Development of Eco-Friendly Soy Protein Fiber: A Comprehensive Critical Review and Prospects
Abstract
1. Introduction
2. Development of Soy Protein Fibers in History
2.1. The Interwar Period: 1937–1939 Second World War
2.2. Soy Protein Fiber during the Second World War: 1939–1945
2.3. Post-World War II Period: 1946–1961
2.4. Contemporary Period: 1995 to Date
3. Soy Protein Structure and Extraction
4. Soy protein Structural Modification
4.1. Denaturation
4.2. Acetylation
4.3. Esterification
4.4. Soy Protein Graft Copolymerization
5. Soy Protein Fiber Spinning Techniques
5.1. Techniques for Spinning Submicron-Scale Soy Protein Fibers
5.1.1. Electrospinning
5.1.2. Solution-Blown Spinning
5.2. Micron-Scale Spinning Techniques
5.2.1. Melt Spinning
5.2.2. Wet Spinning
6. Challenges and Opportunities
6.1. Thermal Stability
6.2. Matrix Plasticization
6.3. Soy Protein Compatibility with Nonpolar Matrices and Functionalization Scope
6.4. Soy Protein Content
6.5. Mechanical Performance
Author Contributions
Funding
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Plastic Pollution Is Growing Relentlessly as Waste Management and Recycling Fall Short, Says OECD. Available online: https://www.oecd.org/environment/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.htm (accessed on 2 June 2023).
- Netravali, A.N.; Chabba, S. Composites get greener. Mater. Today 2003, 6, 22–29. [Google Scholar] [CrossRef]
- Rahimizadeh, A.; Kalman, J.; Henri, R.; Fayazbakhsh, K.; Lessard, L. Recycled Glass Fiber Composites from Wind Turbine Waste for 3D Printing Feedstock: Effects of Fiber Content and Interface on Mechanical Performance. Materials 2019, 12, 3929. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans. IUCN. 2017. Available online: https://portals.iucn.org/library/node/46622 (accessed on 2 June 2023).
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 608826. [Google Scholar] [CrossRef]
- USDA ERS—Market Outlook. Available online: https://www.ers.usda.gov/topics/crops/soybeans-and-oil-crops/market-outlook/ (accessed on 3 June 2023).
- Association IS. Soybean Processing Growth Is Crushing It. Available online: https://www.iasoybeans.com/newsroom/article/isr-january-2023-soybean-processing-growth-is-crushing-it (accessed on 5 June 2023).
- Soy Crush Rapidly Expands, Bringing Opportunity and Worries. DTN Progressive Farmer. 2023. Available online: https://www.dtnpf.com/agriculture/web/ag/news/business-inputs/article/2022/05/13/soy-crush-rapidly-expands-bringing (accessed on 5 June 2023).
- Toshiji, K.; Ryohei, I. Process for Manufacturing Artificial Fiber from Protein Contained in Soybean. U.S. Patent US2198538A, 23 April 1940. Available online: https://patents.google.com/patent/US2198538A/en (accessed on 5 June 2023).
- Boyer, R.A.; Atkinson, W.T.; Robinette, C.F. Artificial Fibers and Manufacture Thereof. U.S. Patent US2377854A, 12 June 1945. Available online: https://patents.google.com/patent/US2377854A/en (accessed on 5 June 2023).
- Huang, H.C.; Hammond, E.G.; Reitmeier, C.A.; Myers, D.J. Properties of fibers produced from soy protein isolate by extrusion and wet-spinning. J. Am. Oil Chem. Soc. 1995, 72, 1453–1460. [Google Scholar] [CrossRef]
- Kolbasov, A.; Sinha-Ray, S.; Joijode, A.; Hassan, M.A.; Brown, D.; Maze, B.; Pourdeyhimi, B.; Yarin, A.L. Industrial-Scale Solution Blowing of Soy Protein Nanofibers. Ind. Eng. Chem. Res. 2016, 55, 323–333. [Google Scholar] [CrossRef]
- Ju, Z.; Lu, G.; Sheng, O.; Yuan, H.; Zhou, S.; Liu, T.; Liu, Y.; Wang, Y.; Nai, J.; Zhang, W.; et al. Soybean Protein Fiber Enabled Controllable Li Deposition and a LiF-Nanocrystal-Enriched Interface for Stable Li Metal Batteries. Nano Lett. 2022, 22, 1374–1381. [Google Scholar] [CrossRef]
- Bhatia, J.K.; Kaith, B.S.; Singla, R.; Mehta, P.; Yadav, V.; Dhiman, J.; Bhatti, M.S. RSM optimized soy protein fibre as a sorbent material for treatment of water contaminated with petroleum products. Desalination Water Treat. 2016, 57, 4245–4254. [Google Scholar] [CrossRef]
- Zhao, H.; Hou, L.; Lan, B.; Lu, Y. Fabrication of conductive soybean protein fiber for electromagnetic interference shielding through electroless copper plating. J. Mater. Sci. Mater. Electron. 2016, 27, 13300–13308. [Google Scholar] [CrossRef]
- Wongkanya, R.; Chuysinuan, P.; Pengsuk, C.; Techasakul, S.; Lirdprapamongkol, K.; Svasti, J.; Nooeaid, P. Electrospinning of alginate/soy protein isolated nanofibers and their release characteristics for biomedical applications. J. Sci. Adv. Mater. Devices 2017, 2, 309–316. [Google Scholar] [CrossRef]
- Gökgönül, G.B.; Sabir, E.C. An Experimental Study on Comparison of Selected Performance Properties of Soybean and Cotton Knitted Fabrics. Çukurova Üniv. Mühendis. Fak. Derg. 2022, 37, 803–812. [Google Scholar] [CrossRef]
- Ferrándiz, M.; Fages, E.; Rojas-Lema, S.; Ivorra-Martinez, J.; Gomez-Caturla, J.; Torres-Giner, S. Development and Characterization of Weft-Knitted Fabrics of Naturally Occurring Polymer Fibers for Sustainable and Functional Textiles. Polymers 2021, 13, 665. [Google Scholar] [CrossRef]
- Materials Market Report. Textile Exchange. 2023. Available online: https://textileexchange.org/knowledge-center/reports/materials-market-report-2023/ (accessed on 7 February 2024).
- Avinc, O.; Yavas, A.; Avinc, O.; Yavas, A. Soybean: For Textile Applications and Its Printing. In Soybean—The Basis of Yield, Biomass and Productivity; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/chapters/53674 (accessed on 7 February 2024).
- Reddy, N.; Yang, Y. Soyprotein fibers with high strength and water stability for potential medical applications. Biotechnol. Prog. 2009, 25, 1796–1802. [Google Scholar] [CrossRef]
- Brooks, M. Substitute Innovation: Rethinking the Failure of Mid-Twentieth Century Regenerated Protein Fibres and Their Legacy. Text Soc. Am. Symp. Proc. 1 September 2014. Available online: https://digitalcommons.unl.edu/tsaconf/930 (accessed on 8 June 2023).
- Aoyagi, W.S.A. History of Soybeans and Soyfoods in Michigan (1853–2021): Extensively Annotated Bibliography and Sourcebook; Soyinfo Center: Lafayette, CA, USA, 2021; 1217p. [Google Scholar]
- Schmitz, J.F.; Erhan, S.Z.; Sharma, B.K.; Johnson, L.A.; Myers, D.J. 17—Biobased Products from Soybeans. In Soybeans; Johnson, L.A., White, P.J., Galloway, R., Eds.; AOCS Press: Champaign, IL, USA, 2008; pp. 539–612. Available online: https://www.sciencedirect.com/science/article/pii/B9781893997646500202 (accessed on 10 June 2023).
- Shurtleff, W.; Aoyagi, A. Henry Ford and His Researchers—History of Their Work with Soybeans, Soyfoods and Chemurgy (1928–2011): Extensively Annotated Bibliography and Sourcebook; Soyinfo Center: Lafayette, CA, USA, 2011; 437p. [Google Scholar]
- Shurtleff, W.; Aoyagi, A. History of Modern Soy Protein Ingredients—Isolates, Concentrates, and Textured Soy Protein Products (1911–2016); Soyinfo Center: Lafayette, CA, USA, 2016; Available online: https://www.soyinfocenter.com/books/190 (accessed on 10 September 2023).
- Shurtleff, W.; Aoyagi, A. History of the Drackett Company’s Work with Soybeans, Soy Protein and Azlon (1937–2020)—SoyInfo Center; Soyinfo Center: Lafayette, CA, USA, 2020; 119p, Available online: https://www.soyinfocenter.com/books/228 (accessed on 15 June 2023).
- United States Office of War Information. Information Digest; Office of Government Reports; United States Office of War Information: Washington, DC, USA, 1942; 282p. [Google Scholar]
- Seymour, R.B. Polymer Science before and after 1899: Notable Developments During the Lifetime of Maurits Dekker. J. Macromol. Sci.—Chem. 1989, 26, 1023–1032. [Google Scholar] [CrossRef]
- Stenton, M.; Houghton, J.A.; Kapsali, V.; Blackburn, R.S. The Potential for Regenerated Protein Fibres within a Circular Economy: Lessons from the Past Can Inform Sustainable Innovation in the Textiles Industry. Sustainability 2021, 13, 2328. [Google Scholar] [CrossRef]
- Berlan, J.P.; Bertrand, J.P.; Lebas, L. The growth of the American ‘soybean complex’. Eur. Rev. Agric. Econ. 1977, 4, 395–416. [Google Scholar] [CrossRef]
- Improvements in or Relating to the Insolubilising Treatment of Films, Filaments, Fibres and Like Shaped Articles Made from Protein Solutions. GB605830A, 30 July 1948. Available online: https://patents.google.com/patent/GB605830A/en?oq=GB+605%2c830 (accessed on 13 June 2023).
- Improvements in the Manufacture and Production of Artificial Filaments, Threads, Bands and the Like. GB614506A, 16 December 1948. Available online: https://patents.google.com/patent/GB614506A/en?oq=GB+605%2c830 (accessed on 14 June 2023).
- Process for Improving the Properties of Protein Spinning Products. GB634812A, 29 March 1950. Available online: https://patents.google.com/patent/GB634812A/en?oq=GB+634%2c812 (accessed on 13 June 2023).
- Wormell, R.L. New Fibres from Proteins; FAO: Rome, Italy, 1954. [Google Scholar]
- Improvements in Regenerated Protein Fibres and Process for Preparation Thereof. GB638356A, 6 July 1950. Available online: https://patents.google.com/patent/GB638356A/en?oq=GB+638%2c356 (accessed on 13 June 2023).
- Improvements in or Relating to a Method for Improving the Strength of Artificial Insolubilised Protein Filaments or Fibres. GB665462A, 1952. Available online: https://patents.google.com/patent/GB665462A/en?oq=GB+665%2c462 (accessed on 13 June 2023).
- Improvements in and Relating to the Production of Artificial Protein Fibres. GB674755A, 7 February 1952. Available online: https://patents.google.com/patent/GB674755A/en?oq=GB+674%2c755 (accessed on 14 June 2023).
- Louis, W.R. Production of Artificial Filaments, Threads, Fibres, Bands, and the Like. U.S. Patent US2775506A, 25 December 1956. Available online: https://patents.google.com/patent/US2775506A/en?oq=GB+605%2c830 (accessed on 14 June 2023).
- Method and Apparatus for Forming Fibers. GB862428A, 8 March 1961. Available online: https://patents.google.com/patent/GB862428A/en?oq=GB+862%2c428 (accessed on 14 June 2023).
- Colton, J.B.; Burns, B.R.; Knapp, F.D. Plastic particles in surface waters of the northwestern atlantic. Science 1974, 185, 491–497. [Google Scholar] [CrossRef]
- Brooks, M.M. 7—Regenerated protein fibres: A preliminary review. In Handbook of Textile Fibre Structure; Eichhorn, S.J., Hearle, J.W.S., Jaffe, M., Kikutani, T., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Shaston, UK, 2009; Volume 2, pp. 234–265. Available online: https://www.sciencedirect.com/science/article/pii/B978184569730350007X (accessed on 16 June 2023).
- Wang, Y. New Materials in Textile Innovation. J. Mater. Process. Des. 2023, 7, 32–40. [Google Scholar]
- Jackman, D.R.; Dixon, M.K.; Condra, J. The Guide to Textiles for Interiors; Portage & Main Press: Winnipeg, MB, Canada, 2003; 248p. [Google Scholar]
- Wang, Q.; Du, Y.; Hu, X.; Yang, J.; Fan, L.; Feng, T. Preparation of alginate/soy protein isolate blend fibers through a novel coagulating bath. J. Appl. Polym. Sci. 2006, 101, 425–431. [Google Scholar] [CrossRef]
- Zhang, X.; Min, B.G.; Kumar, S. Solution spinning and characterization of poly(vinyl alcohol)/soybean protein blend fibers. J. Appl. Polym. Sci. 2003, 90, 716–721. [Google Scholar] [CrossRef]
- Mu, B.; Xu, H.; Li, W.; Xu, L.; Yang, Y. Spinnability and rheological properties of globular soy protein solution. Food Hydrocoll. 2019, 90, 443–451. [Google Scholar] [CrossRef]
- Deng, S.; Cheng, J.; Guo, X.; Jiang, L.; Zhang, J. Fiber Spinning of Polyacrylonitrile Grafted Soy Protein in an Ionic Liquid/DMSO Mixture Solvent. J. Polym. Environ. 2014, 22, 17–26. [Google Scholar] [CrossRef]
- Zhang, M.; Reitmeier, C.A.; Hammond, E.G.; Myers, D.J. Production of Textile Fibers from Zein and a Soy Protein-Zein Blend. Cereal Chem. 1997, 74, 594–598. [Google Scholar] [CrossRef]
- Guzdemir, O.; Ogale, A.A. Influence of Spinning Temperature and Filler Content on the Properties of Melt-Spun Soy Flour/Polypropylene Fibers. Fibers 2019, 7, 83. [Google Scholar] [CrossRef]
- Guzdemir, O.; Lukubira, S.; Ogale, A.A. Soy-filled polyethylene fibers for modified surface and hydrophilic characteristics. J. Appl. Polym. Sci. 2018, 135, 46609. [Google Scholar] [CrossRef]
- Wong, D.W.S. (Ed.) Proteins. In Mechanism and Theory in Food Chemistry, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 55–122. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The Shape and Structure of Proteins. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26830/ (accessed on 2 July 2023).
- Kinsella, J.E. Functional properties of soy proteins. J. Am. Oil Chem. Soc. 1979, 56 Pt 1, 242–258. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, T.; Jiang, L. Soy Protein: Molecular Structure Revisited and Recent Advances in Processing Technologies. Annu. Rev. Food Sci. Technol. 2021, 12, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Witte, N.H. Chapter 7—Soybean Meal Processing and Utilization. In Practical Handbook of Soybean Processing and Utilization; Erickson, D.R., Ed.; AOCS Press: Champaign, IL, USA, 1995; pp. 93–116. Available online: https://www.sciencedirect.com/science/article/pii/B9780935315639500115 (accessed on 18 June 2023).
- Ly, Y.T.P.; Johnson, L.A.; Jane, J. Soy Protein As Biopolymer. In Biopolymers from Renewable Resources; Kaplan, D.L., Ed.; Macromolecular Systems—Materials Approach; Springer: Berlin/Heidelberg, Germany, 1998; pp. 144–176. [Google Scholar] [CrossRef]
- Lusas, E.W.; Rhee, K.C. Chapter 8—Soy Protein Processing and Utilization. In Practical Handbook of Soybean Processing and Utilization; Erickson, D.R., Ed.; AOCS Press: Champaign, IL, USA, 1995; pp. 117–160. Available online: https://www.sciencedirect.com/science/article/pii/B9780935315639500127 (accessed on 20 June 2023).
- Mäkinen, O.E.; Zannini, E.; Koehler, P.; Arendt, E.K. Heat-denaturation and aggregation of quinoa (Chenopodium quinoa) globulins as affected by the pH value. Food Chem. 2016, 196, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, L.; Ren, Z.; Weng, W. Preparation and characterization of soy protein isolate films by pretreatment with cysteine. Food Chem. X 2023, 18, 100735. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Adachi, K.; Schwartz, E. Stabilizing effect of various organic solvents on protein. J. Biol. Chem. 1978, 253, 6423–6425. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Khare, S.K. Protective role of salt in catalysis and maintaining structure of halophilic proteins against denaturation. Front. Microbiol. 2014, 5, 80043. [Google Scholar] [CrossRef] [PubMed]
- Joyce, A.M.; Kelly, A.L.; O’Mahony, J.A. Controlling denaturation and aggregation of whey proteins during thermal processing by modifying temperature and calcium concentration. Int. J. Dairy Technol. 2018, 71, 446–453. [Google Scholar] [CrossRef]
- Schön, A.; Clarkson, B.R.; Siles, R.; Ross, P.; Brown, R.K.; Freire, E. Denatured state aggregation parameters derived from concentration dependence of protein stability. Anal. Biochem. 2015, 488, 45–50. [Google Scholar] [CrossRef]
- Zheng, Z.; Xin, C.; Li, Y. Numerical study on mechanisms of soy protein as a functional modifier for polymer materials. Model. Simul. Mater. Sci. Eng. 2019, 27, 085010. [Google Scholar] [CrossRef]
- Yue, L.; Meng, Z.; Yi, Z.; Gao, Q.; Mao, A.; Li, J. Effects of Different Denaturants on Properties and Performance of Soy Protein-Based Adhesive. Polymers 2019, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- O′Flynn, T.D.; Hogan, S.A.; Daly, D.F.M.; O′Mahony, J.A.; McCarthy, N.A. Rheological and Solubility Properties of Soy Protein Isolate. Molecules 2021, 26, 3015. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghasemzadeh, S.; Kotliar, A.M.; Kumar, S.; Presnell, S.; Williams, L.D. Fibers from soybean protein and poly(vinyl alcohol). J. Appl. Polym. Sci. 1999, 71, 11–19. [Google Scholar] [CrossRef]
- Renkema, J.M.S.; Gruppen, H.; van Vliet, T. Influence of pH and ionic strength on heat-induced formation and rheological properties of soy protein gels in relation to denaturation and their protein compositions. J. Agric. Food Chem. 2002, 50, 6064–6071. [Google Scholar] [CrossRef] [PubMed]
- El-Adawy, T.A. Functional properties and nutritional quality of acetylated and succinylated mung bean protein isolate. Food Chem. 2000, 70, 83–91. [Google Scholar] [CrossRef]
- Barman, B.G.; Hansen, J.R.; Mossey, A.R. Modification of the physical properties of soy protein isolate by acetylation. J. Agric. Food Chem. 1977, 25, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Rhee, J.S. Effects of Acetylation on Physicochemical Properties of J1s Soy Protein. J. Food Biochem. 1989, 13, 187–199. [Google Scholar] [CrossRef]
- Allen, K.A. Study of Mechanical and Thermal Properties of Soy Flour Elastomers. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2014. Available online: https://ui.adsabs.harvard.edu/abs/2014PhDT.......173A (accessed on 26 July 2023).
- Foulk, J.A.; Bunn, J.M. Properties of compression-molded, acetylated soy protein films. Ind. Crops Prod. 2001, 14, 11–22. [Google Scholar] [CrossRef]
- Sitohy, M.; Osman, A. Antimicrobial activity of native and esterified legume proteins against Gram-negative and Gram-positive bacteria. Food Chem. 2010, 120, 66–73. [Google Scholar] [CrossRef]
- Wang, T.; Yi, K.; Li, Y.; Wang, H.; Fan, Z.; Jin, H.; Xu, J. Esterified Soy Proteins with Enhanced Antibacterial Properties for the Stabilization of Nano-Emulsions under Acidic Conditions. Molecules 2023, 28, 3078. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, S.; Sutar, A.K.; Maharana, T. Graft copolymerization of Soy Protein Isolate with Polylactide via Ring Opening Polymerization. IOP Conf. Ser. Mater. Sci. Eng. 2018, 410, 012011. [Google Scholar] [CrossRef]
- Feng, B.; Wang, D.; Li, Y.; Qian, J.; Yu, C.; Wang, M.; Luo, D.; Wei, S. Mechanical Properties of a Soy Protein Isolate–Grafted–Acrylate (SGA) Copolymer Used for Wood Coatings. Polymers 2020, 12, 1137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, G.; Pang, J.; Su, L. Synthesis of renewable soybean protein and acrylate copolymers via ATRP in ionic liquid. Ind. Crops Prod. 2022, 180, 114720. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, H.; Mu, B.; Xu, L.; Yang, Y. Biodegradable soy protein films with controllable water solubility and enhanced mechanical properties via graft polymerization. Polym. Degrad. Stab. 2016, 133, 75–84. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Kalia, S.; Kumar, A.; Mittal, H. Peroxide Treatment of Soy Protein Fibers Followed by Grafting of Poly(methyl acrylate) and Copolymers. J. Renew. Mater. 2013, 1, 302–310. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Anton, F. Process and Apparatus for Preparing Artificial Threads. U.S. Patent US1975504A, 2 October 1934. Available online: https://patents.google.com/patent/US1975504A/en (accessed on 5 February 2024).
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less harmful acidic degradation of poly(lacticco-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int. J. Nanomed. 2006, 1, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.K.; Chandler, R.T.; Mundhekar, A.; Khoo, N.; Pruitt, H.M.; Kucik, D.F.; Parks, D.A.; Kevil, C.G.; Barnes, S.; Patel, R.P. Revealing anti-inflammatory mechanisms of soy isoflavones by flow: Modulation of leukocyte-endothelial cell interactions. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H908–H915. [Google Scholar] [CrossRef] [PubMed]
- Har-el, Y.E.; Gerstenhaber, J.A.; Brodsky, R.; Huneke, R.B.; Lelkes, P.I. Electrospun soy protein scaffolds as wound dressings: Enhanced reepithelialization in a porcine model of wound healing. Wound Med. 2014, 5, 9–15. [Google Scholar] [CrossRef]
- Visakh, P.M.; Nazarenko, O.B. Soy Protein-Based Blends, Composites and Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2017; 275p. [Google Scholar]
- Santin, M.; Ambrosio, L. Soybean-based biomaterials: Preparation, properties and tissue regeneration potential. Expert Rev. Med. Devices 2008, 5, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Tokudome, Y.; Nakamura, K.; Kage, M.; Todo, H.; Sugibayashi, K.; Hashimoto, F. Effects of soybean peptide and collagen peptide on collagen synthesis in normal human dermal fibroblasts. Int. J. Food Sci. Nutr. 2012, 63, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Khabbaz, B.; Solouk, A.; Mirzadeh, H. Polyvinyl alcohol/soy protein isolate nanofibrous patch for wound-healing applications. Prog. Biomater. 2019, 8, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Phelan, M.A.; Kruczek, K.; Wilson, J.H.; Brooks, M.J.; Drinnan, C.T.; Regent, F.; Gerstenhaber, J.A.; Swaroop, A.; Lelkes, P.I.; Li, T. Soy Protein Nanofiber Scaffolds for Uniform Maturation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium. Tissue Eng. Part C Methods 2020, 26, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, S.; Ghorbani, M.; Mahmoodzadeh, F. Silver sulfadiazine-loaded electrospun ethyl cellulose/polylactic acid/collagen nanofibrous mats with antibacterial properties for wound healing. Int. J. Biol. Macromol. 2020, 162, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Popov Pereira da Cunha, M.D.; Aldana, A.A.; Abraham, G.A. Photo-crosslinked soy protein-based electrospun scaffolds. Mater. Lett. X 2021, 12, 100115. [Google Scholar] [CrossRef]
- Popov Pereira da Cunha, M.D.; Ponce, A.G.; Abraham, G.A. Effect of thermal treatments and UV radiation on green soy protein isolated crosslinked electrospun mats. J. Appl. Polym. Sci. 2023, 140, e53777. [Google Scholar] [CrossRef]
- Lin, L. Electrospun Soy Protein-based Scaffolds for Skin Tissue Engineering and Wound Healing. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2011. [Google Scholar]
- Ramji, K.; Shah, R.N. Electrospun soy protein nanofiber scaffolds for tissue regeneration. J. Biomater. Appl. 2014, 29, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Dai, Q.; Wang, Z.; Luo, X.; Xue, Y.; Yu, F. Toughening of electrospun poly(l-lactic acid) nanofiber scaffolds with unidirectionally aligned halloysite nanotubes. J. Mater. Sci. 2015, 50, 1435–1445. [Google Scholar] [CrossRef]
- Cho, D.; Nnadi, O.; Netravali, A.; Joo, Y.L. Electrospun Hybrid Soy Protein/PVA Fibers. Macromol. Mater. Eng. 2010, 295, 763–773. [Google Scholar] [CrossRef]
- Delyanee, M.; Solouk, A.; Akbari, S.; Daliri Joupari, M. Engineered hemostatic bionanocomposite of poly(lactic acid) electrospun mat and amino-modified halloysite for potential application in wound healing. Polym. Adv. Technol. 2021, 32, 3934–3947. [Google Scholar] [CrossRef]
- Doustdar, F.; Ramezani, S.; Ghorbani, M.; Mortazavi Moghadam, F. Optimization and characterization of a novel tea tree oil-integrated poly (ε-caprolactone)/soy protein isolate electrospun mat as a wound care system. Int. J. Pharm. 2022, 627, 122218. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, L.; Zhou, Z.; Wu, X.; Wang, Y. Preparation and Properties of Electrospun Soy Protein Isolate/Polyethylene Oxide Nanofiber Membranes. ACS Appl. Mater. Interfaces 2012, 4, 4331–4337. [Google Scholar] [CrossRef] [PubMed]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Mahto, S.K. Soy protein isolate supplemented silk fibroin nanofibers for skin tissue regeneration: Fabrication and characterization. Int. J. Biol. Macromol. 2020, 160, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Doustdar, F.; Ghorbani, M. ZIF-8 enriched electrospun ethyl cellulose/polyvinylpyrrolidone scaffolds: The key role of polyvinylpyrrolidone molecular weight. Carbohydr. Polym. 2022, 291, 119620. [Google Scholar] [CrossRef] [PubMed]
- Sampath Kumar, N.; Santhosh, C.; Vathaluru Sudakaran, S.; Deb, A.; Raghavan, V.; Venugopal, V.; Bhatnagar, A.; Bhat, S.; Andrews, N.G. Electrospun polyurethane and soy protein nanofibres for wound dressing applications. IET Nanobiotechnol. 2017, 12, 94–98. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Ghorbani, M.; Ramazani, S. Preparation and characterization of TiO2NPs and betanin loaded zein/sodium alginate nanofibers. Food Packag. Shelf Life 2020, 24, 100504. [Google Scholar] [CrossRef]
- Tansaz, S.; Liverani, L.; Vester, L.; Boccaccini, A.R. Soy protein meets bioactive glass: Electrospun composite fibers for tissue engineering applications. Mater. Lett. 2017, 199, 143–146. [Google Scholar] [CrossRef]
- Jaberifard, F.; Ramezani, S.; Ghorbani, M.; Arsalani, N.; Mortazavi Moghadam, F. Investigation of wound healing efficiency of multifunctional eudragit/soy protein isolate electrospun nanofiber incorporated with ZnO loaded halloysite nanotubes and allantoin. Int. J. Pharm. 2023, 630, 122434. [Google Scholar] [CrossRef] [PubMed]
- White, C.; DiStefano, T.; Olabisi, R. The influence of substrate modulus on retinal pigment epithelial cells. J. Biomed. Mater. Res. A 2017, 105, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Marcone, M.F.; Barbut, S.; Lim, L.T. Electrospun soy protein isolate-based fiber fortified with anthocyanin-rich red raspberry (Rubus strigosus) extracts. Food Res. Int. 2013, 52, 467–472. [Google Scholar] [CrossRef]
- Vega-Lugo, A.C.; Lim, L.T. Electrospinning of Soy Protein Isolate Nanofibers. J. Biobased Mater. Bioenergy 2008, 2, 223–230. [Google Scholar] [CrossRef]
- Vega-Lugo, A.C.; Lim, L.T. Controlled release of allyl isothiocyanate using soy protein and poly(lactic acid) electrospun fibers. Food Res. Int. 2009, 42, 933–940. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Behrens, A.M.; Sandler, A.D.; Kofinas, P. A Review of the Fundamental Principles and Applications of Solution Blow Spinning. ACS Appl. Mater. Interfaces 2016, 8, 34951–34963. [Google Scholar] [CrossRef] [PubMed]
- Kolbasov, A.; Sinha-Ray, S.; Yarin, A.L.; Pourdeyhimi, B. Heavy metal adsorption on solution-blown biopolymer nanofiber membranes. J. Membr. Sci. 2017, 530, 250–263. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, M.W.; An, S.; Sinha-Ray, S.; Khansari, S.; Joshi, B.; Hong, S.; Hong, J.H.; Kim, J.J.; Pourdeyhimi, B.; et al. Antibacterial activity of photocatalytic electrospun titania nanofiber mats and solution-blown soy protein nanofiber mats decorated with silver nanoparticles. Catal. Commun. 2013, 34, 35–40. [Google Scholar] [CrossRef]
- Penconek, A.; Kasak, D.; Moskal, A. Soy Protein Nanofibers Obtained by Solution Blow Spinning. Processes 2023, 11, 2310. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Khansari, S.; Yarin, A.L.; Pourdeyhimi, B. Effect of Chemical and Physical Cross-Linking on Tensile Characteristics of Solution-Blown Soy Protein Nanofiber Mats. Ind. Eng. Chem. Res. 2012, 51, 15109–15121. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Zhang, Y.; Yarin, A.L.; Davis, S.C.; Pourdeyhimi, B. Solution Blowing of Soy Protein Fibers. Biomacromolecules 2011, 12, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Khansari, S.; Sinha-Ray, S.; Yarin, A.L.; Pourdeyhimi, B. Stress-strain dependence for soy-protein nanofiber mats. J. Appl. Phys. 2012, 111, 044906. [Google Scholar] [CrossRef]
- Guzdemir, O. Melt-Spinning and Properties of Soy-Filled Polyethylene, Polypropylene, and Poly-(Lactic Acid) Fibers. Diss. 1 August 2019. Available online: https://tigerprints.clemson.edu/all_dissertations/2439 (accessed on 19 July 2023).
- Naphade, C.; Han, I.; Lukubira, S.; Ogale, A.; Rieck, J.; Dawson, P. Prediction of Mold Spoilage for Soy/Polyethylene Composite Fibers. Int. J. Polym. Sci. 2015, 2015, e176826. [Google Scholar] [CrossRef]
- Güzdemir, Ö.; Bermudez, V.; Kanhere, S.; Ogale, A.A. Melt-spun poly(lactic acid) fibers modified with soy fillers: Toward environment-friendly disposable nonwovens. Polym. Eng. Sci. 2020, 60, 1158–1168. [Google Scholar] [CrossRef]
- Boyer, R.A. Soybean Protein Fibers Experimental Production. Ind. Eng. Chem. 1940, 32, 1549–1551. [Google Scholar] [CrossRef]
- Croston, C.B.; Evans, C.D.; Smith, A.K. Zein Fibers. Ind. Eng. Chem. 1945, 37, 1194–1198. [Google Scholar] [CrossRef]
- Sudha, T.B.; Thanikaivelan, P.; Ashokkumar, M.; Chandrasekaran, B. Structural and thermal investigations of biomimetically grown casein-soy hybrid protein fibers. Appl. Biochem. Biotechnol. 2011, 163, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gu, L. Hydrolyzed polyacrylonitrile-blend-soy protein hydrogel fibers: A study of structure and dynamic pH response. Polym. Int. 2009, 58, 66–73. [Google Scholar] [CrossRef]
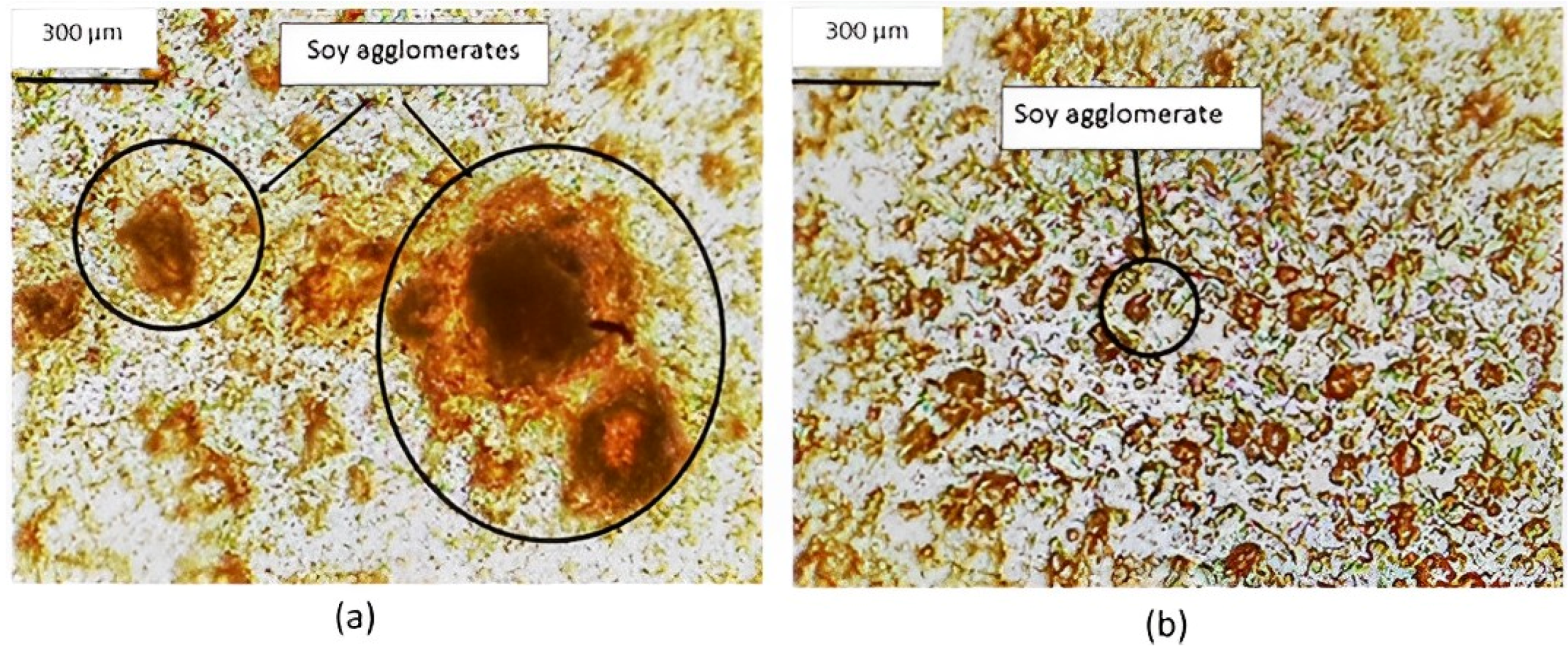
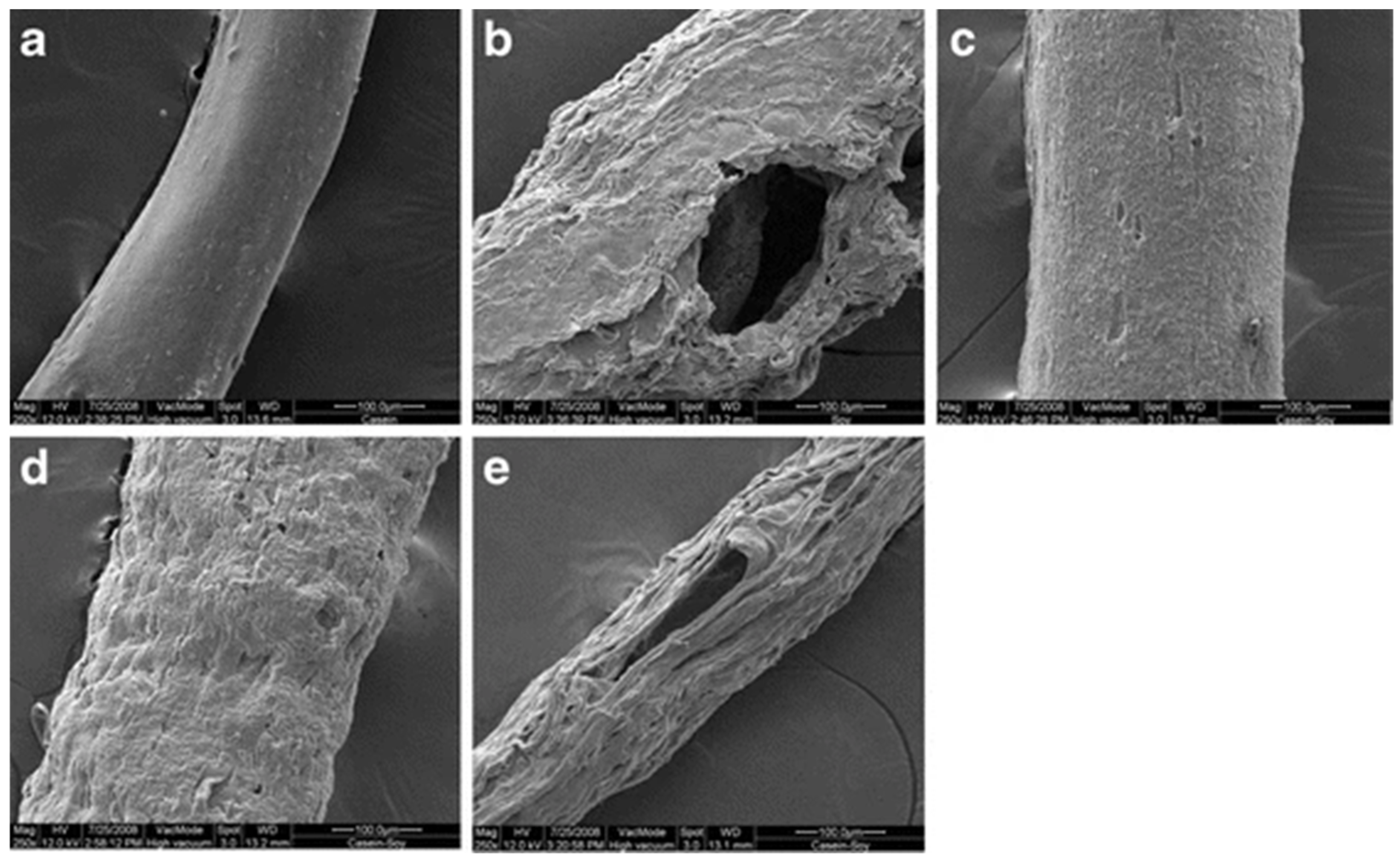
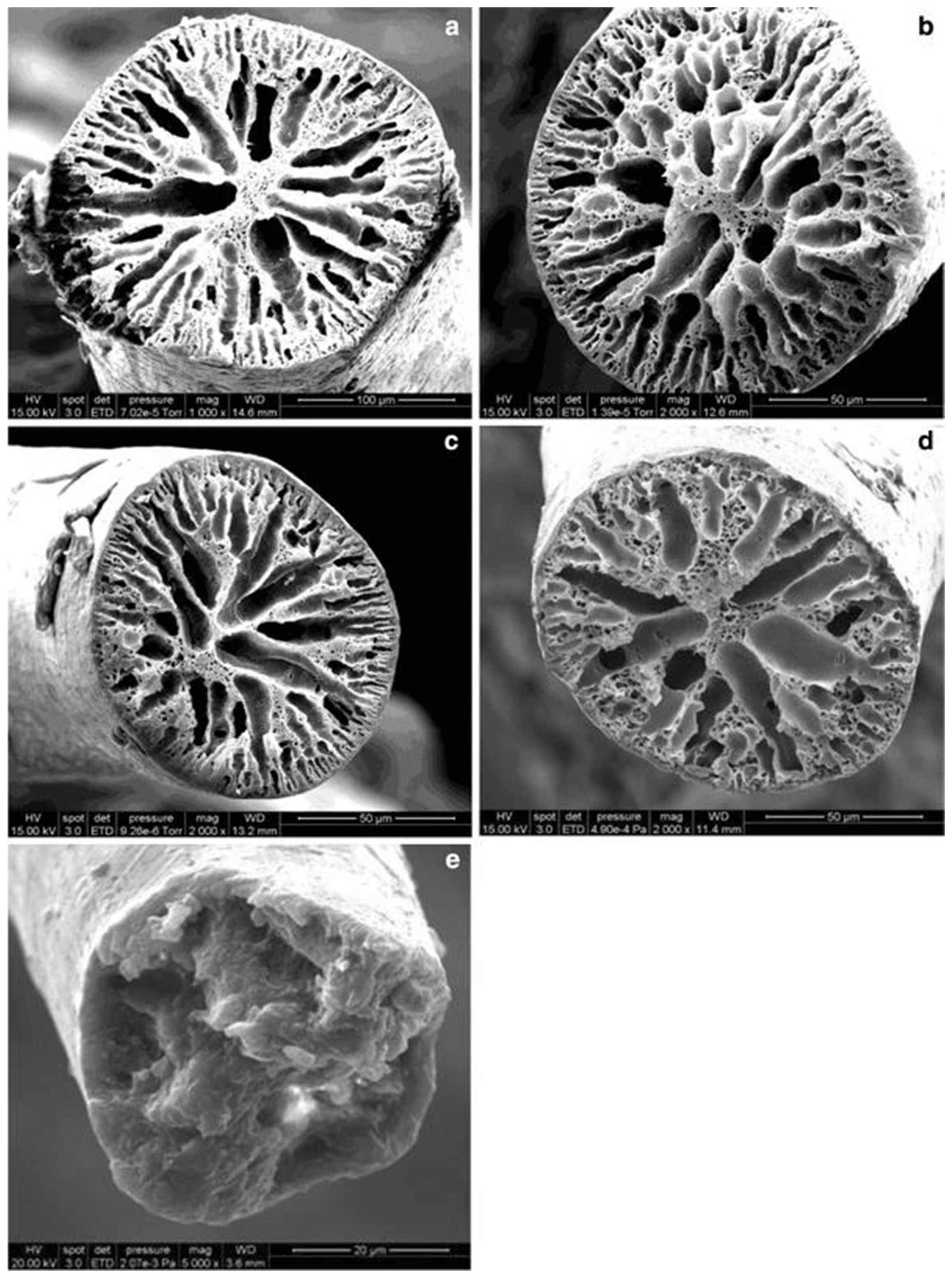
| Fiber Type and Diameter or Linear Density | Spinning Type and Extrusion Temp. | Raw Material and Fiber Conditioning | Tensile Modulus (MPa) | Tensile Strength (MPa) | Tenacity (cN/dtex) | Elongation at Break (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Denaturing Mode and Conditions | Coagulation Bath Composition and Temperature | Post-Spinning Treatment | |||||||
| 10 wt.% soy protein/alginate Diameter < 80 µm | Wet spinning at 25 °C | 10 wt.% sodium hydroxide aqueous solution at room temp. | 10 wt.% calcium chloride + 1 wt.% HCL + 0 wt.% ethanol | 20% drawing | - | - | 1.41 (dry), 0.34 (wet) | 21.7 (dry) 46.4 (wet) | [50] |
| 10 wt.% soy protein/PVA Neither diameter nor linear density provided | Wet spinning at 70 °C | Aqueous solvent of urea + sodium sulfite + 85 °C heating | Sodium sulfate and ammonium sulfate in water with 1 M sulfuric acid | Glutaric dialdehyde crosslinking followed by heating at 190 °C at 20 MPa stress | 5300 ± 300 | 260 ± 11 | - | 11 ± 0.6 | [51] |
| Regenerated 100% soy protein, Diameter of 50–150 µm | Wet spinning | 8 M urea solution with 1% (w/w) sodium sulfite, soy protein solution aged 96 h at room temperature | 10% (w/w) sodium sulfite and 10% (w/w) acetic acid fibers remain in the bath for 30 min | - | 6500 ± 1700 | 145 ± 10 | - | 8 ± 2 | [26] |
| Regenerated 100% soy protein fiber, Diameter < 368 µm | Wet spinning | Sodium hydroxide aqueous solution at pH 12.1 | 4% hydrochloric acid solution containing 3.3% sodium chloride, 3.3% zinc chloride, and 3.3% calcium chloride | 25% glutaraldehyde and drawing to 170% as-spun length | - | - | 0.638 and 0.73 at 0.65 and 1 water activity levels | 3.1 and 59.7 at 0.65 and 1 water activity levels | [16] |
| 45 wt.% soy protein/15 wt.% glycerol/ 40 wt.% water, Diameter < 368 µm | Melt spinning at 96 °C and 20 rpm | 96 °C temp + 20 rpm in a twin-screw extruder | - | glutaraldehyde + acetic anhydride and drawing to 150% as-spun length | - | - | 0.53 and 0.24 at 0.65 and 1 water activity levels | 9.2 and 39.3 at 0.65 and 1 water activity levels | [16] |
| Regenerated 100% soy protein, Diameter 50 µm | Wet spinning | 8 M urea solution with 1.1% (w/w) sodium sulfite, heated for 2 h at 80 °C, solution aged for 2 days | Citric acid, sodium sulfate, zinc sulfate, and water (1:1:0.1:8 w/w), bath’s pH 2.2 | - | 523 g/tex (4801 MPa) | - | 0.9 | 5–9 | [52] |
| 10 wt.% SPI-g-PAN, Diameter < 11 µm | Wet spinning at 70 °C | DMSO + BMIMCI at 75 °C | Water and ethanol (1:1) constant temperature at 4 °C | - | 1478.4 ± 185 | 512.6 ± 76.9 | - | 11.87 ± 1.1 | [53] |
| 45 wt.% soy protein/15 wt.% glycerol/ 40 wt.% water, Diameter < 368 µm | Melt spinning at 96 °C and 20 rpm | 96 °C temp + 20 rpm in a twin-screw extruder | - | 89 wt.% water/9.5 wt.% ethanol/1.5 wt.% 1,4-benzoquinone | 2552.04 ± 238.68 g/tex (11 rh) 2570.4 ± 330.48 g/tex (65 rh) 293.76 ± 55.08 g/tex (100 rh) | - | 0.354 at 11% rh 0.337 at 65% rh 0.053 at 100 rh | - | [54] |
| 15 wt.% soy flour/PP + monoglyceride compatibilizer | Melt spinning at 190 °C | 190 °C temp + 100 rpm in twin screw for 2 min | - | 100 draw-down ratio | 914 ± 164 | 74 ± 7 | - | 268 ± 57 | [55] |
| 23 wt.% soy flour/7 wt.% monoglyceride/70 wt.% LLDPE, Diameter 45 ± 11 μm | Melt spinning at 140 °C | 140 °C temp + 100 rpm twin screw extruder | - | - | 615 ± 38 | 57.0 ± 8.0 | - | 280 ± 29 | [56] |
| Structural Modification Techniques | Advantages | Disadvantages |
|---|---|---|
| Denaturation | Increased compatibility with hydrophobic thermoplastic matrices due to exposure of hydrophobic groups buried deep within the coiled soy protein structure. | Soy protein aggregation post-denaturation due to new protein–protein interactions. These aggregates can plug the spinnerets or the screens/filters during the fiber formation. Exposure of soy protein’s hydrophobic groups can aid the processibility in melt spinning; however, in the case of wet spinning, the solubility of the protein in aqueous solvents can decline. |
| Acetylation | Acetylation makes soy protein less polar and, hence, more hydrophobic. Induced hydrophobic character can increase soy protein’s compatibility with nonpolar matrices, amplifying its processibility in melt spinning. | The increased hydrophobic character of acetylated soy protein can compromise their solubility in aqueous solvents during wet spinning. |
| Esterification | Subdued soy protein brittleness post-esterification. | The increased isoelectric point of esterified soy protein necessitates using environmentally harmful, highly acidic, or basic conditions for its solubility, which is vital for preparing wet-spinnable dopes. |
| Graft polymerization | The highly tunable nature of soy protein due to an abundance of chemical moieties enables its grafting with select monomers. Strong linkages can then be established between the polymeric matrices or solvents and grafted soy proteins, enhancing the mechanical properties of the resultant spun fibers. | Limited grafting monomer choices. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, M.; Li, A.; Moore, M.; Ford, E.; Theyson, T.; Seyam, A.-F.M. Development of Eco-Friendly Soy Protein Fiber: A Comprehensive Critical Review and Prospects. Fibers 2024, 12, 31. https://doi.org/10.3390/fib12040031
Tahir M, Li A, Moore M, Ford E, Theyson T, Seyam A-FM. Development of Eco-Friendly Soy Protein Fiber: A Comprehensive Critical Review and Prospects. Fibers. 2024; 12(4):31. https://doi.org/10.3390/fib12040031
Chicago/Turabian StyleTahir, Muneeb, Ang Li, Marguerite Moore, Ericka Ford, Thomas Theyson, and Abdel-Fattah M. Seyam. 2024. "Development of Eco-Friendly Soy Protein Fiber: A Comprehensive Critical Review and Prospects" Fibers 12, no. 4: 31. https://doi.org/10.3390/fib12040031
APA StyleTahir, M., Li, A., Moore, M., Ford, E., Theyson, T., & Seyam, A.-F. M. (2024). Development of Eco-Friendly Soy Protein Fiber: A Comprehensive Critical Review and Prospects. Fibers, 12(4), 31. https://doi.org/10.3390/fib12040031








