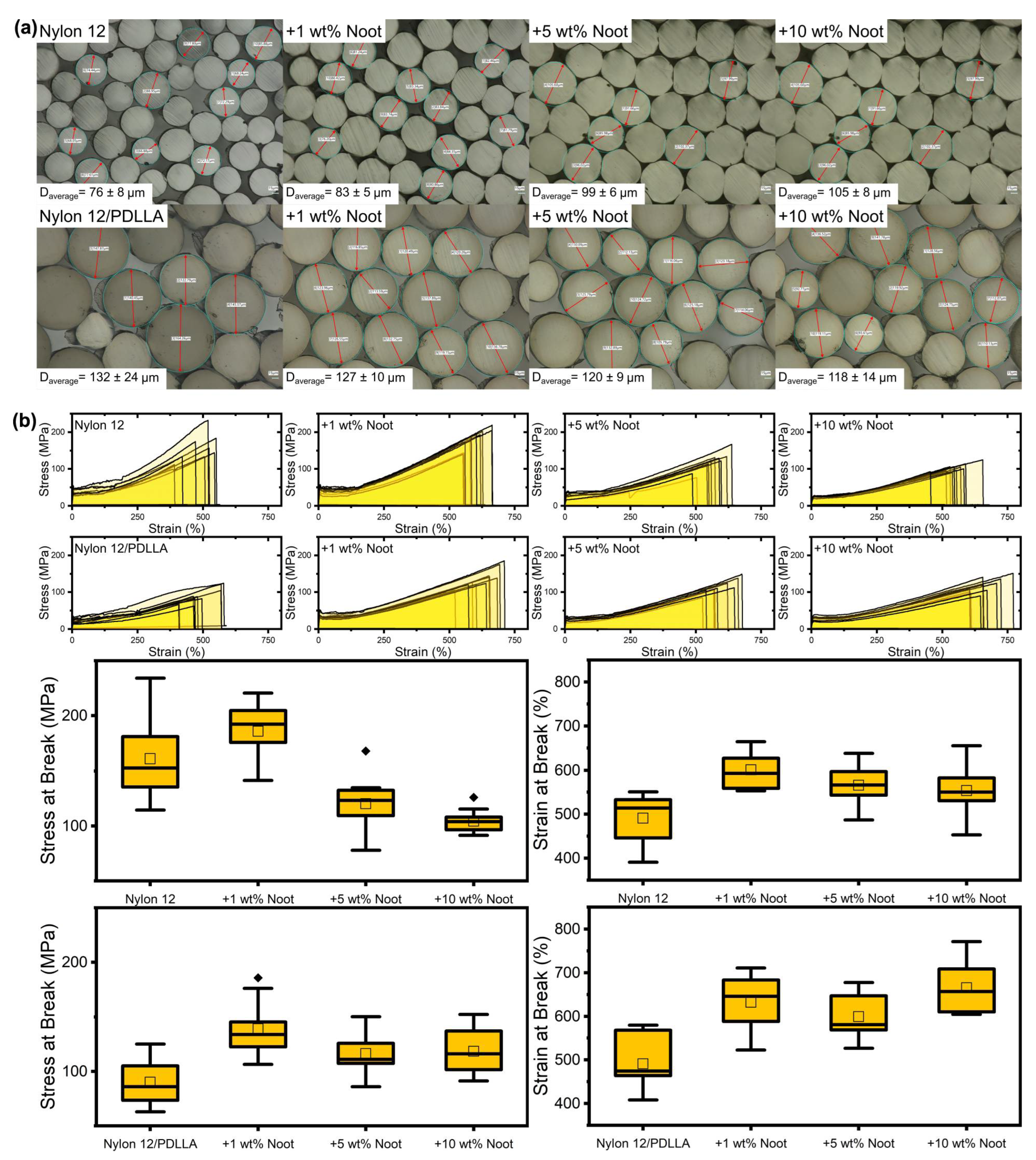
- Nylon 12/PDLLA filaments maintained similar tensile properties up to 10 wt% nootkatone loading whereas Nylon 12 demonstrated decreasing properties at >1 wt% nootkatone.
- The chemical affinity present between PDLLA and nootkatone hinders volatile diffusion of nootkatone during Nylon 12 fabric manufacturing.
- The PDLLA domains are suspected to behave as reservoirs for excess nootkatone to prevent its role as a defect within the Nylon 12 matrix.
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Compounding and Fabric Manufacturing
2.2. Characterization Techniques
2.3. Live Mosquito Bioassay
3. Results and Discussion
3.1. Characterization of Nootkatone-Infused Pellets
3.2. Spinning, Knitting, and Mosquito Bioassay of Nootkatone-Infused Fabrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Daverage | average diameter |
| DSC | Differential Scanning Calorimetry |
| DTG | Differential Thermogravimetric Analysis |
| HSPs | Hansen Solubility Parameters |
| PDLLA | poly-D,L-lactic acid |
| PLA | polylactic acid |
| PLLA | poly-L-lactic acid |
| Tg | glass transition temperature |
| TGA | thermogravimetric analysis |
| Tm | melting temperature |
References
- Katz, T.M.; Miller, J.H.; Hebert, A.A. Insect repellents: Historical perspectives and new developments. J. Am. Acad. Dermatol. 2008, 58, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Lupi, E.; Hatz, C.; Schlagenhauf, P. The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp.—A literature review. Travel Med. Infect. Dis. 2013, 11, 374–411. [Google Scholar] [CrossRef]
- Orsborne, J.; DeRaedt Banks, S.; Hendy, A.; Gezan, S.A.; Kaur, H.; Wilder-Smith, A.; Lindsay, S.W.; Logan, J.G. Personal Protection of Permethrin-Treated Clothing against Aedes aegypti, the Vector of Dengue and Zika Virus, in the Laboratory. PLoS ONE 2016, 11, e0152805. [Google Scholar] [CrossRef]
- Pennetier, C.; Chabi, J.; Martin, T.; Chandre, F.; Rogier, C.; Hougard, J.-M.; Pages, F. New protective battle-dress impregnated against mosquito vector bites. Parasites Vectors 2010, 3, 81. [Google Scholar] [CrossRef]
- Frances, S.P.; Watson, K.; Constable, B.G. Comparative toxicity of permethrin- and bifenthrin-treated cloth fabric for Anopheles farauti and Aedes aegypti. J. Am. Mosq. Control Assoc. 2003, 19, 275–278. [Google Scholar]
- Sholdt, L.L.; Schreck, C.E.; Mwangelwa, M.I.; Nondo, J.; Siachinji, V.J. Evaluations of permethrin-impregnated clothing and three topical repellent formulations of deet against tsetse flies in Zambia. Med. Vet. Entomol. 1989, 3, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, M.F.; Funkhouser, S.W.; Lin, F.-C.; Fine, J.; Juliano, J.J.; Apperson, C.S.; Meshnick, S.R. Long-lasting Permethrin Impregnated Uniforms: A Randomized-Controlled Trial for Tick Bite Prevention. Am. J. Prev. Med. 2014, 46, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Schofield, S.; Crane, F.; Tepper, M. Good Interventions That Few Use: Uptake of Insect Bite Precautions in a Group of Canadian Forces Personnel Deployed to Kabul, Afghanistan. Mil. Med. 2012, 177, 209–215. [Google Scholar] [CrossRef]
- Chen-Hussey, V.; Behrens, R.; Logan, J.G. Assessment of methods used to determine the safety of the topical insect repellent N,N-diethyl-m-toluamide (DEET). Parasites Vectors 2014, 7, 173. [Google Scholar] [CrossRef]
- Ryan, J.J.; Casalini, R.; Orlicki, J.A.; Lundin, J.G. Controlled release of the insect repellent picaridin from electrospun nylon-6,6 nanofibers. Polym. Adv. Technol. 2020, 31, 3039–3047. [Google Scholar] [CrossRef]
- Fulton, A.C.; Thum, M.D.; Jimenez, J.; Camarella, G.; Cilek, J.E.; Lundin, J.G. Long-Term Insect Repellent Electrospun Microfibers from Recycled Poly(ethylene terephthalate). Acs Appl. Mater. Inter. 2023, 15, 44722–44730. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.; da Silva, M.R.M.; de Oliveira de Siqueira, L.B.; Rodrigues, R.A.S.; Bodjolle-d’Almeida, L.; dos Santos, E.P.; Ricci-Júnior, E. Trends in insect repellent formulations: A review. Int. J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Bonadies, I.; Longo, A.; Rupp, H.; Di Lorenzo, M.L.; Androsch, R. Sustainable Electrospun Poly(l-lactic acid) Fibers for Controlled Release of the Mosquito-Repellent Ethyl Butylacetylaminopropionate (IR3535). ACS Appl. Polym. Mater. 2023, 5, 4838–4848. [Google Scholar] [CrossRef]
- Jimenez, J.; Cilek, J.E.; Schluep, S.M.; Lundin, J.G. Designing thermoreversible gels for extended release of mosquito repellent. J. Mater. Chem. B 2024, 12, 9249–9257. [Google Scholar] [CrossRef]
- Sibanda, M.; Focke, W.; Braack, L.; Leuteritz, A.; Brünig, H.; Tran, N.H.A.; Wieczorek, F.; Trümper, W. Bicomponent fibres for controlled release of volatile mosquito repellents. Mater. Sci. Eng. C 2018, 91, 754–761. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Focke, W.W.; Tewo, R.K.; Androsch, R.; Kruger, T. Mosquito-repellent controlled-release formulations for fighting infectious diseases. Malar. J. 2021, 20, 165. [Google Scholar] [CrossRef]
- Muñoz, V.; Buffa, F.; Molinari, F.; Hermida, L.G.; García, J.J.; Abraham, G.A. Electrospun ethylcellulose-based nanofibrous mats with insect-repellent activity. Mater. Lett. 2019, 253, 289–292. [Google Scholar] [CrossRef]
- Misni, N.; Nor, Z.M.; Ahmad, R. Repellent effect of microencapsulated essential oil in lotion formulation against mosquito bites. J. Vector Borne Dis. 2017, 54, 44–53. [Google Scholar] [CrossRef]
- Thum, M.D.; Weise, N.K.; Casalini, R.; Fulton, A.C.; Purdy, A.P.; Lundin, J.G. Incorporation of N,N,-diethyl-meta-toluamide within electrospun nylon-6/6 nanofibers. J. Appl. Polym. Sci. 2022, 139, e53237. [Google Scholar] [CrossRef]
- Bonadies, I.; Longo, A.; Androsch, R.; Jehnichen, D.; Gobel, M.; Di Lorenzo, M.L. Biodegradable electrospun PLLA fibers containing the mosquito-repellent DEET. Eur. Polym. J. 2019, 113, 377–384. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Longo, A. N,N-Diethyl-3-methylbenzamide (DEET): A mosquito repellent as functional plasticizer for poly(l-lactic acid). Thermochim. Acta 2019, 677, 180–185. [Google Scholar] [CrossRef]
- Mapossa, A.B.; López-Beceiro, J.; Díaz-Díaz, A.M.; Artiaga, R.; Moyo, D.S.; Mphateng, T.N.; Focke, W.W. Properties of Mosquito Repellent-Plasticized Poly(lactic acid) Strands. Molecules 2021, 26, 5890. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Brünig, H.; Focke, W.; Boldt, R.; Androsch, R.; Leuteritz, A. Melt-Spun Poly(D,L-lactic acid) Monofilaments Containing N,N-Diethyl-3-methylbenzamide as Mosquito Repellent. Materials 2021, 14, 638. [Google Scholar] [CrossRef]
- Murariu, M.; Arzoumanian, T.; Paint, Y.; Murariu, O.; Raquez, J.-M.; Dubois, P. Engineered polylactide (PLA)–polyamide (PA) blends for durable applications: 1. PLA with high crystallization ability to tune up the properties of PLA/PA12 blends. Eur. J. Mater. 2023, 3, 1–36. [Google Scholar] [CrossRef]
- ASTM D3822; Standard Test Method for Tensile Properties of Single Textile Fibers. ASTM International: West Conshohocken, PA, USA, 2020.
- Jiang, S.; Yang, L.; Bloomquist, J.R. High-throughput screening method for evaluating spatial repellency and vapour toxicity to mosquitoes. Med. Vet. Entomol. 2019, 33, 388–396. [Google Scholar] [CrossRef]
- Owusu, H.F.; Müller, P. How important is the angle of tilt in the WHO cone bioassay? Malar. J. 2016, 15, 243. [Google Scholar] [CrossRef]
- Sungkapreecha, C.; Iqbal, N.; Gohn, A.M.; Focke, W.W.; Androsch, R. Phase behavior of the polymer/drug system PLA/DEET. Polymer 2017, 126, 116–125. [Google Scholar] [CrossRef]
- Liang, J.-Z. The elastic behaviour during capillary extrusion of LDPE/LLDPE blend melts. Polym. Test. 2002, 21, 69–74. [Google Scholar] [CrossRef]
- Ji, B.-H.; Wang, C.-G.; Wang, Y.-X. Effect of jet stretch on polyacrylonitrile as-spun fiber formation. J. Appl. Polym. Sci. 2007, 103, 3348–3352. [Google Scholar] [CrossRef]
- Banjo, A.D.; Agrawal, V.; Auad, M.L.; Celestine, A.-D.N. Moisture-induced changes in the mechanical behavior of 3D printed polymers. Compos. Part. C Open Access 2022, 7, 100243. [Google Scholar] [CrossRef]
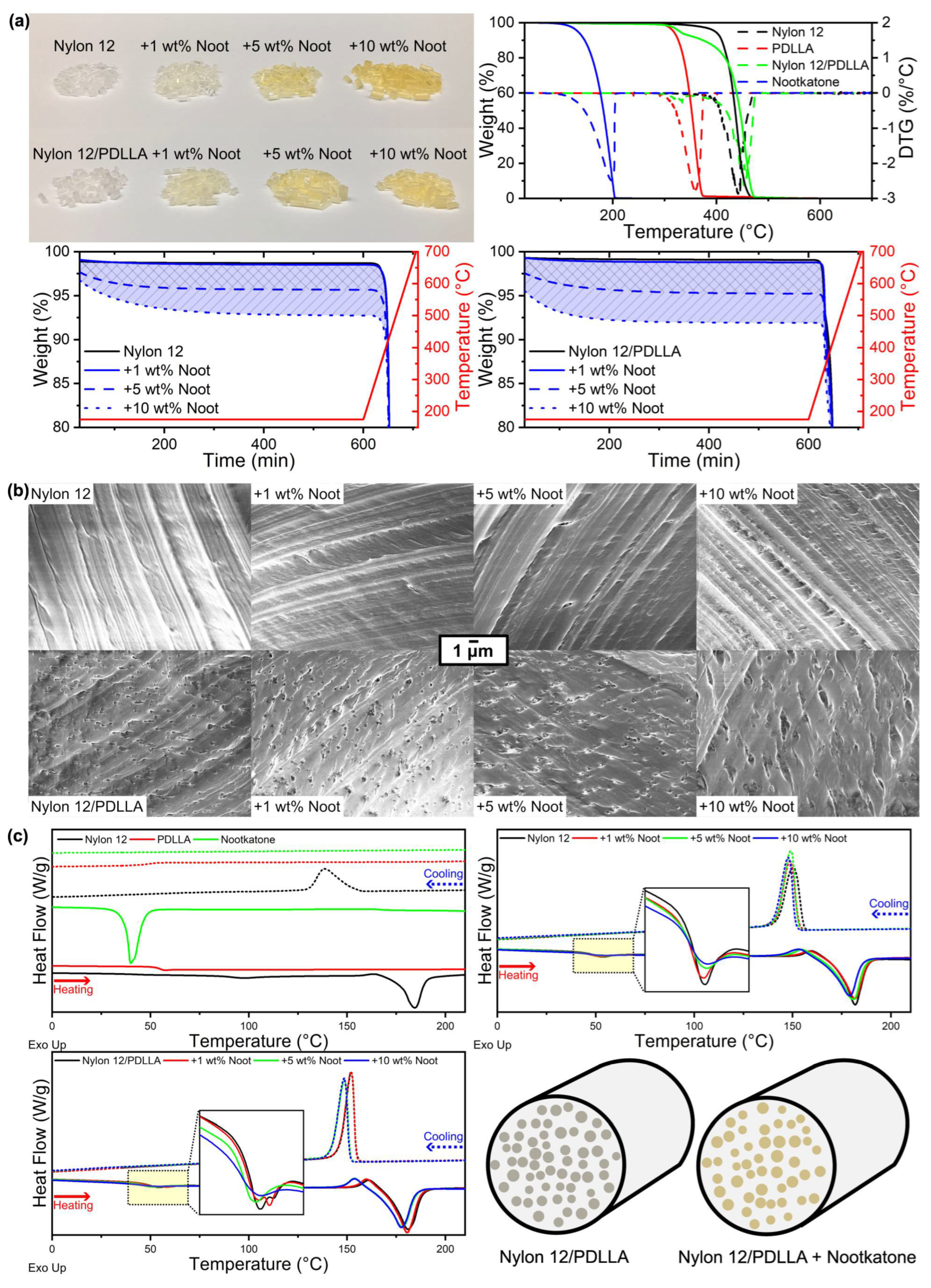
| Polymeric System | Nootkatone Loading (%) | Theoretical Nootkatone Content (%) | Nootkatone Content (%) | Nootkatone Retention (%) |
|---|---|---|---|---|
| Nylon 12 | 0 | 0 | 0 | --- |
| 1 | 1.0 | 0.2 | 19% | |
| 5 | 4.8 | 3.1 | 65% | |
| 10 | 9.1 | 6.1 | 67% | |
| Nylon 12/PDLLA | 0 | 0 | 0 | --- |
| 1 | 1.0 | 0.3 | 29% | |
| 5 | 4.8 | 3.9 | 82% | |
| 10 | 9.1 | 7.2 | 80% |
| Polymeric System | Nootkatone Loading (%) | Zone 1 (°C) | Zone 2 (°C) | Zone 3 (°C) | Zone 4 (°C) | Feed Rate (rpm) | Take-Up (m/min) |
|---|---|---|---|---|---|---|---|
| Nylon 12 | 0 | 115 | 160 | 185 | 205 | 4 | 20 |
| 1 | 150 | 180 | 210 | 215 | 4 | 20 | |
| 5 | 160 | 180 | 190 | 210 | 6 | 20 | |
| 10 | 160 | 180 | 190 | 210 | 6 | 20 | |
| Nylon 12/PDLLA | 0 | 115 | 160 | 185 | 205 | 7 | 15 |
| 1 | 115 | 180 | 185 | 205 | 6 | 15 | |
| 5 | 115 | 160 | 180 | 200 | 7 | 18 | |
| 10 | 115 | 160 | 180 | 190 | 7 | 20 |
| Polymeric System | Nootkatone Loading (%) | Theoretical Nootkatone Content (%) | Nootkatone Content (%) | Nootkatone Retention (%) |
|---|---|---|---|---|
| Nylon 12 Knit | 0 | 0 | 0 | --- |
| 1 | 1.0 | 0.2 | 18% | |
| 5 | 4.8 | 3.8 | 79% | |
| 10 | 9.1 | 5.3 | 59% | |
| Nylon 12/PDLLA Knit | 0 | 0 | 0 | --- |
| 1 | 1.0 | 1.1 | 113% | |
| 5 | 4.8 | 4.1 | 87% | |
| 10 | 9.1 | 6.7 | 73% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez, J.; Orlando, J.A.; Cilek, J.E.; Lundin, J.G. Poly-D,L-Lactic Acid as a Compatibilizer for Nootkatone-Embedded Nylon 12 Fabric Manufacturing. Fibers 2025, 13, 74. https://doi.org/10.3390/fib13060074
Jimenez J, Orlando JA, Cilek JE, Lundin JG. Poly-D,L-Lactic Acid as a Compatibilizer for Nootkatone-Embedded Nylon 12 Fabric Manufacturing. Fibers. 2025; 13(6):74. https://doi.org/10.3390/fib13060074
Chicago/Turabian StyleJimenez, Javier, Joseph A. Orlando, James E. Cilek, and Jeffrey G. Lundin. 2025. "Poly-D,L-Lactic Acid as a Compatibilizer for Nootkatone-Embedded Nylon 12 Fabric Manufacturing" Fibers 13, no. 6: 74. https://doi.org/10.3390/fib13060074
APA StyleJimenez, J., Orlando, J. A., Cilek, J. E., & Lundin, J. G. (2025). Poly-D,L-Lactic Acid as a Compatibilizer for Nootkatone-Embedded Nylon 12 Fabric Manufacturing. Fibers, 13(6), 74. https://doi.org/10.3390/fib13060074