Highlights
What are the main findings?
- The fibres of the William banana peduncle are extracted by three methods and chemical analysis by Van Soest’s dry biomass fractionation method has shown that its fibers have a cellulose content of over 70%.
- Thermogravimetric analysis shows that banana peduncle fibers are thermally stable at 82 °C, and X-ray diffraction reveals a crystallinity rate of over 50%.
What is the implication of the main finding?
- The method of extracting fibers from dew is advantageous and economical; chemical analysis results show that WBPF is a good candidate for the production of non-woven fabrics.
- In the case of textiles developed from banana peduncle fibers, they can be used in applications that can withstand temperatures of around 80 °C without damage and crystallinity levels predispose the fibers to better mechanical properties.
Abstract
This study deals with the physical, chemical, and thermal properties of William banana peduncle fibers in order to consider the possibility of using these new fibers in textile applications. The samples were collected in Cameroon, in the Littoral region, Njombe Penja district (agri-food industry). The fibers were extracted by three methods, including Water Retting (WR), Dew Retting (DR), and Mechanical Extraction (ME). The various resulting fibers were characterized by X-ray Diffraction (XRD), Thermogravimetric Analysis (TGA), Fourier-Transform Infrared Spectroscopy (FT-IR) and Scanning Electron Microscopy (SEM), respectively. The FTIR analysis confirmed the lignocellulosic structure of the fibers and revealed that the three extraction methods had not affected the chemical nature of the fibers. The extraction methods also had no significant impact on density and moisture content. Scanning electron microscopy showed bands of fibers bundles on all samples. Thermogravimetric analysis (TGA) showed that the fibers extracted were thermally stable at 82 °C. X-ray diffraction (XRD) analysis showed crystallinity levels ranging from 58.24% for (WR), 54.83% for (DR), and 69.53% for (ME). The results obtained on the chemical composition show that the extracted fibers consist mainly of 71.8%, 73.6%, and 74.8% cellulose for WR, DR, and ME, respectively, making them suitable for textile applications.
1. Introduction
Tropical and sub-tropical countries grow bananas in large quantities. Approximately 13% of the weight of the banana bunch harvested represents waste (peduncle, pseudo-stem, and leaves), which is generally thrown away or incinerated [1]. The banana plant is a monocotyledonous plant belonging to the Musaceae family (order Zingiberales), of which nearly 70 species have been discovered [2]. Subsistence varieties are derived from the hybridization of Musa acuminata (AA) and Musa balbisiana (BB) [3]. The banana itself is one of the most popular fruits and one of the most important in diets due to its high nutrient content [4]. It is considered to be the third most consumed food [5]. Dessert bananas, marketed worldwide, are almost all hybrids of Musa acuminata with a triploid character, called AAA [6]. Plantain bananas (Musa AAB) and other bananas that can be used for cooking (Musa ABB) are also triploid and come from AA-B hybridization [5,7].
In Africa, Cameroon ranks 6th among banana-producing countries. According to the Cameroon Banana Association (ASSOBACAM), Cameroon exported 216,103 tons of dessert bananas from MBÔH Plantation Limited (BPL), Haut Penja Plantations (PHP), and Cameroon Development Corporation (CDC), all of the Grande Nain or William varieties. Figure 1 shows the different dessert banana harvesting sectors.
Figure 1.
Main banana production sectors in Cameroon.
The peduncle is the part of the banana plant that supports the inflorescence and links it to the rhizomes and fruit. The work of Kamdem et al. [8] showed that the ‘Williams Cavendish’ cultivar is taller, more robust, and produces more waste. Numerous works in the literature show that fibers can be extracted from banana peduncles by biological, chemical, and mechanical retting. For example, the work of the authors of [9,10,11] on water-extracted banana peduncle fibers showed cellulose contents of 73.20%, 66.43%, and 72.90%, hemicellulose contents of 10.85%, 13.72%, and 11.01%, lignin contents of 15.32%, 16.85%, and 15.11%, moisture contents of 9.01%, 12.05%, 93.6%, and densities of 972 kg/m3, 942 kg/m3, and 990 kg/m3. The work of Pitchayya et al. [12] on banana peduncle fibers extracted manually and then with water showed a cellulose content of 79.13%, a lignin content of 12.3%, a moisture content of 7.51%, and a density of 0.85 kg/m3. The work of Pretti et al. [13] studied the physical and chemical properties of mechanically extracted fibers from the Grande Nain, Poovan, Monthan, and Nendran cultivars, revealing that the cellulose content of the Nendran cultivar was 60.41% higher, the hemicellulose content 10.20% higher, and the lignin content 17.56% higher. Zara et al. [14] worked on the analysis of certain textile properties of plantain stalk fibers and their results showed a fiber moisture content in the range 1.56–11.24%. Overall, the work presented in the literature on banana peduncle fibers shows interesting physical and chemical properties. Nevertheless, retting with water causes water pollution, a foul odor, and the development of microorganisms that can degrade the fibers and consequently influence their properties [15]. In addition, chemical extraction is energy-intensive and requires the use of chemical products, making it expensive to obtain the fibers [12]. In addition, some authors carry out post-treatment of these fibers with a view to improving their properties [12]. To our knowledge, very few authors have conducted studies on the extraction of banana peduncle fibers using dew extraction. It seems interesting to apply this extraction technique to assess its effect on the properties of the fibers and compare it with other extraction methods. The objective of this study is to investigate the effect of tree extraction methods on the chemical properties of William banana peduncle fibers.
The physical–chemical and thermal properties of William banana peduncle fibers extracted by water retting, in dew, and by rolling will therefore be assessed. Physical–chemical characterization such as density, moisture content, chemical composition, Fourier-transform infrared spectroscopy (FT-IR), X-ray diffraction, and thermogravimetric analysis (TGA) of William banana peduncle fibers obtained by the three methods will be evaluated and compared with those of other plant fibers studied in the literature.
2. Materials and Methods
2.1. Origin of Samples
The William banana peduncle fiber is collected in the Littoral region, Mungo Department, and Njombe Penja Arrondissement, more specifically in the Kumbe sector of the PHP company, an agri-food industry located in Cameroon.
Banana peduncles were collected during the rainy season (from July to September). They are obtained after cutting the bunch from the mature pseudo stem (approximately 6 months old). Once the stems have been separated from the bunch, they are collected and stored in the laboratory (at approximately 27 °C) for water retting and mechanical extraction. Another peduncle is left on the banks of a watercourse not far from the collection area for dew extraction.
2.2. Methods for Extracting Fibers from the Peduncle of William Banana (WBPF)
The William banana peduncle was cleaned with a knife to remove the green skin, regardless of the extraction method used. The following three fibers extraction methods were considered: water-retted, dew-retted, and laminated. The following codification (Table 1) was adopted for the different extraction processes.

Table 1.
Coding of WBPF.
The methods for extracting fibers from the William banana peduncle mentioned above have been described in previous work by (Anafack et al. 2023) [16] and are in agreement with many works in the literature [17,18,19,20].
2.3. Physical–Chemical Characterization of William Peduncle Fibers
The various fibers obtained underwent physical and chemical characterization, including chemical composition, scanning electron microscopy, moisture content, density, thermogravimetric analysis, X-ray diffraction, and Fourier-transform infrared spectroscopy to determine the lignocellulosic content, morphology, moisture absorption, crystalline phases, and functional groups of the fibers, respectively.
2.3.1. Study of the Chemical Composition of the Fiber
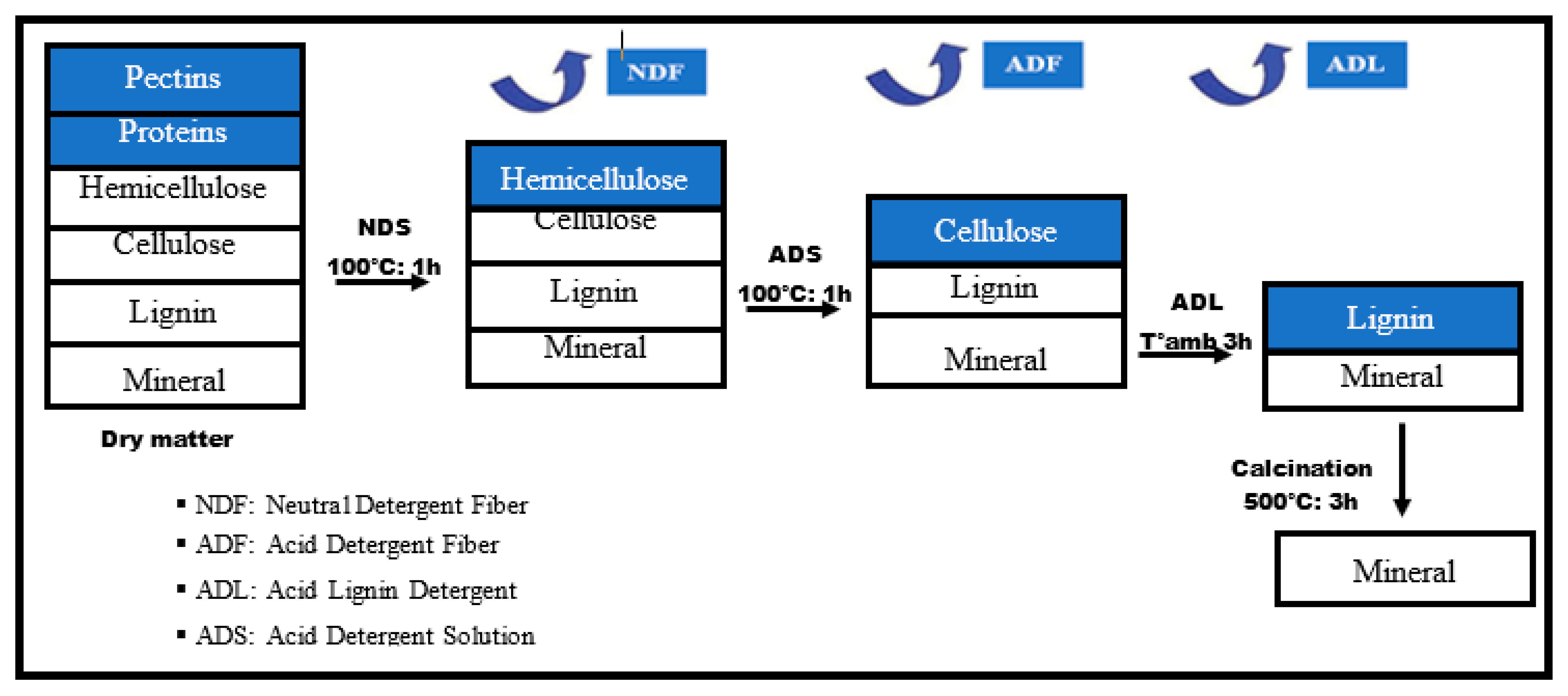
Van Soest’s dry biomass fractionation method [21,22,23] was used in this work to determine the percentage of cellulose, hemicellulose, and lignin in accordance with the French standard XP U44-162. Before testing the sample must be in powder form; to achieve this, the fibers were dried at 105 °C for 24 h in an oven, then cut, crushed, and sieved to obtain a very fine powder [21]. The constituents are determined sequentially by treatment with neutral detergent, acid, and sulfuric acid.
Figure 2 shows the principle of this method, which is carried out in four stages, with different experimental conditions for each stage.
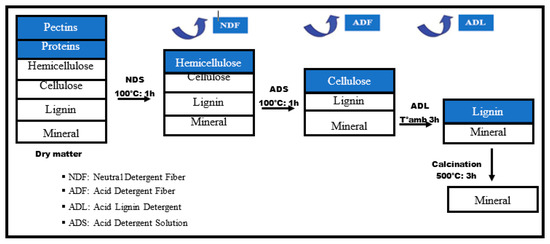
Figure 2.
Schematic diagram of the fractionation of lignocellulosic biomass [21].
2.3.2. Scanning Electron Microscopy (SEM) Study of Extracted Fibers
The fibers were observed using a scanning electron microscope (JOEL JSM-IT100) at magnifications of ×200, ×300, and ×500. Five fibers samples were pre-coated with a gold film to make them conductive. The fibers samples were analyzed at different acceleration voltages between 15 and 20 kV.
2.3.3. Study of the Density and Moisture Content of WBPF
Fiber density is determined by using a pycnometer and the successive weighing method in accordance with ASTM D3800-99 (2010) and with a methanol solution. The fiber bundles are first introduced into desiccators filled with silica cells to remove the residual water present in the fiber bundles [9]. An analytical balance with a resolution of 0.001 g was used for this purpose. The density is calculated by Equation (1) as follows:
where is the density of methanol (0.791 g/cm3), is the mass of the dried fiber sample, is the mass of the empty pycnometer, is the mass of the pycnometer containing methanol up to the lower meniscus of the gauge point, and is the mass of the pycnometer containing methanol and dried fibers.
The test took place in an environment with a temperature of 25 °C ± 2 °C with 65% ± 2% relative humidity. The fibers previously dried for 24 h in the oven at a temperature of 105 °C were tied into small bundles of about 0.5 g (±0.05) constant mass, as mentioned in the work of Ebanda et al. [24] and Athalie et al. [25]. These authors specify that in a jar saturated with sodium chloride (NaCl), the relative humidity is approximately 75% [26]. The moisture uptake () of banana peduncle fibers is determined by Equation (2), which is as follows:
where
- : Moisture absorption in %;
- Mi: Initial mass in the anhydrous state;
- Mf: Final mass.
2.3.4. Thermogravimetric Analysis (TGA)
A NETZSCH STA 449F3 thermogravimetric analyzer (TGA) was used. A 100 mg sample was placed in an aluminum crucible and then introduced into the apparatus. The sample was subjected to a temperature rise from 20 to 900 °C, while maintaining a constant heating rate of 5 °C/min and an isotherm at 900 °C for 30 min in air. The device provides mass loss as a function of temperature.
2.3.5. X-Ray Diffraction (XRD) Study
The material analyzed was finely ground, homogenized, and then oven dried at 60 °C for 6 h. X-ray diffraction patterns of the fibers were recorded by X-ray diffractometry. An Empyrean diffractometer with radiation (CuKα = 1.5418 Å) was used. An angle interval of 2θ from 4° to 75° was scanned with a step size of 0.026261 at 8.67 s using a graphite monochromator, a voltage of 45 kV, and a current of 40 mA. The crystallinity ratio of the William banana peduncle fibers was obtained as shown in Equation (3) [27], which is as follows:
where IC % is the crystallinity index, Aam is the area of the amorphous phase, and Acr is the area of the crystalline phase.
2.3.6. Study Using Fourier-Transform Infrared Spectroscopy (FTIR)
The functional groups present in the banana stem fibers were analyzed using a BRUKER IR spectrometer. A mass of 2 mg of fine powder from the banana peduncle fibers extracted by the three methods was introduced into the diamond/ZnSe crystal and a manual force of approximately 150 N was exerted on the sample to ensure contact. The sample was scanned 5 times, and each spectrum was obtained after 32 scans at a resolution of between 4000 cm−1 and 500 cm−1 [28,29].
3. Results and Discussion
3.1. Study of Fiber Morphology
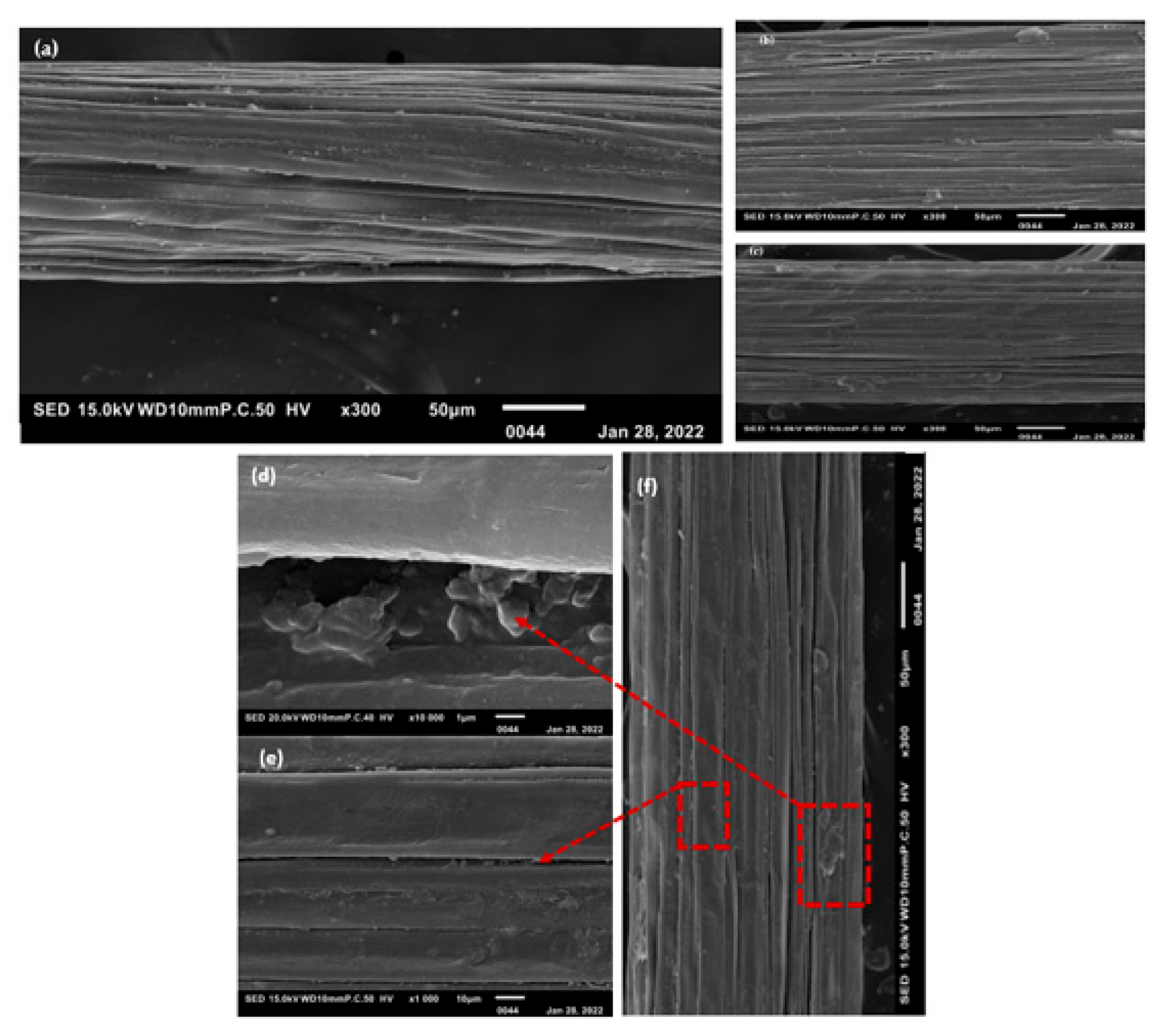
The fibers of the William banana peduncle were observed using a scanning electron microscope (SEM). Five fibers for each extraction method were observed using a scanning electron microscope (SEM), and the images are shown in Figure 3.
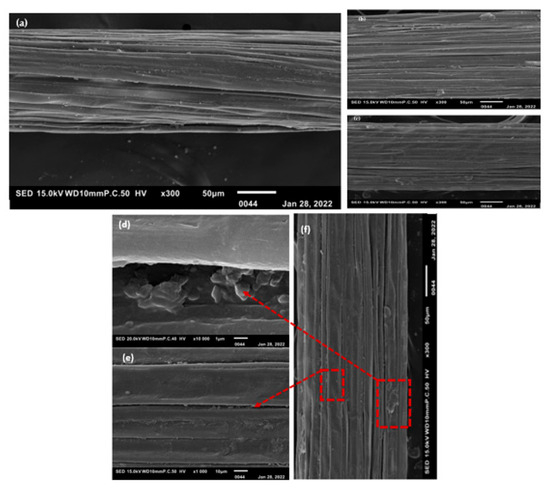
Figure 3.
SEM images of longitudinal views of three extraction methods: (a) WR; (b) DR; (c) ME; (d) pectins; (e) bundle banana; and (f) expansion of fibers [16].
This figure shows that fibers extracted by rolling still contain impurities (pectins), unlike fibers extracted by retting, which are visibly smooth. [10]. The final fibers are more visible due to the retting action on the pectins that bind the fibers together (Figure 3b). We can see that the longitudinal structure is in the form of small flat ribbons, whatever the extraction method (Figure 3a).
3.2. Analysis of the Chemical Composition, Density, and Moisture Content of WBPF
The density, moisture content, and chemical composition of William banana peduncle fibers were determined and the results obtained are presented in Table 2. This table shows that the average density of the fibers studied is almost identical (0.934 g/cm3), which is much lower than the fibers found in the work of Baley et al. [30] for which they had obtained densities of the order of 2.6 g/cm3, 0.89 g/m3, 0.97 g/m3, 1.30 g/m3, 1.45 g/m3, and 0.942 g/m3, respectively, for the fibers of Nendran banana peduncle, flax, jute, alfa, and the fibers of Musa acuminata peduncle [10,29,30]. The low density from the William banana peduncle fiber (WBPF) predisposes the fiber for industrial use as a reinforcement for bio-sourced composites. Additional contributions could improve the adhesion properties and make the fiber lightweight, improving its lightness properties [31].

Table 2.
Comparison of the physical–chemical properties of William banana peduncle fibers with other natural fibers found in the literature.
With regard to the moisture content of extracted fibers, the moisture content of water-retted and laminated fibers was similar (9.62% and 8.10%, respectively), while the moisture content of dew-retted fibers was low (6.66%). This may be due to the weather conditions and the colonization of the fibers by fungi during the retting process. This low moisture content of dew-retted fibers seems to be of interest for textile applications. The quantity of water present in the fibers of the William banana peduncle extracted by the three methods was confirmed by thermogravimetric analysis. A comparison of the moisture content of the dew-retted fibers with the moisture content of natural fibers in the literature shows that they are lower than the fibers from Nendran (9.01%), Musa acuminata (12.05%), and Red banana (9.36%). Comparing the chemical composition of the extracted fibers, it was found that their chemical nature (cellulose, hemicellulose, and lignin) was not affected by the different methods. However, the extraction methods had a significant effect on the lignin content of the WBPF. Water-retted fibers have a higher lignin content (8.6%) compared with the lignin content of dew-retted fibers (5.7%). The work of Praveen et al. [10] has shown that such a high lignin content protects the fibers against biological attack (bacteria and fungi). Compared to the three extraction methods, the cellulose content of the first two, ME and DR (74.8% and 73.6%), appears to be identical. According to the work of Yogesha et al. [32], this increase predisposes the fibers to better mechanical properties. However, water extraction gives a slightly lower result. Nevertheless, regardless of the extraction method, it can be noted that these fibers have a high cellulose content compared to certain natural fibers found in the literature, in particular Musa acuminata fibers (66.43%), Nendran fibers (60.41%), Poovan banana fibers (56.24%), and Abyssinia banana fibers (52.14%) [10,13,31,32]. In terms of chemical composition, it can be concluded that all fibers extracted by the three methods are very good candidates for use in various industrial applications, particularly in the textile industry.
3.3. Analysis by Fourier-Transform Infrared Spectrometry (FTIR)
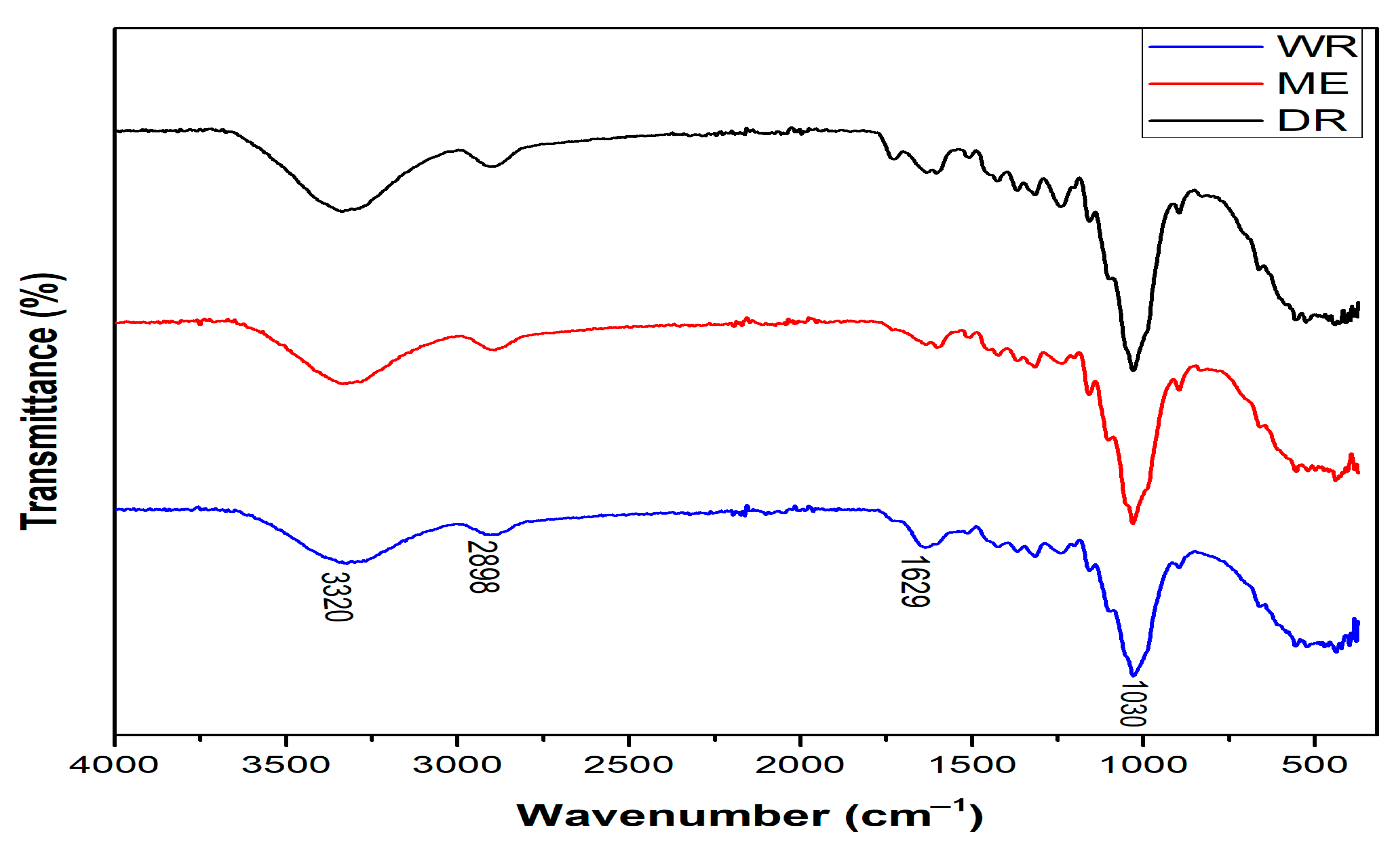
Figure 4 and Table 3 show, respectively, the spectra and FTIR vibration bands of William banana peduncle fibers obtained by three methods, identifying the functional groups present in the molecular structure of the fibers. From the spectra of WR, DR, and ME fibers, the vibration bands observed around 3320 cm−1 are attributable to OH stretches of α-cellulose and the hydrogen-bonded cellulose structure [33]. Those located at 2898 cm−1 correspond to the C-H stretching of CH and CH2 cellulose and hemicellulose in fibers [29,34]. The peaks also observed at 1629 cm−1 are attributable to stretching of of the amides present in lignin and hemicellulose. Finally, the peaks located at 1030 cm−1 are attributed to C-OH stretching of lignin [35]. This result confirms the lignocellulosic structure of WR, DR, and ME fibers and also correlates with their chemical composition. In addition, the extraction methods do not affect the chemical composition of the extracted fibers.
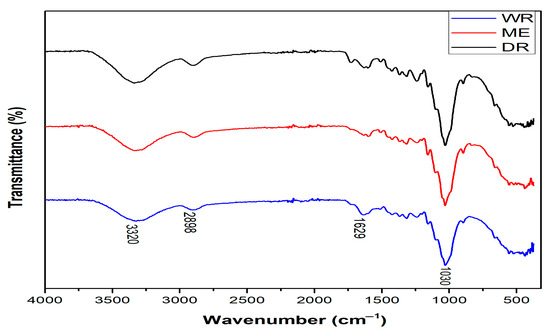
Figure 4.
FTIR spectrum of WR, DR, and ME extracted from William banana peduncle fibers.

Table 3.
FT-IR peak positions and chemical functional groups of William banana peduncle fibers.
3.4. Thermogravimetric Analysis (ATG)
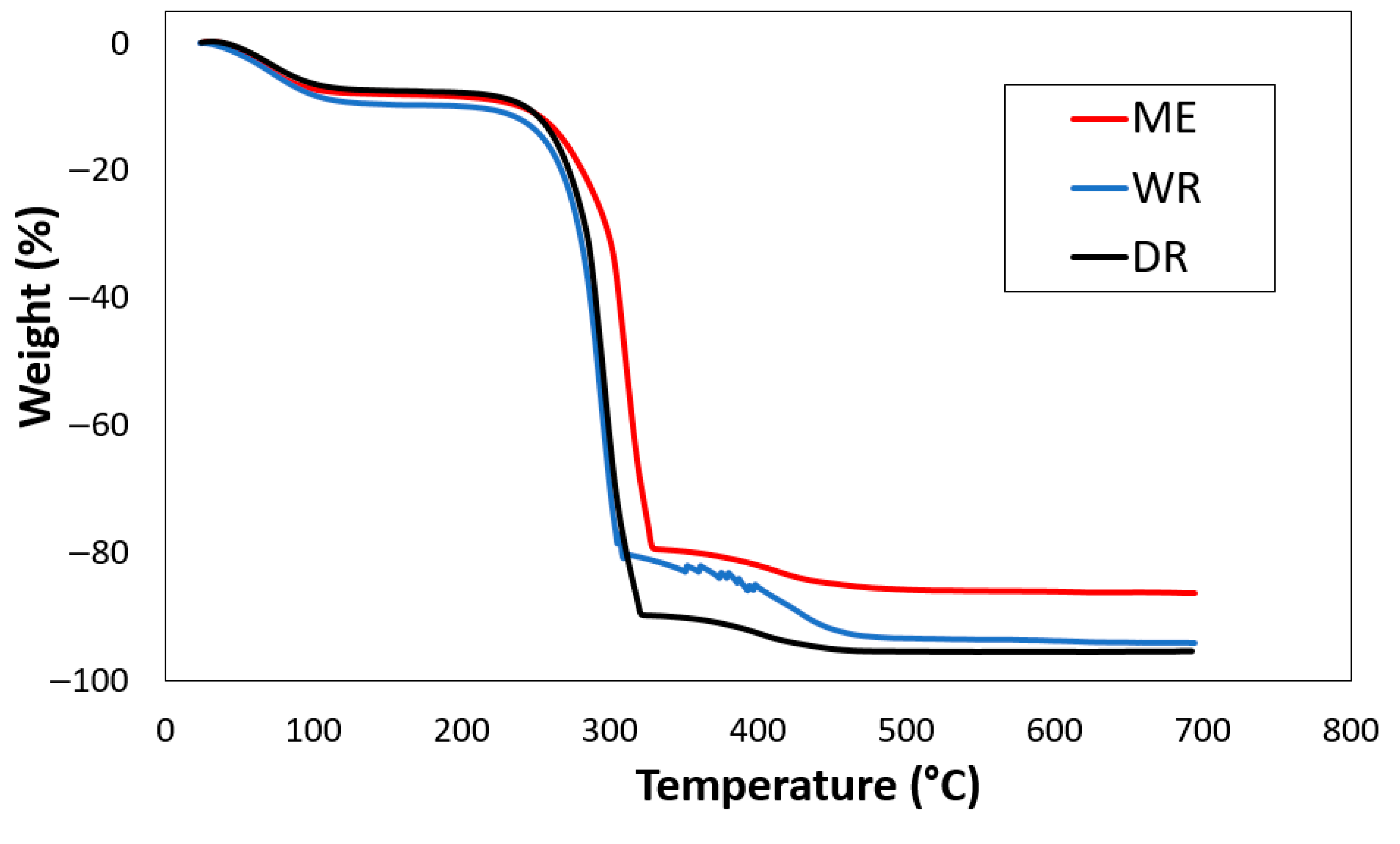
The combined thermograms (ATGs) of William banana peduncle fibers extracted by water retting, in the dew, and by rolling are shown in Figure 5. According to the various thermograms obtained (Figure 5), degradation occurred in the following three stages: The first stage of moisture evaporation takes place at 82 °C for water retting fibers, at 76 °C for dew retting fiber, and at 66 °C for fibers extracted by rolling. These degradations are due to the vaporization of moisture and the elimination of moisture from the fiber [38], which are (9.66%) for water retting fibers, (6.68%) for dew retting fibers, and (8.15%) for fibers extracted by rolling.
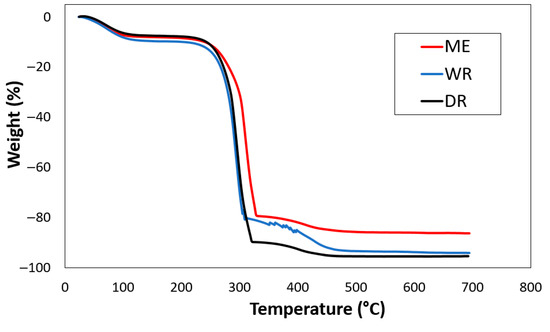
Figure 5.
Thermogram of extracted by WR, DR, and ME fibers.
The second stage of degradation, which occurs at around 301 °C for water retting fibers, 319 °C for dew retting fibers, and 316 °C for fibers extracted by lamination, marks the decomposition of cellulose and hemicellulose. [39]. Mass losses of 74.51%, 49.97%, and 70.76% were recorded for water retting fibers, dew retting fibers, and fibers extracted by lamination, respectively. The final phase of cellulose degradation occurs from the temperature ranges of 426 °C for water retting fibers, 439 °C for dew retting fibers, and 457 °C for fibers extracted by lamination, with respective mass losses of (12.90%), (19.96%), and (4.80%) which corresponds to the degradation of wax as well as lignin and which leaves ash as residue [39]. Table 4 shows the thermal degradation values for the extracted fibers. These different interpretations correlate with the results of the chemical composition and FTIR analysis and show that fabrics made from William banana peduncle fibers can be ironed at temperatures of up to 82 °C.

Table 4.
Thermal degradation values of William banana peduncle fibers.
3.5. X-Ray Diffraction Analysis
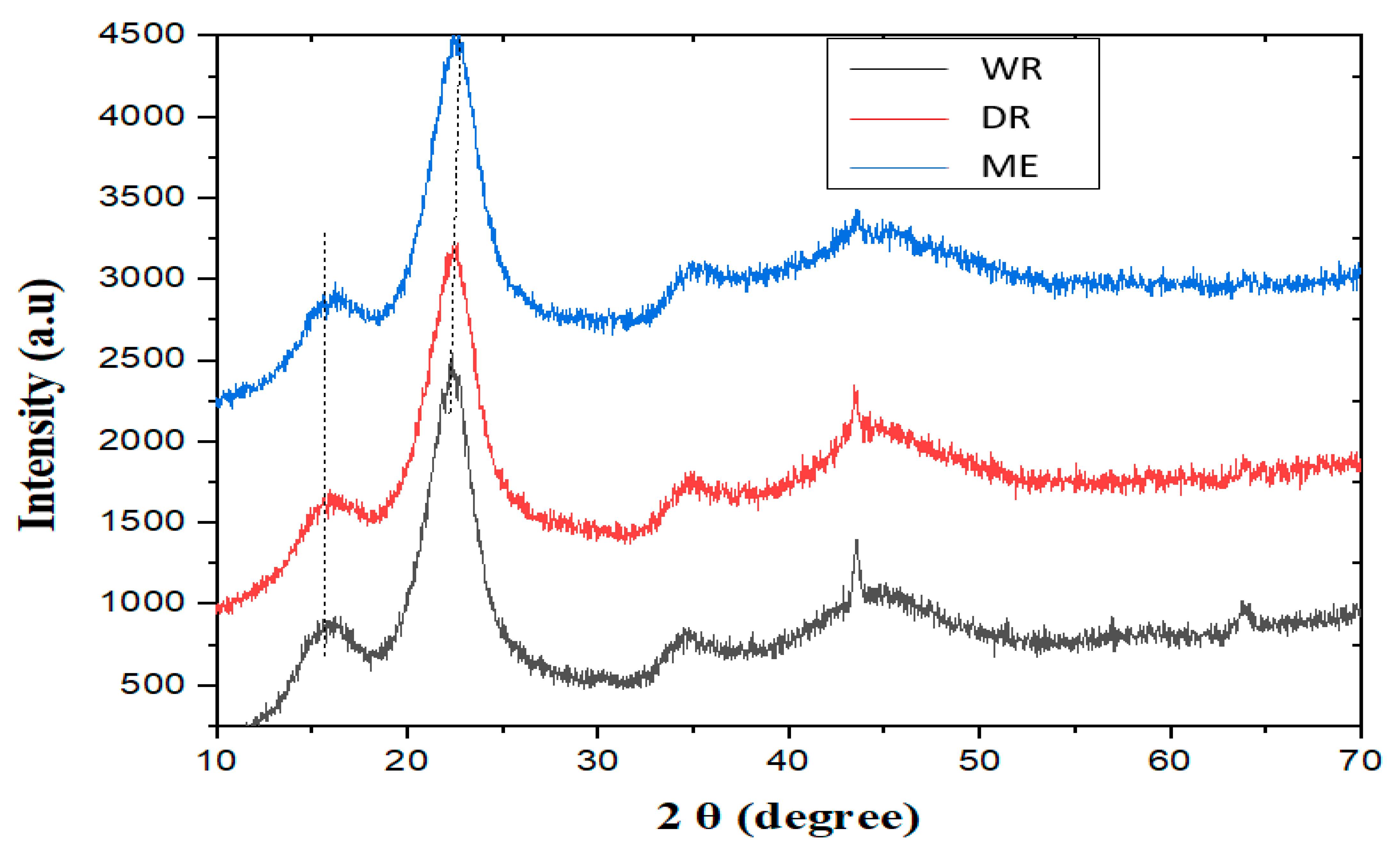
Figure 6 shows the diffractograms of the fibers extracted by the three methods. The first peaks observed at around 2θ = 16.09° correspond to the different amorphous fractions. On the other hand, the second peaks observed at around 2θ = 22.67° correspond to the crystallographic planes of the cellulose. These results are similar to those found by Belouadah et al. [40] and Dallel [41].
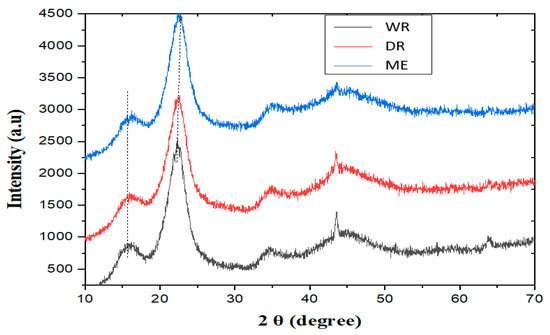
Figure 6.
X-ray diffractograms of William banana peduncle fibers.
A comparison of the crystallinity index values for William banana peduncle fibers with those in the literature (Table 5) shows that the crystallinity index for dew retting fibers is higher (54.83%) than for Nendran peduncle fibers (53.30%) and Musa acuminata peduncle fibers (36.47%). The value of the crystallinity index of the fibers extracted by lamination is more significant (69.53%) than those obtained with water and dew retting fibers (58.24 and 54.83%, respectively), which is in line with the results of the chemical composition of the fibers. Furthermore, according to the literature, its crystallinity rates predispose William banana peduncle fibers to better mechanical properties [42]. This is also confirmed in previous work by Anafack et al. (2023) [16].

Table 5.
Comparison of the crystallinity indices of William peduncle fibers with those found in the literature.
4. Conclusions
This study focused on the physical, chemical, and thermal properties of William banana peduncle fibers, with a view to their use in textile applications. The samples were collected and extracted biologically (WR and DR) and mechanically (ME). The analysis of the physical characterization shows that the densities of the WBPF are almost the same, at around 0.93%, and that the moisture content of the WR is 9.62%, 6.66% for DR, and 8.1% for ME.
Analysis of the chemical composition shows that the fibers of the William banana peduncle are sufficiently rich in cellulose, with contents in the order of 71.8%, 73.6% and 74.8% for WR, DR, and ME, respectively. Scanning electron microscopy showed flat fibers bundles regardless of the extraction method used. FTIR analysis confirmed the lignocellulosic structure of the fibers and revealed that the three extraction methods had not affected the chemical nature of the fibers. TGA showed thermal stability at around 82 °C. This also indicates that the extracted fibers can be used in the design of clothing with a maximum ironing temperature of 82°C. XRD analysis shows that the crystallinity rates of WBPF are approximately 58.24%, 54.83%, and 69.53% for WR, DR, and ME, respectively. These crystallinity rates predispose William banana peduncle fibers to better mechanical properties. A comparison of the dew extraction method with other methods commonly used in the literature shows that it is cost-effective and economical (because it requires little water and does not require the use of containers), and that the fibers obtained are promising candidates for the textile industry. We suggest that future researchers focus on developing these fibers for use in nonwoven production given the cellulose content obtained.
Author Contributions
S.M.A.: Conceptualization, Investigation, Methodology, Analysis of results, Validation, Writing. P.W.M.H.: Investigation, Methodology, Supervision, Reading. J.-Y.D.: Investigation, Methodology, Supervision, Correction. O.H.: Supervision, Correction, Validation. R.N.S.T.: Supervision, Correction, Validation. H.T.D.: Conceptualization, Methodology, Analysis of results, Reading, Validation. M.B.: Supervision, Correction, Validation. E.N.: Supervision, Correction, Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pazmiño-Hernandez, M.; Moreira, C.M.; Pullammanappallil, P. Feasibility assessment of waste banana peduncle as feedstock for biofuel production. Biofuels 2019, 10, 473–484. [Google Scholar] [CrossRef]
- Perrier, X.; De Langhe, E.; Donohue, M.; Lentfer, C.; Vrydaghs, L.; Bakry, F.; Carreel, F.; Hippolyte, I.; Horry, J.-P.; Jenny, C.; et al. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc. Natl. Acad. Sci. USA 2011, 108, 11311–11318. [Google Scholar] [CrossRef] [PubMed]
- Lassois, L.; Busogoro, J.-P.; Jijakli, H. La banane: De son origine à sa commercialisation. Biotechnol. Agron. Soc. Environ. 2009, 13, 575–586. [Google Scholar]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic compounds within banana peel and their potential uses: A review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Dury, S.; Bricas, N.; Tchango-tchango, J.; Temple, L.; Bikoi, A. The determinants of urban plantain consumption in Cameroon. Food Qual. Prefer. 2008, 13, 81–88. [Google Scholar] [CrossRef]
- Emaga, T.H.; Andrianaivo, R.H.; Wathelet, B.; Tchango, J.T.; Paquot, M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007, 103, 590–600. [Google Scholar] [CrossRef]
- FAO. La Situation Mondiale de L’alimentation et de L’agriculture: Investir Dans L’agriculture Pour un Avenir Meilleur; FAO: Rome, Italy, 2012. [Google Scholar]
- Kamdem, I.; Tomekpe, K.; Thonart, P. Production potentielle de bioéthanol, de biométhane et de pellets à partir des déchets de biomasse lignocellulosique du bananier (Musa spp.) au Cameroun. Biotechnol. Agron. Soc. Environ. 2011, 15, 471–483. [Google Scholar]
- Manimaran, P.; Pillai, G.P.; Vignesh, V.; Prithiviraj, M. Characterization of natural cellulosic fibers from Nendran Banana Peduncle plants. Int. J. Biol. Macromol. 2020, 162, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Durai, P.N.; Viswalingam, K. Suitability Assessment of Musa Acuminate Peduncles Fiber for Fabrication of Green Composites. J. Nat. Fibers 2022, 19, 14866–14879. [Google Scholar] [CrossRef]
- Manimaran, P.; Sanjay, M.R.; Senthamaraikannan, P.; Jawaid, M.; Saravanakumar, S.S.; George, R. Synthesis and characterization of cellulosic fiber from red banana peduncle as reinforcement for potential applications. J. Nat. Fibers 2019, 16, 768–780. [Google Scholar] [CrossRef]
- Pillai, G.P.; Manimaran, P.; Vignesh, V. Physico-chemical and Mechanical Properties of Alkali-Treated Red Banana Peduncle Fiber. J. Nat. Fibers 2020, 18, 2102–2111. [Google Scholar] [CrossRef]
- Preethi, P.; Balakrishna Murthy, G. Physical and Chemical Properties of Banana Fiber Extracted from Commercial Banana Cultivars Grown in Tamilnadu State. Agrotechnology 2013, 8, 1–3. [Google Scholar] [CrossRef]
- Haman, Z.; Korgaï, D.; Mejouyo, H.P.W.; Justin, B.T.D.; Hambate, G.V. Analysis of Some Technological Properties of Textile Fibers From the Banana Tree Stalk. Can. J. Pure Appl. Sci. 2022, 16, 5467–5473. [Google Scholar]
- Lyu, P.; Zhang, Y.; Wang, X.; Hurren, C. Degumming methods for bast fibers—A mini review. Ind. Crops Prod. 2021, 174, 114158. [Google Scholar] [CrossRef]
- Anafack, S.M.; Harzallah, O.; Nkemaja, E.D.; Huisken, P.W.M.; Drean, J.Y.; Murugesh, B.K. Effects of extraction techniques on textile properties of William banana peduncle fibers. Ind. Crops Prod. 2023, 201, 116912. [Google Scholar] [CrossRef]
- Bleuze, L.; Chabbert, B.; Lashermes, G.; Recous, S. Hemp harvest time impacts on the dynamics of microbial colonization and hemp stems degradation during dew retting. Ind. Crops Prod. 2020, 145, 112122. [Google Scholar] [CrossRef]
- Martin, M.A.N. Contribution à L’étude de Paramètres Influençant Les Propriétés Mécaniques de Fibers Élémentaires de Lin: Corrélation Avec Les Propriétés de Matériaux Composites. Ph.D. Thesis, Université Européenne de Bretagne, Rennes, France, 2015. [Google Scholar]
- Chabbert, B.; Padovani, J.; Djemiel, C.; Ossemond, J.; Lemaître, A.; Yoshinaga, A.; Hawkins, S.; Grec, S.; Beaugrand, J.; Kurek, B. Multimodal assessment of flax dew retting and its functional impact on fibers and natural fiber composites. Ind. Crops Prod. 2020, 148, 112255. [Google Scholar] [CrossRef]
- Amel, B.A.; Paridah, M.T.; Sudin, R.; Anwar, U.; Hussein, A.S. Effect of fiber extraction methods on some properties of kenaf bast fiber. Ind. Crops Prod. 2013, 46, 117–123. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Wine, R.H. Use of Detergents in the Analysis of Fibrous Feeds. IV. Determination of Plant Cell-Wall Constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- Guitet, A. Adaptation de la Méthode “Fractionnement Biochimique Selon Van Soest” Appliqué Aux Échantillons de Composts via un Plan D’experience. Master’s Thesis, 2012. Available online: https://theses.hal.science/tel-01153478/file/BETENE_EBANDA_2012CLF22298.pdf (accessed on 10 July 2025).
- Peltre, C.; Dignac, M.; Derenne, S.; Houot, S. Change of the chemical composition and biodegradability of the Van Soest soluble fraction during composting: A study using a novel extraction method. Waste Manag. 2010, 30, 2448–2460. [Google Scholar] [CrossRef] [PubMed]
- Ebanda, F.B. Etude des Propriétés Mécaniques et Thermiques du Plâtre Renforcé de Fibers Végétales Tropicales. Ph.D. Thesis, Université Blaise Pascal—Clermont-Ferrand II, Aubière, France, 2012. [Google Scholar]
- Atalie, D.; Gideon, R.K. Extraction and characterization of Ethiopian palm leaf fibers. Res. J. Text. Appar. 2018, 22, 15–25. [Google Scholar] [CrossRef]
- Betene, A.D.O.; Betene, F.E.; Martoïa, F.; Dumont, P.J.J.; Atangana, A.; Noah, P.M.A. Physico-Chemical and Thermal Characterization of Some Lignocellulosic Fibres: Ananas comosus (AC), Neuropeltis acuminatas (NA) and Rhecktophyllum camerunense (RC). J. Miner. Mater. Charact. Eng. 2020, 8, 205–222. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.P.; Saravanakumar, S.S. Isolation and characterization of cellulose fibers from Thespesia populnea barks: A study on physicochemical and structural properties. Int. J. Biol. Macromol. 2019, 129, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, V.; Balaji, A.N.; Karthikeyan, M.K.V. Extraction and characterization of new cellulosic fibers from Indian mallow stem: An exploratory investigation. Int. J. Polym. Anal. Charact. 2016, 21, 504–512. [Google Scholar] [CrossRef]
- Baley, C. Fibers naturelles de renfort pour matériaux composites. Tech. L’ingénieur 2020, 33, 1–37. [Google Scholar]
- Madhu, P.; Sanjay, M.R.; Senthamaraikannan, P.; Pradeep, S.; Saravanakumar, S.S.; Yogesha, B. A review on synthesis and characterization of commercially available natural fibers: Part-I. J. Nat. Fibers 2018, 16, 1132–1144. [Google Scholar] [CrossRef]
- Asim, M.; Paridah, M.T.; Chandrasekar, M.; Shahroze, R.M.; Jawaid, M.; Nasir, M.; Siakeng, R. Thermal stability of natural fibers and their polymer composites. Iran. Polym. J. 2020, 29, 625–648. [Google Scholar] [CrossRef]
- Atiqah, A.; Jawaid, M.; Ishak, M.R.; Sapuan, S.M. Effect of Alkali and Silane Treatments on Mechanical and Interfacial Bonding Strength of Sugar Palm Fibers with Thermoplastic Polyurethane. J. Nat. Fibers 2018, 15, 251–261. [Google Scholar] [CrossRef]
- NagarajaGanesh, B.; Muralikannan, R. Extraction and characterization of lignocellulosic fibers from Luffa cylindrica fruit. Int. J. Polym. Anal. Charact. 2016, 21, 259–266. [Google Scholar] [CrossRef]
- Senthamaraikannan, P.; Kathiresan, M. Characterization of raw and alkali treated new natural cellulosic fiber from Coccinia grandis L. Carbohydr. Polym. 2018, 186, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, N.; Balasubramaniam, S.; Venkataraman, V.; Manickam, R.; Nagarajan, R.; Oluwarotimi, I.S. Effect of cellulosic filler loading on mechanical and thermal properties of date palm seed/vinyl ester composites. Int. J. Biol. Macromol. 2020, 147, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Palai, B.K.; Sarangi, S.K. Characterization of Untreated and Alkalized Eichhornia Crassipes Fibers and Its Composites. J. Nat. Fibers 2020, 19, 3809–3824. [Google Scholar] [CrossRef]
- Palai, B.K.; Sarangi, S.K.; Mohapatra, S.S. Investigation of Physiochemical and Thermal Properties of Eichhornia Crassipes Fibers. J. Nat. Fibers 2019, 18, 1320–1331. [Google Scholar] [CrossRef]
- Belouadah, Z.; Ati, A.; Rokbi, M. Optimisation Des Méthodes D ’extraction Et Caractérisation Mécanique De La Fiber Alfa En Vue De Son Application Comme Renfort Des Matériaux Composites. J. Mater. Process. Environ. 2014, 2, 51–57. [Google Scholar]
- Dallel, M. Evaluation du Potentiel Textile Des Fibers D’alfa (Stipa tenacissima L.): Caractérisation Physico-Chimique de la Fibre au Fil. Ph.D. Thesis, Université de Haute Alsace, Mulhouse, France, 2013. [Google Scholar]
- Sanjay, M.R.; Madhu, P.; Jawaid, M.; Senthamaraikannan, P.; Senthil, S.; Pradeep, S. Characterization and properties of natural fiber polymer composites: A comprehensive review. J. Clean. Prod. 2018, 172, 566–581. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).