Eco-Friendly Extraction of Curcumin from Turmeric and Dyeability of Textile Fibers
Abstract
Highlights
- Degradation of the turmeric matrix by chemical (acids, oxidants, enzymes) or physical (ultrasound, microwaves) agents breaks carbohydrate cross-links, releasing starch, amylose, amylopectin, and consequently curcumin.
- Microwave-assisted extraction and dyeing with eco-friendly additives (ethanol, kojic acid) achieve high curcumin uptake, especially on polyacrylonitrile and cellulose acetate fibers, which exhibit excellent color fastness (grade 5).
- Microwave-assisted extraction is an efficient, eco-friendly method achieving 78.35 ± 2.62% curcumin recovery, suitable for sustainable natural dyeing.
- The strong affinity and durability of curcumin on synthetic fibers (fastness grade 5) suggest hydrophobic interactions and microwave effects enhance dyeing performance and color retention.
Abstract
1. Introduction
- The conventional methods of extracting curcumin from turmeric powder are based on the action of simple chemical substances (acids, oxidants, enzymes), which degrade the points of connection between curcumin and the other components included in the turmeric matrix. At certain temperatures and durations, these substances swell the starch until the macromolecular chain splits at several points, releasing amylose, amylopectin, maltose, or glucose.
- Unconventional/modern methods for curcumin extraction involve the use of microwaves or ultrasound; these methods are faster and more effective than classical methods [16,17,18,19,20,21,22]. Typically, the extraction of curcumin can be performed in the presence or absence of a solvent. Ethanol, ethyl acetate, acetone, benzene, dichloromethane, or dimethyl sulfoxide can be used as solvents. Curcumin is strongly non-polar, which is why it can also dissolve in non-polar solvents. In this sense, the following hydrophobic products can be used: sunflower oil, soybean oil, and linseed oil [18].
- The UV–VIS method provides information about the chemical composition and concentration of active substances in the extract, indicating the absorption peaks specific to the compounds present;
- The CIELab method provides information about the color of the extract, being useful for monitoring the visual appearance and correlating the color with the quality of the product. Although this method is specific only to solid supports, it has been adapted and applied to liquid extracts under controlled conditions.
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction Processes
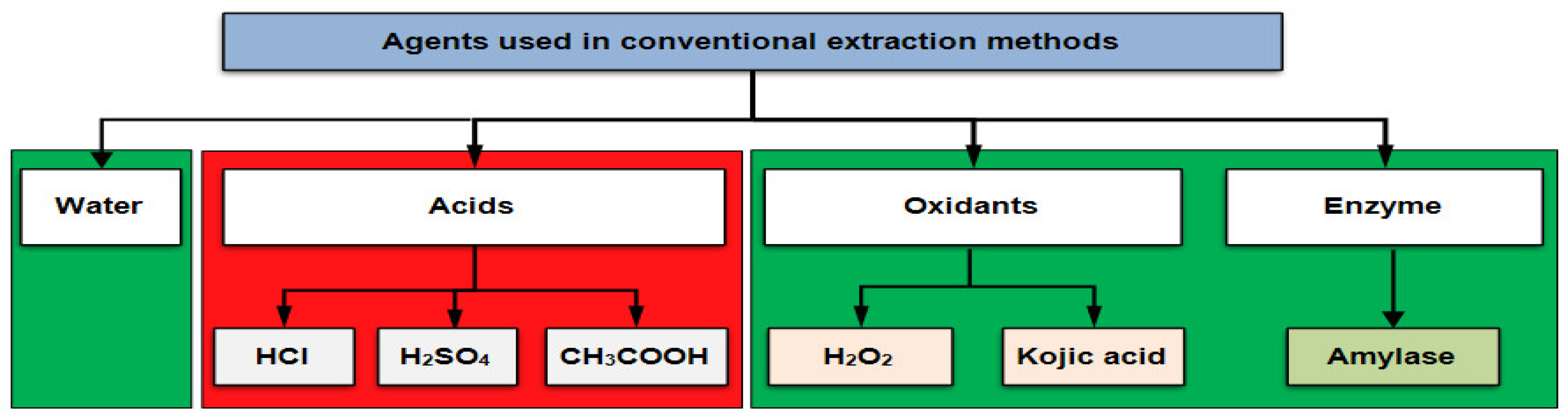
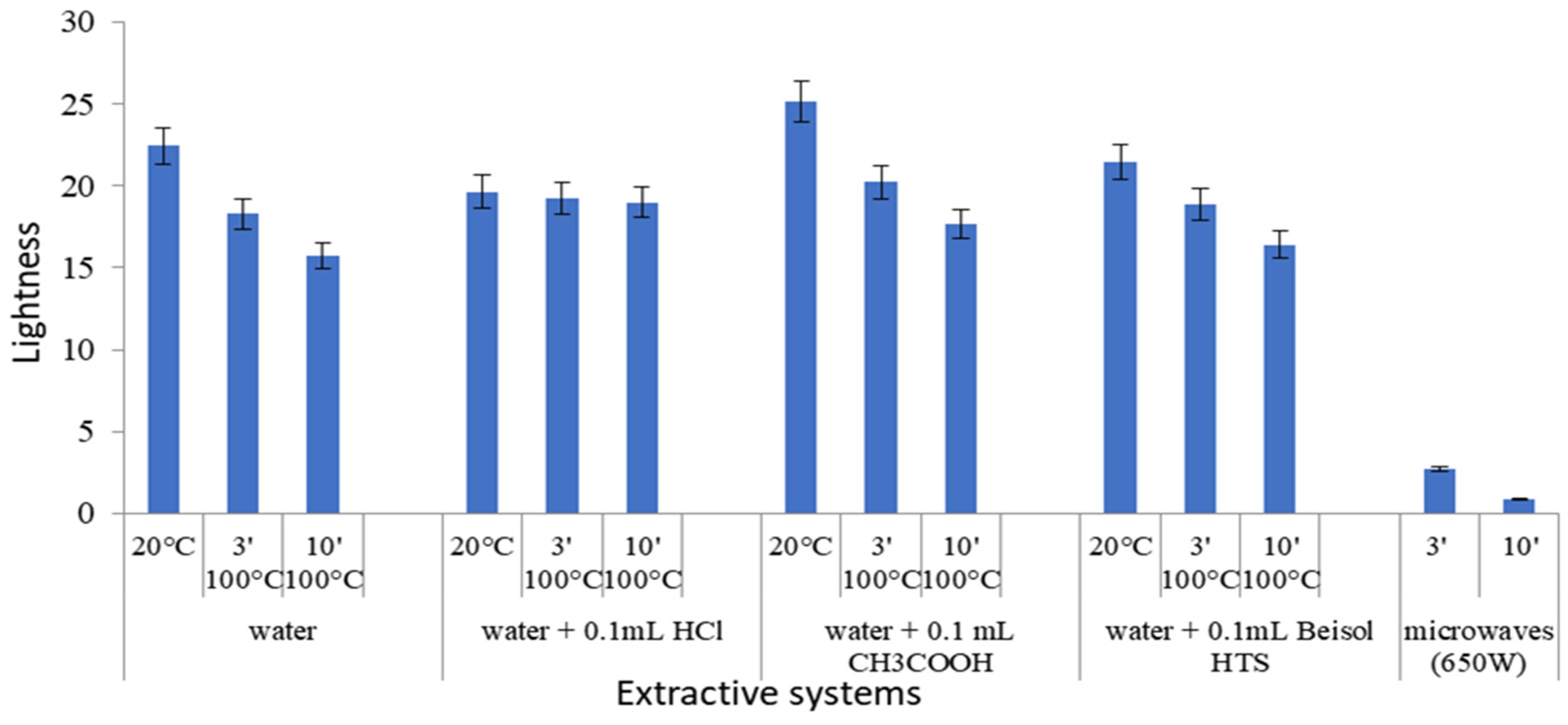
- Classical extraction methods are based on extraction in water, in an acidic medium, in an oxidizing medium, or in the presence of enzymes. It is the first time that oxidizing agents (such as H2O2 and kojic acid) are used in the extraction process of a natural dye. In these methods, the amount of 0.1 g of turmeric powder is used, to which 100 mL of water + 0.1 mL of starch degradation agent (acids/oxidants/enzyme) is added, at different temperatures and contact times, imposed by the extraction method adopted (Figure 1). HCl, H2SO4, and CH3COOH are used as acids. The green areas in Figure 1 indicate the eco-friendly agents used in the extraction processes, such as: water, oxidants (H2O2 and Kojic acid), and the amylase type enzyme (Beisol HTS, a bacterial amylase that resists temperatures close to boiling).
- 2.
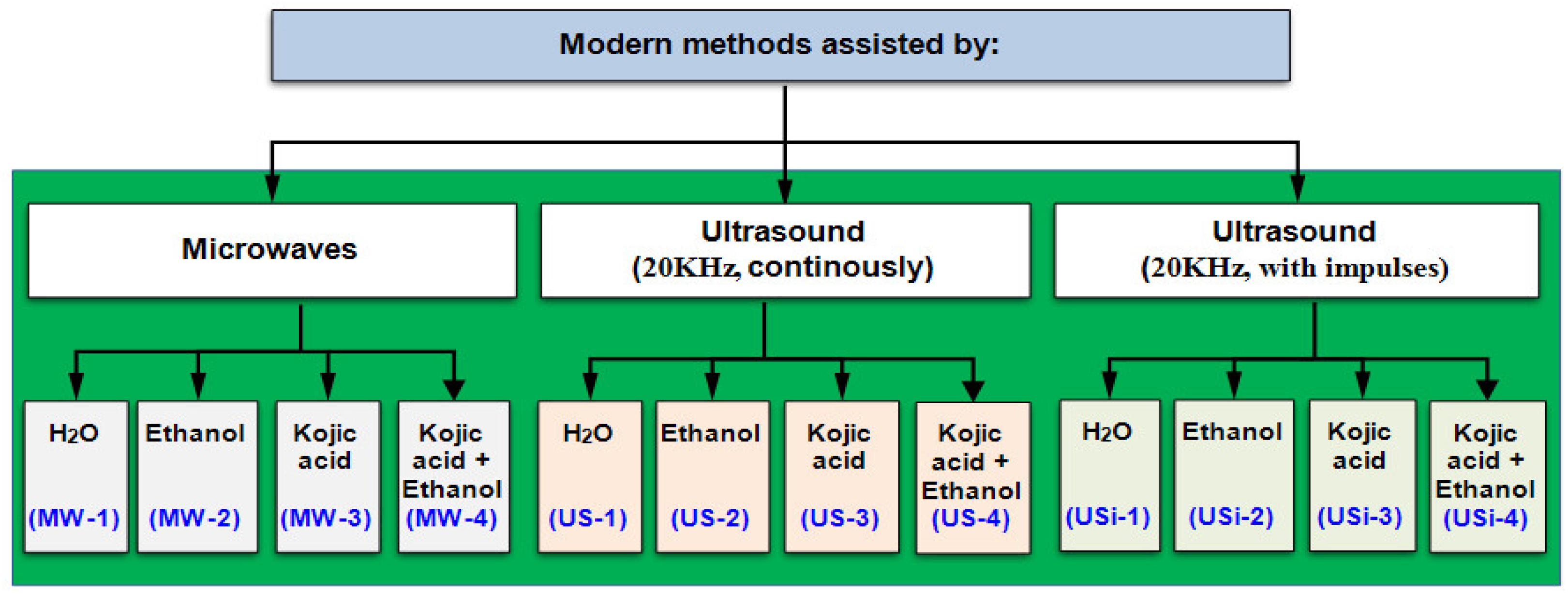
- Modern extraction methods are based on the use of physical stimuli (MW and US), in the presence of eco-friendly agents (water, ethanol, and kojic acid).
- Microwave-assisted extraction method: frequency of 2450 MHz, power 650–750 W, for 10 min (coding MW-1 to MW-4);
- Ultrasound-assisted extraction method: 20 KHz, US emitted continuously for 10 min (coding US-1 to US-4):
- Ultrasound-assisted extraction method: 20 KHz, US emitted intermittently (impulses every 2 s), for 10 min (coding USi-1 to USi-4).
2.3. Methods of Analysis
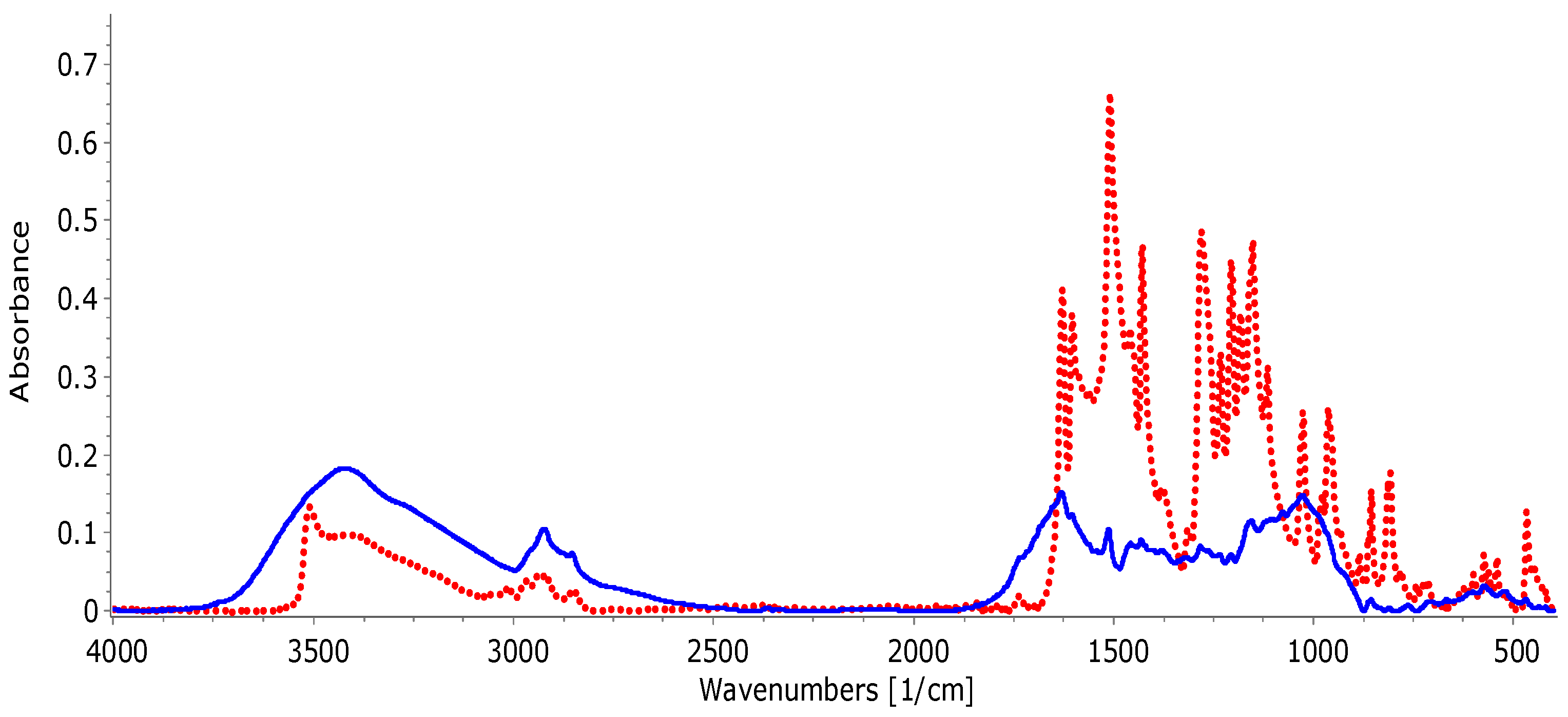
- FTIR analysis to highlight the presence of curcumin in turmeric powder; FTIR analysis is performed on a FTIR IR Affinity-1 Schimadzu spectrophotometer (Japan); the spectra are recorded in the range 4000–400 cm−1 (with 250 scans). After recording, IR absorption spectra are overlaid using Spectragryph software, version 1.2.
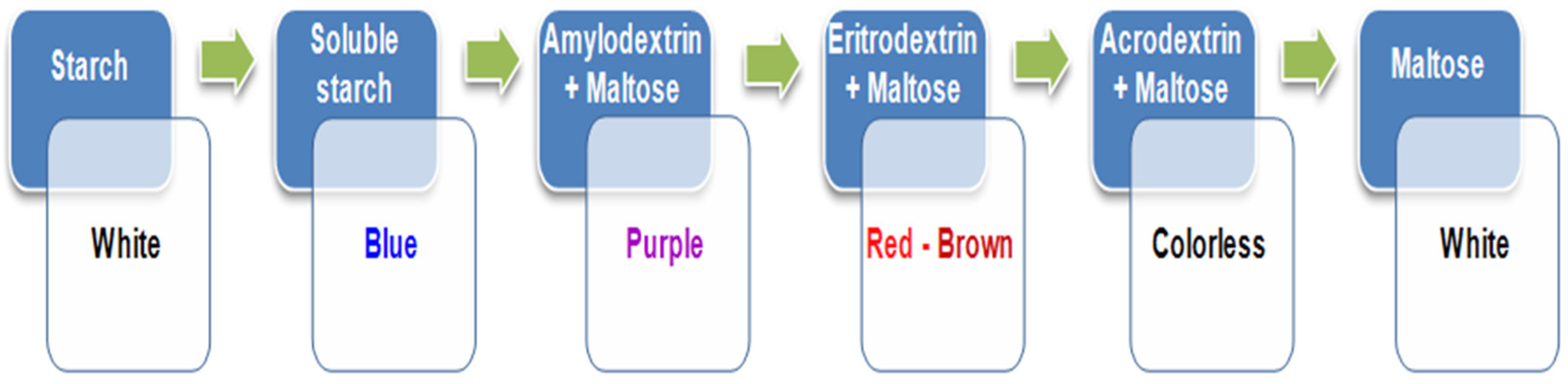
- Iodometric method: the qualitative analysis of the extract involves highlighting starch, amylose, amylopectin, and maltose using the iodine test, because their presence equates to the degradation of the turmeric matrix and the release of curcumin.
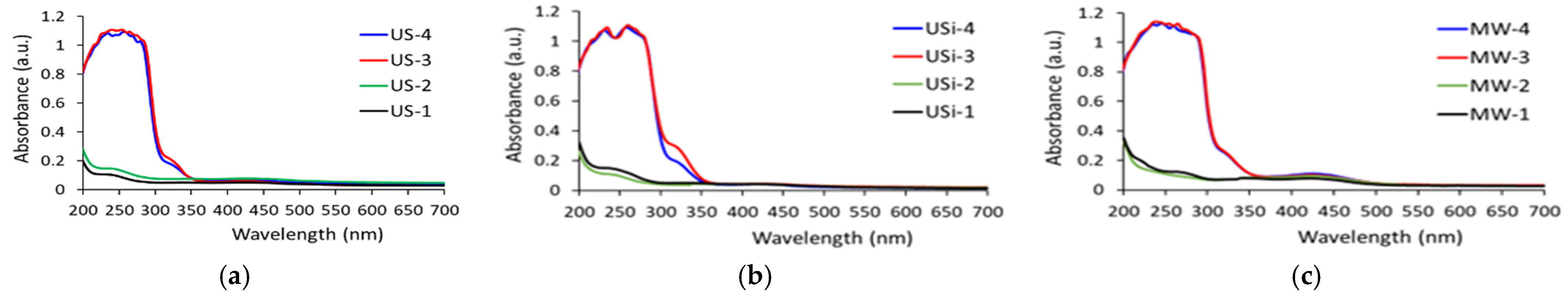
- UV–VIS analysis to characterize the extracts. For this analysis, a Shimadzu UVmini-1240 UV–VIS spectrophotometer (Shimadzu Corporation, Kyoto, Japan) is used, which allows the measurement of absorbance in the 0–5 a.u. range using a cell of 3 mL volume. The cuvettes used in the experiments are made of quartz, with a length of 1 cm. The efficiency of ultrasound and microwaves in extraction processes can be calculated using the maximum values of UV–VIS absorbances, related to wavelengths from 420 nm, according to Equation (1):
- 4.
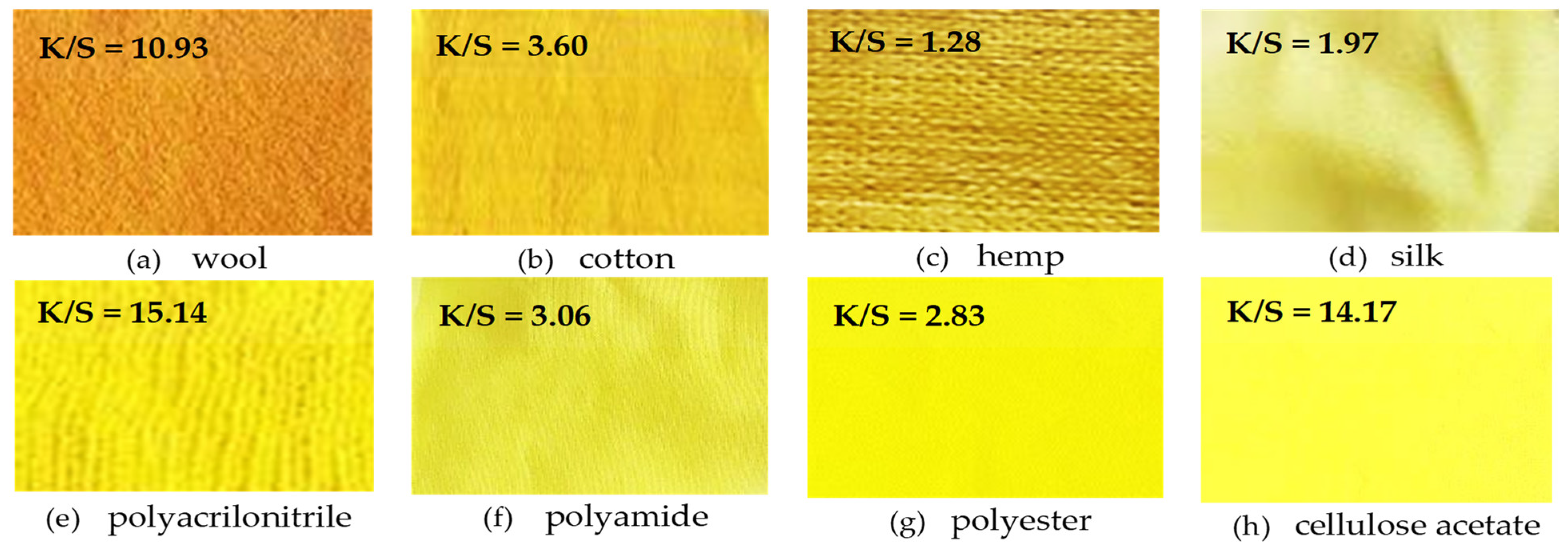
- Dyeing of textile fibers: 1 g of textile material and the mixture of 100 mL water + 0.1 g turmeric powder + 0.1 mL ethanol and 0.1 g kojic acid are subjected to microwave action (750 W, 10 min) when the extraction steps are carried out simultaneously with the dyeing. The textile materials used are wool, cotton, hemp, natural silk, polyacrylonitrile, polyamide, polyester, and cellulose acetate fibers.
- -
- ISO 105-C06 test [33] for the evaluation of color fastness to washing of dyed textiles (at 40 °C, for 30 min, hydromodulus 50:1). The tests are carried out on a textile washing tester manufactured by Thwing-Albert in the United States of America.
- -
- ISO 105-X12 test: Textiles—Color fastness test—Rubbing resistance is used for the evaluation of resistance to rubbing (dry and wet) [34]. This test evaluates the color fastness and integrity of dyed or colored fabrics. The rubbing action is carried out on a Pattering Tester (Computext, Budapest, Hungary).
- 5.
- Colorimetric measurements of extracts and dyed textile supports. For this purpose, a DataColor Check II Plus spectrophotometer (DataColor AG, Luzern, Switzerland) is used to determine the color strength (K/S) and the CIELab colors quantities: lightness (L*), position on the red–green axis (a*), position on the yellow–blue axis (b*), and chroma/saturation (C*).
- Measurement of the color of liquid extracts: For the determination of the color of liquid extracts, 30 mL volumes are used, introduced into a transparent glass beaker, with smooth walls, used consistently for all samples. The container is placed on a white fundal, to ensure a uniform optical background. The measuring head of the spectrophotometer is applied perpendicularly to the side wall of the beaker, at the same point for all measurements. The outer surface of the container is cleaned before each determination, to prevent unwanted reflections.
- Measurement of the color of dyed textile materials: The textile samples were conditioned for 24 h in a standardized atmosphere (20 ± 2 °C and 65 ± 5% relative humidity), then placed on a flat white surface (same for all samples), with the fabric completely smoothed, to eliminate the influences of wrinkles or texture on the measurement. The measuring head of the apparatus is applied perpendicularly to the textile surface, in at least five different points, representative of the color uniformity. For each sample, the arithmetic mean of the values obtained is calculated.
- 6.
- Statistical analysis: All experimental determinations are carried out in five independent replicates (n = 5). Results are expressed as arithmetic mean ± percentage error, in order to evaluate the precision of the applied methods.
3. Results
3.1. Mechanism
3.2. FTIR Analysis
3.3. Qualitative Analysis of the Extract
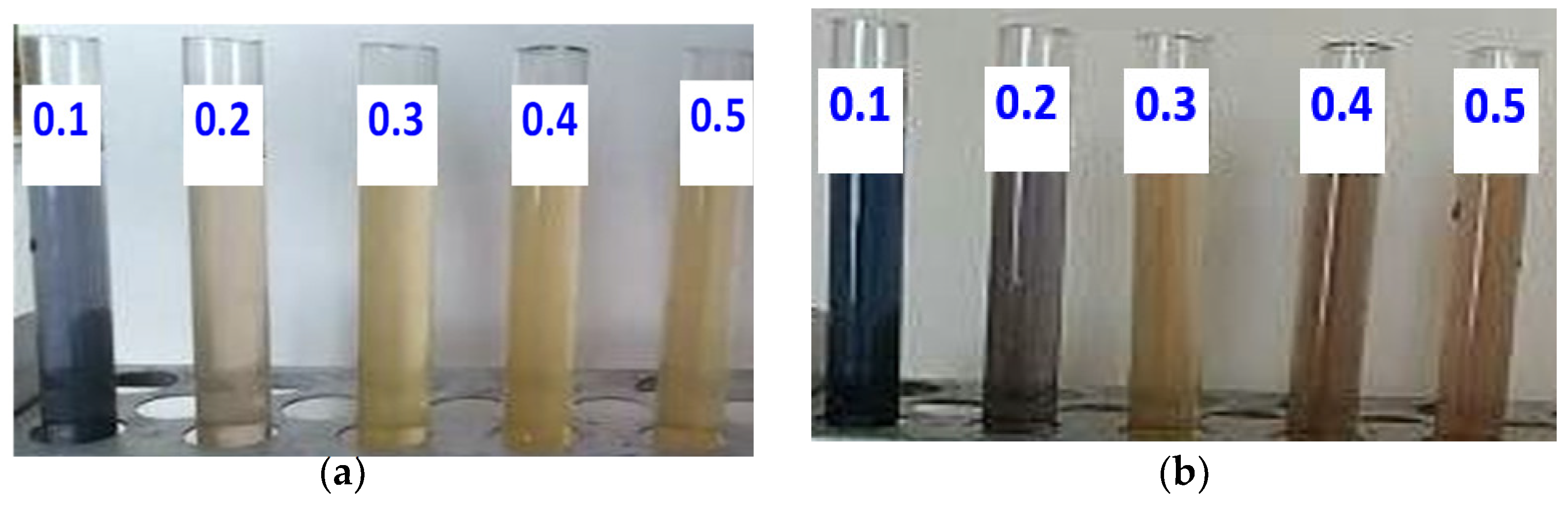
3.3.1. Extraction of Curcumin from Turmeric Using H2SO4 and H2O2
3.3.2. Extraction of Curcumin from Turmeric Using Kojic Acid
3.3.3. Enzyme Assisted Extraction
- cellulases—to hydrolyze cellulose and release cellobiose and glucose molecules when the hydrolysis is complete;
- hemicelluloses—to hydrolyze hemicelluloses that produce simple sugars or oligosaccharides;
- α—amylases—to degrade starch; it acts on the 1–4 glycosidic bonds producing hydrolysis into dextrin fragments and finally into maltose molecules;
- glucoamylases—to hydrolyze the glycosidic bond in starch and dextrin;
- mixture of xylanases + cellulases—to degrade the linear polysaccharide xylan into xylose and cellulose into cellobiose and glucose, respectively;
- pectinases—to hydrolyze the different types of pectins, releasing uronic acids;
- proteases—to hydrolyze proteins that release peptides, amino acids.
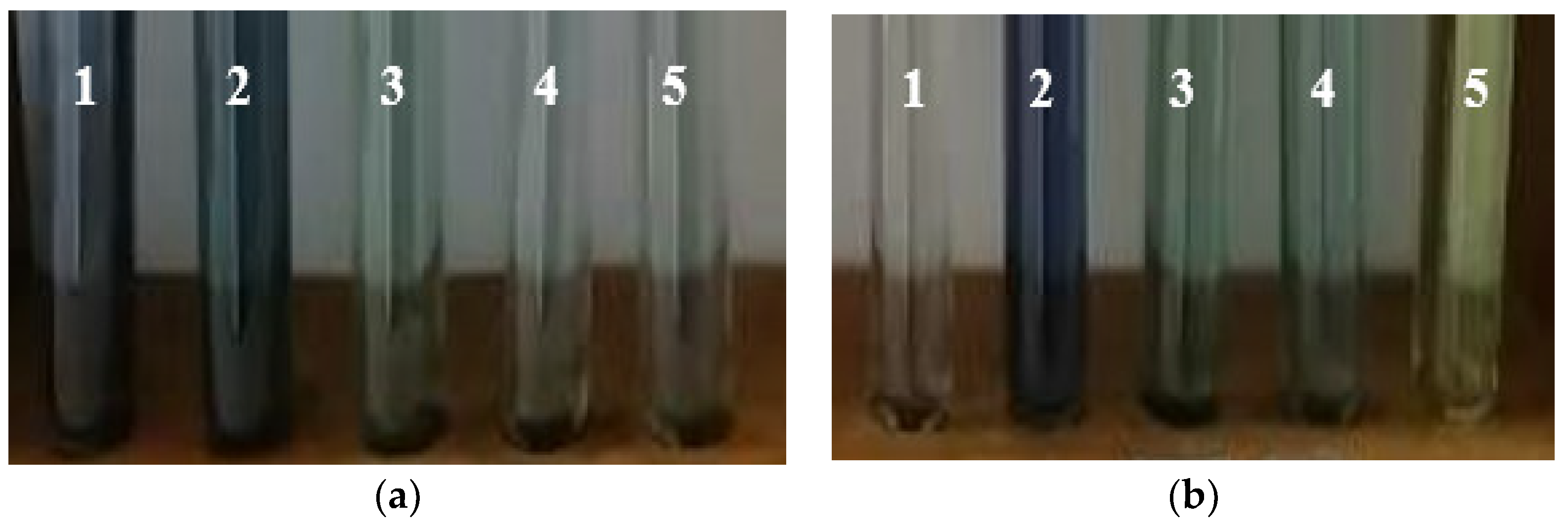
3.3.4. Unconventional Extractions Assisted by Microwaves or Ultrasound
Microwave Assisted Extraction; Comparisons with Classical Methods
Extraction Mechanism—Assisted by Ultrasound
Extraction Mechanism—Assisted by Microwaves
3.4. Characterization of the Extracts by Colorimetric Measurements
3.4.1. CIELab Values of Extracts Obtained by Applying Conventional Methods
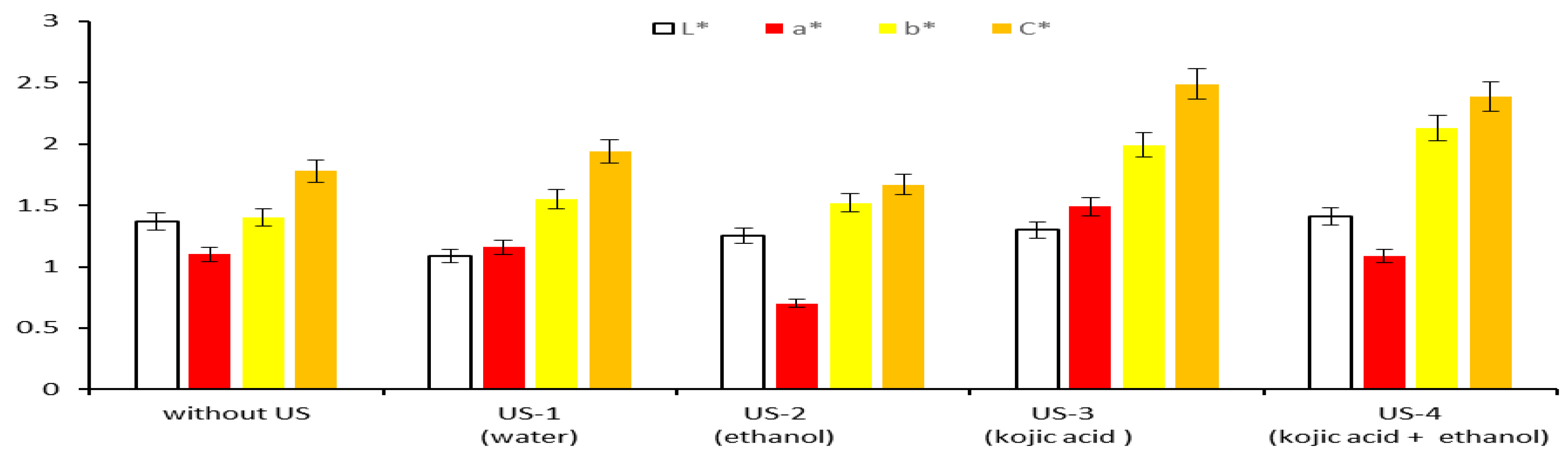
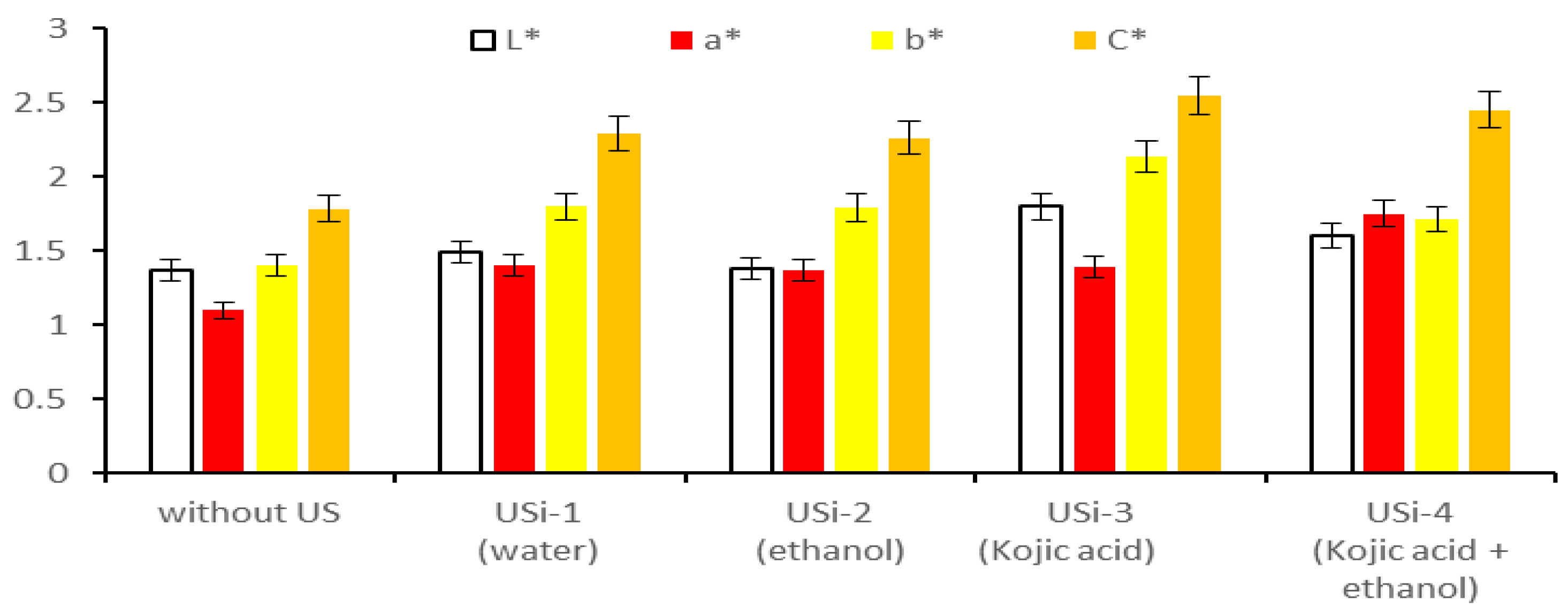
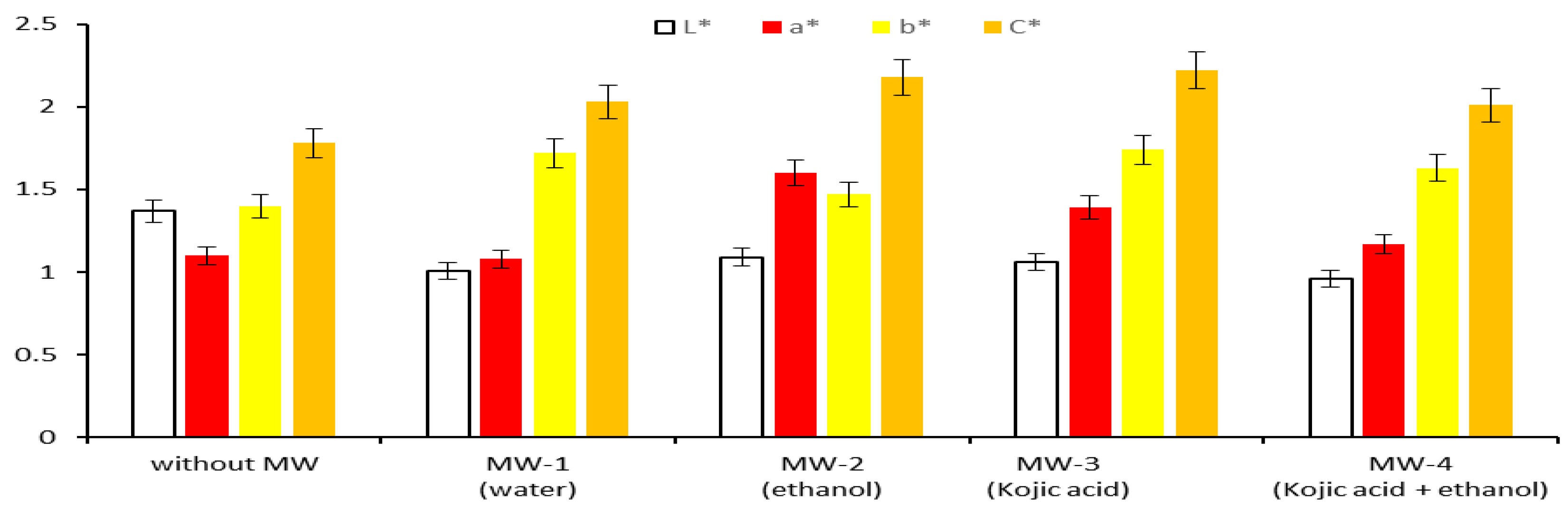
3.4.2. CIELab Values of Extracts Obtained by Applying Modern Methods
- The extracts are more colored, having lower lightness values (L* ranging 1.09 to 0.96) than the reference extract (without MW, L* = 1.37);
- The yellow color with reddish hues is more pronounced (b* ranging 1.47 to 1.74 and a* varying 1.08 to 1.60) than that of the reference extract (b* = 1.4 and a* = 1.1);
- Higher chroma/saturation for each extract obtained in the presence of microwaves (C* ranging 2.01 to 2.22), compared to the saturation of the reference extract (C* = 1.78).
4. Discussion
4.1. Efficiency of Ultrasound and Microwave in Extraction Processes of Curcumin from Turmeric
4.2. Color Characteristics of the Dyed Textile Materials
4.3. Fastness Properties
5. Limitations
- -
- A significant limitation of this study is the lack of direct monitoring of the surface temperature of the textile material during the simultaneous extraction and dyeing process assisted by microwaves. While the temperature of the extraction solution was measured (reaching approximately 54 °C after the first minute and 98 °C at the end of the 10 min process), the surface temperature of the textile materials could not be monitored due to the constraints of the microwave equipment used. This limitation restricts the ability to fully isolate thermal from non-thermal effects in the process.
- -
- Although UV–VIS analysis confirmed the presence of curcumin in turmeric extracts, using the characteristic absorption signal at 420 nm and 425 nm, the main limitation of this study is the absence of a selective analytical technique, such as HPLC, that would have allowed for a more specific identification of curcumin.
- -
- One limitation of the present study lies in the absence of a true control dyeing experiment using separately pre-extracted curcumin. Although blind baths without textile support were included to observe the extractive capacity of the system, this does not fully isolate the dyeing efficiency from the extraction process. Future studies should include dyeing trials with standardized curcumin extracts, applied independently, in order to better distinguish between the extraction and fixation mechanisms involved under microwave-assisted conditions.
- -
- Although the dyeing process was conducted in a neutral pH environment to minimize pH-related effects on curcumin, the lack of direct pH monitoring during both the extraction and dyeing processes may limit the comprehensive understanding of how pH influences curcumin stability and dyeing behavior. Future studies could incorporate real-time pH monitoring to better assess the impact of pH variations on both extraction efficiency and dyeing outcomes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004, 87, 44–50. [Google Scholar]
- Jia, S.; Sun, Y.; Li, L.; Wang, R.; Xiang, Y.; Li, S.; Zhang, Y.; Jiang, H.; Du, Z. Discrimination of turmeric from different origins in China by MRM-based curcuminoid profiling and multivariate analysis. Food Chem. 2021, 338, 127794. [Google Scholar] [CrossRef] [PubMed]
- Chungkrang, L. Environmental friendly synthesis of natural dyes and pigments. Int. J. Chem. Stud. 2020, 8, 2398–2402. [Google Scholar] [CrossRef]
- Yusuf, M.; Shabbir, M.; Mohammad, F. Natural Colorants: Historical, Processing and Sustainable Prospects. Nat. Prod. Bioprospect. 2017, 7, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Kamble, K.J.; Ingale, V.M.; Kaledhonkar, D.P. Comparative study of curcumin extraction from turmeric varieties grown in Maharashtra. Afr. J. Food Sci. 2011, 5, 780–789. [Google Scholar] [CrossRef]
- Thangavel, K.; Dhivya, K. Determination of curcumin, starch and moisture content in turmeric by Fourier transform near infrared spectroscopy (FT-NIR). Eng. Agric. Environ. Food 2019, 12, 264–269. [Google Scholar] [CrossRef]
- Sharma, A.; Dikshit, M.; Tiwari, R.C.; Sharma, V.B. Haridra-Curcumin the active principle. World J. Pharm. Res. 2021, 10, 357–362. [Google Scholar] [CrossRef]
- Bagchi, A. Extraction of Curcumin. IOSR J. Environ. Sci. Toxicol. Food Technol. (IOSR-JESTFT) 2012, 1, 01–16. [Google Scholar] [CrossRef]
- Chignell, C.; Bilski, P.; Reszka, Z.; Motten, A.; Sik, R.; Dahl, T. Spectral and photochemical properties of curcumin. Photochem. Photobiol A Chem. 1994, 59, 295–302. [Google Scholar] [CrossRef]
- BeMiller, J.N. (Ed.) Starches: Molecular and Granular Structures and Properties. In Carbohydrate Chemistry for Food Scientists, 3rd ed.; AACC International Press: St. Paul, MN, USA, 2019; pp. 159–189. [Google Scholar]
- Maniglia, B.C.; Silveira, T.M.G.; Tapia-Blacido, D.R. Starch isolation from turmeric dye extraction residue and its application in active film production. Int. J. Biol. Macromol. 2022, 202, 508–519. [Google Scholar] [CrossRef]
- Seung, D. Amylose in starch: Towards an understanding of biosynthesis, structure and function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Eggleston, G.; Triplett, A. New Starch Methodology to Measure Both Soluble and Insoluble Starch. Adv. Sugar Crop Proc. Conv. 2016, 1, 150–177. [Google Scholar]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Enzyme-assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin. Ind. Crops Prod. 2017, 95, 686–694. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Moreschi, S.R.M.; Meireles, M.A.A. Effects of supercritical fluid extraction on Curcuma longa L. and Zingiber officinale R. starches. Carbohydr. Polym. 2006, 63, 340–346. [Google Scholar] [CrossRef]
- Popescu, V.; Astanei, D.-G.; Burlica, R.; Popescu, A.; Munteanu, C.; Ciolacu, F.; Ursache, M.; Ciobanu, L.; Cocean, A. Sustainable and cleaner microwave-assisted dyeing process for obtaining eco-friendly and fluorescent acrylic knitted fabrics. J. Clean. Prod. 2019, 232, 451–461. [Google Scholar] [CrossRef]
- Quoc, L.P.T. Ultrasound-assisted extraction of phenolic compounds from red turmeric (Curcuma zanthorrhiza). Bull. Transilv. Univ. 2019, 12, 107–116. [Google Scholar] [CrossRef]
- Creţu, R.; Dima, C.; Bahrim, G.; Dima, Ş. Improved solubilization of curcumin with a microemulsification formulation. Ann. Univ. Dunarea Galati Fascicle VI-Food Technol. 2011, 35, 46–55. [Google Scholar]
- Dandekar, D.V.; Gaikar, V.G. Microwave assisted extraction of curcuminoids from curcuma longa. Sep. Sci. Technol. 2002, 37, 2669–2690. [Google Scholar] [CrossRef]
- Manasa, P.S.L.; Kamble, A.D.; Chilakamarthi, U. Various Extraction Techniques of Curcumin—A Comprehensive Review. ACS Omega 2023, 8, 34868–34878. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Dadsena, U. Extraction and evaluation of dyeing quality of natural curcumin. J. Innov. Appl. Pharma. Sci. (JIAPS) 2017, 2, 01–03. [Google Scholar]
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Batool, F.; Adeel, S.; Naveed, M.; Ali, A.; Mia, R.; Assiri, M.A. Green extraction and application of yellow natural curcumin colorant from Curcuma aromatica rhizomes for silk dyeing. Sci. Rep. 2024, 14, 13032. [Google Scholar] [CrossRef] [PubMed]
- Dass, P.M.; Joshua, Y.; Atoshi, M.A.; Emmanuel, E. Extraction, optimization and evaluation of turmeric root (Curcuma longan) dyeing conditions on cotton fabric. Int. J. Multidiscip. Res. Growth Eval. 2023, 4, 930–935. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, M.; Youm, K.; Kumar, S.; Koh, J.; Hong, K.H. Sustainable one-bath natural dyeing of cotton fabric using turmeric root extract and chitosan biomordant. J. Clean. Prod. 2023, 382, 135303. [Google Scholar] [CrossRef]
- Awode, A.U.; Dalyop, G.M.; Olatidoye, S.D.; Tijani, S.; Kalu, I.H.; Adeyanju, O. Extraction, Chemical Modification and Characterization of Turmeric Dye (Curcuma longa) and its Application on Cotton Fabric. Int. J. Res. Innov. Appl. Sci. 2023, 8, 124–128. [Google Scholar] [CrossRef]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Frone, A.N.; Nicolae, C.A.; Raut, I.; Constantin, M.; Grapin, M. Multifunctional Finishing of Cotton Fabric with Curcumin Derivatives Coatings Obtained by Sol–Gel Method. Gels 2023, 9, 369. [Google Scholar] [CrossRef]
- Sadaf, S.A.; Hassan, S.K.; Ahmad, Z.; Butt, M.N. Extraction and application of turmeric dye on silk fabric. J. Innov. Sci. 2024, 10, 1–6. [Google Scholar] [CrossRef]
- Do, K.L.; Ahsan, T.; Wahab, A.; Tayyab, M.; Yin, X.; Pan, N.; Huang, T.; Mushtaq, A.; Su, M. Bioactive Silk Revolution: Harnessing Curcuminoid Dye and Chitosan for Superior Antimicrobial Defence and UV Shielding. Pharmaceutics 2024, 16, 1510. [Google Scholar] [CrossRef]
- Palaskar, S.S.; Kale, R.D.; Deshmukh, R.R. Application of natural yellow (curcumin) dye on silk to impart multifunctional finishing and validation of dyeing process using BBD model. Color Res. Appl. 2021, 46, 1301–1312. [Google Scholar] [CrossRef]
- Onal, A.; Tombul, K.C.; Nached, S. Investigation of Dyeing Properties of Different Fabric Species with Curcuma Longa Extracts. Rev. Roum. Chim. 2020, 65, 983–988. [Google Scholar] [CrossRef]
- Lykidou, S.; Pashou, M.; Vouvoudi, E.; Nikolaidis, N. Study on the Dyeing Properties of Curcumin on Natural and Synthetic Fibers and Antioxidant and Antibacterial Activities. Fibers Polym. 2021, 22, 3336–3342. [Google Scholar] [CrossRef]
- ISO 105-C06-2010; Textiles—Tests for Colour Fastness—Part C06: Colour Fastness to Washing of Dyed Textiles. International Organization for Standardization (ISO): Geneva, Switzerland, 2010.
- ISO 105-X12-2016; Textiles—Color Fastness—Test for Rubbing Resistance (Dry and Wet). International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- Yallapua, M.M.; Jaggi, M.; Chauhana, S.C. Beta-Cyclodextrin-curcumin selfassembly enhances curcumin delivery in prostate cancer cells. Colloids Surf. B 2010, 79, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hu, W.; Su, W. Molecularly imprinted poly (methacrylamide-comethacrylic acid) composite membranes for recognition of curcumin. Anal. Chim. Acta 2008, 615, 54–62. [Google Scholar] [CrossRef]
- Karimi, N.; Ghanbarzadeh, B.; Hamishehkar, H.; Mehramuz, B.; Kafil, H.S. Antioxidant, antimicrobial and physicochemical properties of turmeric extract-loaded nanostructured lipid carrier (NLC). Colloid Interfac. Sci. 2018, 22, 18–24. [Google Scholar] [CrossRef]
- Hossain, M.K.; Firoz Pervez, M.; Mia, M.N.H.; Mortuza, A.A.; Rahaman, M.S.; Karim, M.R.; Islam, J.M.M.; Ahmed, F.; Khan, M.A. Effect of dye extracting solvents and sensitization time on photovoltaic performance of natural dye sensitized solar cells. Results Phys. 2017, 7, 1516–1523. [Google Scholar] [CrossRef]
- Krishna Mohan, P.R.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water soluble complexes of curcumin with cyclodextrins: Characterization by FT-Raman spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Kanchana Devi, M. UV-Vis Spectroscopic Investigation on Hydrolysis of Starch and Starch-Drug Interaction. Asian J. Chem. 2007, 19, 3363–3374. [Google Scholar]
- Muniglia, L.; Claisse, N.; Baudelet, P.-H.; Ricochon, G. Enzymatic Aqueous Extraction (EAE). In Alternative Solvents for Natural Products Extraction; Chemat, F., Vian, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 167–205. [Google Scholar]
- Kurmudle, N.; Kagliwal, L.D.; Bankar, S.B.; Singhal, R.S. Enzyme-assisted extraction for enhanced yields of turmeric oleoresin and its constituents. Food Biosci. 2013, 3, 36–41. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Singhal, R.S. Enzyme-Assisted Extraction of Bioactives. In Food Bioactives: Extraction and Biotechnology Applications; Puri, M., Ed.; Springer: Cham, Switzerland, 2017; pp. 171–201. [Google Scholar] [CrossRef]
- Sivakumar, V.; Lakshmi Anna, J.; Vijayeeswarri, J.; Swaminathan, G. Ultrasound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrason. Sonochem. 2009, 16, 782–789. [Google Scholar] [CrossRef]
- Oancea, S.; Perju, M.; Olosutean, H. Influence of enzyme-aided extraction and ultrasonication on the phenolics content and antioxidant activity of Paeonia officinalis L. petals. J. Serb. Chem. Soc. 2020, 85, 845–856. [Google Scholar] [CrossRef]
- Ghafoor, K.; Yong-hee, C.; Yeong Jeon, J.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis Vinifera) seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2007, 67, 160–172. [Google Scholar] [CrossRef]
- Liang, H.; Wang, W.; Xu, J.; Zhang, Q.; Shen, Z.; Zeng, Z.; Li, Q. Optimization of ionic liquid based microwave-assisted extraction technique for curcuminoids from Curcuma longa L. Food Bioprod. Process. 2017, 104, 57–65. [Google Scholar] [CrossRef]
- Kumar, S. Organic Chemistry. Spectroscopy of Organic Compounds; University Amritsar: Amritsar, India, 2006; pp. 10–64. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ruhanea, T.A.; Tauhidul Islam, M.; Saifur Rahaman, M.; Bhuiyan, M.M.H.; Islam, J.M.M.; Newaz, M.K.; Khan, K.A.; Khan, M.A. Photo current enhancement of natural dye sensitized solar cell by optimizing dye extraction and its loading period. Optik 2017, 149, 174–183. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Extraction of bioactive compound curcumin from turmeric (Curcuma Longa L.) Via Different Routes: A comparative study. Pak. J. Biotechnol. 2016, 13, 173–180. [Google Scholar]
- Li, M.; Ngadi, M.O.; Ma, Y. Optimisation of pulsed ultrasonic and microwave-assisted extraction for curcuminoids by response surface methodology and kinetic study. Food. Chem. 2014, 165, 29–34. [Google Scholar] [CrossRef]
- Wakte, P.S.; Sachin, B.S.; Patil, A.A.; Mohato, D.M.; Band, T.H.; Shinde, D.B. Optimization of microwave, ultra-sonic and supercritical carbon dioxide assisted extraction techniques for curcumin from Curcuma longa. Sep. Purif. Technol. 2011, 79, 50–55. [Google Scholar] [CrossRef]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power Ultrasound Uses and Applications, 2nd ed.; Woodhead Publishing: London, UK, 2002. [Google Scholar] [CrossRef]
- Savun-Hekimoglu, B.A. Review on Sonochemistry and Its Environmental Applications. Acoustics 2020, 2, 766–775. [Google Scholar] [CrossRef]
- Kerboua, K.; Merouani, S.; Hamdaoui, O.; Alghyamah, A.; Islam, M.H.; Hansen, H.E.; Pollet, B.G. How do dissolved gases affect the sonochemical process of hydrogen production? An overview of thermodynamic and mechanistic effects—On the “hot spot theory”. Ultrason. Sonochem. 2021, 72, 105422. [Google Scholar] [CrossRef]
- Brenner, T.; Kiessler, B.; Radosta, S.; Arndt, T. Processing surface sizing starch using oxidation, enzymatic hydrolysis and ultrasonic treatment methods—Preparation and application. Carbohydr. Polym. 2016, 138, 273–279. [Google Scholar] [CrossRef]
- Suslick, K.; Flannigan, S.D.J. Inside a collapsing bubble—Sonoluminescence and the conditions during cavitation. Annu. Rev. Phys. Chem. 2008, 59, 659–683. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Eddingsaas, N.C.; Flannigan, D.J.; Hopkins, S.D.; Xu, H. The Chemical History of a Bubble. Acc. Chem. Res. 2018, 51, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Kubrakova, I.V.; Toropchenova, E.S. Microwave heating for enhancing efficiency of analytical operations. Inorg. Mater. 2008, 44, 1509–1519. [Google Scholar] [CrossRef]




| Extractive Systems | Codes for Extractions Assisted by: | ||
|---|---|---|---|
| US | USi | MW | |
| 100 mL water | US-1 | USi-1 | MW-1 |
| 100 mL water + 0.1 mL ethanol | US-2 | USi-2 | MW-2 |
| 100 mL water + 0.1 g kojic acid | US-3 | USi-3 | MW-3 |
| 100 mL water + 0.1 g kojic acid + 0.1 mL ethanol | US-4 | USi-4 | MW-4 |
| Extractive Systems | λmaxim for curcumin/λmaxim for amylose | ||
|---|---|---|---|
| US | USi | MW | |
| 100 mL water | 425/- | 415/- | 420/560 |
| 100 mL water + 0.1 mL ethanol | 430/- | 420/- | 425/590 |
| 100 mL water + 0.1 g kojic acid | 420/- | 420/590 | 425/585 |
| 100 mL water + 0.1 g kojic acid + 0.1 mL ethanol | 425/590 | 420/575, 590 | 425/590 |
| Extractive System | Extract Absorbance ± Standard Deviation | ||
|---|---|---|---|
| US | USi | MW | |
| 100 mL water (without additives) | 0.1540 ± 0.0051 | 0.2238 ± 0.0075 | 0.2283 ± 0.0076 |
| 100 mL water + 0.1 mL ethanol | 0.2284 ± 0.0076 | 0.2331 ± 0.0078 | 0.2801 ± 0.0093 |
| 100 mL water + 0.1 g kojic acid | 0.1819 ± 0.0061 | 0.2318 ± 0.0077 | 0.3043 ± 0.0100 |
| 100 mL water + 0.1 mL ethanol + 0.1 g kojic acid | 0.1987 ± 0.0066 | 0.2302 ± 0.0077 | 0.3336 ± 0.0100 |
| Extractive System | US | USi | MW |
|---|---|---|---|
| 100 mL water (without additives) | 53.11 ± 1.78 | 68.73 ± 2.27 | 68.37 ± 2.29 |
| 100 mL water + 0.1 mL ethanol | 68.38 ± 2.29 | 69.02 ± 2.31 | 74.22 ± 2.48 |
| 100 mL water + 0.1 g kojic acid | 60.30 ± 2.02 | 68.84 ± 2.30 | 76.27 ± 2.55 |
| 100 mL water + 0.1 mL ethanol + 0.1 g kojic acid | 63.66 ± 2.13 | 68.63 ± 2.30 | 78.35 ± 2.62 |
| Sample | CIELab Colorimetric Values 1 | Color Strength K/S | Color Codes | ||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | RGB 2 | HEX 3 | ||
| Wool | 69.76 ± 0.38 | 10.87 ± 0.06 | 67.55 ± 0.38 | 68.42 ± 0.38 | 10.93 ± 0.06 | 224, 138, 29 | #e08a1d |
| Cotton | 81.05 ± 0.45 | 5.40 ± 0.03 | 66.93 ± 0.37 | 67.15 ± 0.37 | 3.60 ± 0.02 | 241, 204, 28 | #f1cc1c |
| Silk | 88.26 ± 0.49 | −8.48 ± 0.05 | 51.70 ± 0.29 | 52.39 ± 0.29 | 1.97 ± 0.01 | 225, 221, 98 | #e1dd62 |
| Hemp | 81.10 ± 0.45 | −1.89 ± 0.01 | 38.11 ± 0.21 | 38.15 ± 0.21 | 1.28 ± 0.01 | 190, 164, 94 | #bea45e |
| Polyacrylonitrile | 81.58 ± 0.45 | −6.13 ± 0.03 | 86.32 ± 0.48 | 86.53 ± 0.48 | 15.15 ± 0.08 | 255, 248, 39 | #fff827 |
| Polyamide | 85.94 ± 0.48 | −11.44 ± 0.06 | 57.87 ± 0.32 | 58.97 ± 0.32 | 3.06 ± 0.01 | 217, 212, 48 | #d9d430 |
| Polyester | 89.70 ± 0.50 | −15.02 ± 0.08 | 59.91 ± 0.33 | 61.75 ± 0.34 | 2.83 ± 0.01 | 248, 247, 7 | #f8f707 |
| Cellulose acetate | 90.39 ± 0.50 | −16.35 ± 0.09 | 90.40 ± 0.50 | 91.87 ± 0.51 | 14.17 ± 0.07 | 255, 255, 69 | #ffff45 |
| Sample | Color Fastness to Washing | Color Fastness to Rubbing | |||
|---|---|---|---|---|---|
| Change in Color | Staining | ||||
| Cotton | Wool | Dry | Wet | ||
| Wool | 4-5 | 4-5 | 4-5 | 4-5 | 4-5 |
| Cotton | 4 | 4 | 4 | 4-5 | 4 |
| Silk | 4-5 | 4-5 | 4-5 | 4-5 | 4 |
| Hemp | 4 | 4 | 4 | 4 | 4 |
| Polyacrylonitrile | 5 | 5 | 5 | 5 | 5 |
| Polyamide | 4-5 | 4-5 | 4-5 | 4-5 | 4 |
| Polyester | 4-5 | 4-5 | 4-5 | 4-5 | 4 |
| Cellulose acetate | 5 | 5 | 5 | 5 | 4-5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, V.; Alexandrescu, A.-D.; Popescu, G.; Vasilache, V. Eco-Friendly Extraction of Curcumin from Turmeric and Dyeability of Textile Fibers. Fibers 2025, 13, 73. https://doi.org/10.3390/fib13060073
Popescu V, Alexandrescu A-D, Popescu G, Vasilache V. Eco-Friendly Extraction of Curcumin from Turmeric and Dyeability of Textile Fibers. Fibers. 2025; 13(6):73. https://doi.org/10.3390/fib13060073
Chicago/Turabian StylePopescu, Vasilica, Ana-Diana Alexandrescu, Gabriel Popescu, and Viorica Vasilache. 2025. "Eco-Friendly Extraction of Curcumin from Turmeric and Dyeability of Textile Fibers" Fibers 13, no. 6: 73. https://doi.org/10.3390/fib13060073
APA StylePopescu, V., Alexandrescu, A.-D., Popescu, G., & Vasilache, V. (2025). Eco-Friendly Extraction of Curcumin from Turmeric and Dyeability of Textile Fibers. Fibers, 13(6), 73. https://doi.org/10.3390/fib13060073






