Abstract
Microfibers are small fiber particles that separate from larger textiles through wear abrasion or home laundering. Pervasive accumulation of synthetic microfibers in the environment is motivating efforts to quantify them, and to gain a better understanding of the factors that lead to their release from garments. Automated imaging systems have been previously employed for the quantification of synthetic and natural microfibers. In the current study, a rayon standard and microfibers sourced from scoured cotton HVI calibration standards were examined with the Fiber Quality Analyzer-360 (FQA) automated imaging system. Mechanically stirred suspensions of six cotton microfiber standards showed significantly lower fiber counts than those obtained with a rayon standard. Probe sonication of the sample suspensions significantly increased observed fiber counts for the cotton standard samples, by 105% on average. Mean length determinations decreased by, on average, 5% for the sonicated samples, an indication that count increases were not due to sample fragmentation. No significant change was observed for the fiber counts or length measurements of the sonicated rayon samples. The sonicated cotton samples showed an average of 95% detection by the FQA. These results highlight the importance of proper microfiber suspension for accurate detection and quantification using the FQA system.
1. Introduction
The accumulation of textile microfibers in the environment is raising concerns related to the ecological impact of apparel products [1,2,3,4,5]. Microfibers, as the term is used in the present study, are small fragments, 1 µm to 5 mm in length, that breakaway from larger garments usually through wear or home laundering. Of these, synthetic microfibers (e.g., fibers of polyester, nylon) are expected to persist indefinitely in the environment, while microfibers from natural fibers (e.g., cotton, wool) or regenerated cellulose fibers (e.g., rayon) have been shown to readily decompose [6,7,8,9]. As market and consumer demands move towards more sustainable products, there is also interest in addressing the generation and accumulation of microfibers. To reach these goals, it is crucial to investigate reliable and fast methods for microfiber quantification.
Microfiber research has evolved from the wider field of microplastic contamination [1,3,10,11,12]. Frias and Nash described microplastics as synthetic solid particles made of a polymeric matrix of a size ranging from 1 µm to 5 mm [13]. Frias and Nash distinguished between primary microplastics, those manufactured in sizes within the defined range, and secondary microplastics, plastics that fragmented into the microplastic range. While a number of published studies have relied on narrower size limits, by revising both the upper and lower size limits [14,15,16], the wider size range proposed by Frias and Nash effectively included most studies reported at the time under the umbrella of microplastic research [13]. Microfiber studies have often adopted the length limitations described by Frias and Nash for microplastics, i.e., a textile fragment that ranges in length from 1 µm to 5 mm.
Several studies have explored the distribution of microplastics in the environment and documented their presence in waterways [10,17], air [18,19], soil [20,21], tap water systems [22], and food sources [23]. While the scale of microfiber environmental contamination is still being assessed, estimates place the number of microplastic particles in ocean systems alone at 24.4 trillion particles, a figure that will only grow with continued use of plastic products that do not readily biodegrade [24]. Microfibers from synthetic textiles are considered a large contributor to microplastic contamination. A study by Boucher and Friot estimated that microfibers from synthetic textiles account for as much as 35% of microplastics found in seas [25]. The accumulation of microfibers and microplastics in the environment also raises concerns related to their potential impact on the health of animals or humans that inadvertently consume them [26,27,28], related both to the microplastics on their own and potential sorption of toxic chemicals to the microplastics [29,30]. Microfiber field contamination studies, particularly those examining contamination in water systems, are often limited to synthetic microfragments; however, several animal ingestion studies have reported the recovery of microfibers from natural fibers [23,31]. Still, the impact of naturally sourced microfibers on environmental contamination is not as well established.
Microfiber research efforts have also focused on understanding factors that contribute to the generation of these microfibers from the routine use of textiles, their home laundering, or from abrasion though wear or tumble drying. A review article by Tan and co-authors covers deformation and fracture mechanisms of a wide range of polymeric fibers [32], which can provide insight into the process by which microfibers are generated. Studies that examined microfiber generation from laundering have also been reported. In an early study, Browne et al. examined the effluent of three commercial top-loading washing machines after washing polyester fleece, blankets, and t-shirts [11]. In that study, single polyester garments were found to release as many as 1900 microfibers in a single washing cycle, with polyester fleece garments releasing almost three times as many microfibers as polyester blankets [11], an early indication that fabric construction impacts microfiber release. Hartline et al. explored microfiber release from polyester and nylon jackets using conventional top-loading and front-loading washing machines [33], and found a garment loss of 0.3% of the unwashed garment weights. Sillanpaa and Sainio considered microfiber shedding of polyester and cotton knit fabrics and observed 0.12% loss in weight for the polyester fabrics and a 0.33% loss for the cotton fabrics [34]. The initial wash released the largest mass and number of microfibers, with additional washings releasing less [34]. In a series of studies, Zambrano and collaborators further characterized the release of microfiber in natural and synthetic textiles following accelerated laboratory washings of the garments and while using a traditional washing machine [6,7,35]. The cellulose-based fabrics (e.g., cotton and rayon knits) released more microfibers than the cotton/polyester blend or the polyester knit. Microfiber release increased at higher washing temperatures and with use of detergents. Notably, knits with higher abrasion resistance, lower hairiness, and higher yarn strength released less microfibers. Tao et al. also examined the release of cotton and polyester microfibers following a tumble dry cycle in a household dryer; the air filter system employed to recover released microfibers recovered a higher number of polyester fibers compared to cotton, which lead to an estimate of ~90,000 polyester microfibers released per kg of fabric and ~70,000 cotton microfibers per kg of fabric [36]. A better understanding of the construction and fiber property parameters that might influence microfiber generation and release could lead to the construction of more durable garments which are able to release fewer microfibers when subjected to routine abrasion or laundering cycles.
Comparison across microfiber generation studies is complicated by experimental discrepancies among these studies, including the type of washing machines used, the use of detergents, washing temperature, finishings, fabric selection, cycle duration, microfiber recovery methods, and microfiber quantification methods, among others. For instance, among the studies regarding microfiber release from laundering, several relied on gravimetric examinations alone [33,37], traditional microscope image analysis [11], automated image analysis systems [6,36], or a combination of multiple methods [34] for the microfiber quantification. Additional methods for examining and quantifying microfibers using Fourier transform infrared [36,38] spectroscopy, Raman microspectroscopy [39], and fluorescence spectroscopy [40] have also been reported. While several companies, associations, and standard organizations are presently considering standardizing methodologies for examinations of microfibers, a standard method still has not been established [41].
The OpTest Fiber Quality Analyzer (FQA; OpTest Equipment, Inc., Hawkesbury, ON, Canada) was used in several of the above studies for the quantification of cotton, rayon, and polyester microfibers [6,35]. The FQA is an automated digital image analysis system routinely used for the examination of fibers in pulp industry samples. To achieve this, a water suspension containing the fiber samples is drawn into the sample inlet where it joins two additional streams of filtered water. This combined sample stream then moves to the optical cell in the system where a digital camera captures images of the individual microfibers as they are illuminated with circular polarized light. The system’s image analysis software, LDA17 V1.4; OpTest Equipment, Inc., Hawkesbury, ON, Canada, then provides a summary of fiber number, average length (arithmetic, length weighted, and weight weighted), fines number, and several additional determinations useful in the pulp industry such as sample coarseness and kink and curl index of the examined sample. The system reports length measurements for fibers from 0.05 mm to 15 mm, a range that covers most of the range of microplastics proposed by Frias and Nash [13] (excluding samples between 1 and 5 µm). Typical sample volumes are a 600 mL suspension, of which only ~500 mL are sampled in ~8 min. Since only a portion of the sample is examined by the FQA, a homogeneous microfiber suspension is necessary to minimize sampling bias. Given its ease of use and relative short analysis time, widespread adoption of automated image analysis systems like the FQA could help standardize microfiber quantification studies. Still, as with any examination method, potential drawbacks need to be further explored. As discussed above, proper sample suspension is needed to avoid sampling bias by the FQA system, yet various textile fibers are known for their limited wettability or hydrophobicity [42,43]. Microfiber aggregation in the water suspension could also present challenges to proper sample suspension. Raw cotton fibers, for example, have a natural wax coating that severely restricts their suspension in water. Indeed, the scouring of cotton-based fabrics is essential for the proper uptake of dyes. Similarly, the chemical treatment of cotton fabrics could also impact their hydrophobicity and their ability to properly suspend in water. Are there steps that need to be taken to ensure the proper suspension of textile microfibers? Additionally, cotton fibers are known to differentially absorb polarized light depending on their level of secondary cell wall development, a principle used in the Cottonscope instrument to estimate the maturity of water-suspended cotton fiber cuttings [44]. Thus, the question of whether the circular polarized light system used in the FQA could suffer from differential detection of microfibers based on their maturity is raised.
In the current study, we further explored the use of a FQA system for quantifying microfiber particles of cotton and rayon. For this study, six cotton microfiber samples were selected to better examine the ability of the FQA system to detect cellulose-based fibers with a range of fiber maturity from 0.73 to 0.92 and were prepared from laboratory scoured cotton fibers. The six cotton samples were selected from micronaire standards available from the Agricultural Marketing Service (AMS); these standards are well-blended and well-characterized which will help limit variables in the study. It is hoped that microfiber studies that center on cotton and cellulose based microfibers can help identify ways in which microfiber generation from their garments can be minimized. Additionally, the range of micronaire and maturity values for the standards will help answer if the FQA instrument is impacted by the maturity or micronaire of the microfiber samples. Rayon was selected since a highly uniform and well characterized microfiber sample of the fiber was provided by the instrument manufacturer. Fiber count and fiber lengths were analyzed using statistical models and compared to results obtained for a rayon standard. Additionally, pulse sonication of the microfiber sample suspensions was explored as a means to improve homogeneous sample suspension.
2. Materials and Methods
2.1. Microfiber Samples
The rayon microfiber standard was purchased from OpTest Equipment Inc. (Hawkesbury, ON, Canada) and used without modification. Cotton microfiber samples were prepared from loose cotton fibers from micronaire calibration standards (AM-32, BM-11, CM-129, DM-9, GM-39, and IM-114) obtained from the Agricultural Marketing Service (AMS). Cotton microfibers samples were prepared using a Thomas Model 4 Wiley Mill (Thomas Scientific, Swedesboro, NJ, USA). Microfibers were prepared using the 30 mesh guard. The microfibers from each micronaire standard were passed through a U.S. Standard sieve with ASTM specification of 325 (opening of 44 microns) once to remove shorter microfibers.
2.2. Cotton Fiber Scouring
For the scouring procedure, 5 g of the cotton sample were selected at random from three different locations of the bulk of each calibration standard. The subsample was then blended three times using a Fiber Blender (Custom Scientific Instruments, Easton, PA, USA). The blended sample was transferred to a 1000 mL beaker containing 750 mL of distilled water. A dilute solution of the surfactant Triton-X 100 (1%; Fisher Scientific, Pittsburgh, PA, USA) was added to the beaker solution to improve the wettability of the cotton. The mixture was stirred for 15 min and heated to 95 °C. Next, sodium hydroxide (50% solution, Fisher Scientific) was added to the mixture to reach a 1.75% concentration. The mixture was left stirring for two hours at 95 °C. The treated cotton was then rinsed three times by transferring to beakers containing ~750 mL of warm, distilled water. After rinsing, the cotton was then treated with a 0.125% solution of acetic acid and stirred for 15 min at room temperature. The scoured fibers were then rinsed thoroughly with distilled water, squeezed to remove excess water, and left to air dry overnight. After drying, the treated cotton fibers were again blended three times using the Fiber Blender to obtain a more consistent sample.
2.3. Microfiber Examination with FQA
Microfibers samples were examined with the FQA. For each examination, 0.0020 g of the microfiber sample was weighted in a 20 mL scintillation vial using an analytical balance (Mettler Toledo XSR205DU). Prior to weighing, microfiber samples were allowed to equilibrate for 24 hrs. in a conditioned room at standard cotton testing conditions (21 °C ± 1 °C, 65% relative humidity ± 2%, per ASTM standard method D1776-20). A total of 15 mL of deionized water was added to each vial, and the sample pulsed for 5 min using a 50 W probe sonicator (Fisherbrand model 50, Fisher Scientific, Pittsburgh, PA, USA). The sonication probe was moved in an up and down motion while immersed in the sample suspension, ensuring all parts of the sample were sonicated. The mixed suspension was transferred to a Nalgene plastic beaker with a metric capacity of 600 mL (Nalge Nunc International, Rochester, NY, USA) while ensuring that the scintillation vial was rinsed with an additional 40 mL of deionized water. An additional 545 mL of water was added to the plastic beaker for a total of 600 mL of water. The suspension was mechanically stirred for 2 additional minutes using a stir plate set at 440 RPM (Thermo Scientific model HP88850100, Frequency 50/60 Hz, Thermo Fisher Scientific, Waltham, MA, USA) which was immediately followed by examination with the FQA. At the beginning of each FQA run, the instrument was flushed three times and degassed once. After each examination, the FQA was further flushed and degassed one additional time. A total of 15 samples were examined for each cotton standard. Examinations were performed over 15 randomized runs of each of the 6 cotton standards. Examinations of the rayon standard followed the same procedure described above for the cotton standards. Non-sonicated examinations followed the same general procedure, but the 5 min sonication step was omitted.
2.4. AFIS Cotton Fiber Property Measurements
Length based on weight, L(w), upper quarter length based on weight, UQL(w), short fiber content based on weight, SFC(w), immature fiber content (IFC), maturity ratio, and fiber fineness were measured using the Advanced Fiber Information System (AFIS, USTER Technologies Inc., Knoxville, TN, USA). AFIS examinations were performed under standard cotton testing conditions (see above). Samples were also moved to standard conditions and allowed to equilibrate for 24 h prior to examination. For each examination, a sliver of the cotton material was hand-prepared by the operator and placed in the AFIS sample holder. Cotton samples were examined in three replicates of 3000 fiber examinations and the reported values represent an average of the three examinations. The standard deviation of the three measurements is given as the error.
2.5. Microfiber Characterization
Optical images of the raw and scoured cotton fibers were acquired using an Hirox digital microscope model KH 8700 (Hirox Co., Ltd., Hackensack, NJ, USA) equipped with a revolver zoom lens attachment (MXG-2500REZ, Hirox Co., Ltd.) set to the mid-range lens at a resolution of 1000X.
2.6. Statistical Analysis
FQA test results were extracted from the reports generated by the FQA instrument for each examination using Excel (Excel for Microsoft 365, Version 2304, Microsoft Corporation, Redmond, Washington, DC, USA). Collected data was then transferred into JMP 16.2 (SAS Institute Inc., Cary, NC, USA). One way analysis of variance (ANOVA) with a significance level (alpha) of 0.05 was performed for all non-sonicated samples (seven groups, n = 105) and for all sonicated samples (seven groups, n = 105). ANOVA is a powerful tool that could indicate if the variance observed between cotton samples is significant. A drawback of ANOVA from a large number of cotton types is that it does not identify which cotton varieties are significantly different. As a result, an all-pair comparisons Tukey honestly significant difference test (HSD) with 0.05 alpha- was also performed. Direct comparison between sonicated and non-sonicated samples was deemed to be informative, hence, each cotton standard was compared individually with and without sonication (two groups, n = 30). A series of bivariate plots with a p-value of 0.05 and linear fits were also calculated to explore possible variable correlations. For the connecting letters reports, groups not connected by the same letter are statistically different.
3. Results
A summary of the AFIS fiber property determinations and micronaire values for the six cotton varieties are displayed in Table 1. The cotton samples represent a wide-range of possible micronaire values from 2.6 to 5.48, Table 1. Micronaire is a measurement of the permeability of air through cotton fibers, which can provide an indication of the maturity and fineness of a cotton sample [45,46,47]. Cotton fiber maturity is a unitless measurement that indicates the degree of secondary cell wall thickness in the fiber relative to the fiber perimeter, with numbers closer to 1 representing more developed/mature fibers [44,48]. Maturity ratio is directly proportional to the degree of cell wall thickening [49]. Micronaire standards were used since there are currently no cotton maturity standards available for purchase from AMS. Fineness provides a measurement of the linear density of the fiber, that is, the mass of fiber expected for a certain length [50]. Fineness is typically reported as mTex, or micrograms per meter of fiber. However, it is important to consider that the micronaire scale, and its relationship to maturity and fineness, is not linear [49]. For example, low micronaire values could be due to a cotton variety having fine fibers or from an immature cotton fiber. Conversely, a high micronaire value could arise from a genetically fine fiber with high secondary cell wall development [49,51]. A range of micronaire values, and more importantly, fineness and maturity ratio values could help elucidate the impact of these fiber characteristics on FQA fiber count and length determinations.

Table 1.
Fiber characteristics of cotton standards. Micronaire values obtained from the AMS standard; additional characteristics were obtained using AFIS instrument. AFIS examinations were made on slivers made from unscoured source fibers.
Rankings of the fineness measurements for the six cotton samples mirror those of the maturity ratio and micronaire values, with GM-39 having the smallest values and IM-114 and AM-32 having the highest values for all three fiber traits. Additional length measurements given by the AFIS are listed in Table 1; L(w), UQL(w), and SFC(w). While these length determinations do not reflect the length of the milled microfiber used in the remainder of the study, they can provide a sense of the length consistency of the source cottons. For example, SFC(w) details the percentage of fibers shorter than 12.70 mm. GM-39 and CM-129 show the highest SFC(w), with 13.2 and 12.4%, respectively. The higher SFC values for these samples are partially a reflection of the shorter average length of these cotton varieties, being 22.01 and 23.62 mm for L(w). The AFIS instrument also provides a percentage of immature fiber content (IFC). Both maturity ratio and IFC are determined by the AFIS instrument measuring the circularity of the examined fibers, an indirect measurement of the level of secondary cell wall in the fibers. While maturity ratio considers the ratio of fibers with a circularity higher than 0.5 over those with circularity of 0.25 or lower, IFC measures the percent of fibers with circularity lower than 0.25 [52].
Mechanically stirred suspensions of the scoured cotton microfiber standards showed significantly lower fiber counts than those obtained with a rayon standard. For these determinations, sample suspensions were examined immediately following mechanical stirring (2 min). All-pairs comparisons (Tukey–Kramer HSD test) showed statistically different values for the fiber counts of each of the cotton standards to the rayon fiber counts. Several of the cotton standards, however, were not statistically different and are represented by matching letters in the fiber count column of Table 2. Optical images of the scoured and unscoured source cotton fibers are shown in Figure S1, and do not appear to show significant impact to the fiber surface morphology following scouring.

Table 2.
Fiber count, arithmetic length mean (Ln), and length weighted mean (LW) for rayon standard and microfiber samples of six cotton samples sourced from AMS micronaire standards. Average determinations reflect the examination of 15 randomized replicates each weighing 2 mg. Connecting letters with an alpha of 0.05 are indicated next to each average. Groups not connected by the same letter are statistically different.
A range of Ln and LW mean length values are observed for the six cotton microfiber samples and the rayon standard as calculated by the FQA system using Equations (1) and (2) as described in the FQA instrument manual. The rayon length determinations from the FQA are comparable to the values of the standard reported by its manufacturer: 0.53 ± 0.02 mm and 0.55 ± 0.02 mm for Ln and LW, respectively. While all cotton microfiber samples were prepared using the same Wiley mill mesh, and dispersed through the same standard sieve, the average recorded length varied in a range from 0.41 to 0.55 mm. Bivariate statistical analysis are summarized in Table 3. Ln showed a strong positive correlation to maturity ratio and fineness, also displayed in Figure 1a,b. Strong negative correlations were observed for IFC and SFC(w). Comparison to L(w) also showed a strong positive correlation. Only UQL(w) showed a reduced correlation to Ln. A combination of these fiber characteristics likely explains the range of microfiber lengths seen for the microfiber samples by the FQA system; shorter, finer, more immature fibers are fragmented into smaller microfibers and more easily pass through the Wiley mill mesh, reducing their mean length measurements.
where i stands for the number of categories, n stands for the fiber number in the specified category, and L stand for the contour length measure for the sample in each category.

Table 3.
Bivariate correlations (Pearson’s r value) for Ln and five fiber properties.
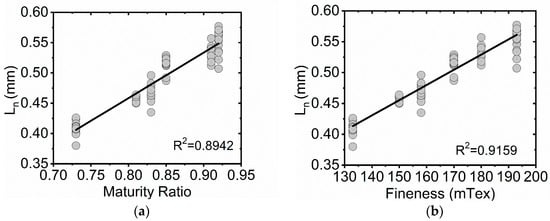
Figure 1.
Bivariate plots of microfiber Ln (arithmetic mean) values for six cotton standards (FQA) versus maturity ratio and fineness determinations (AFIS). (a) Ln as a function of maturity ratio for 90 mechanically stirred cotton microfiber suspensions. (b) Ln as a function of fineness for 90 mechanically stirred cotton microfiber suspensions. A linear fit (black trace) is displayed in each plot.
The statistically different fiber counts for the cotton standards and Rayon samples are not surprising since factors like the linear density and mean fiber length impact how many microfibers would be expected in a 2 mg sample of the cotton microfibers. Still, the high percent coefficient of variation (%CV) for the cotton microfiber measurements (19%, on average) raises concerns that the microfibers were not being properly sampled by the FQA. In contrast, the %CV for the rayon standard was 4%. Visual examination of the suspended cotton standard microfiber samples following mechanical stirring revealed aggregates of microfibers near the surface of the sampling beaker, Figure 2a. As discussed above, the fiber suspension inlet (clear tube in Figure 2b) uptakes the suspension until the water level reaches the second longest metal probe in the assembly, while the longest probe serves to detect the presence of the sample beaker. When 600 mL of a water suspension is placed in the sample beaker, the system examines about 500 mL of the sample, at which point the water level reaches the second probe, and the examination stops. Since the sample suspension inlet uptakes sample from the bottom of the container, samples that aggregate on the surface of the sample beaker are being undercounted. Given the aggregates observed in the cotton microfiber samples, steps need to be taken to maximize proper sample suspension prior to FQA examination.
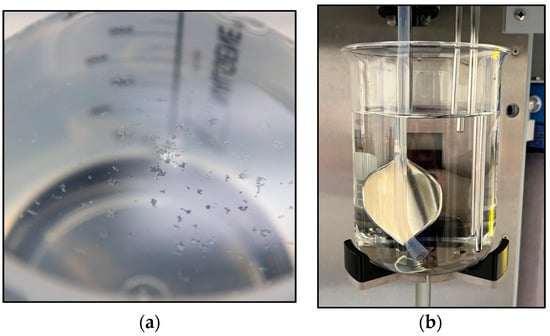
Figure 2.
(a) Cotton microfiber aggregates observed near the surface of a mechanically stirred microfiber suspension. (b) Sample holder for the FQA system displaying the sample inlet and probes for detecting the sample beaker and water level.
Probe sonication significantly increased the fiber counts detected by the FQA for the six cotton standards, Table 4. On average, the cotton standards saw a 105% increase in fiber counts following sonication. One-way ANOVA tests comparing the sonicated fiber counts of each cotton standard to its non-sonicated counterpart showed a significant difference for all fiber counts. Notably, no aggregates of the cotton microfibers were observed following sonication and mechanical stirring. It is important to note that not all fiber counts were significantly impacted by the additional step; sonication of the rayon standard resulted in only a 2% fiber count increase. Bivariate statistical analysis supports negative correlations for the sonicated fiber counts and the maturity ratio of the sonicated cotton standards, fineness, and Lw, Table 3 and Figure 3a,b. Additionally there was strong positive correlation to IFC and SFC(w).

Table 4.
Fiber count, arithmetic length mean (Ln), length weighted length mean (LW), and percent detection for microfiber samples of rayon standard and six cotton samples sourced from AMS micronaire standards following probe sonication. Average determinations reflect the examination of 15 randomized replicates, each weighing 2 mg. Connecting letters with an alpha of 0.05 are indicated next to each average. Groups not connected by the same letter are statistically different.
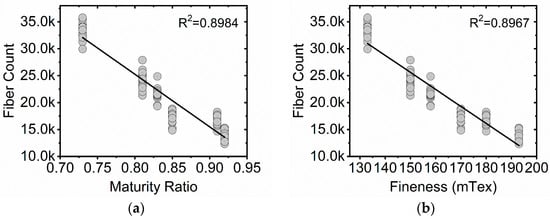
Figure 3.
Bivariate plots of fiber counts values for six cotton standards (FQA) versus maturity ratio and fineness determinations (AFIS). (a) Fiber count as a function of maturity ratio for 90 sonicated cotton microfiber suspensions. (b) Fiber count as a function of fineness for 90 sonicated cotton microfiber suspensions. A linear fit (black trace) is displayed in each plot.
While the changes in Ln measurements for the sonicated vs. non-sonicated cotton standard samples were small (five of the cotton showed a reduction in length of 5% or less, while one sample saw a small increase in length of 2%), one-way ANOVA pair tests revealed the differences to be significant for CM-129, DM-9, GM-39, and IM-114. Plots displaying the relative distribution of Ln values for six cotton standards as measured with the FQA instrument with and without sonication are shown in Figure 4, and except for small discrepancies, the histograms of the sonicated samples show similar relative distributions to their corresponding non-sonicated counterparts. Similarly, LW determinations showed small changes over the non-sonicated measurements. The limited change in fiber length determinations and the similarity in Ln distribution plots strongly suggest that the changes in fiber counts observed following sonication were not due to widespread damage or fragmentation of the microfibers by the probe sonicator.
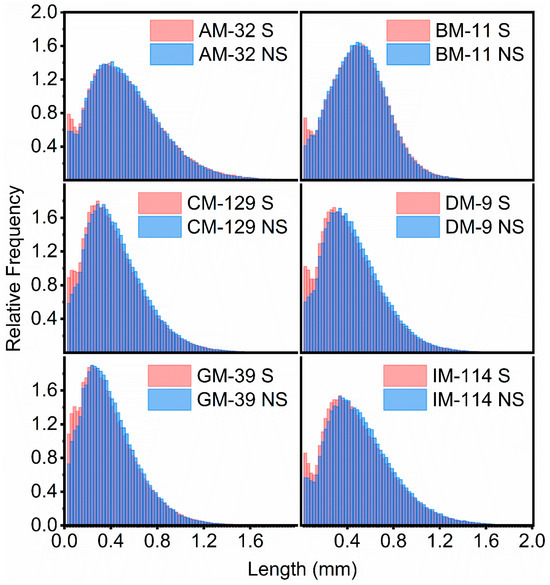
Figure 4.
Relative distribution of microfiber length (Ln) values for six cotton standards as measured with the FQA instrument: AM-32 (top left), BM-11 (top right), CM-129 (middle left), DM-9 (middle right), GM-39 (bottom left), and IM-114 (bottom right). Each relative distribution displays the length (Ln) measurements observed for 15 microfiber samples (2 mg) that were sonicated prior to examination (light red histograms) or without sonication (light blue histograms). The darker blue histogram columns represent areas of overlap between the sonicated and non-sonicated distributions.
The overall weight of the microfiber sample being sampled by the FQA system can be estimated based on the total length of microfibers detected by the instrument, Equation (3). The system achieved, on average, 95% of the expected detection for all the sonicated cotton standards with detection levels that ranged between 88% for AM-32 and 107% for GM-39, Figure 5 and Table 4. While this estimate relies on AFIS measurement of fineness, it can provide a sense of the detection efficiency for each of the cotton microfiber standards. ANOVA tests show considerable overlap among five of the microfiber samples, with two groups of four being represented by the same letter, Table 4. GM-39, however, stands as being significantly different than all other cotton samples in its percent detection. A plotting of the percent microfiber weight detected by the FQA as a function of maturity ratio, Figure 5b, shows a Pearson’s correlation value of −0.6619. Notably, GM-39 has a higher relative frequency for the shortest microfibers than the other five cotton standards.
where
and
where equals percent microfiber weight detected, equals total microfiber weight, and equals total microfiber length.
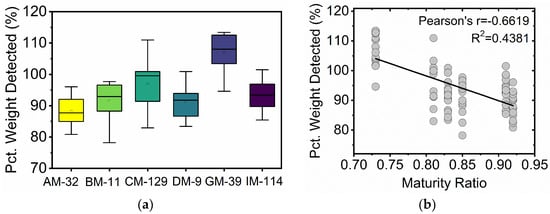
Figure 5.
(a) Box plot analysis depicting the percent weight detected for the six cotton microfiber standards. (b) Percent weight detected as a function of the maturity ratio for 90 sonicated cotton microfiber suspensions.
While the FQA experiment report details the length of all detected fibers, the reliability of the instrument for shorter fibers is greatly reduced. For example, fibers longer than 0.07 mm are reported in increments of 0.001 mm, while fiber shorter than 0.07 mm are reported in increments of 0.007 mm. This instrument limitation is not unique to the FQA; lower reliability is expected of many instruments at their lower and higher detection limits. It is also unclear if this limitation severe impacts the observed results in this study, but it allows for the possibility that the higher percent detection for GM-39 could in part be due to the FQA assigning longer fiber lengths for fibers shorter than 0.07 mm in the sample. It is reasonable to expect such a bias to impact GM-39 the most given its relatively higher incidence of very short microfibers, Figure 4. Taken together, these results suggests that while the FQA system appears biased towards detection of microfibers from GM-39, it is unclear if the higher detection was influenced by sample maturity or the relatively higher incidence of very short microfibers (shorter than 0.07 mm). Percent detection for the other fibers did not appear dependent on fiber maturity.
4. Conclusions
The FQA system can be used for the quick examination of cotton and rayon microfiber samples in a water suspension, an analysis that provides important measurements such as microfiber counts and mean length measurements. Sample preparation was minimal; it comprised probe sonication for 5 min followed by mechanical stirring. The pulse sonication step was first explored in the present study, and it increased the fiber count of the cotton standard microfibers examined on average by 105%. ANOVA tests confirmed that the observed fiber count increases were statistically significant for all the cotton standards examined. Only a small change in microfiber lengths was registered for the cotton samples. This observation coupled with the overlapping proportional length distribution of the microfibers suggests that the significant increases in fiber counts observed were not the result of widespread sample fragmentation following sonication. On average, the FQA system appeared to detect 95% of the microfibers in the 90 cotton standard microfiber suspensions. While the detection levels for five of the samples ranged between 88 and 96%, a range of 8 percentage points, the GM-39 sample saw a statistically higher detection percentage of 107%.
While previous reports have documented the use of the FQA system for the characterization of an array of natural and synthetic textile microfibers, the current study highlights the need for proper microfiber suspension to obtain more reliable microfiber counts from the system. As research efforts to quantify microfibers generated from textile garments further expand, it will be important to undertake similar validation steps to identify possible limitations of these quantification systems and methods. Such considerations should not be unique to examinations with the FQA system; proper suspension of synthetic and natural microfibers in water is essential for their proper sampling and quantification.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fib12100081/s1, Figure S1: Optical microscopy images of six cotton micronaire standards following scouring and raw (no scouring).
Author Contributions
M.S.C. conceptualized the project, designed the methodology, performed or supervised the experiments, executed the statistical analysis, and is the principal author of the manuscript; C.D.D. conceptualized and designed the project, provided research materials, provided critical review and editing of the manuscript, and supervised planning and execution of the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors acknowledge Mary Ann Ankeny, Phoebe Zito, Matthew Lebar and Soojin Kwon for insightful comments on the manuscript. Acknowledgements are also made to Holly King for AFIS examinations and Chanel Fortier for performing FQA microfiber determinations. The findings and conclusions in this publication are those of the author(s) and should not be construed to represent any official USDA or U.S. Government determination or policy.
Conflicts of Interest
The authors declare no conflicts of interest. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture (USDA). USDA is an equal opportunity provider and employer.
References
- Devi, A.; Hansa, A.; Gupta, H.; Syam, K.; Upadhyay, M.; Kaur, M.; Lajayer, B.A.; Sharma, R. Microplastics as an emerging menace to environment: Insights into their uptake, prevalence, fate, and sustainable solutions. Environ. Res. 2023, 229, 115922. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.; Laitala, K.; Klepp, I.G. Microfibres from apparel and home textiles: Prospects for including microplastics in environmental sustainability assessment. Sci. Total Environ. 2019, 652, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.; Nowack, B.; Mitrano, D.M. Polyester Textiles as a Source of Microplastics from Households: A Mechanistic Study to Understand Microfiber Release during Washing. Environ. Sci. Technol. 2017, 51, 7036–7046. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Hong, S.H.; Eo, S. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Cheng, J.J.; Venditti, R.A. Microfibers generated from the laundering of cotton, rayon and polyester based fabrics and their aquatic biodegradation. Mar. Pollut. Bull. 2019, 142, 394–407. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Venditti, R.A. Impact of dyes and finishes on the aquatic biodegradability of cotton textile fibers and microfibers released on laundering clothes: Correlations between enzyme adsorption and activity and biodegradation rates. Mar. Pollut. Bull. 2021, 165, 112030. [Google Scholar] [CrossRef]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Anselmi, S.; Provenza, F.; Bentivoglio, T.; Picerno, G.; Cavallo, A.; Renzi, M. Marine Biodegradability and Ecotoxicity of MWool® Recycled Wool Fibers: A Circular-Economy-Based Material. Oceans 2023, 4, 114–131. [Google Scholar] [CrossRef]
- Belzagui, F.; Gutiérrez-Bouzán, C.; Álvarez-Sánchez, A.; Vilaseca, M. Textile microfibers reaching aquatic environments: A new estimation approach. Environ. Pollut. 2020, 265, 114889. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.P.W.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Ryan, P.G. A Brief History of Marine Litter Research. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar]
- Barrows, A.P.W.; Cathey, S.E.; Petersen, C.W. Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Wei, N.; Song, Z.; Li, D. Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: Implications for human health. Environ. Int. 2019, 132, 105127. [Google Scholar] [CrossRef]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef] [PubMed]
- Isobe, A.; Azuma, T.; Cordova, M.R.; Cózar, A.; Galgani, F.; Hagita, R.; Kanhai, L.D.; Imai, K.; Iwasaki, S.; Kako, S.i.; et al. A multilevel dataset of microplastic abundance in the world’s upper ocean and the Laurentian Great Lakes. Microplast. Nanoplast. 2021, 1, 16. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017. [Google Scholar]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. 2018, 25, 36046–36063. [Google Scholar] [CrossRef] [PubMed]
- Biamis, C.; Driscoll, K.O.; Hardiman, G. Microplastic toxicity: A review of the role of marine sentinel species in assessing the environmental and public health impacts. Case Stud. Chem. Environ. Eng. 2021, 3, 100073. [Google Scholar] [CrossRef]
- Akanyange, S.N.; Lyu, X.; Zhao, X.; Li, X.; Zhang, Y.; Crittenden, J.C.; Anning, C.; Chen, T.; Jiang, T.; Zhao, H. Does microplastic really represent a threat? A review of the atmospheric contamination sources and potential impacts. Sci. Total Environ. 2021, 777, 146020. [Google Scholar] [CrossRef]
- Sørensen, L.; Rogers, E.; Altin, D.; Salaberria, I.; Booth, A.M. Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions. Environ. Pollut. 2020, 258, 113844. [Google Scholar] [CrossRef]
- Sharma, M.D.; Elanjickal, A.I.; Mankar, J.S.; Krupadam, R.J. Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2020, 398, 122994. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Tan, C.J.; Andriyana, A.; Ang, B.C.; Wong, D. Mechanical deformation and fracture mechanisms of polymeric fibres from the perspective of fractography—A review. Eur. Polym. J. 2020, 137, 109924. [Google Scholar] [CrossRef]
- Hartline, N.L.; Bruce, N.J.; Karba, S.N.; Ruff, E.O.; Sonar, S.U.; Holden, P.A. Microfiber Masses Recovered from Conventional Machine Washing of New or Aged Garments. Environ. Sci. Technol. 2016, 50, 11532–11538. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Sainio, P. Release of polyester and cotton fibers from textiles in machine washings. Environ. Sci. Pollut. Res. 2017, 24, 19313–19321. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Venditti, R.A. Impact of dyes and finishes on the microfibers released on the laundering of cotton knitted fabrics. Environ. Pollut. 2021, 272, 115998. [Google Scholar] [CrossRef]
- Tao, D.; Zhang, K.; Xu, S.; Lin, H.; Liu, Y.; Kang, J.; Yim, T.; Giesy, J.P.; Leung, K.M.Y. Microfibers Released into the Air from a Household Tumble Dryer. Environ. Sci. Technol. Lett. 2022, 9, 120–126. [Google Scholar] [CrossRef]
- Pirc, U.; Vidmar, M.; Mozer, A.; Kržan, A. Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ. Sci. Pollut. Res. 2016, 23, 22206–22211. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Kuczera, M.; Mintenig, S.; Lorenz, C.; Gerdts, G. Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ. Chem. 2015, 12, 563–581. [Google Scholar] [CrossRef]
- Zhao, S.; Danley, M.; Ward, J.E.; Li, D.; Mincer, T.J. An approach for extraction, characterization and quantitation of microplastic in natural marine snow using Raman microscopy. Anal. Methods 2017, 9, 1470–1478. [Google Scholar] [CrossRef]
- Scircle, A.; Cizdziel, J.V. Detecting and Quantifying Microplastics in Bottled Water using Fluorescence Microscopy: A New Experiment for Instrumental Analysis and Environmental Chemistry Courses. J. Chem. Educ. 2020, 97, 234–238. [Google Scholar] [CrossRef]
- Ramasamy, R.; Subramanian, R.B. Synthetic textile and microfiber pollution: A review on mitigation strategies. Environ. Sci. Pollut. Res. 2021, 28, 41596–41611. [Google Scholar] [CrossRef]
- Volgare, M.; De Falco, F.; Avolio, R.; Castaldo, R.; Errico, M.E.; Gentile, G.; Ambrogi, V.; Cocca, M. Washing load influences the microplastic release from polyester fabrics by affecting wettability and mechanical stress. Sci. Rep. 2021, 11, 19479. [Google Scholar] [CrossRef]
- Allam, O.G. Recent Developments on Wettability Treatment of Wool and Polyester Textiles. Egypt. J. Chem. 2019, 62, 1185–1195. [Google Scholar] [CrossRef]
- Rodgers, J.; Delhom, C.; Fortier, C.; Thibodeaux, D. Rapid measurement of cotton fiber maturity and fineness by image analysis microscopy using the Cottonscope®. Text. Res. J. 2011, 82, 259–271. [Google Scholar] [CrossRef]
- Montalvo, J.G. Relationships Between Micronaire, Fineness, and Maturity. Part I. Fundamentals. J. Cotton Sci. 2005, 9, 81–88. [Google Scholar]
- Hequet, E.F.; Wyatt, B.; Abidi, N.; Thibodeaux, D.P. Creation of a Set of Reference Material for Cotton Fiber Maturity Measurements. Text. Res. J. 2006, 76, 576–586. [Google Scholar] [CrossRef]
- Lord, E. 2—Air Flow Through Plugs of Textile Fibres. J. Text. Inst. Trans. 1956, 47, T16–T47. [Google Scholar] [CrossRef]
- Thibodeaux, D.P.; Rajasekaran, K. Development of New Reference Standards for Cotton Fiber Maturity. J. Cotton. Sci. 1999, 3, 188–193. [Google Scholar]
- Paudel, D.R.; Hequet, E.F.; Abidi, N. Evaluation of cotton fiber maturity measurements. Ind. Crops. Prod. 2013, 45, 435–441. [Google Scholar] [CrossRef]
- Leitgeb, D.J.; Wakeham, H. Cotton Quality and Fiber Properties: Part V: Effects of Fiber Fineness. Text. Res. J. 1956, 26, 543–552. [Google Scholar] [CrossRef]
- Hsieh, Y.-L. Structural Development of Cotton Fibers and Linkages to Fiber Quality. In Cotton Fibers; Basra, A.S., Ed.; Hawthorne Press Inc.: New York, NY, USA, 1999; pp. 137–144. [Google Scholar]
- Kim, H.J.; Rodgers, J.; Delhom, C.; Cui, X. Comparisons of methods measuring fiber maturity and fineness of Upland cotton fibers containing different degrees of fiber cell wall development. Text. Res. J. 2014, 84, 1622–1633. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).