Aloe vera Rind Valorization to Improve the Swelling Capacity of Commercial Acrylic Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Vegetal Waste
2.3. Nanofiber Obtention
2.3.1. Obtention of Native Nanofibers
2.3.2. Nanofibers Production by Acid Treatment Combined with Ultrasound
2.4. Characterization of the Nanofibers
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Infrared Analysis (FITR)
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. Transmission Electron Microscopy (TEM)
2.5. Hydrogels Reinforcement
2.5.1. Swelling Study
2.5.2. Scanning Electron Microscopy (SEM)
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
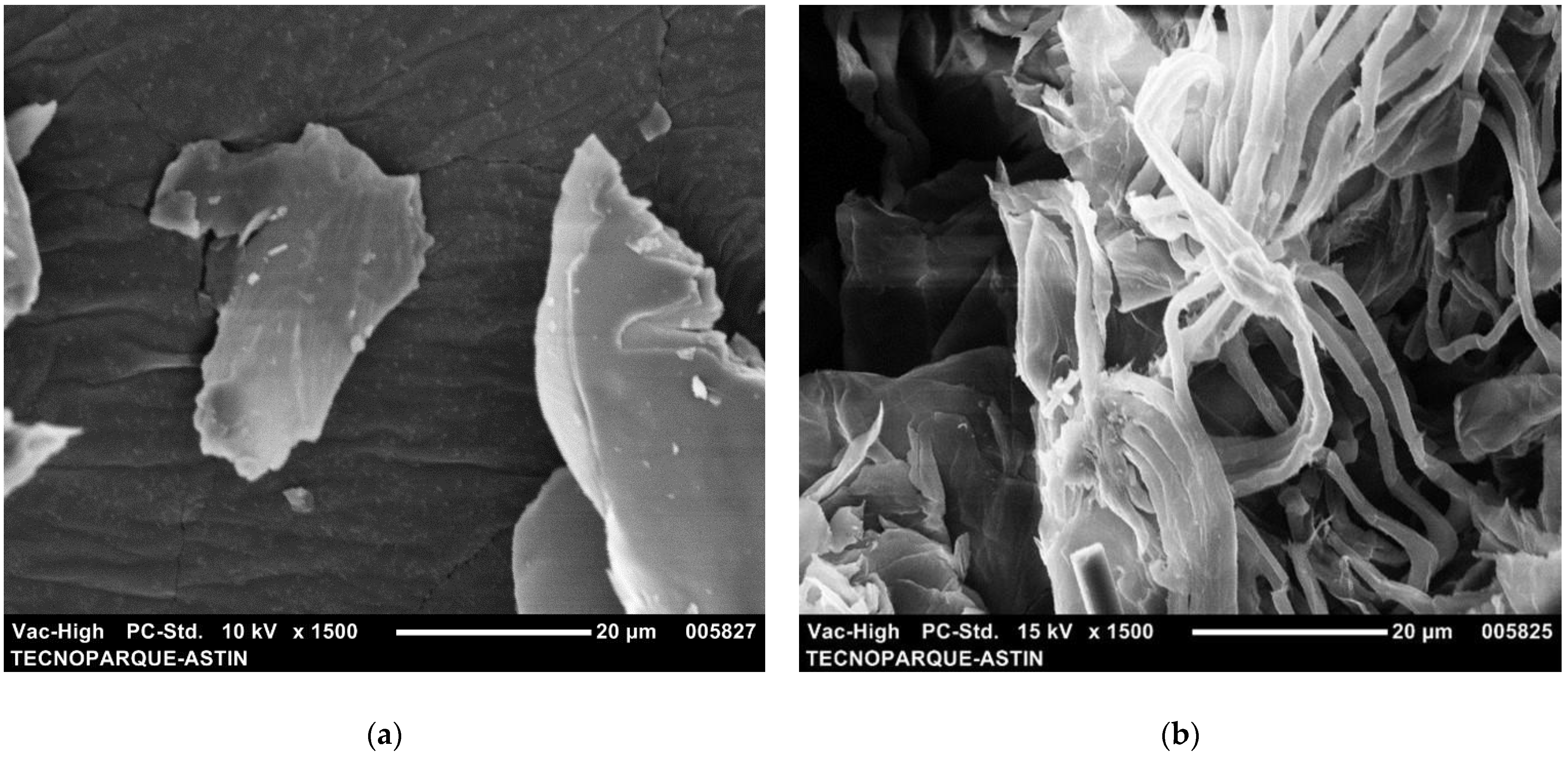
3.1. Morphology of Cellulose Nanofibers
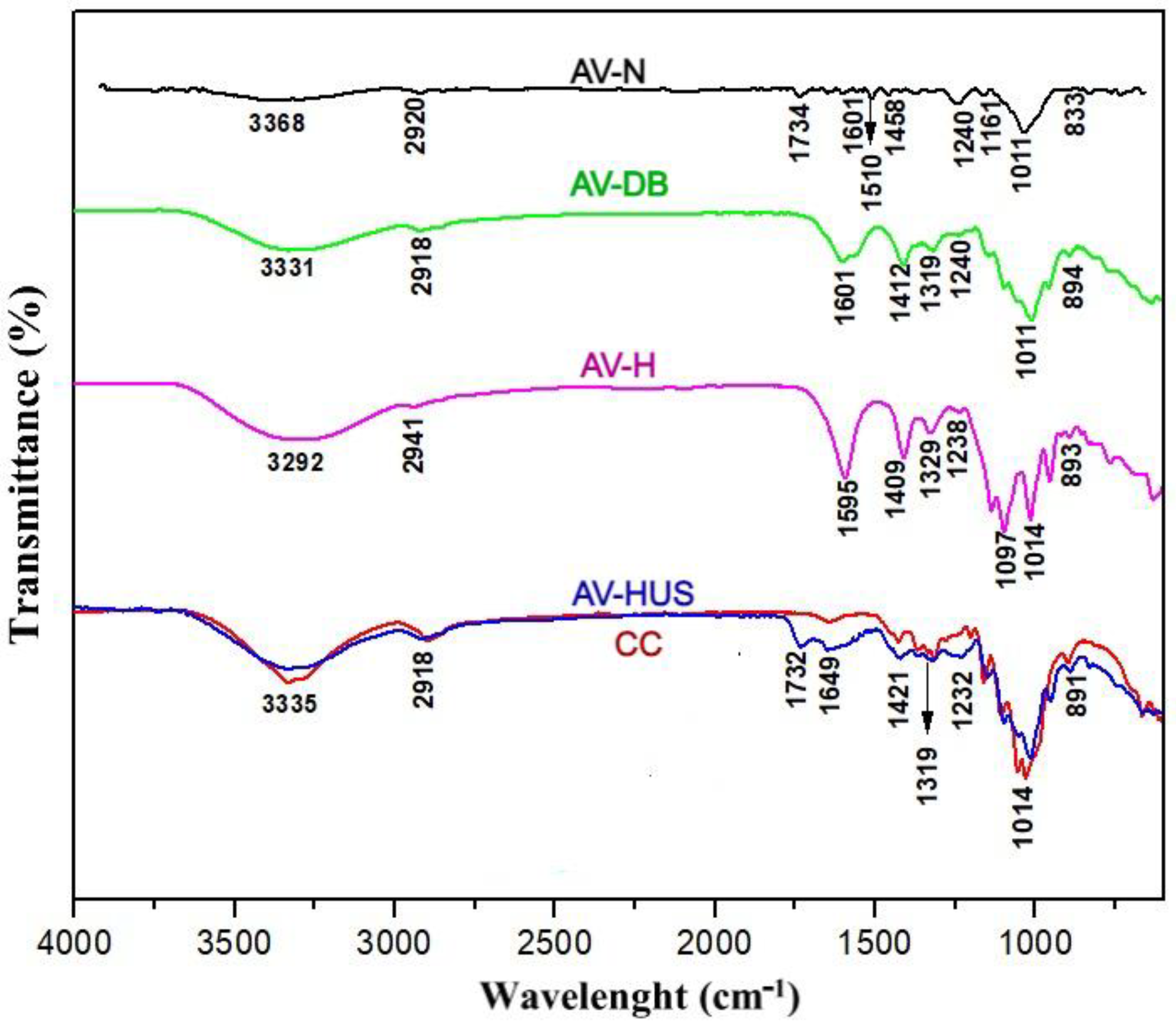
3.2. Functional Groups of Cellulose Nanofibers
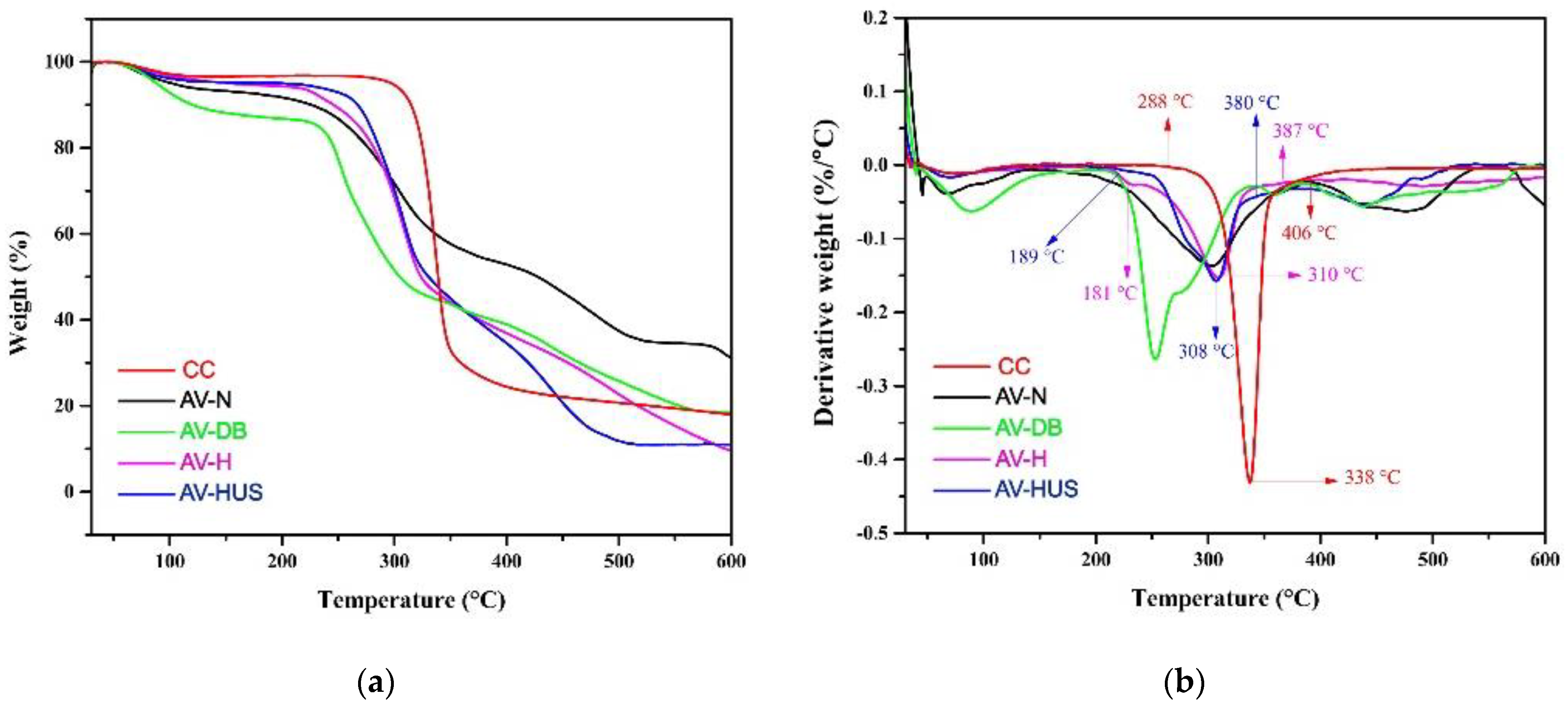
3.3. Thermal Stability Analysis of Cellulose Nanofibers
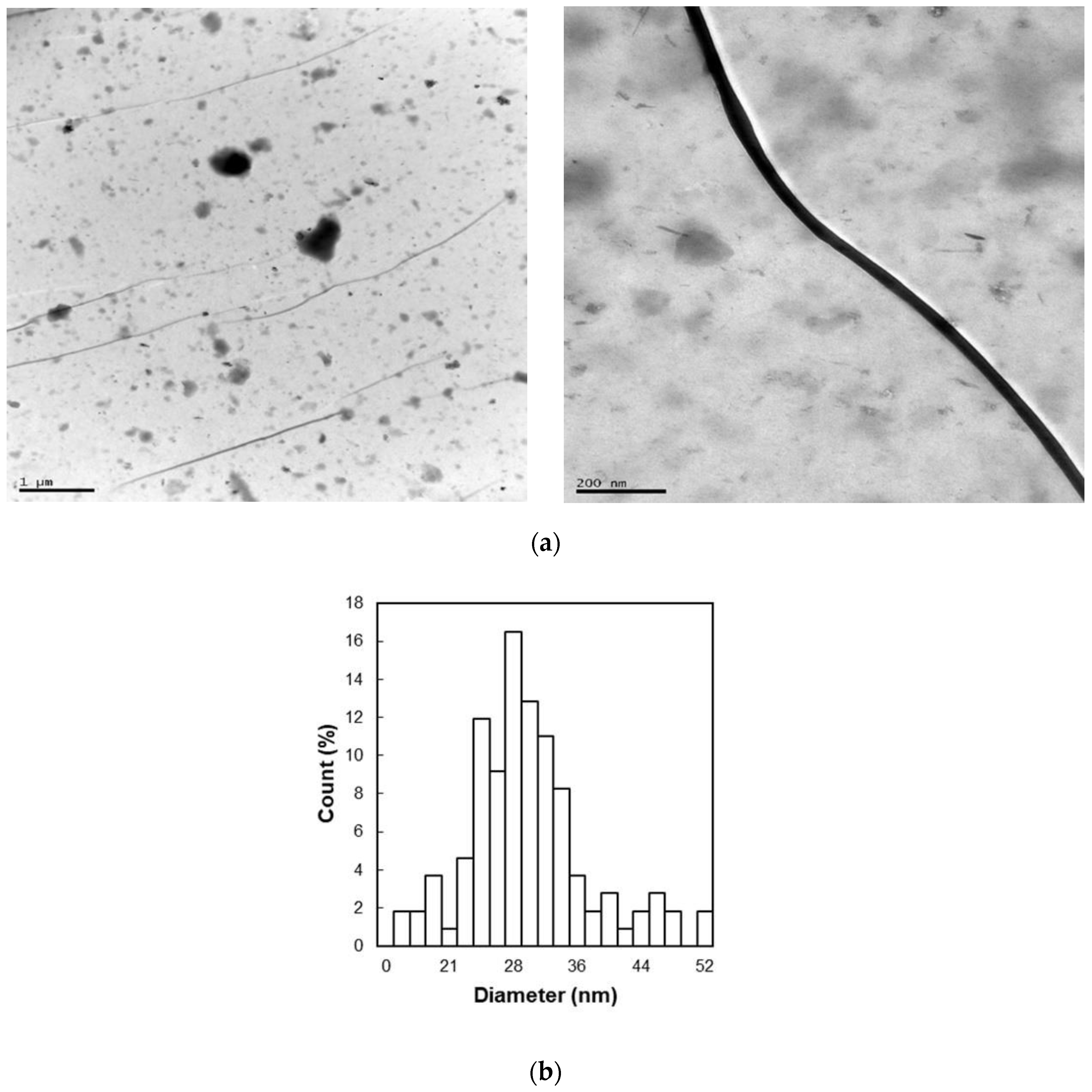
3.4. Particle Size Analysis of Cellulose Nanofibers
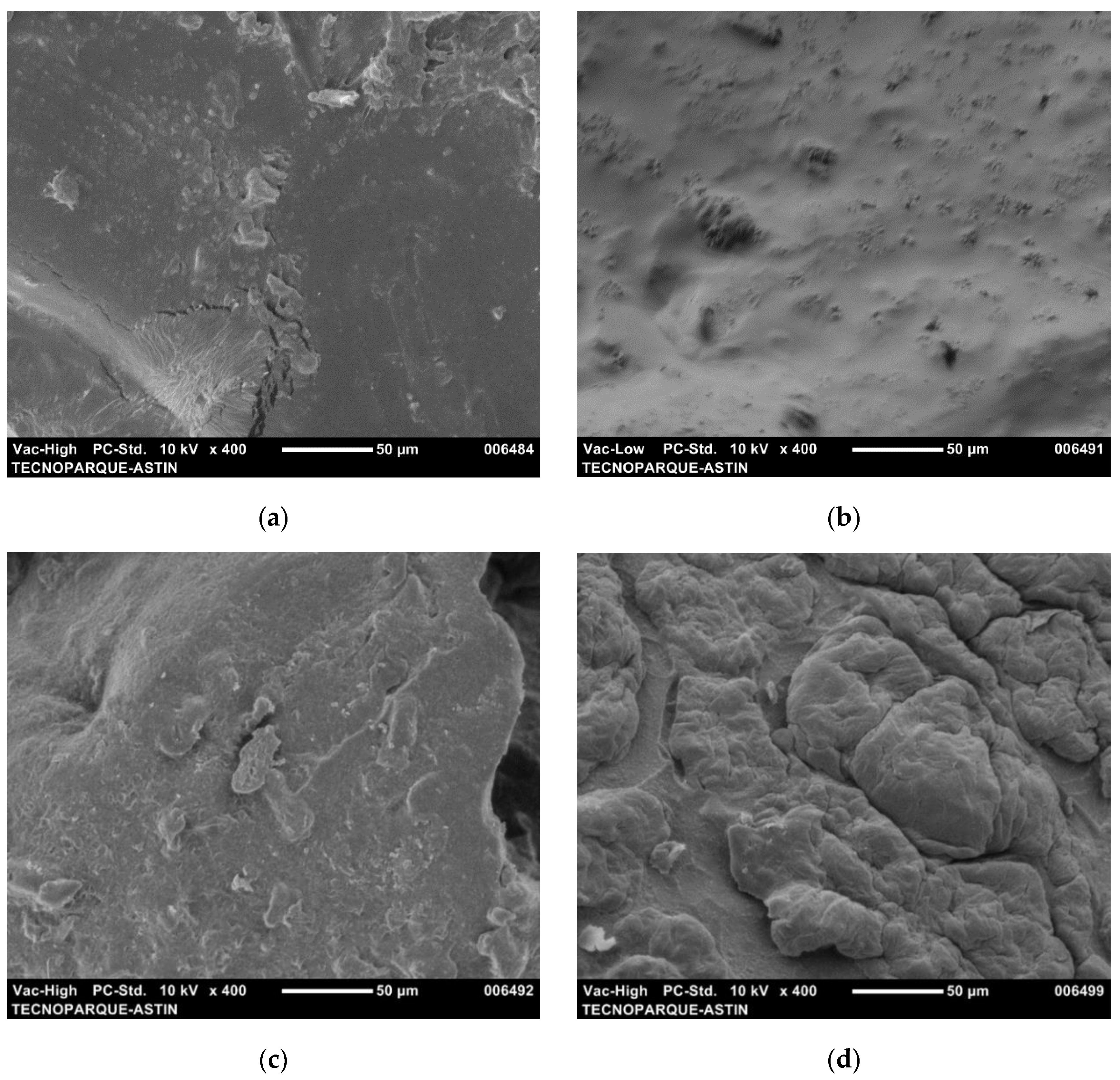
3.5. Morphology of Reinforced Hydrogels
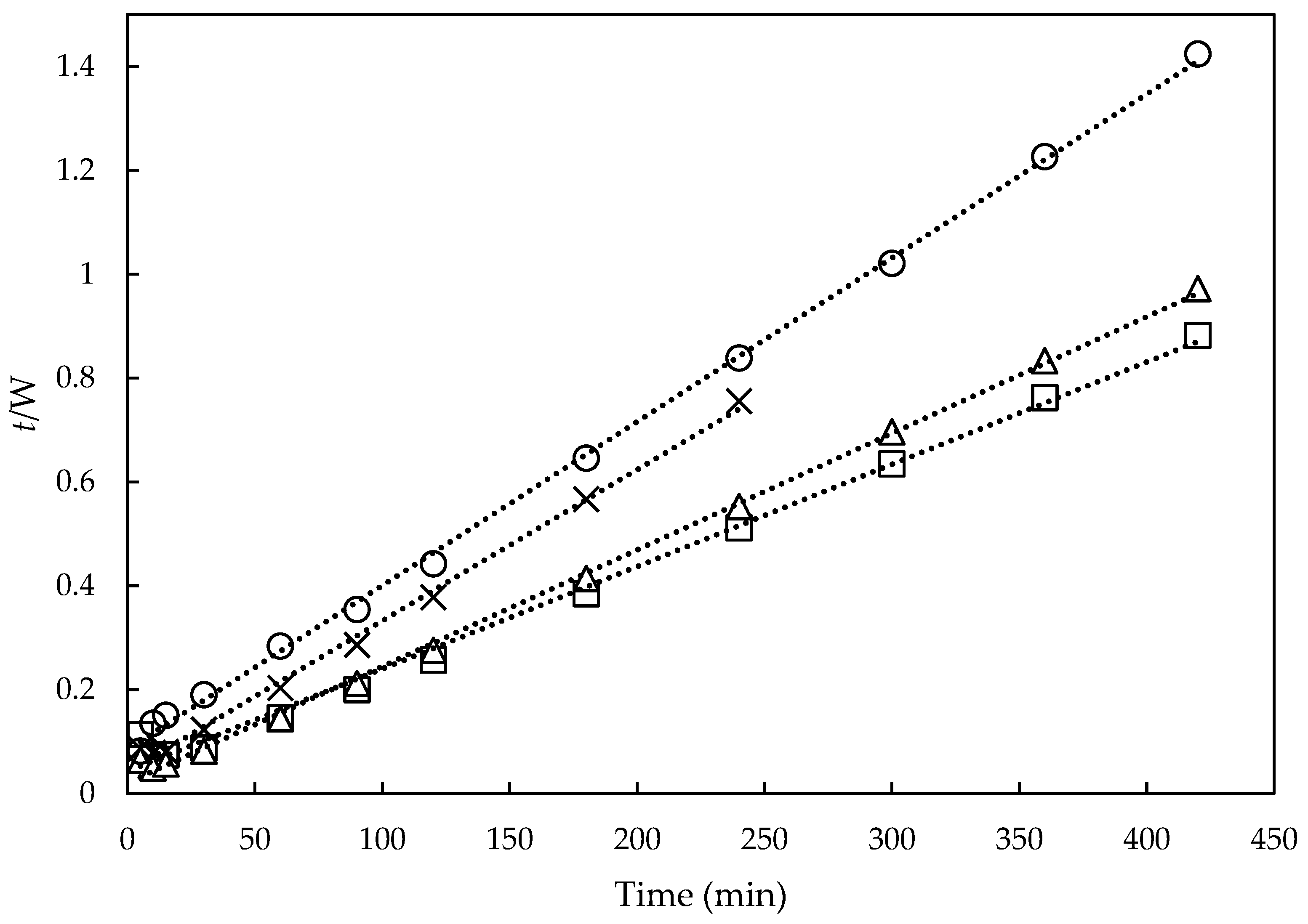
3.6. Swelling Capacity and Kinetics of Reinforced Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohd Kanafi, N.; Abdul Rahman, N.; Rosdi, N.H.; Bahruji, H.; Maarof, H. Hydrogel nanofibers from carboxymethyl sago pulp and its controlled release studies as a methylene blue drug carrier. Fibers 2019, 7, 56. [Google Scholar] [CrossRef]
- Cakir Hatir, P. Light-induced hydrogels derived from poly(ethylene glycol) and acrylated methyl ricinoleate as biomaterials. J. Appl. Polym. Sci. 2022, 139, e52754. [Google Scholar] [CrossRef]
- Ling, Y.; Chen, L.; Huang, M.; Zhou, C.; Yang, L.; Niu, H.; Su, L.; Yang, Y.; Pirraco, R.P.; Reis, R.L.; et al. A novel method for the preparation of poly (acrylamide−co−acrylonitrile) upper critical solution temperature thermosensitive hydrogel by the partial dehydration of acrylamide grafted polypropylene sheets. Gels 2022, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-containing wound dressing: Antimicrobial and healing effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef]
- Xie, Y.; Guan, Q.; Guo, J.; Chen, Y.; Yin, Y.; Han, X. Hydrogels for exosome delivery in biomedical applications. Gels 2022, 8, 328. [Google Scholar] [CrossRef]
- Goulis, P.; Kartsonakis, I.A.; Charitidis, C.A. Synthesis and characterization of a core-shell copolymer with different glass transition temperatures. Fibers 2020, 8, 71. [Google Scholar] [CrossRef]
- Wu, R.; Niamat, R.; Sansbury, B.; Borjigin, M. Fabrication and evaluation of multilayer nanofiber-hydrogel meshes with a controlled release property. Fibers 2015, 3, 296–308. [Google Scholar] [CrossRef]
- Jin, S.; Kim, Y.; Son, D.; Shin, M. Tissue adhesive, conductive, and injectable cellulose hydrogel ink for on-skin direct writing of electronics. Gels 2022, 8, 336. [Google Scholar] [CrossRef]
- Lubasova, D.; Netravali, A.N. A novel method for electrospinning nanofibrous 3-D structures. Fibers 2020, 8, 27. [Google Scholar] [CrossRef]
- Guancha-Chalapud, M.A.; Serna-Cock, L.; Tirado, D.F. Hydrogels are reinforced with Colombian fique nanofibers to improve techno-functional properties for agricultural purposes. Agriculture 2022, 12, 117. [Google Scholar] [CrossRef]
- Pérez-Blanco, C.D.; Hrast-Essenfelder, A.; Perry, C. Irrigation technology and water conservation: A review of the theory and evidence. Rev. Environ. Econ. Policy 2020, 14, 216–239. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects the 2017 Revision: Key Findings and Advance Tables; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Serna Cock, L.; Guancha-Chalapud, M.A. Natural fibers for hydrogels production and their applications in agriculture. Acta Agronómica 2017, 66, 495–505. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.; Pereira, A.; Fajardo, A.; Rubira, A.; Muniz, E. Superabsorbent hydrogel composite made of cellulose nanofibrils and chitosan-graft-poly(acrylic acid). Carbohydr. Polym. 2012, 87, 2038–2045. [Google Scholar] [CrossRef]
- Markets and MarketsTM Research Private Ltd. Hydrogel Market. Available online: https://www.marketsandmarkets.com/Market-Reports/hydrogel-market-181614457.html (accessed on 5 June 2022).
- Joseph, O.O.; Babaremu, K.O. Agricultural waste as a reinforcement particulate for aluminum metal matrix composite (AMMCs): A review. Fibers 2019, 7, 33. [Google Scholar] [CrossRef]
- Cheng, S.; Panthapulakkal, S.; Sain, M.; Asiri, A. Aloe vera rind cellulose nanofibers-reinforced films. J. Appl. Polym. Sci. 2014, 131, 40592. [Google Scholar] [CrossRef]
- Ramezani Kakroodi, A.; Cheng, S.; Sain, M.; Asiri, A. Mechanical, thermal, and morphological properties of nanocomposites based on polyvinyl alcohol and cellulose nanofiber from Aloe vera rind. J. Nanomater. 2014, 2014, 903498. [Google Scholar] [CrossRef]
- Guancha-Chalapud, M.A.; Gálvez, J.; Serna-Cock, L.; Aguilar, C.N. Valorization of Colombian fique (Furcraea bedinghausii) for production of cellulose nanofibers and its application in hydrogels. Sci. Rep. 2020, 10, 11637. [Google Scholar] [CrossRef]
- Guancha-Chalapud, M.A.; Serna-Cock, L.; Tirado, D.F. Valorization of pineapple residues from the colombian agroindustry to produce cellulose nanofibers. Appl. Sci. 2022, 12, 6956. [Google Scholar] [CrossRef]
- Zhong, K.; Zheng, X.-L.; Mao, X.-Y.; Lin, Z.-T.; Jiang, G.-B. Sugarcane bagasse derivative-based superabsorbent containing phosphate rock with water–fertilizer integration. Carbohydr. Polym. 2012, 90, 820–826. [Google Scholar] [CrossRef]
- Karadağ, E.; Bariş Üzüm, Ö.; Saraydin, D. Water uptake in chemically crosslinked poly(acrylamide-co-crotonic acid) hydrogels. Mater. Des. 2005, 26, 265–270. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.; Neto, A.G.V.C.; Pereira, A.G.B.; Fajardo, A.R.; Radovanovic, E.; Rubira, A.F.; Muniz, E.C. Nanocomposites based on poly(acrylamide-co-acrylate) and cellulose nanowhiskers. Eur. Polym. J. 2012, 48, 454–463. [Google Scholar] [CrossRef]
- Schott, H. Swelling kinetics of polymers. J. Macromol. Sci. Part B 1992, 31, 1–9. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.-Y.; Li, C.; Shupe, T.F.; Hu, T.; Qi, J.; De Hoop, C.F. Characterization of microwave liquefied bamboo residue and its potential use in the generation of nanofibrillated cellulosic fiber. ACS Sustain. Chem. Eng. 2016, 4, 3477–3485. [Google Scholar] [CrossRef]
- Célino, A.; Fréour, S.; Jacquemin, F.; Casari, P. The hygroscopic behavior of plant fibers: A review. Front. Chem. 2014, 1, 43. [Google Scholar] [CrossRef] [PubMed]
- Julie Chandra, C.S.; George, N.; Narayanankutty, S.K. Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohydr. Polym. 2016, 142, 158–166. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Zhang, P.P.; Tong, D.S.; Lin, C.X.; Yang, H.M.; Zhong, Z.K.; Yu, W.H.; Wang, H.; Zhou, C.H. Effects of acid treatments on bamboo cellulose nanocrystals. Asia-Pacific J. Chem. Eng. 2014, 9, 686–695. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.; De Hoop, C.F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Song, Y.K.; Leng Chew, I.M.; Yaw Choong, T.S.; Tan, J.; Tan, K.W. Isolation of Nanocrystalline Cellulose from oil palm empty fruit bunch—A response surface methodology study. MATEC Web Conf. 2016, 60, 04009. [Google Scholar] [CrossRef]
- Vieyra, H.; Figueroa-López, U.; Guevara-Morales, A.; Vergara-Porras, B.; San Martín-Martínez, E.; Aguilar-Mendez, M.Á. Optimized monitoring of production of cellulose nanowhiskers from Opuntia ficus-indica (nopal cactus). Int. J. Polym. Sci. 2015, 2015, 871345. [Google Scholar] [CrossRef]
- Udeni Gunathilake, T.; Ching, Y.; Chuah, C. Enhancement of curcumin bioavailability using nanocellulose reinforced chitosan hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, T.; Ko, H.-U.; Kim, H.C.; Kim, J.W.; Kim, J. Swelling behavior of polyacrylamide–cellulose nanocrystal hydrogels: Swelling kinetics, temperature, and pH effects. Materials 2019, 12, 2080. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Aponte, A.A.; Cárdenas-Nieto, J.D.; Tirado, D.F. Aloe vera gel drying by Refractance Window®: Drying kinetics and high-quality retention. Foods 2021, 10, 1445. [Google Scholar] [CrossRef] [PubMed]

| Hydrogel | * Weq | ** Wt |
|---|---|---|
| AV-R0 | 310 ± 9 a | 324 |
| AV-R3 | 476 ± 7 b | 508 |
| AV-R5 | 432 ± 7 c | 446 |
| AV-R10 | 295 ± 13 d | 317 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guancha-Chalapud, M.A.; Serna-Cock, L.; Tirado, D.F. Aloe vera Rind Valorization to Improve the Swelling Capacity of Commercial Acrylic Hydrogels. Fibers 2022, 10, 73. https://doi.org/10.3390/fib10090073
Guancha-Chalapud MA, Serna-Cock L, Tirado DF. Aloe vera Rind Valorization to Improve the Swelling Capacity of Commercial Acrylic Hydrogels. Fibers. 2022; 10(9):73. https://doi.org/10.3390/fib10090073
Chicago/Turabian StyleGuancha-Chalapud, Marcelo A., Liliana Serna-Cock, and Diego F. Tirado. 2022. "Aloe vera Rind Valorization to Improve the Swelling Capacity of Commercial Acrylic Hydrogels" Fibers 10, no. 9: 73. https://doi.org/10.3390/fib10090073
APA StyleGuancha-Chalapud, M. A., Serna-Cock, L., & Tirado, D. F. (2022). Aloe vera Rind Valorization to Improve the Swelling Capacity of Commercial Acrylic Hydrogels. Fibers, 10(9), 73. https://doi.org/10.3390/fib10090073







