Durable Hydrophilic PVDF Hollow Fiber Membrane for Dissolved Organics Separation from High-Salinity Produced Water
Abstract
Highlights
- Durable hydrophilic PVDF hollow fiber membranes were formulated and fabricated with lithium chloride (LiCl) and polyvinylpyrrolidone (PVP) as synergistic pore agents.
- The hydrophilic PVDF hollow fiber membrane showed a double-skinned macrovoids-free structure.
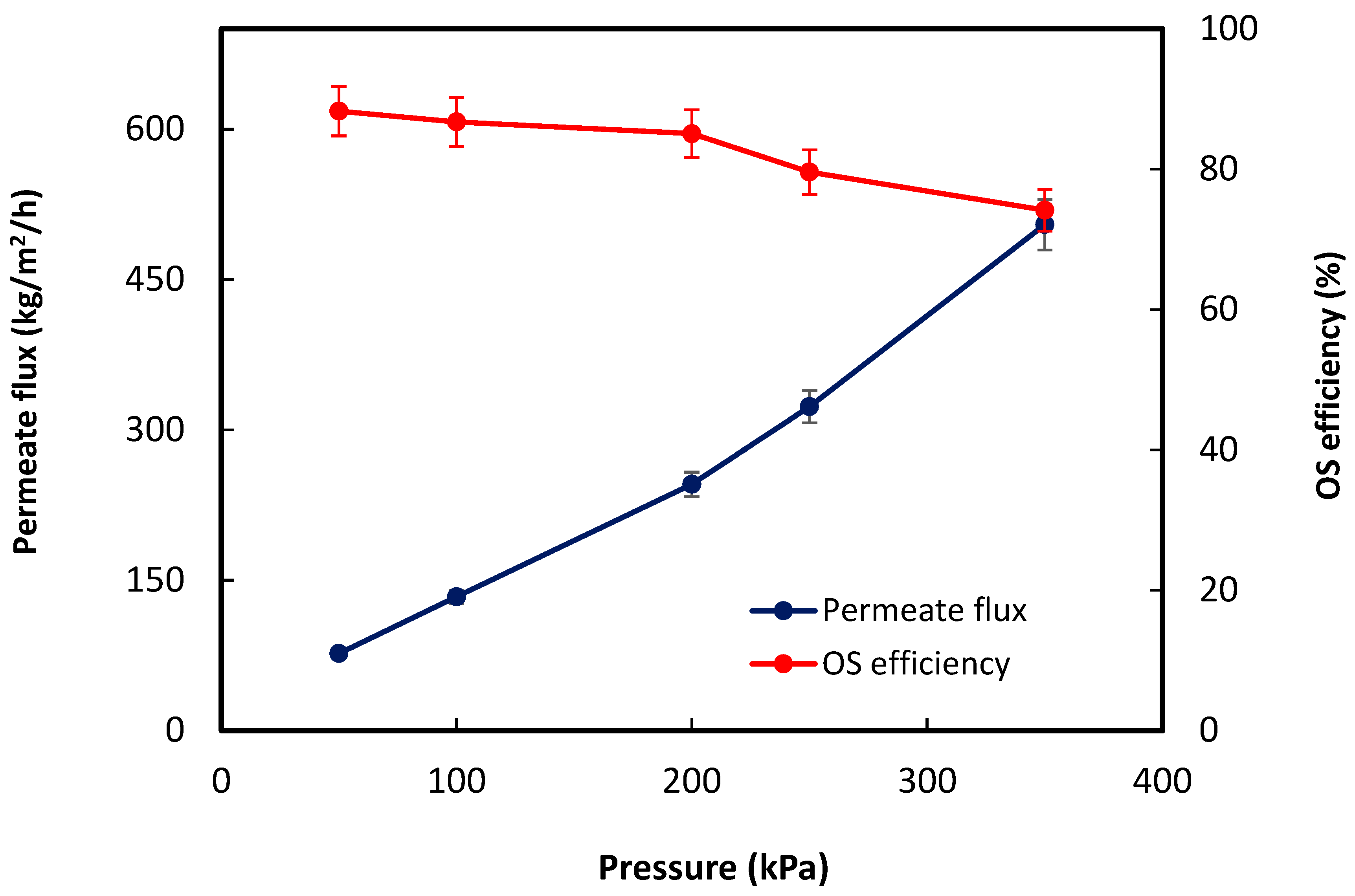
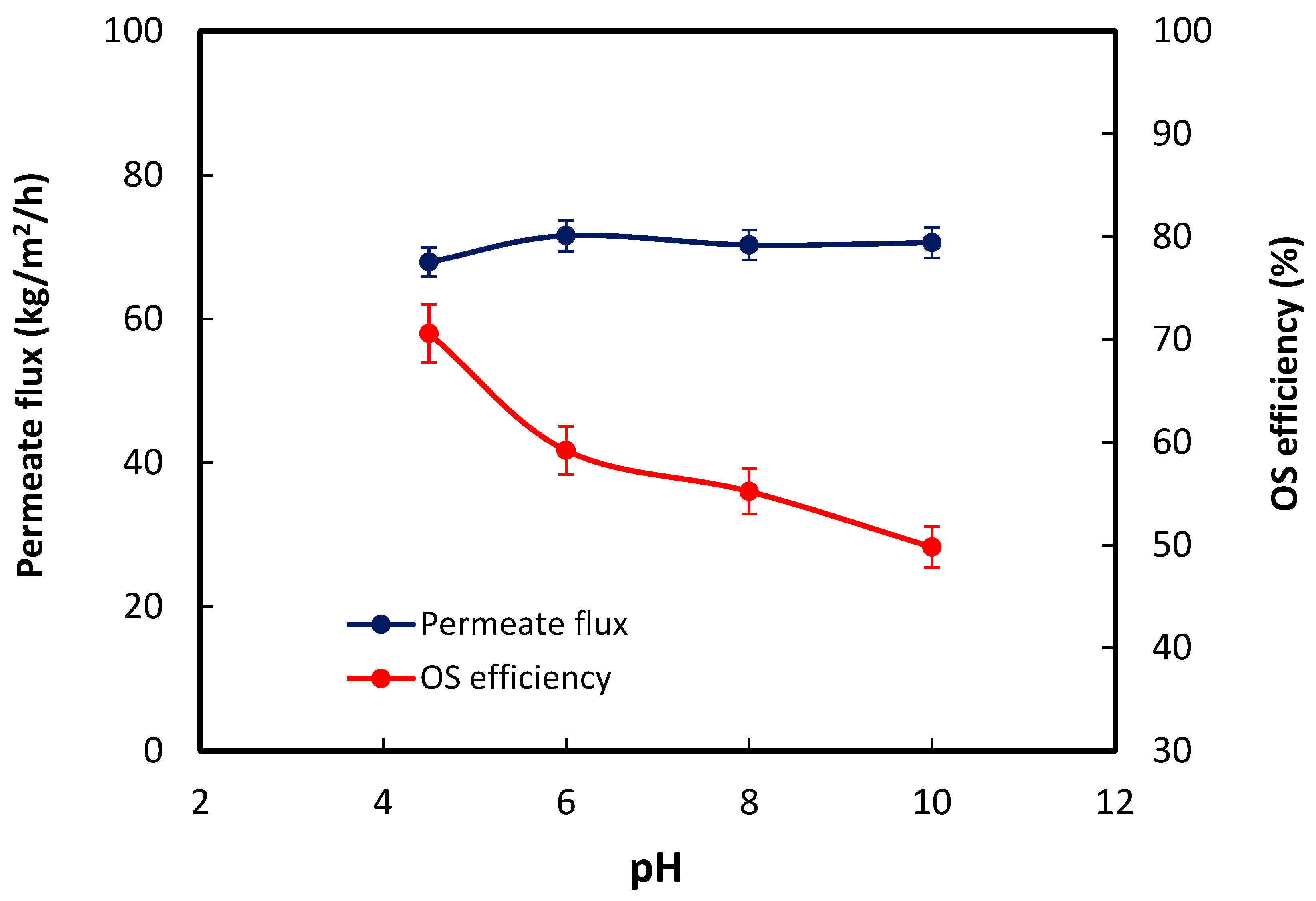
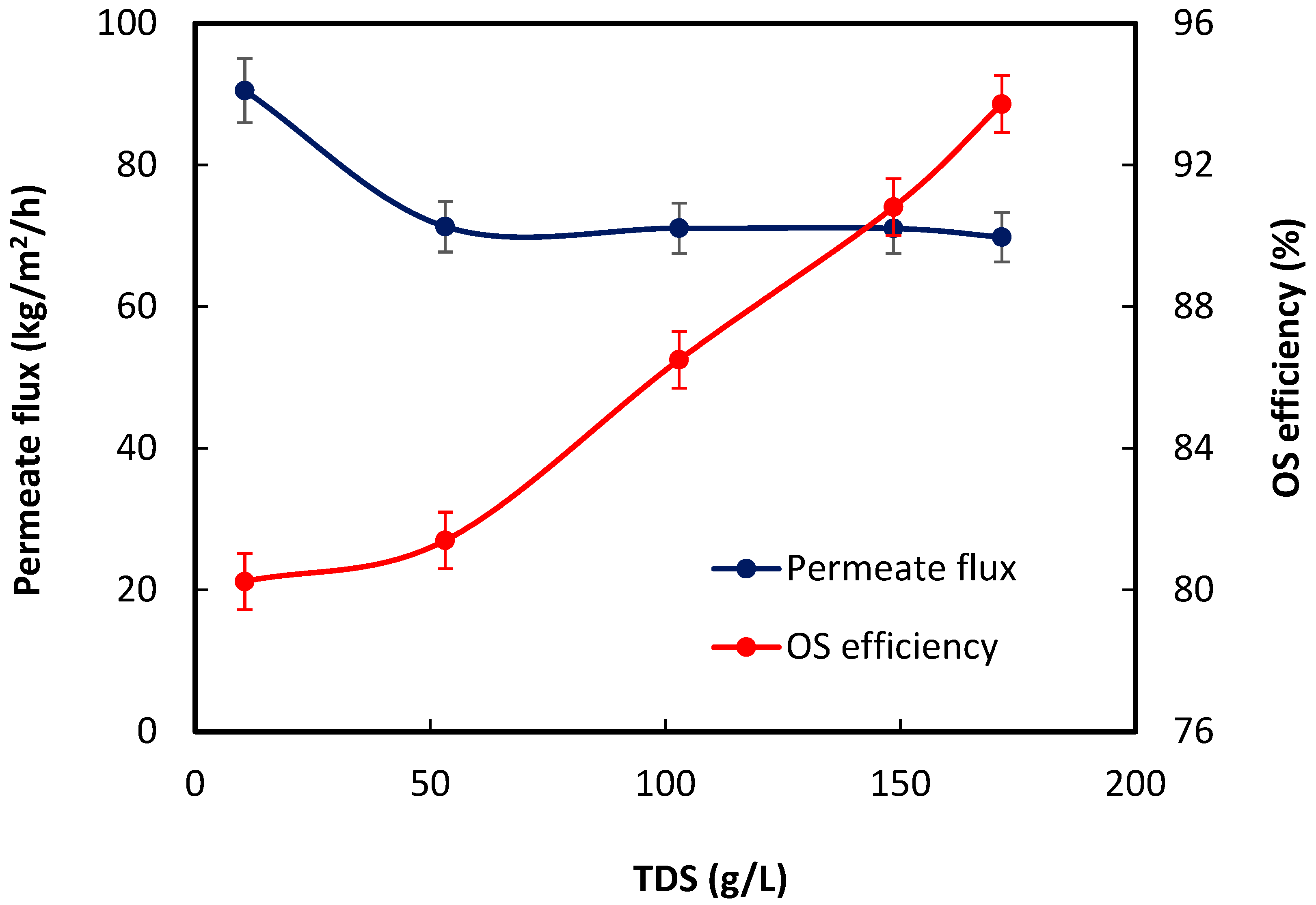
- Both produced water salinity and pH affected the organics removal efficiency.
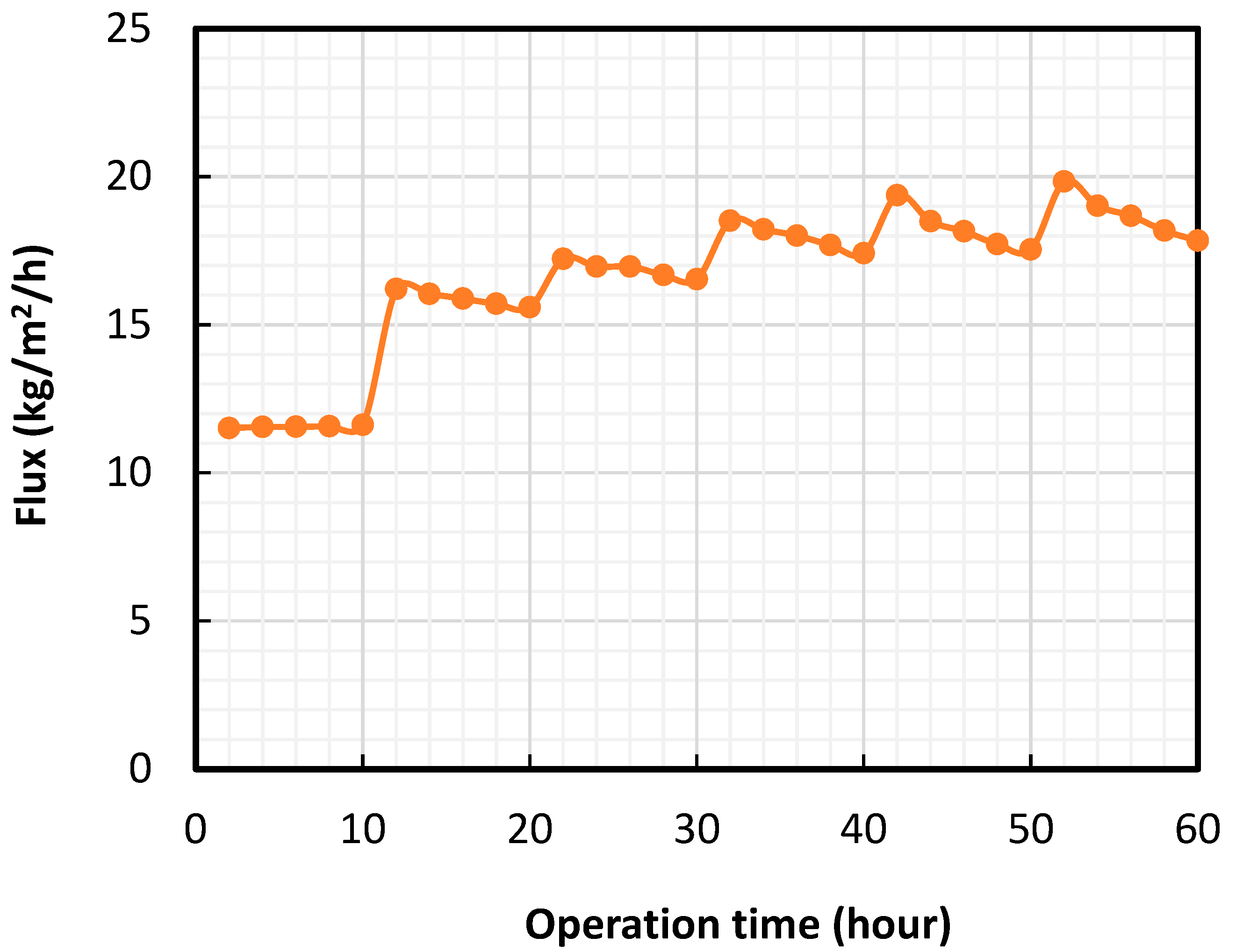
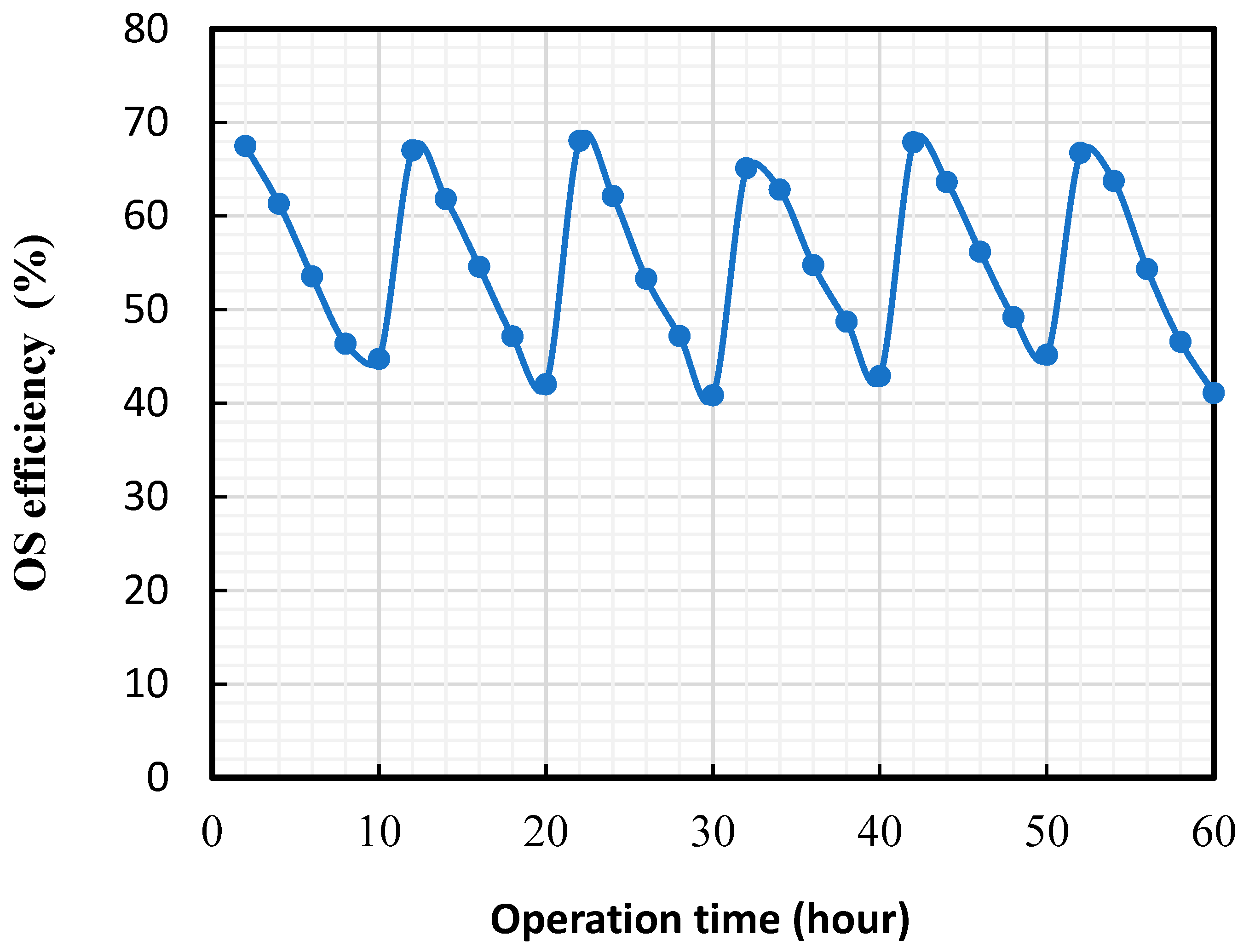
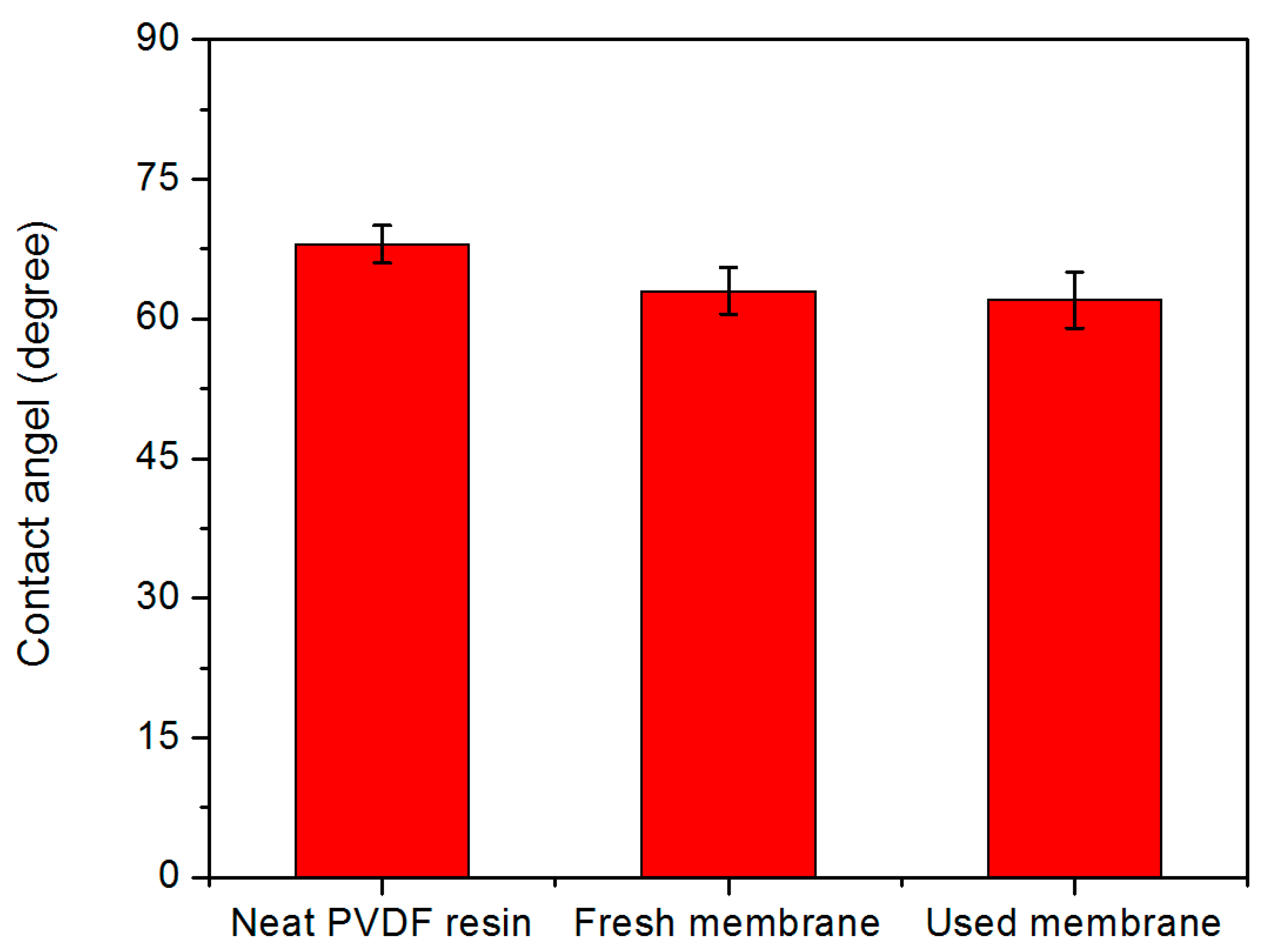
- The membrane showed a near-100% recovery of organics separation efficiency due to consistent membrane hydrophilicity.
- The results provided a portable and durable strategy for organic separation from high-salinity produced water.
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Dope Solution Preparation
2.3. Hollow Fiber Membrane Fabrication
2.4. Membrane Characterization
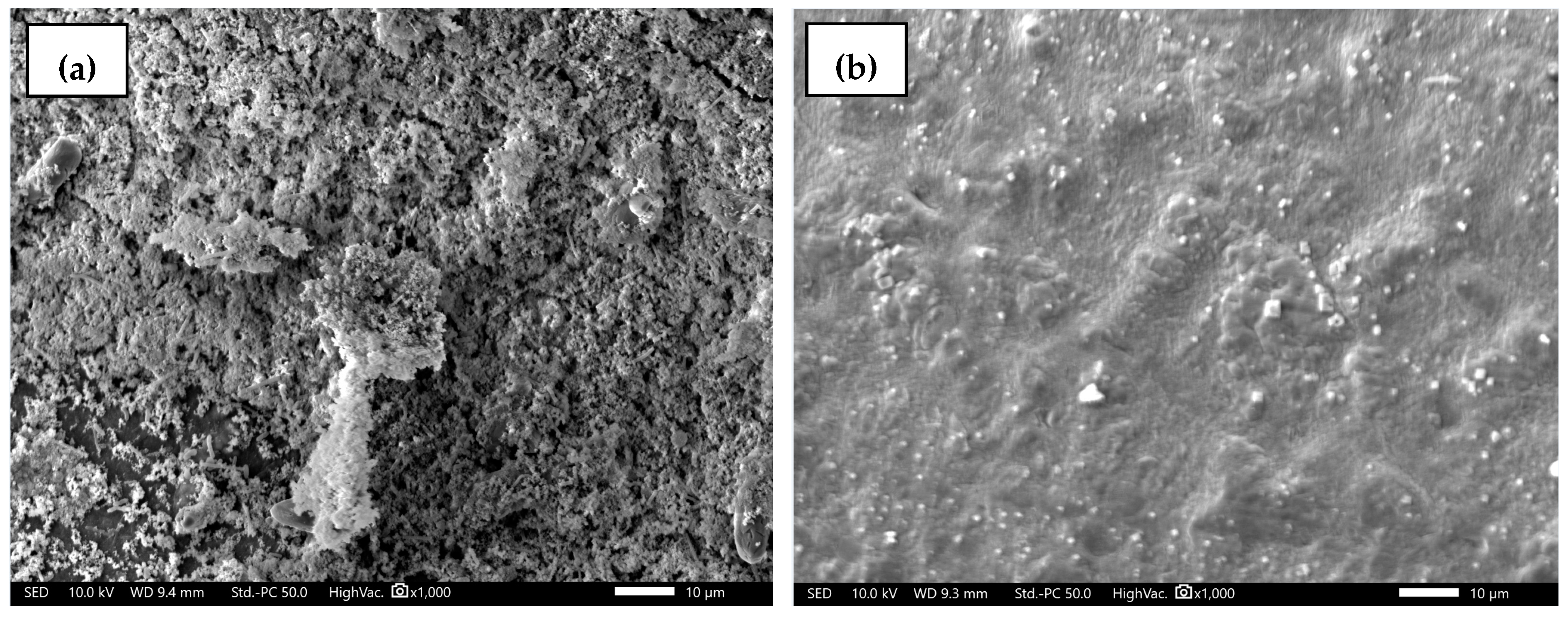
2.4.1. Membrane Morphology
2.4.2. Contact Angle
2.4.3. Porosity and Pore Size
2.5. Experimental Setup for Organics Separation (OS)
2.6. Long-Term OS Experiments
3. Results and Discussion
3.1. Membrane Characterization
3.2. OS Performance Evaluation
3.3. Cyclic UF Experiments Using Real Oilfield-Produced Water as the Feed Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dardor, D.; Al-Maas, M.; Minier-Matar, J.; Janson, A.; Sharma, R.; Hassan, M.K.; Al-Maadeed, M.A.A.; Adham, S. Protocol for Preparing Synthetic Solutions Mimicking Produced Water from Oil and Gas Operations. ACS Omega 2021, 6, 6881–6892. [Google Scholar] [CrossRef]
- Rahman, W.U.; Khan, M.D.; Khan, M.Z.; Halder, G. Anaerobic biodegradation of benzene-laden wastewater under mesophilic environment and simultaneous recovery of methane-rich biogas. J. Environ. Chem. Eng. 2018, 6, 2957–2964. [Google Scholar] [CrossRef]
- Shokrollahzadeh, S.; Golmohammad, F.; Naseri, N.; Shokouhi, H.; Arman-mehr, M. Chemical Oxidation for Removal of Hydrocarbons from Gas–Field Produced Water. Procedia Eng. 2012, 42, 942–947. [Google Scholar] [CrossRef]
- Sinha, S.; Roy, D.; Roy, O.; Neogi, S.; De, S. Removal of organic contaminants from flowback water using Fenton process. J. Water Process Eng. 2022, 47, 102680. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, E.; Cui, X.; Zhu, W.; Han, X.; Yu, G.; Wang, Y. Removal of organic compounds from shale gas fracturing flowback water by an integrated electrocoagulation and electro-peroxone process. Sep. Purif. Technol. 2021, 265, 118496. [Google Scholar] [CrossRef]
- Jaynes, W.F.; Vance, G.F. BTEX Sorption by Organo-Clays: Cosorptive Enhancement and Equivalence of Interlayer Complexes. Soil Sci. Soc. Am. J. 1996, 60, 1742–1749. [Google Scholar] [CrossRef]
- Nicholas, E.R.; Cath, T.Y. Evaluation of sequencing batch bioreactor followed by media filtration for organic carbon and nitrogen removal in produced water. J. Water Process Eng. 2021, 40, 101863. [Google Scholar] [CrossRef]
- Freire, D.D.; Cammarota, M.C.; Santanna, G.L., Jr. Biological treatment of oil field wastewater in a sequencing batch reactor. Environ. Technol. 2001, 22, 1125–1135. [Google Scholar] [CrossRef]
- Mousa, I.E. Total petroleum hydrocarbon degradation by hybrid electrobiochemical reactor in oilfield produced water. Mar. Pollut. Bull. 2016, 109, 356–360. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Willershausen, D.; Ashaghi, K.S.; Engel, L.; Placido, L.; Mund, P.; Bolduan, P.; Czermak, P. Investigations on the use of different ceramic membranes for efficient oil-field produced water treatment. Desalination 2010, 250, 991–996. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Samuel, O.; Othman, M.H.D.; Kamaludin, R.; Sinsamphanh, O.; Abdullah, H.; Puteh, M.H.; Kurniawan, T.A.; Li, T.; Ismail, A.F.; Rahman, M.A.; et al. Oilfield-produced water treatment using conventional and membrane-based technologies for beneficial reuse: A critical review. J. Environ. Manag. 2022, 308, 114556. [Google Scholar] [CrossRef]
- Badrnezhad, R.; Beni, A.H. Ultrafiltration membrane process for produced water treatment: Experimental and modeling. J. Water Reuse Desalination 2013, 3, 249–259. [Google Scholar] [CrossRef]
- Liu, N.; Li, L.; McPherson, B.; Lee, R. Removal of organics from produced water by reverse osmosis using MFI-type zeolite membranes. J. Membr. Sci. 2008, 325, 357–361. [Google Scholar] [CrossRef]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Modification and characterization of ultrafiltration membranes for treatment of produced water. J. Membr. Sci. 2011, 373, 178–188. [Google Scholar] [CrossRef]
- Zha, S.; Zhang, G.; Dawson, N.; Yu, J.; Liu, N.; Lee, R. Study of PVDF/Si-R hybrid hollow fiber membranes for removal of dissolved organics from produced water by membrane adsorption. Sep. Purif. Technol. 2016, 163, 290–299. [Google Scholar] [CrossRef]
- Rajabzadeh, S.; Liang, C.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Effect of additives on the morphology and properties of poly(vinylidene fluoride) blend hollow fiber membrane prepared by the thermally induced phase separation method. J. Membr. Sci. 2012, 423–424, 189–194. [Google Scholar] [CrossRef]
- Abed, M.R.M.; Kumbharkar, S.C.; Groth, A.M.; Li, K. Ultrafiltration PVDF hollow fibre membranes with interconnected bicontinuous structures produced via a single-step phase inversion technique. J. Membr. Sci. 2012, 407–408, 145–154. [Google Scholar] [CrossRef]
- Shi, L.; Wang, R.; Cao, Y.; Liang, D.T.; Tay, J.H. Effect of additives on the fabrication of poly(vinylidene fluoride-co-hexafluropropylene) (PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Membr. Sci. 2008, 315, 195–204. [Google Scholar] [CrossRef]
- Wang, D.; Li, K.; Teo, W.K. Porous PVDF asymmetric hollow fiber membranes prepared with the use of small molecular additives. J. Membr. Sci. 2000, 178, 13–23. [Google Scholar] [CrossRef]
- Khayet, M.; Feng, C.Y.; Khulbe, K.C.; Matsuura, T. Preparation and characterization of polyvinylidene fluoride hollow fiber membranes for ultrafiltration. Polymer 2002, 43, 3879–3890. [Google Scholar] [CrossRef]
- Han, M.-J.; Nam, S.-T. Thermodynamic and rheological variation in polysulfone solution by PVP and its effect in the preparation of phase inversion membrane. J. Membr. Sci. 2002, 202, 55–61. [Google Scholar] [CrossRef]
- Fontananova, E.; Jansen, J.C.; Cristiano, A.; Curcio, E.; Drioli, E. Effect of additives in the casting solution on the formation of PVDF membranes. Desalination 2006, 192, 190–197. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, X.; Gusnawan, P.; Zhang, G.; Yu, J. Crosslinked PVDF based hydrophilic-hydrophobic dual-layer hollow fiber membranes for direct contact membrane distillation desalination: From the seawater to oilfield produced water. J. Membr. Sci. 2021, 619, 118802. [Google Scholar] [CrossRef]
- Zha, S.; Gusnawan, P.; Zhang, G.; Liu, N.; Lee, R.; Yu, J. Experimental study of PES/SiO2 based TFC hollow fiber membrane modules for oilfield produced water desalination with low-pressure nanofiltration process. J. Ind. Eng. Chem. 2016, 44, 118–125. [Google Scholar] [CrossRef]
- Zha, S.; Yu, J.; Zhang, G.; Liu, N.; Lee, R. Polyethersulfone (PES)/cellulose acetate butyrate (CAB) composite hollow fiber membranes for BTEX separation from produced water. RSC Adv. 2015, 5, 105692–105698. [Google Scholar] [CrossRef]
- Gryta, M.; Karakulski, K.; Morawski, A.W. Purification of oily wastewater by hybrid UF/MD. Water Res. 2001, 35, 3665–3669. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 427–437. [Google Scholar] [CrossRef]
- Monnard, P.A.; Deamer, D.W. Membrane self-assembly processes: Steps toward the first cellular life. Anat. Rec. 2002, 268, 196–207. [Google Scholar] [CrossRef]
- Elzo, D.; Huisman, I.; Middelink, E.; Gekas, V. Charge effects on inorganic membrane performance in a cross-flow microfiltration process. Colloids Surf. A Physicochem. Eng. Asp. 1998, 138, 145–159. [Google Scholar] [CrossRef]
- Yi, X.S.; Yu, S.L.; Shi, W.X.; Sun, N.; Jin, L.M.; Wang, S.; Zhang, B.; Ma, C.; Sun, L.P. The influence of important factors on ultrafiltration of oil/water emulsion using PVDF membrane modified by nano-sized TiO2/Al2O3. Desalination 2011, 281, 179–184. [Google Scholar] [CrossRef]
- Shields, A.; Sharma, R.; McLin, K.; Bjornen, K.; Jenneman, G.; Freeman, J.; Mesa, W. Three Years of Water Treatment in the Permian: A ConocoPhillips Case History. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, San Antonio, TX, USA, 20–22 July 2015. [Google Scholar]
- Cob, S.S.; Hofs, B.; Maffezzoni, C.; Adamus, J.; Siegers, W.G.; Cornelissen, E.R.; Güner, F.E.G.; Witkamp, G.J. Silica removal to prevent silica scaling in reverse osmosis membranes. Desalination 2014, 344, 137–143. [Google Scholar]




| Parameters | Value |
|---|---|
| Dope Composition (wt.%) | PVDF/PVP/LiCl/NMP (18/9/3/70) |
| Bore fluid (wt.%) | NMP/water (30/70) |
| External coagulant | Tap water |
| Dope flow rate (mL/min) | 2 |
| Bore flow rate (mL/min) | 0.5 |
| Length of the air gap (cm) | 7 |
| Take-up speed | free fall |
| Properties | Thickness (um) | MWCO (kDa) | Mean Pore Size (nm) | Porosity (%) |
|---|---|---|---|---|
| Values | 219 | 600 | 62.7 | 76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oppong, S.; He, Z.; Fernandez, G.T.; Zhang, G.; Yu, J. Durable Hydrophilic PVDF Hollow Fiber Membrane for Dissolved Organics Separation from High-Salinity Produced Water. Fibers 2025, 13, 31. https://doi.org/10.3390/fib13030031
Oppong S, He Z, Fernandez GT, Zhang G, Yu J. Durable Hydrophilic PVDF Hollow Fiber Membrane for Dissolved Organics Separation from High-Salinity Produced Water. Fibers. 2025; 13(3):31. https://doi.org/10.3390/fib13030031
Chicago/Turabian StyleOppong, Samuel, Zongjie He, Gabriela Torres Fernandez, Guoyin Zhang, and Jianjia Yu. 2025. "Durable Hydrophilic PVDF Hollow Fiber Membrane for Dissolved Organics Separation from High-Salinity Produced Water" Fibers 13, no. 3: 31. https://doi.org/10.3390/fib13030031
APA StyleOppong, S., He, Z., Fernandez, G. T., Zhang, G., & Yu, J. (2025). Durable Hydrophilic PVDF Hollow Fiber Membrane for Dissolved Organics Separation from High-Salinity Produced Water. Fibers, 13(3), 31. https://doi.org/10.3390/fib13030031






