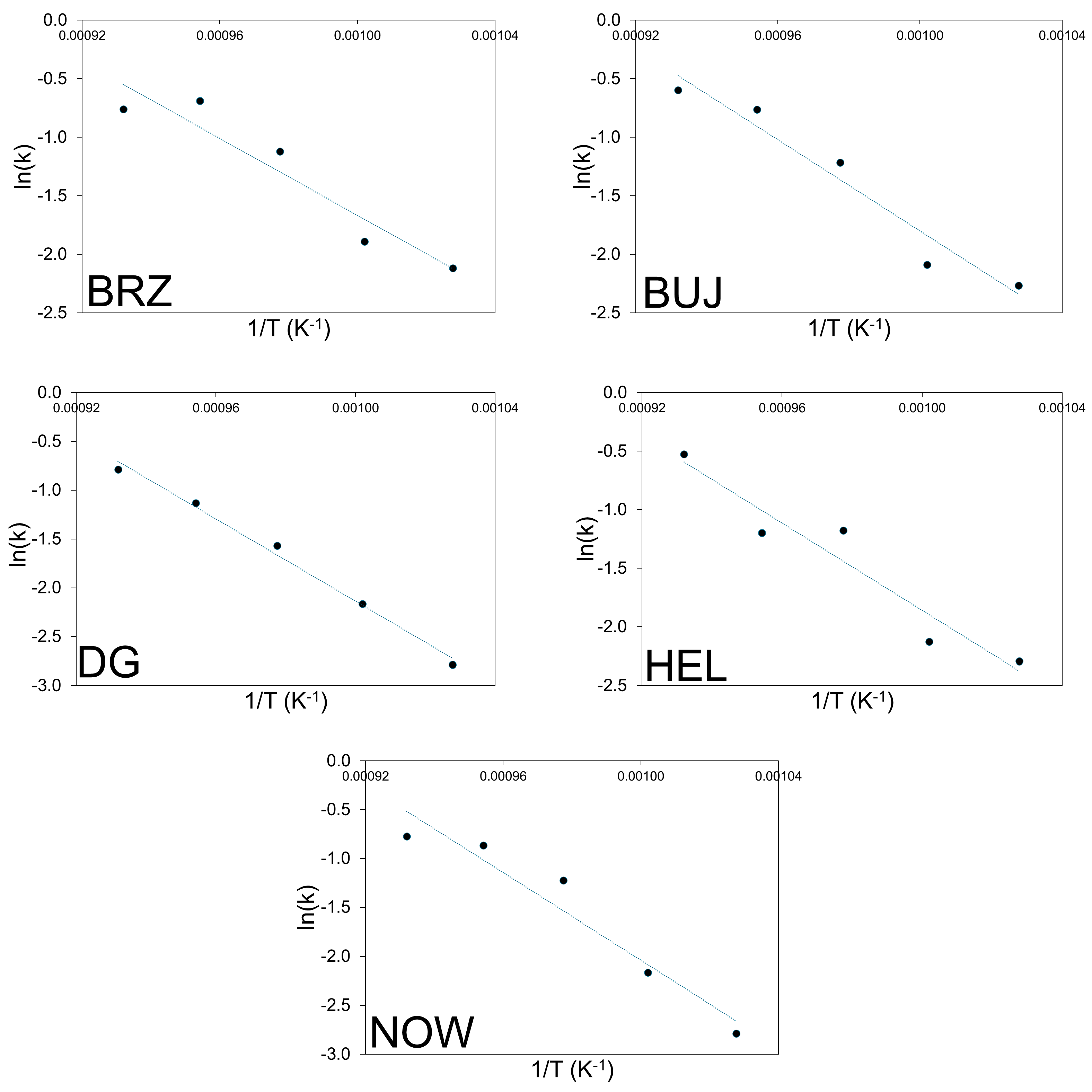Highlights
What are the main findings?
- The kinetic aspects connected with the thermal decomposition of cement–asbestos materials from Poland were studied.
- The exsitu experiments performed confirm the considerable similarity of the cement–asbestos thermal decomposition process among the samples.
What are the implications of the main findings?
- Asbestos waste is dangerous; however, it can be effectively transformed by thermal methods, mitigating its environmental impact.
- The results obtained may be useful for the design of thermal methods for asbestos waste disposal technology.
Abstract
For many years, countries around the world have been struggling with the problem of storing asbestos waste, especially in, those countries where the production and use of asbestos products have been legally banned. Following the adoption of plans for cleaning up asbestos waste, countries are struggling with the problem of its disposal, which mainly involves storing it in specialist landfills. At the same time, scientists are looking for alternatives to this type of “disposal” of asbestos by developing methods for degrading the harmful fibers. Particular attention has been paid to methods based on the thermal treatment of this waste, which results in hazardous asbestos fibers being thermally decomposed. This work focuses on the kinetic study of the thermal decomposition process of cement–asbestos using an exsitu thermal treatment. The results obtained made it possible to interpret this thermal transformation kinetically. Kinetic analysis of the isothermal data using an Avrami–Erofeev model yielded values for the overall reaction order. On this basis, the value of the apparent activation energy of the thermal decomposition process of the tested cement–asbestos samples was obtained, which was approximately 140–180 kJ mol−1.
1. Introduction
Asbestos is a general name for materials that include fibrous varieties of silicate minerals from the serpentine and amphibole groups, which, from a chemical point of view, are hydrated silicates of various metals, mainly Mg, Ca, Fe, or Na. They are characterized by a fibrous structure as well as good strength and chemical properties [1]. Their production and use began at the beginning of the 20th century, and the development of their use included almost 3000 products. It was a popular material for industries such as chemicals, buildings, and transport, but it was mainly used in construction to produce cement–asbestos boards and roofing [1,2]. In Poland, an estimated 85% of asbestos was used to produce building materials due to its cheap and easy production [3].
Unfortunately, during the production and use of asbestos materials, their harmful properties came to light. This mainly affected the health of workers working in asbestos extraction, its processing, and in the treatment of finished products [4,5,6]. In the 1930s and 1940s, attention was paid to the undesirable properties of asbestos materials, and by the 1970s, significant scientific evidence had been obtained linking asbestos to lung cancer and mesothelioma, leading to asbestos being officially classified as a human carcinogen in 1987 [4,5,6]. The fibrous structure characteristic of asbestos plays a significant role here. On the one hand, it provides excellent properties to finished materials; on the other hand, it harms human health. Concern for the population’s health led to increasing legal regulations in many countries prohibiting the production and, later, the use of materials containing asbestos [7,8,9].
The fastest disposal method for harmful asbestos materials has been to dismantle and store them securely in specialist hazardous-waste landfills. However, this solution should not be considered definitive in an era of growing ecological awareness. Firstly, it involves the waste of materials, 80% of which is the cement matrix, the waste of usable space due to their conversion into landfills, and the exposure of the environment to secondary pollution [10,11]. In connection with the above, asbestos removal methods and recycling have begun to be created and developed over recent years. Detailed data on this matter are provided by review articles from recent years [12,13,14]. The main idea of these removal methods is to destroy the harmful fibrous structure of asbestos to avoid the need for landfilling and to transform it into a reusable material. For this purpose, various methods have been proposed which can be divided into chemical, thermal, mechanical, or biological methods [12,13,14]. Of course, each method has its advantages and disadvantages, which significantly influence the level of interest in a specific method. Much attention has been paid to thermal methods due to their relatively easy implementation on a larger industrial scale. Thermal treatment methods are particularly useful because of their relatively lower cost, smaller impact on the environment and human health, and non-toxic waste production compared with other asbestos disposal processes. Finally, the products obtained after the thermal treatment process can be used as secondary raw materials and are easily recycled in different technologies [12,13,14]. More precisely, thermal treatment aims to transform asbestos into an inert material using a temperature range in which asbestos fibers become unstable and transform into new crystalline phases. Thanks to the use of elevated temperatures and the dehydration, dihydroxylation, and decarbonization processes occurring under their influence, we obtain a material that does not exhibit carcinogenic properties [15] due to the crystalline transformation of asbestos into harmless phases and degradation of the fibrous form [16,17,18,19,20,21]. A similar situation occur in the case of cement–asbestos materials; however, the thermal process is more complicated in light of the presence of a multi-phase cementitious matrix [17,21,22,23,24,25,26,27,28,29]. Its presence influences the thermal decomposition course of the whole system and, ultimately, on the creation of new mineral phases [22,23]. The chemical composition of raw cement–asbestos samples may vary to some extent, especially in the context of the main oxide contents, i.e., CaO and SiO2. In turn, the variable CaO/SiO2 ratio influences the formation of the final mineral phase composition after thermal treatment, which results from the triple equilibrium system CaO-SiO2-MgO as well as the presence of a liquid phase during thermal treatment [24].
Kinetic studies in the context of the entire thermal decomposition process for cement–asbestos waste are scarce and not widely available to readers. This study aims to analyze the thermal decomposition process of cement–asbestos waste from a kinetic point of view. In this study, the kinetic parameters of this overall process are determined for the first time. This has been achieved by ex-situ isothermal treatment of cement–asbestos samples in relation to the sample mass loss occurring during heat treatment.
2. Materials and Methods
In this study, five samples of corrugated cement–asbestos roofing boards were used. The specimens were collected from different regions in the south of Poland and were named BRZ, BUJ, DG, HEL, and NOW, respectively. There is no detailed information about the origin of the materials, the time of installation on the roof, or their service life. However, it can be said with certainty that they were exposed to external weather conditions for many years. Before the proper kinetic study, the main physical properties of the cement–asbestos panels were measured. The hydrostatic weighing method allowed us to determine the samples’ open porosity and apparent density, as well as their water absorbability. Each parameter was determined from at least three parallel samples.
Microstructure analysis was performed by SEM using a Mira 3 electron microscope (Tescan, Brno, Czech Republic) equipped with an EDS system provided by AZtec Automated 3.1 software (Oxford Instruments, Abingdon, UK). The analysis was performed at an accelerating voltage of 15 kV in the back-scattered electron mode. The samples were covered by a graphite layer by the Quorum Q150R ES device (Quorum Technologies, Laughton, UK). The chemical analysis, enriched with the L.O.I. (loss on ignition; isothermal calcination at 1025 °C), of the samples was performed using a Panalytical Magix PW-2424 spectrometer (Malvern PANalytical, Almelo, The Netherlands) based on the fused cast-bead method described in the PN-EN ISO 12677:2011 standard [30]. The phase analysis was obtained by X-ray powder diffraction using a PANalytical X’pert Pro diffractometer with CuKα radiation and a Ni filter, 40 kV, 30 mA X’Celerator detector (Malvern PANalytical, Almelo, The Netherlands). The diffraction patterns were quantitatively analyzed via Rietveld refinement (using 15 wt% anatase (TiO2) as an internal calibration standard) using the HighScore Plus software (version 5.2; Malvern PANalytical, Almelo, The Netherlands) and the ICDD PDF-5+ database.
For this kinetic study, these five materials were used in ground form with a grain size of less than 0.5 mm. Samples were crushed in the wet state by the BB50 jaw crusher (Retsch, Haan, Germany). The kinetic study of the considered cement–asbestos samples was performed by an ex-situ technique, where the cement–asbestos waste material (with a starting amount of material of 5 g) was isothermally treated in a porcelain crucible at selected temperatures—700, 725, 750, 775 and 800 °C—and recording changes in the mass at time intervals until a constant mass was obtained. At each temperature considered, different heating times were used to complete the thermal decomposition process. Isothermal treatment was conducted in an LT9/14 electric laboratory furnace (Nabertherm, Lilienthal, Germany) equipped with Controller B510 to set the heating curve. The weighing operation was carried out on an ALN220G laboratory analytical balance (Axis, Gdańsk, Poland).
3. Results and Discussion
3.1. Cement–Asbestos Sample Characterisation
The presence of asbestos fibers in the tested material was obvious. In the crushed material, the presence of both larger, thicker tufts and bundles as well as single fibers was observed (Figure 1). The observed bundles had a tendency to be divided into narrower elementary fibers. Most of the observed fibers had a tendency to be flexible and wavy, which indicated the presence of white asbestos (chrysotile). Only in the case of the BUJ and HEL samples were needle-shaped fibers with a lack of flexibility observed. This may indicate the presence of an amphibole variety of asbestos.

Figure 1.
SEM images of cement–asbestos samples with the presence of a characteristic fibrous form of asbestos: (a) HEL sample; (b) DG sample.
The main physical properties of the cement–asbestos samples are presented in Table 1. Most of the tested cement–asbestos samples were characterized by a relatively high value of open porosity, which changed from ~15% to close to 30%, and water absorption at the level of ~10%. These samples also had a similar apparent density of c.a. 1.9 g cm−3. Only in the case of one sample (NOW) did these properties differ significantly. Its lowest apparent density as well as highest open porosity and water absorption may indicate severe aging and corrosion of the material.

Table 1.
Main physical properties of the cement–asbestos samples.
The main chemical composition and structural characterization of the samples have been reported in a previous work [21]. Here, we can summarize that from a chemical point of view, the cement–asbestos materials were composed mainly of calcium and silicon compounds accompanied by significant loss on ignition. This is in accordance with the general properties of cement–asbestos roofing, which are mainly due to the presence of cement matrix in the waste [17,21,22]. The average chemical composition of the cement–asbestos samples is presented in Table 2. In turn, the quantitative phase composition is shown in Table 3. The cement–asbestos samples were multi-component systems. Primarily, phases typical of an aged and weathered cementitious matrix were identified in the considered samples. Note the significant amount of X-ray amorphous phase caused by many years of aging of the samples and exposure to external factors, which caused gradual degradation of the material. Moreover, products of cement hydration possess a low degree of crystallinity, which also influences the amount of amorphous fraction [22]. It is worth noting that—apart from the most common white asbestos i.e., chrysotile—in two of the samples, the amphibole variety of asbestos (so-called blue asbestos; crocidolite) was also identified.

Table 2.
Average chemical composition of the cement–asbestos samples (in wt%).

Table 3.
Mineralogical composition of the cement–asbestos samples (in wt%).
Qualitatively, the course of the thermal decomposition process of cement–asbestos samples is well described [17,21,23,24,25,26,27,28,29]. Cement–asbestos is a multi-phase system, so its thermal decomposition cannot be described by one reaction. This process is multi-stage and can be summarized in tabular form as in Table 4, which describes the typical thermal effects recorded during thermal analysis measurements of cement–asbestos samples [17,21,23,24,25,26,27,28,29,31,32] and of white asbestos (chrysotile) in particular.

Table 4.
Progress of the cement–asbestos thermal decomposition process [17,21,23,24,25,26,27,28,29,31,32].
3.2. Kinetic Study
The kinetic issues discussed in the article may be extremely useful when designing thermal methods for asbestos waste recycling technology during re-scaling at an industrial level. The kinetics of a chemical/physical process can be expressed by the general equation dα/dt = k·f(α), where α is the degree of conversion of the reaction/process, k is the rate constant at a given temperature, and f(α) is the kinetic model function, which is usually an empirical function that is dependent on the mechanism of the reaction.
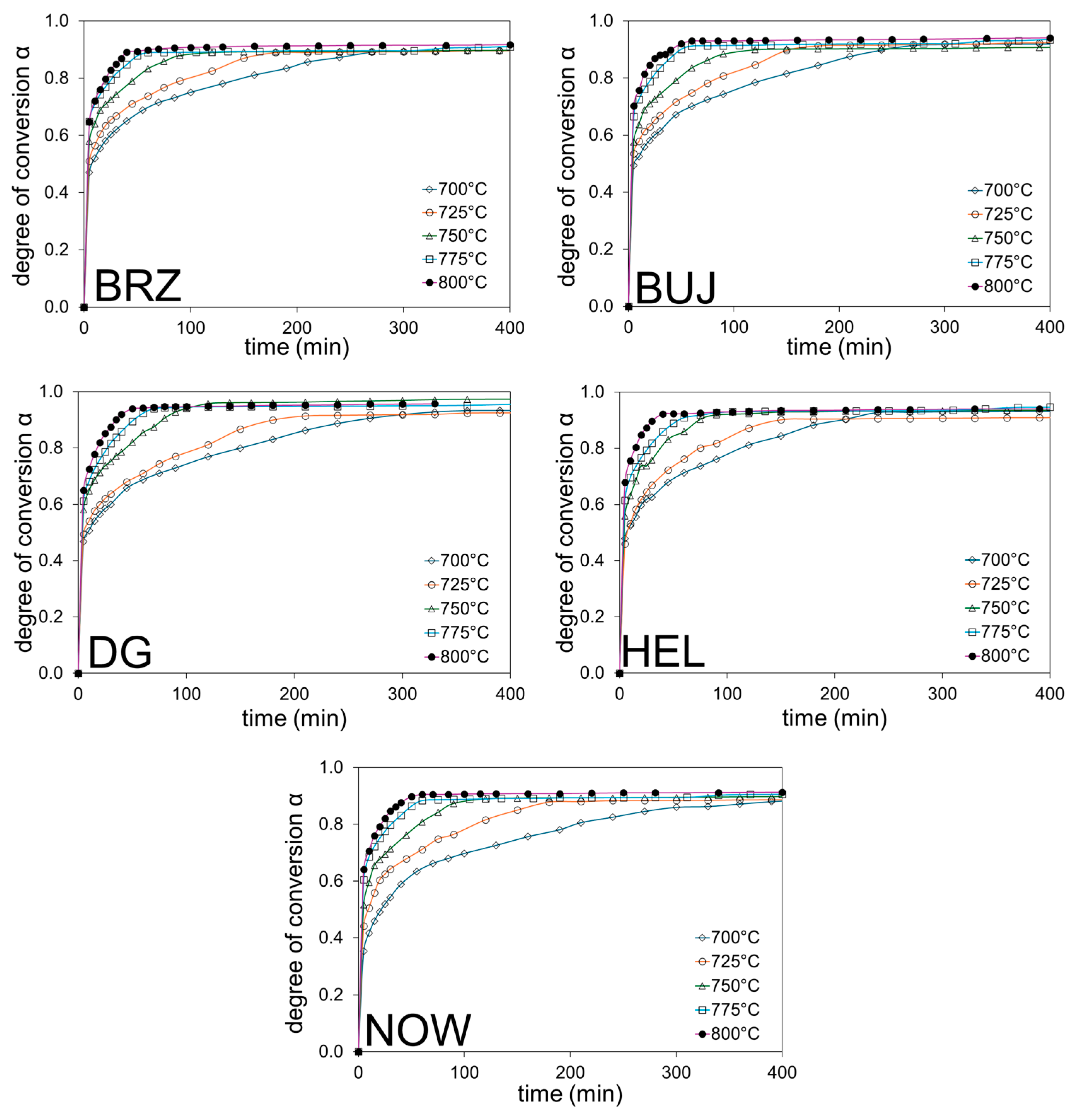
The subject of this research is the process of thermal decomposition of cement–asbestos materials, which consists of several stages and results from the multi-phase nature of the reacting system (Table 3 and Table 4). It is, therefore, not possible to express this process by a simple chemical reaction; rather, it is better to consider the process as a whole. Because volatile components like H2O and CO2 (the main gaseous decomposition products) are released from the system during the thermal treatment of cement–asbestos materials, the degree of conversion α of this whole process can be expressed as the mass loss in relation to the base value, i.e., the value resulting from the loss on ignition or the value of the total mass change from the thermogravimetric analysis. In Figure 2, the variations of α vs. time under ex-situ isothermal calcination of the tested cement–asbestos samples are reported. Of course, the higher the temperature, the faster the maximum conversion rate for all the tested samples was achieved.
Figure 2.
Variations with time under isothermal conditions for the degree of conversion α, determined by the difference in mass loss of the cement–asbestos samples.
The slowest decomposition process was observed for the lowest assumed calcination temperature, i.e., 700 °C. Based on the results obtained, the thermal decomposition process of cement–asbestos connected with asbestos neutralization took place after c.a. 30 min for the highest calcination temperature (800 °C). In turn, for the lowest assumed temperature of thermal treatment, i.e., 700 °C, the same effect was achieved over a significantly longer period, taking approximately 5 h (300 min). In this respect, there were no significant differences for the tested samples, with all of them showing similar recorded thermal decompositions.
It is possible to employ a general empirical rate equation in which the function f(α) is expressed, in some general manner, with a variable parameter that reflects the rate-controlling mechanism. Analysis of the kinetic curves was performed using the classical model for heterogeneous solid-state reactions. A good establishment data-analysis procedure is the Avrami–Erofeev method [33], which assumes that α = 1 − exp[(kt)n], where k is the kinetic constant and n is reaction order, and it is indicative of the mechanism controlling the reaction/process rate.
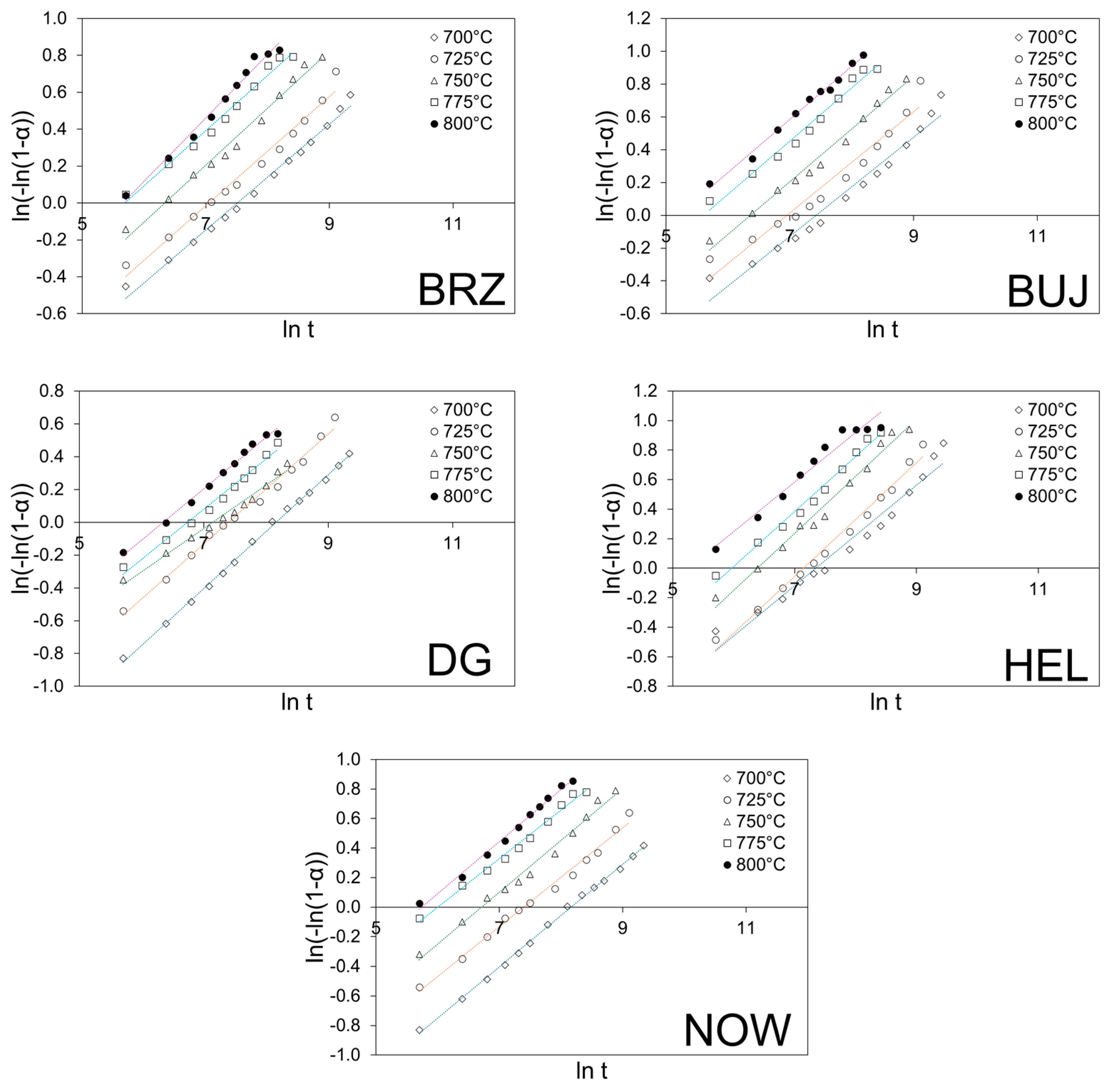
Logarithmic transformation of the above equation can be used to plot the graph ln(−ln(1 − α)) versus ln(t). This corresponds to the linearized form of the Avrami–Erofeev equation. The values of k (rate coefficient) and n (reaction order) can be calculated from the intercept and slope of the graph ln(−ln(1 − α)) vs. ln t, i.e., the so-called ln-ln plot. This is an important issue because it allows for the interpretation of the reaction mechanism, while the rate coefficient can be used in the Arrhenius equation with T (in Kelvin) and R (gas constant) as follows: k = A·exp[−Ea/RT]. The plot of ln(k) vs. 1/T yields the apparent activation energy (Ea) from the slope of the linear curve and the pre-exponential factor (A) from the line intercept. The corresponding ln-ln plots connected with the experimental data shown in Figure 2 are reported in Figure 3. The kinetic constants (k) are reported in an Arrhenius plot in Figure 4, while Table 5 and Table 6 report the calculated values of n, k, and Ea, respectively.
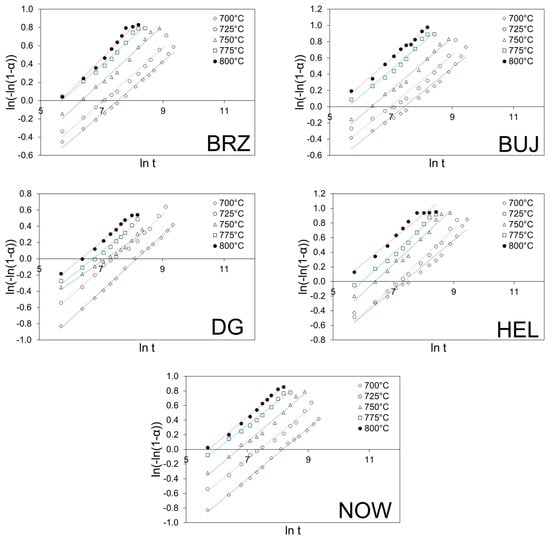
Figure 3.
Ln-ln plots of the corresponding data from the thermal decomposition of the cement–asbestos samples.
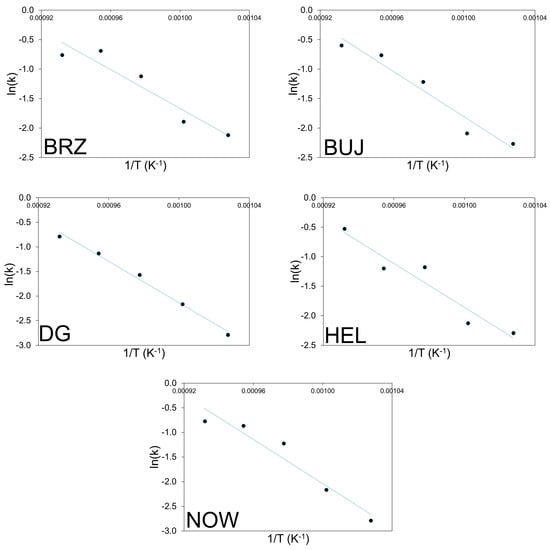
Figure 4.
Arrhenius plots of the thermal decomposition process of the cement–asbestos samples.

Table 5.
Calculated kinetic parameters for the decomposition process of the tested cement–asbestos samples.

Table 6.
Values of the apparent activation energy obtained from the Arrhenius plot.
The ln-ln curves (Figure 3) show nearly the same slope and are parallel, which indicate that the mechanism is the same for the thermal decomposition process in all the samples in the assumed temperature range. The calculated values of reaction order for each sample and calcination temperature were close to 0.3 (Table 5), and they were in quite good linear correlation. This fractional order reaction suggests a complex mechanism for the whole decomposition process. The calculated values of the rate coefficients increase with increasing thermal treatment, which was in line with expectations. For the lowest calcination temperature, the rate-coefficient value was close to 0.1, while for the highest temperature, it was five times larger.
The activation energy associated with the cement–asbestos thermal decomposition process changed from ~140 kJ mol−1 (for the BRZ sample) to ~185 kJ mol−1 (for the NOW sample). The average value of Ea was calculated at the level of 163 ± 19 kJ mol−1. The calculated activation energy is very similar to the Ea value measured for calcite decomposition [34]. This is not surprising, since one of the main mineral components of cement–asbestos is calcium carbonate in the form of calcite. However, it should be emphasized that within the temperature range for calcite thermal decomposition, thermal decomposition of asbestos, mainly chrysotile, also occurs. Hence, what is measured is the resultant effect of parallel processes taking place within the material.
4. Conclusions
The process of thermal decomposition of cement–asbestos from the kinetic point of view was studied. In the present study, isothermal calcination at different temperatures from 700 to 800 °C was used to investigate the kinetics of the thermal decomposition process of five cement–asbestos samples from Poland. The tested materials were characterized by differential chemical and mineralogical compositions. From a chemical point of view, all the cement–asbestos samples were primarily composed of calcium oxide and silicon oxide. These samples were also characterized by a high value of loss on ignition. From a mineralogical point of view, the tested materials were multi-phase. In the tested materials, nine different mineral phases were identified, occurring in different proportions. In addition, the tested cement–asbestos samples were characterized by a significant content of the X-ray amorphous phase, which was often above 50% by weight.
Ex-situ experiments were carried out at temperature values which allowed for the decomposition of asbestos minerals, especially chrysotile (white asbestos). From the mass-change measurements taken at selected temperatures during the thermal treatment, the graph of the conversion degree associated with the decomposition of cement–asbestos versus time was determined. The heterogeneous solid-state reaction analyzed by the Avrami–Erofeev model was the kinetic model that showed a fairly good fit with the experimental results. The empirical activation energy values were evaluated from the Arrhenius plot, and these achieved values close to 140–180 kJ mol−1. Differences in the activation energy could be associated with the variable phase composition of the samples. However, due to the presence of large amounts of the amorphous phase, it was difficult to find any correlation. The issues raised and discussed in the article may be extremely useful when designing thermal methods for asbestos waste treatment and recycling on an industrial scale, especially in the context of cement–asbestos. This study allows for the determination of the best conditions for the deactivation of cement–asbestos waste by thermal methods. For the considered samples, thermal treatment at 800 °C for 30 min was sufficient to complete the thermal decomposition process for cement–asbestos waste. However, it should be borne in mind that other types of asbestos may be presented in the cement–asbestos products, which would require adjusting the course of the thermal process and the temperature needed for their decomposition.
Author Contributions
Conceptualization, R.K. and A.G.; methodology, R.K and A.G.; software, A.G.; validation, R.K., M.K. and A.G.; formal analysis, R.K. and A.G.; investigation, R.K., M.K. and A.G.; resources, R.K.; data curation, R.K.; writing—original draft preparation, R.K.; writing—review and editing, M.K. and A.G.; visualization, R.K., M.K. and A.G.; supervision, R.K.; project administration, R.K.; funding acquisition, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in whole by the National Science Centre, Poland under “Sonata 17” grant number UMO-2021/43/D/ST5/00356.
Data Availability Statement
The data presented in the study are openly available. The experimental data that support the findings of this study are available in the RepOD repository with the identifier https://doi.org/10.18150/XCDVVX as well as https://doi.org/10.18150/B55A4G.
Acknowledgments
The authors would like to thank Elwira Cieślińska and Maria Pyka for their help in preparing the samples for testing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gualtieri, A.F. Mineral Fibers: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; European Mineralogical Union: Dublin, UK, 2017. [Google Scholar]
- Gualtieri, A.F. Recycling asbestos-containing material (ACM) from construction and demolition waste (CDW). In Handbook of Recycled Concrete and Demolition Waste; Woodhead Publishing: Sawston, UK, 2013; pp. 500–525. [Google Scholar]
- Kusiorowski, R.; Lipowska, B.; Kujawa, M.; Gerle, A. Problem of asbestos-containing wastes in Poland. Clean. Waste Syst. 2023, 4, 1000085. [Google Scholar] [CrossRef]
- Musk, A.W.; de Klerk, N.; Reid, A.; Hui, J.; Franklin, P.; Brims, F. Asbestos-related diseases. Tuberc. Lung Dis. 2020, 24, 562–567. [Google Scholar] [CrossRef]
- Stayner, L.; Welch, L.S.; Lemen, R. The worldwide pandemic asbestos-related diseases. Annu. Rev. Public Health 2013, 34, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.; Kang, D.; Paek, D. Environmental exposure to asbestos and the risk of lung cancer: A systematic review and meta-analysis. J. Occup. Environ. Med. 2022, 79, 207–214. [Google Scholar] [CrossRef]
- Paglietti, F.; Malinconico, S.; della Stafa, B.C.; Bellagamba, S.; De Simone, P. Classification and management of asbestos-containing waste: European legislation and the Italian experience. Waste Manag. 2016, 50, 130–150. [Google Scholar] [CrossRef]
- Li, J.; Dong, Q.; Yu, K.; Liu, L. Asbestos and asbestos waste management in the Asian-Pacific region: Trends, challenges and solutions. J. Clean. Prod. 2014, 81, 218–226. [Google Scholar] [CrossRef]
- Szymańska, D.; Lewandowska, A. Disposal of asbestos and products containing asbestos in Poland. J. Mater. Cycles Waste Manag. 2019, 21, 345–355. [Google Scholar] [CrossRef]
- Wallis, S.L.; Emmett, E.A.; Hardy, R.; Casper, B.B.; Blanchon, D.J.; Testa, J.R.; Menges, C.W.; Gonneau, C.; Jerolmack, D.J.; Seiphoori, A.; et al. Challenging global waste management—Bioremediation to detoxify asbestos. Front. Environ. Sci. 2020, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Promentilla, M.A.B.; Peralta, G.L. An evaluation of landfill disposal of asbestos-containing waste and geothermal residues within a risk-assessment framework. J. Mater. Cycles Waste Manag. 2003, 5, 0013–0021. [Google Scholar] [CrossRef]
- Spasiano, D.; Pirozzi, F. Treatments of asbestos containing wastes. J. Environ. Manag. 2017, 204, 82–91. [Google Scholar] [CrossRef]
- Paolini, V.; Tomassetti, L.; Segreto, M.; Borin, D.; Liotta, F.; Torre, M.; Petracchini, F. Asbestos treatment technologies. J. Mater. Cycles Waste Manag. 2019, 21, 205–226. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Giacobbe, C.; Sardisco, L.; Saraceno, M.; Gualtieri, M.L.; Lusvardi, G.; Cavenati, C.; Zanatto, I. Recycling of the product of thermal inertization of cement–asbestos for various industrial applications. Waste Manag. 2011, 31, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Takata, A.; Yamauchi, H.; Yamashita, K.; Aminaka, M.; Hitomi, T.; Toya, T.; Kohyama, N. Mesothelioma carcinogenesis of chrysotile and forsterite compared and validated by intraperitoneal injection in rat. Ind. Health 2025, 63, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Zaremba, T.; Krząkała, A.; Piotrowski, J.; Garczorz, D. Study on the thermal decomposition of chrysotile asbestos. J. Therm. Anal. Calorim. 2010, 101, 479–485. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J.; Gerle, A. Thermal decomposition of asbestos-containing materials. J. Therm. Anal. Calorim. 2013, 113, 179–188. [Google Scholar] [CrossRef]
- Kim, C.; Kim, Y.; Roh, Y. Thermal decomposition and phase transformation of chrysotile in asbestos-containing waste. Minerals 2025, 15, 344. [Google Scholar] [CrossRef]
- Poniatowska, A.; Andrzejewska-Górecka, D.; Macherzyński, B.; Kisiel, M. Thermal decomposition of asbestos fiber from asbestos cement wastes. Rocz. Ochr. Środowiska 2019, 21, 855–867. [Google Scholar]
- Bloise, A.; Catalano, M.; Barrese, E.; Gualtieri, A.F.; Bursi Gandolfi, N.; Capella, S.; Belluso, E. TG/DSC study of the thermal behaviour of hazardous mineral fibres. J. Therm. Anal. Calorim. 2016, 123, 2225–2239. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Gerle, A.; Kujawa, M.; Śliwa, A.; Adamek, J. Charaterisation of asbestos-containing wastes by thermal analysis. J. Therm. Anal. Calorim. 2024, 149, 10681–10694. [Google Scholar] [CrossRef]
- Viani, A.; Gualtieri, A.F.; Secco, M.; Peruzzo, L.; Artioli, G.; Cruciani, G. Crystal chemistry of cement-asbestos. Am. Mineral. 2013, 98, 1095–1105. [Google Scholar] [CrossRef]
- Viani, A.; Gualtieri, A.F.; Pollastri, S.; Rinaudo, C.; Croce, A.; Urso, G. Crystal chemistry of the high-temperature product of transformation of cement-asbestos. J. Hazard. Mater. 2013, 248–249, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Kusiorowski, R.; Gerle, A.; Kujawa, M.; Antonovič, V.; Boris, R. Structural characterisation of end-of-life cement-asbestos materials from Lithuania. Fibers 2024, 12, 37. [Google Scholar] [CrossRef]
- Belardi, C.; Piga, L. Influence of calcium carbonate on the decomposition of asbestos contained in end-of-life products. Thermochim. Acta 2013, 573, 220–228. [Google Scholar] [CrossRef]
- Dias, C.M.R.; Cincotto, M.A.; Savastano, H., Jr.; John, V.M. Long-term aging of fiber-cement corrugated sheets—The effect of carbonation, leaching and acid rain. Cem. Concr. Compos. 2008, 30, 255–265. [Google Scholar] [CrossRef]
- Vergani, F.; Galimberti, L.; Marian, N.M.; Giorgetti, G.; Viti, C.; Capitani, G. Thermal decomposition of cement–asbestos at 1100 °C: How much “safe” is “safe”? J. Mater. Cycles Waste Manag. 2022, 24, 297–310. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Cavenati, C.; Zanatto, I.; Meloni, M.; Elmi, G.; Gualtieri, M.L. The transformation sequence of cement-asbestos slates up to 1200 ◦C and safe recycling of the reaction product in stoneware tile mixtures. J. Hazard. Mater. 2008, 152, 563–570. [Google Scholar] [CrossRef]
- Iwaszko, J.; Lubas, M.; Sitarz, M.; Zajemska, M.; Nowak, A. Production of vitrified material from hazardous asbestos-cement waste and CRT glass cullet. J. Clean. Prod. 2021, 317, 128345. [Google Scholar] [CrossRef]
- EN ISO 12677:2011; Chemical Analysis of Refractory Products by X-ray Fluorescence (XRF)—Fused Cast-Bead Method. ISO: Geneva, Switzerland, 2011.
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Geological Institute of Hungary: Budapest, Hungary, 2011. [Google Scholar]
- Strzałkowska, E. Thermal Analysis of Minerals, Rocks and Mineral Waste Materials; Silesian University of Technology: Gliwice, Poland, 2019. (In Polish) [Google Scholar]
- Bamford, C.H.; Tipper, C.F.H. Comprehensive Chemical Kinetics; Elsevier: Alpharetta, GA, USA, 1980. [Google Scholar]
- Halikia, I.; Zoumpoulakis, L.; Christodoulou, E.; Prattis, D. Kinetic study of the thermal decomposition of calcium carbonate by isothermal methods of analysis. Eur. J. Miner. Process. Environ. Protect. 2001, 1, 89–102. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).