Polyaniline-Coated Electrospun Polyacrylonitrile Nanofibers for Effective Short-Chain PFAS (GenX) Removal from Water
Abstract
Highlights
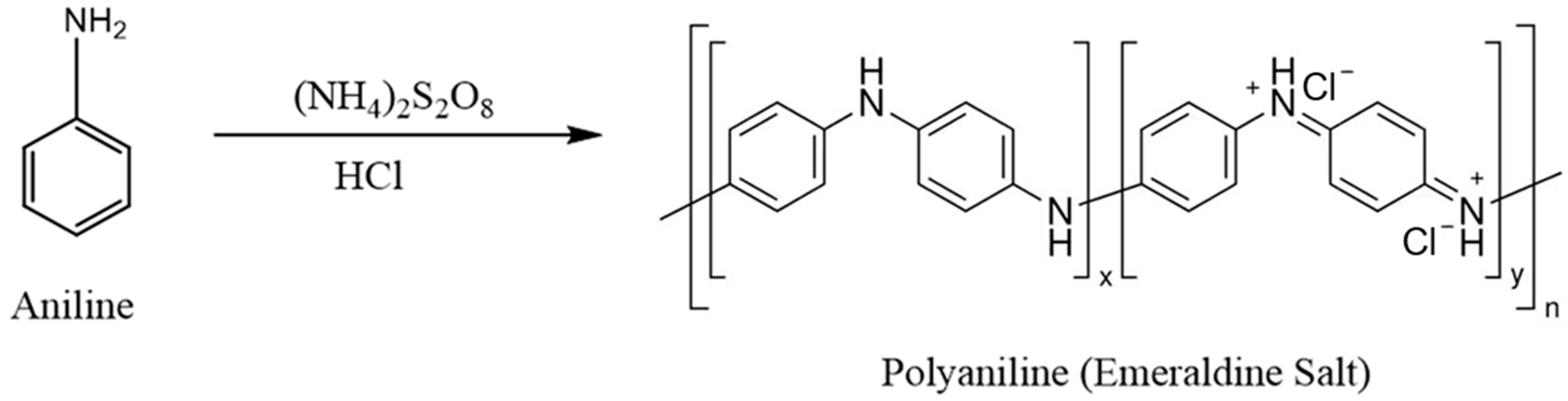
- Electrospun polyacrylonitrile (ESPAN) nanofibers were coated with polyaniline (PANI) through in situ polymerization.
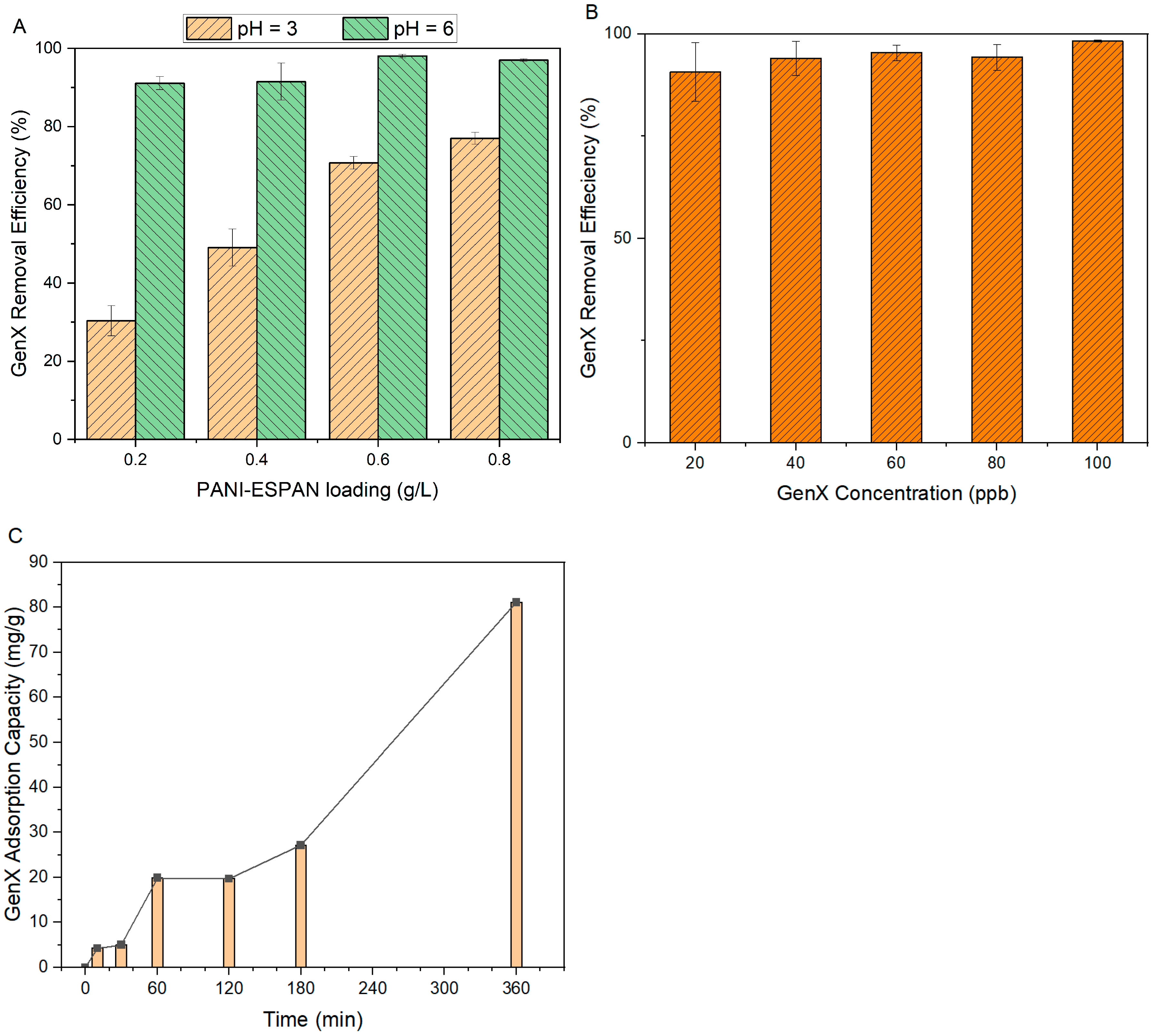
- The PANI-coated ESPAN nanofibrous adsorbent (PANI-ESPAN) demonstrated excellent adsorption capabilities for a short-chain PFAS (GenX) and could remove nearly all GenX (>98%) from a 100 ppb aqueous solution at pH = 6 and 0.6 g/L loading, with a contact time of 12 h.
- The GenX adsorption mechanism of PANI-ESPAN at pH = 6 can mainly be attributed to non-Coulomb interactions (hydrophobic, dipole–dipole, and hydrogen bonding), while Coulomb interactions (electrostatic interaction) also played a role in the adsorption.
- By coupling the unique properties of PANI with ESPAN nanofibers, this research demonstrated a versatile and efficient solution to address current short-chain PFAS contamination issues in the practice of water purification.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ESPAN and PANI-Coated ESPAN Nanofibrous Adsorbents
2.3. Characterization of PANI-ESPAN
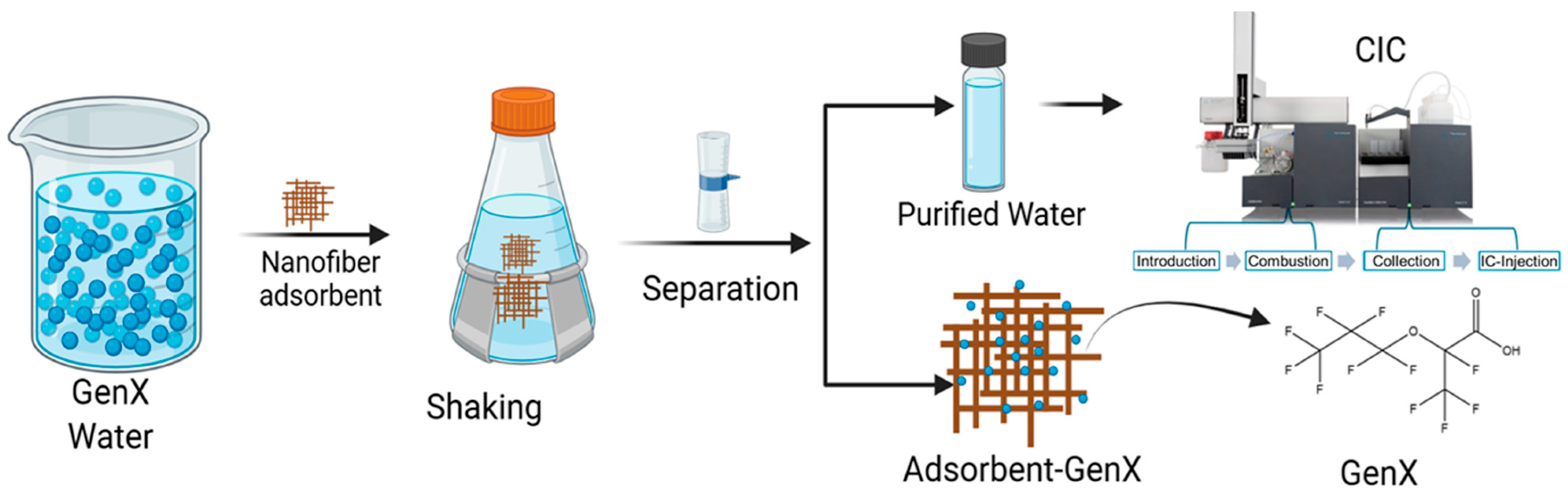
2.4. GenX Adsorption
2.5. GenX Analysis
3. Results and Discussion
3.1. Characterization of PANI-ESPAN Nanofibrous Adsorbent
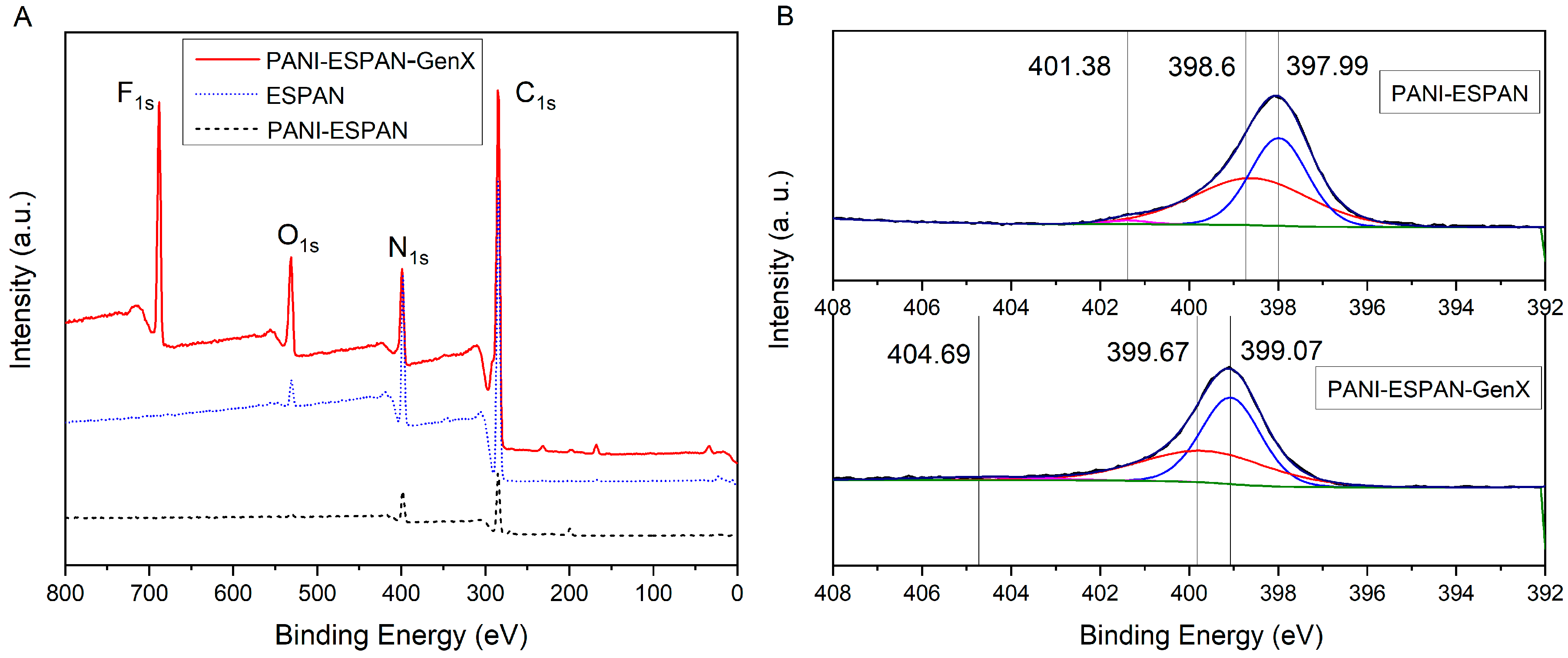
3.1.1. PANI Coating
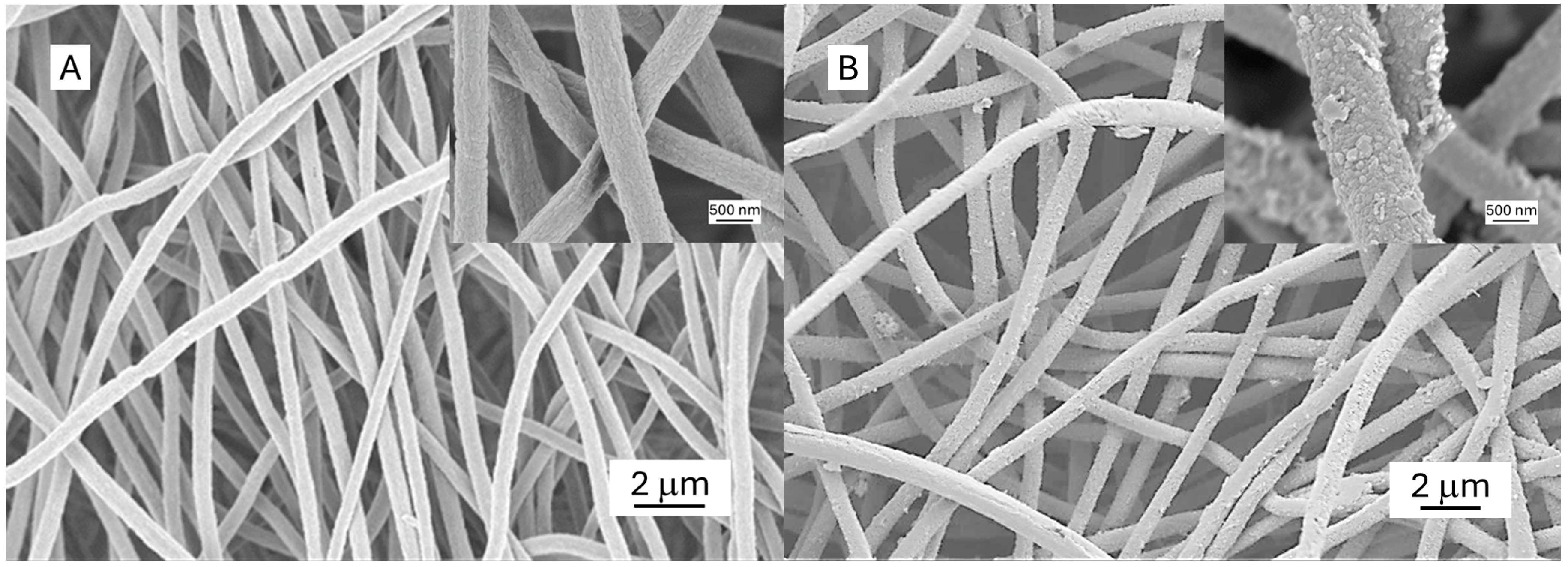
3.1.2. Morphology
3.1.3. Specific Surface Area and Pore Volume
3.1.4. Surface Charge
3.2. Performance of PANI-ESPAN Nanofibrous Adsorbent in GenX Removal
3.2.1. GenX Adsorption on PANI-ESPAN Nanofibrous Adsorbent
3.2.2. Effect of pH
3.2.3. Effect of Adsorbent Loading
3.2.4. Effect of GenX Concentration
3.2.5. Effect of Contact Time
3.3. GenX Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amen, R.; Ibrahim, A.; Shafqat, W.; Hassan, E.B. A Critical Review on PFAS Removal from Water: Removal Mechanism and Future Challenges. Sustainability 2023, 15, 16173. [Google Scholar] [CrossRef]
- Brendel, S.; Fetter, E.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-Chain Perfluoroalkyl Acids: Environmental Concerns and a Regulatory Strategy Under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel Treatment Technologies for PFAS Compounds: A Critical Rreview. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of Poly- and Perfluoroalkyl Substances (PFAS) from Water by Adsorption: Role of PFAS Chain Length, Effect of Organic Matter and Challenges in Adsorbent Regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Chaleshtari, Z.A.; Foudazi, R. A Review on Per- and Polyfluoroalkyl Substances (PFAS) Remediation: Separation Mechanisms and Molecular Interactions. ACS ES&T Water 2022, 2, 2258–2272. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A Review of PFAS Adsorption from Aqueous Solutions: Current Approaches, Engineering Applications, Challenges, and Opportunities. Environ. Pollut. 2023, 321, 121138. [Google Scholar] [CrossRef]
- Kang, S.B.; Wang, Z.; Zhang, W.; Kim, K.-Y.; Won, S.W. Removal of Short- and Long-Chain PFAS from Aquatic Systems Using Electrostatic Attraction of Polyethylenimine-Polyvinyl Chloride Electrospun Nanofiber Adsorbent. Sep. Purif. Technol. 2023, 326, 124853. [Google Scholar] [CrossRef]
- Vu, C.T.; Wu, T. Recent Progress in Adsorptive Removal of Per- and Poly-Fluoroalkyl Substances (PFAS) from Water/Wastewater. Crit. Rev. Environ. Sci. Technol. 2022, 52, 90–129. [Google Scholar] [CrossRef]
- Wang, W.; Maimaiti, A.; Shi, H.; Wu, R.; Wang, R.; Li, Z.; Qi, D.; Yu, G.; Deng, S. Adsorption Behavior and Mechanism of Emerging Perfluoro-2-Propoxypropanoic Acid (GenX) on Activated Carbons and Resins. Chem. Eng. J. 2019, 364, 132–138. [Google Scholar] [CrossRef]
- Ji, W.; Xiao, L.; Ling, Y.; Ching, C.; Matsumoto, M.; Bisbey, R.P.; Helbling, D.E.; Dichtel, W.R. Removal of GenX and Perfluorinated Alkyl Substances from Water by Amine-Functionalized Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 12677–12681. [Google Scholar] [CrossRef]
- Huang, P.-J.; Hwangbo, M.; Chen, Z.; Liu, Y.; Kameoka, J.; Chu, K.-H. Reusable Functionalized Hydrogel Sorbents for Removing Long- and Short-Chain Perfluoroalkyl Acids (PFAAs) and GenX from Aqueous Solution. ACS Omega 2018, 3, 17447–17455. [Google Scholar] [CrossRef] [PubMed]
- Maske, V.A.; Kokate, A.M.; More, P.A.; Salunkhe, R.S.; More, A.P. A Comprehensive Review of Polyacrylonitrile Membranes: Modifications and Applications. Polym. Bull. 2024, 81, 16415–16455. [Google Scholar] [CrossRef]
- Mantripragada, S.; Deng, D.; Zhang, L. Remediation of GenX from Water by Amidoxime Surface-Functionalized Electrospun Polyacrylonitrile Nanofibrous Adsorbent. Chemosphere 2021, 283, 131235. [Google Scholar] [CrossRef] [PubMed]
- Olshansky, Y.; Gomeniuc, A.; Chorover, J.; Abrell, L.; Field, J.A.; Hatton, J.; He, J.; Sierra-Alvarez, R. Tailored Polyanilines Are High-Affinity Adsorbents for Per- and Polyfluoroalkyl Substances. ACS EST Water 2022, 2, 1402–1410. [Google Scholar] [CrossRef]
- Mohammad, N.; Atassi, Y. Enhancement of Removal Efficiency of Heavy Metal Ions by Polyaniline Deposition on Electrospun Polyacrylonitrile Membranes. Water Sci. Eng. 2021, 14, 129–138. [Google Scholar] [CrossRef]
- Mohammad, N.; Atassi, Y. Adsorption of Methylene Blue onto Electrospun Nanofibrous Membranes of Polylactic Acid and Polyacrylonitrile Coated with Chloride Doped Polyaniline. Sci. Rep. 2020, 10, 13412. [Google Scholar] [CrossRef]
- Shakiba, M.; Nabavi, S.R.; Emadi, H.; Faraji, M. Development of a Superhydrophilic Nanofiber Membrane for Oil/Water Emulsion Separation via Modification of Polyacrylonitrile/Polyaniline Composite. Polym. Adv. Technol. 2021, 32, 1301–1316. [Google Scholar] [CrossRef]
- Trchova, M.; Sedenkova, I.; Tobolkova, E.; Stejskal, J. FTIR Spectroscopic and Conductivity Study of the Thermal Degradation of Polyaniline Films. Polym. Degrad. Stab. 2004, 86, 179–185. [Google Scholar] [CrossRef]
- Liu, H.; Hu, X.B.; Wang, J.Y.; Boughton, R.I. Structure, Conductivity, and Thermopower of Crystalline Polyaniline Synthesized by the Ultrasonic Irradiation Polymerization Method. Macromolecules 2022, 35, 9414–9419. [Google Scholar] [CrossRef]
- Graves, D.A.; Thodoroviez, L.B.; de Oliveira, T.C.; Cividanes, L.S.; Ferreira, N.G.; Almeida, D.A.L.; Goncalves, E.S. Enhanced electrochemical properties of polyaniline (PANI) films electrodeposited on carbon fiber felt (CFF): Influence of monomer/acid ratio and deposition time parameters in energy storage applications. Electrochim. Acta 2023, 454, 142388. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Luo, X.; Guo, H.; Wang, N.; Su, D.; Zhang, C.; Liu, T.; Wang, G.; Cui, L. Polyaniline engineering defect-induced nitrogen doped carbo-supported Co3O4 hybrid composite as a high-efficiency electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2020, 526, 146626. [Google Scholar] [CrossRef]
- Mantripragada, S.; Dong, M.; Zhang, L. Sustainable filter/adsorbent materials from cellulose-based electrospun nanofibrous membranes with soy protein coating for high-efficiency GenX fluorocarbon remediation from water. Cellulose 2023, 30, 7063–7078. [Google Scholar] [CrossRef]
- Olshansky, Y.; Gomeniuc, A.; Chorover, J.; Abrell, L.; Field, J.A.; Hatton, J.; Sierra-Alvarez, R. Synthesis and Characterization of Customizable Polyaniline-Derived Polymers and Their Application for Perfluorooctanoic Acid Removal from Aqueous Solution. ACS EST Water 2021, 1, 1438–1446. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahan, I.; Tani, E.A.; Patel, H.V.; Zhao, R.; Zhang, L. Polyaniline-Coated Electrospun Polyacrylonitrile Nanofibers for Effective Short-Chain PFAS (GenX) Removal from Water. Fibers 2025, 13, 42. https://doi.org/10.3390/fib13040042
Jahan I, Tani EA, Patel HV, Zhao R, Zhang L. Polyaniline-Coated Electrospun Polyacrylonitrile Nanofibers for Effective Short-Chain PFAS (GenX) Removal from Water. Fibers. 2025; 13(4):42. https://doi.org/10.3390/fib13040042
Chicago/Turabian StyleJahan, Israt, Easmin Ara Tani, Harsh V. Patel, Renzun Zhao, and Lifeng Zhang. 2025. "Polyaniline-Coated Electrospun Polyacrylonitrile Nanofibers for Effective Short-Chain PFAS (GenX) Removal from Water" Fibers 13, no. 4: 42. https://doi.org/10.3390/fib13040042
APA StyleJahan, I., Tani, E. A., Patel, H. V., Zhao, R., & Zhang, L. (2025). Polyaniline-Coated Electrospun Polyacrylonitrile Nanofibers for Effective Short-Chain PFAS (GenX) Removal from Water. Fibers, 13(4), 42. https://doi.org/10.3390/fib13040042





