Simple Synthesis of Fe3O4@-Activated Carbon from Wastepaper for Dispersive Magnetic Solid-Phase Extraction of Non-Steroidal Anti-Inflammatory Drugs and Their UHPLC–PDA Determination in Human Plasma
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Standard
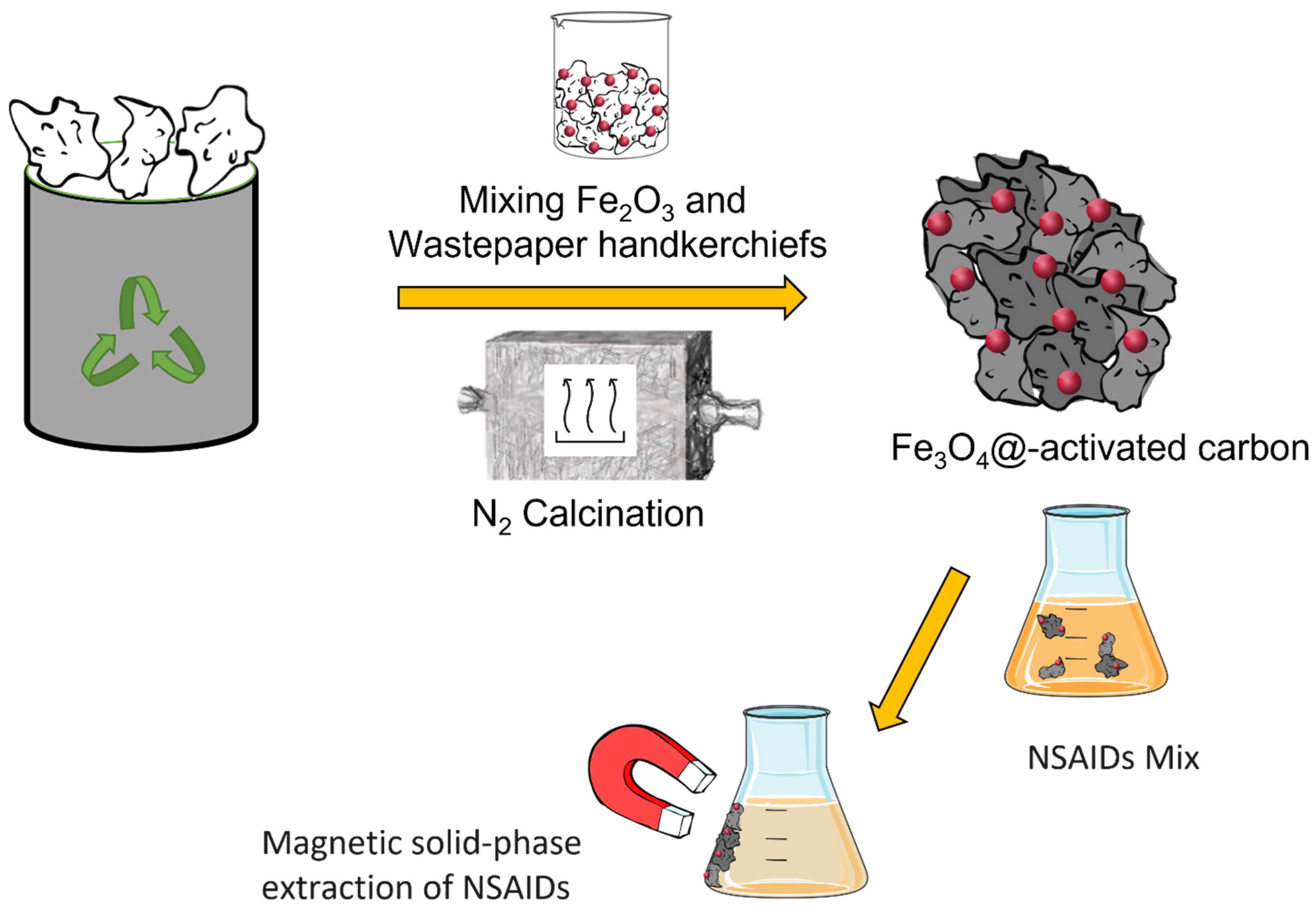
2.2. Preparation of “Fe3O4@-Activated Carbon”
2.3. Characterisation
2.4. Dispersive Magnetic Solid-Phase Extraction
2.5. UHPLC Apparatus and Operating Conditions
3. Results and Discussion
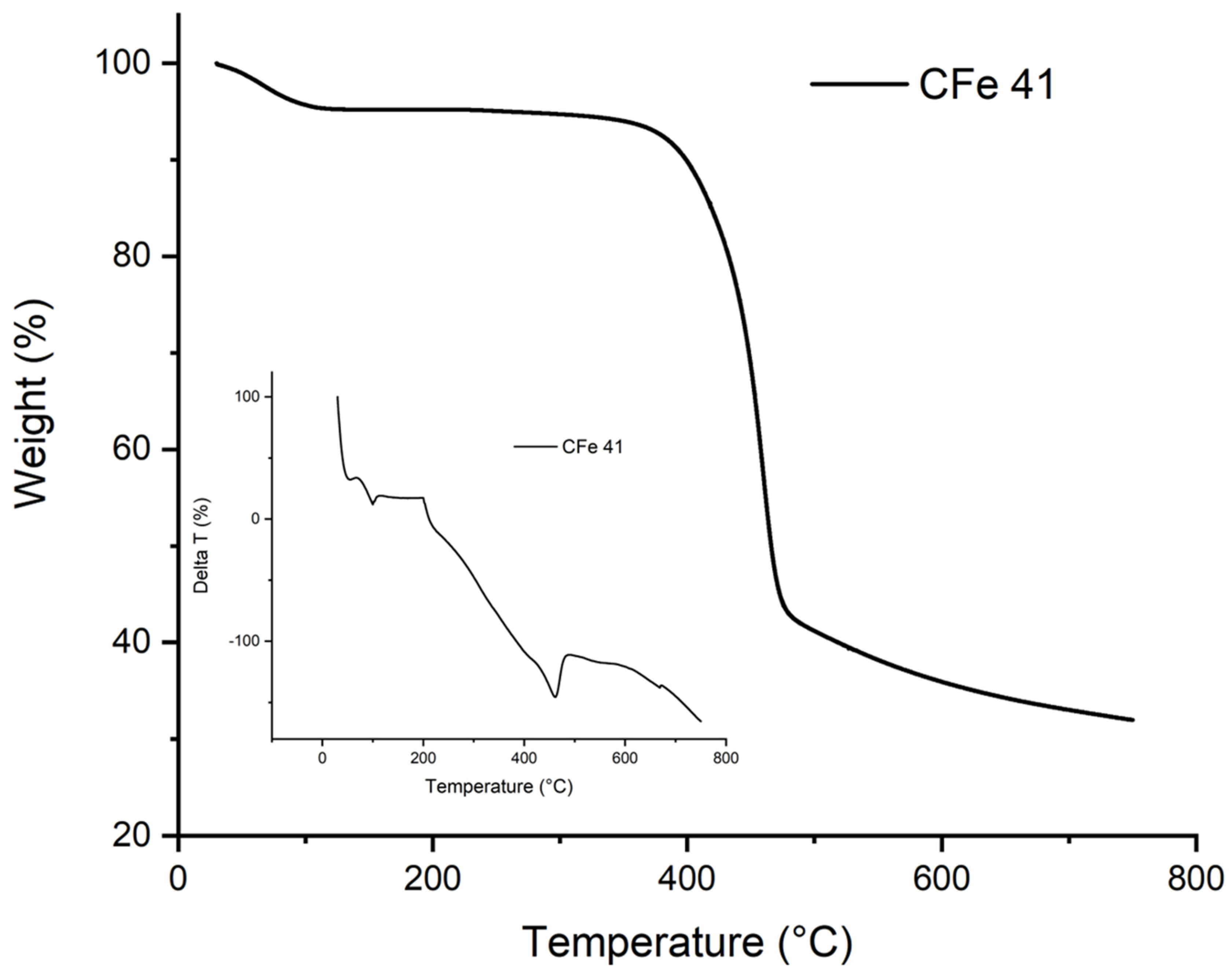
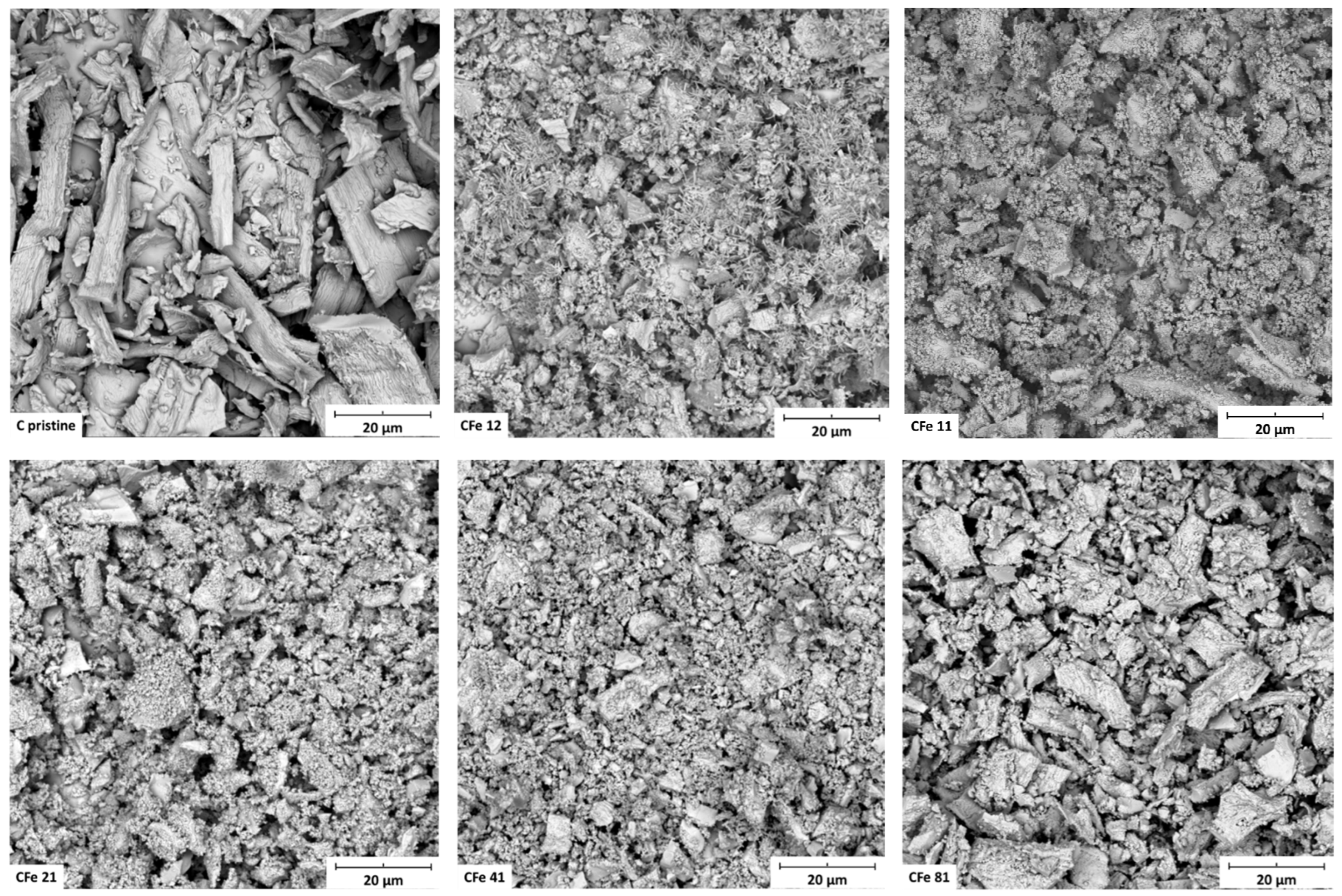
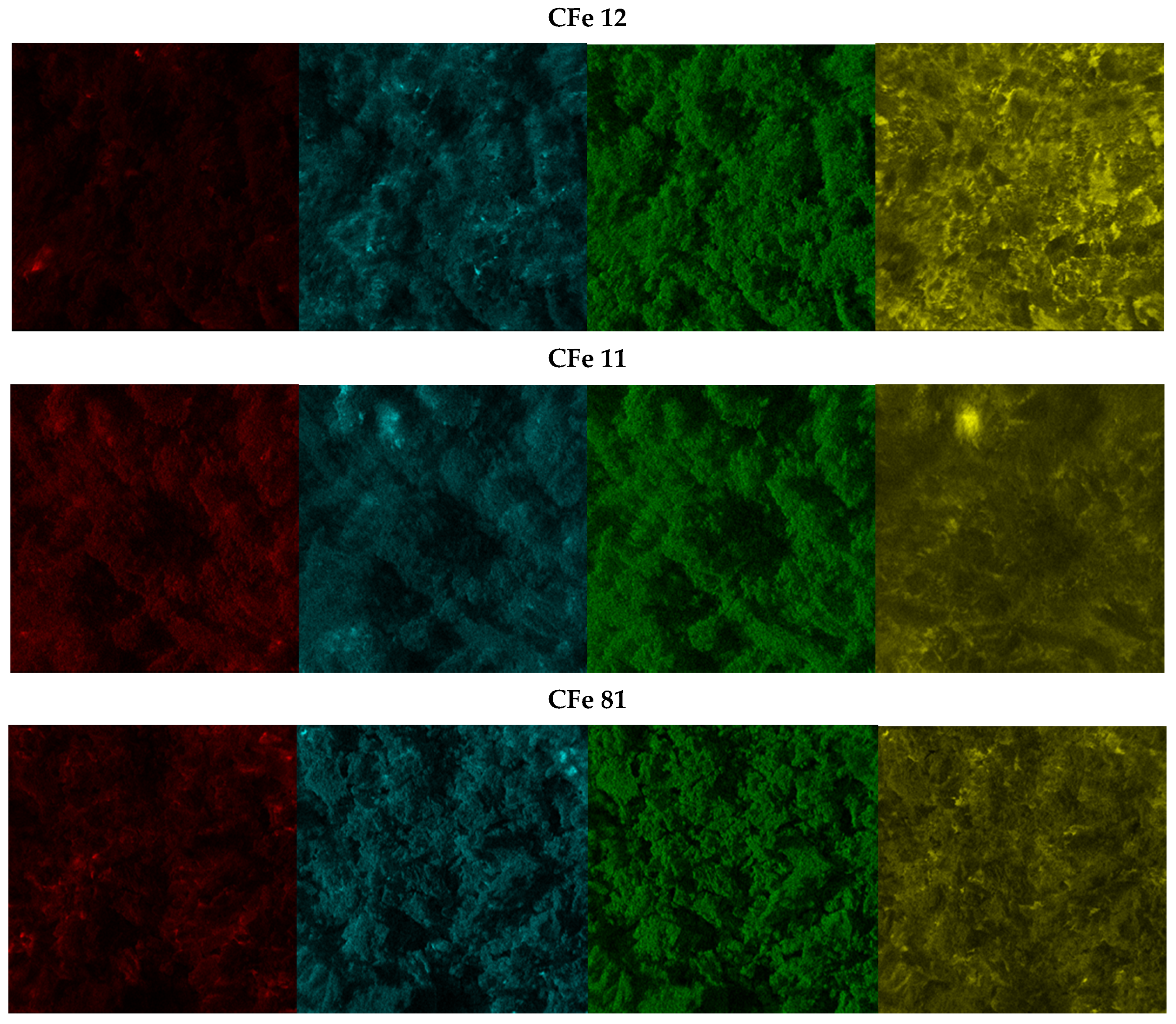
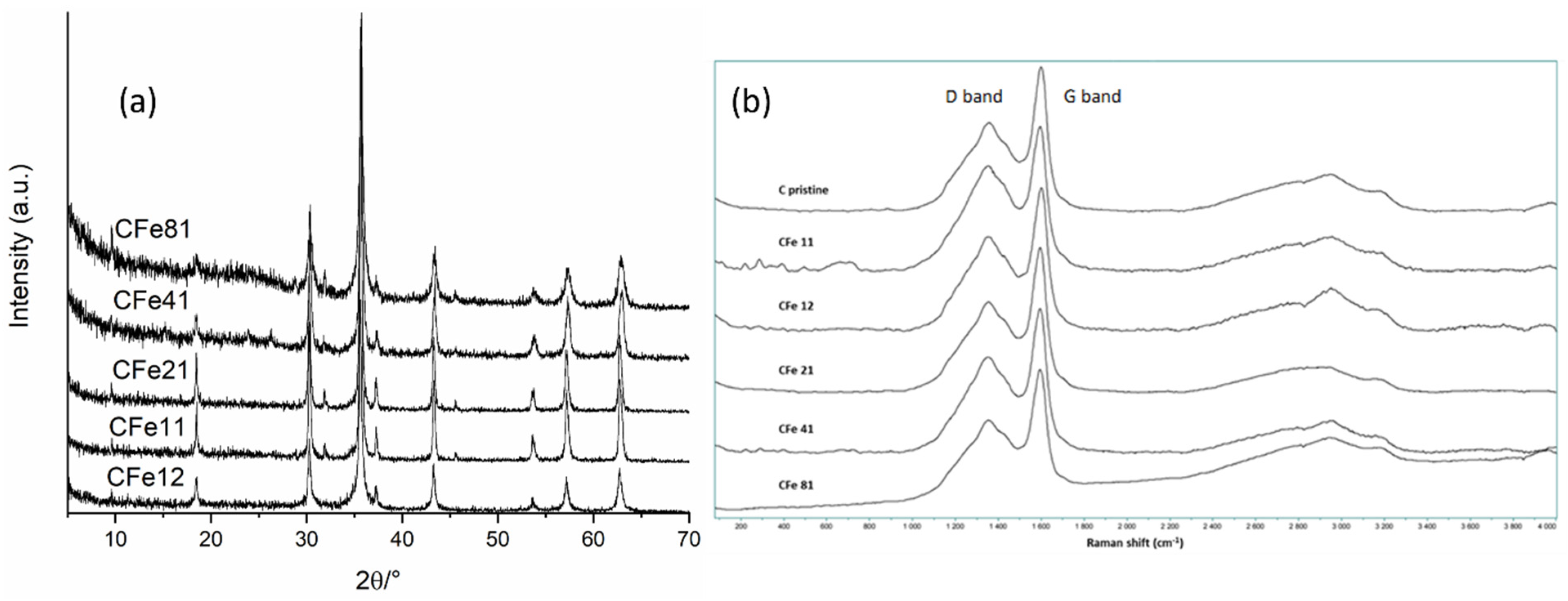
3.1. Materials Characterisation
3.2. NSADs Analysis
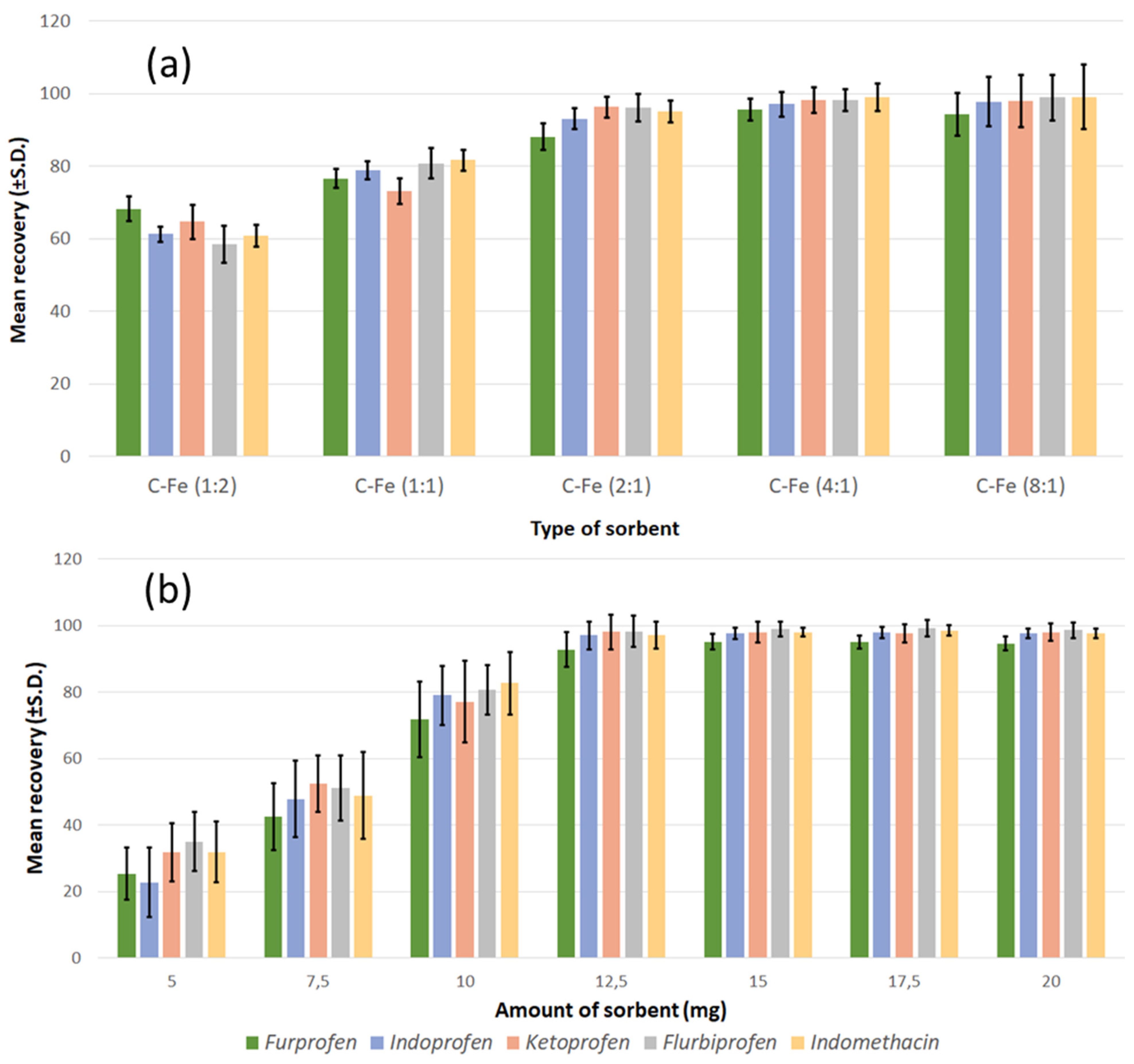
3.2.1. Dispersive Magnetic Solid-Phase Extraction and Optimisation of Conditions
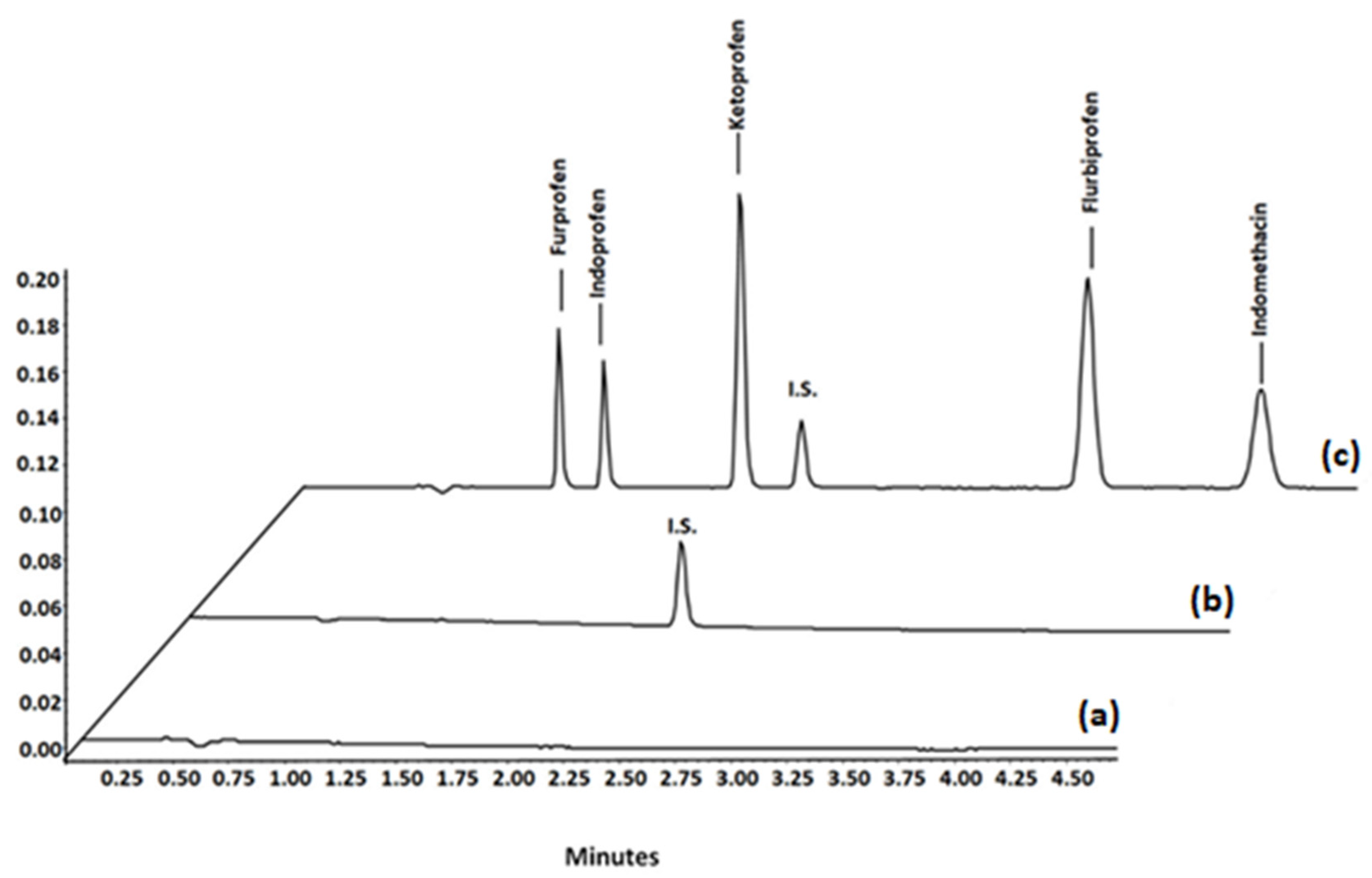
3.2.2. Method Validation
3.3. Comparison with Other Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paper Production—and Waste—To Double. Available online: https://www.theworldcounts.com/stories/paper-waste-facts (accessed on 2 June 2022).
- Li, M.; Du, H.; Kuai, L.; Huang, K.; Xia, Y.; Geng, B. Scalable Dry Production Process of a Superior 3D Net-Like Carbon-Based Iron Oxide Anode Material for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2017, 56, 12649–12653. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, D.; Zhu, S.; Chen, P.; Zhu, G.T.; Jiang, X.; Di, S. Eco-friendly and facile one-step synthesis of a three dimensional net-like magnetic mesoporous carbon derived from wastepaper as a renewable adsorbent. RSC Adv. 2019, 9, 12419–12427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Y.; Liu, X.; Zhang, L. Green construction of Fe3O4@GC submicrocubes for highly sensitive magnetic dispersive solid-phase extraction of five phthalate esters in beverages and plastic bottles. Food Chem. 2019, 277, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Yu, J.; Zhu, G.; Zhu, S. Net-like mesoporous carbon nanocomposites for magnetic solid-phase extraction of sulfonamides prior to their quantitation by UPLC-HRMS. Microchim. Acta 2020, 187, 112. [Google Scholar] [CrossRef]
- Mashile, P.P.; Nomngongo, P.N. Magnetic Cellulose-Chitosan Nanocomposite for Simultaneous Removal of Emerging Contaminants: Adsorption Kinetics and Equilibrium Studies. Gels 2021, 7, 190. [Google Scholar] [CrossRef] [PubMed]
- Streete, P.J. Rapid high-performance liquid chromatographic methods for the determination of overdose concentrations of some non-steroidal anti-inflammatory drugs in plasma or serum. J. Chromatogr. B Biomed. Sci. Appl. 1989, 495, 179–193. [Google Scholar] [CrossRef]
- Burgess, R.R. Protein precipitation techniques. Methods Enzymol. 2009, 463, 331–342. [Google Scholar] [PubMed]
- Almeida, H.F.D.; Marrucho, I.M.; Freire, M.G. Removal of Non-Steroidal Anti-Inflammatory Drugs from Aqueous Environments with Reusable Ionic-Liquid-based Systems. ACS Sustain. Chem. Eng. 2017, 5, 2428–2436. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.R.M.; de Santana, F.J.M.; Bonato, P.S. Stereoselective determination of the major ibuprofen metabolites in human urine by off-line coupling solid-phase microextraction and high-performance liquid chromatography. Anal. Chim. Acta 2005, 538, 25–34. [Google Scholar] [CrossRef]
- Abdel-Rehim, M. Recent advances in microextraction by packed sorbent for bioanalysis. J. Chromatogr. A 2010, 1217, 2569–2580. [Google Scholar] [CrossRef]
- D’Archivio, A.A.; Maggi, M.A.; Ruggieri, F.; Carlucci, M.; Ferrone, V.; Carlucci, G. Optimisation by response surface methodology of microextraction by packed sorbent of non steroidal anti-inflammatory drugs and ultra-high performance liquid chromatography analysis of dialyzed samples. J. Pharm. Biomed. Anal. 2016, 125, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J.; Lord, H.L. Handbook of Sample Preparation; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Piovesana, S.; Laganà, A. Recent application of magnetic solid-phase extraction for sample preparation. Chromatographia 2019, 82, 1251–1274. [Google Scholar] [CrossRef]
- Pardasani, D.; Kanaujia, P.K.; Purohit, A.K.; Shrivastava, A.R.; Dubey, D.K. Magnetic multi-walled carbon nanotubes assisted dispersive solid phase extraction of nerve agents and their markers from muddy water. Talanta 2011, 86, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kolaei, M.; Dashtian, K.; Rafiee, Z.; Ghaedi, M. Ultrasonic-assisted magnetic solid phase extraction of morphine in urine samples by new imprinted polymer-supported on MWCNT-Fe3O4-NPs: Central composite design optimization. Ultrason. Sonochem. 2016, 33, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, I.; Fernandes, C. Magnetic solid phase extraction for determination of drugs in biological matrices. Trends Anal. Chem. 2017, 89, 41–52. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Liu, T. A magnetic cellulose-based carbon fiber hybrid as a dispersive solid-phase extraction material for the simultaneous detection of six bisphenol analogs from environmental samples. Analyst 2018, 143, 3100–3106. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Jin, R.; Luo, C.; Song, C.; Hu, Y.; Cheng, H. Synthesis of polydopamine-functionalized magnetic graphene and carbon nanotubes hybrid nanocomposites as an adsorbent for the fast determination of 16 priority polycyclic aromatic hydrocarbons in aqueous samples. J. Sep. Sci. 2018, 41, 1847–1855. [Google Scholar] [CrossRef]
- Thongprapai, P.; Cheewasedtham, W.; Chong, K.F.; Rujiralai, T. Selective magnetic nanographene oxide solid-phase extraction with high-performance liquid chromatography and fluorescence detection for the determination of zearalenone in corn samples. J. Sep. Sci. 2018, 41, 4348–4354. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lin, F.; Yang, X.; Wang, B.; Lu, X.; Chen, Q.; Ye, F.; Zhao, S. Facile synthesis of magnetic carbon nanotubes derived from ZIF-67 and application to magnetic solid-phase extraction of profens from human serum. Talanta 2020, 207, 120284. [Google Scholar] [CrossRef] [PubMed]
- Soylak, M.; Ozalp, O.; Uzcan, F. Magnetic nanomaterials for the removal, separation and preconcentration of organic and inorganic pollutants at trace levels and their practical applications: A review. Trends Environ. Anal. Chem. 2021, 29, e00109. [Google Scholar] [CrossRef]
- Maroni, F.; Bruni, P.; Suzuki, N.; Aihara, Y.; Gabrielli, S.; Carbonari, G.; Agostini, M.; Branchi, M.; Ferrari, S.; Navarra, M.A.; et al. Highly Stable Fe3O4/C Composite: A Candidate Material for All Solid-State Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 070556. [Google Scholar] [CrossRef]
- Liu, S.; Yao, K.; Fu, L.H.; Ma, M.G. Selective synthesis of Fe3O4, g-Fe2O3, and a-Fe2O3 using cellulose-based composites as precursors. RSC Adv. 2016, 6, 2135–2140. [Google Scholar] [CrossRef]
- Maroni, F.; Gabrielli, S.; Palmieri, A.; Marcantoni, E.; Croce, F.; Nobili, F. High cycling stability of anodes for lithium-ion batteries based on Fe3O4 nanoparticles and poly(acrylic acid) binder. J. Power Sources 2016, 332, 79–87. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Q.; Wang, Y.; Li, Z.; Li, Z.; Yuan, Q. New insights into the structure–performance relationships of mesoporous materials in analytical science. Chem. Soc. Rev. 2018, 47, 8766–8803. [Google Scholar] [CrossRef]
- Schuepfer, D.B.; Badaczewski, F.; Guerra-Castro, J.M.; Hofmann, D.M.; Heiliger, C.; Smarsly, M.; Klar, P.J. Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon 2020, 161, 359–372. [Google Scholar] [CrossRef]
- Yamagishi, T.; Yamauchi, S.; Suzuki, K.; Suzuki, T.; Kurimoto, Y.; Takayama, T.; Yoichi Sakai, Y. Mössbauer and Raman spectroscopic characterization of iron and carbon in iron-loaded Japanese cypress charcoal. J. Wood Sci. 2020, 66, 82. [Google Scholar] [CrossRef]
- Guidance for Industry-Bioanalytical Method Validation; FDA Food and Drug Administration of the United States, US Department of Health and Human Services, Center for Drug Evaluation and Research and Center for Veterinary Medicine: Rockville, MD, USA, 2003.
- Guideline on bioanalytical method validation. In 2012 ICH International Conferences on Harmonization (ICH), Q2(R1): Text on Validation of Analytical Procedures, US FDA Federal Register; European Medicines Agency: London, UK, 2005.
- Locatelli, M.; Ferrone, V.; Cifelli, R.; Barbacane, R.C.; Carlucci, G. Microextraction by packed sorbent and high performance liquid chromatography determination of seven non-steroidal anti-inflammatory drugs in human plasma and urine. J. Chromatogr. A 2014, 1367, 1–8. [Google Scholar] [CrossRef]
- Espinosa-Mansilla, A.; Munoz De La Pena, A.; Canada-Canada, F.; Gonzalez Gomez, D. Determination of fluoroquinolones and non steroidal anti-inflammatory drugs in urine by extractive spectrophotometry and photoinduced spectrofluorimetry using multivariate calibration. Bioanal. Chem. 2005, 347, 275–286. [Google Scholar]
- Fan, W.; Mao, X.; He, M.; Chen, B.; Hu, B. Development of novel sol–gel coatings by chemically bonded ionic liquids for stir bar sorptive extraction—Application for the determination of NSAIDS in real samples. Anal. Bioanal. Chem. 2014, 406, 7261–7273. [Google Scholar] [CrossRef]
- Toledo-Neira, C.; Alvarez Lueje, A. Ionic liquids for improving the extraction of NSAIDs in water samples using dispersive liquid-liquid microextraction by high performance liquid chromatography-diode array- fluorescence detection. Talanta 2015, 134, 619–626. [Google Scholar] [CrossRef]
- Ferrone, V.; Carlucci, M.; Ettorre, V.; Cotellese, R.; Palumbo, P.; Fontana, A.; Siani, G.; Carlucci, G. Dispersive magnetic solid phase extraction exploiting magnetic graphene nanocomposite coupled with UHPLC-PDA for simultaneous determination of NSAIDs in human plasma and urine. J. Pharm. Biomed. Anal. 2018, 161, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Ulfa, M.; Prasetyoko, D.; Bahruji, H.; Nugraha, R.E. Green Synthesis of Hexagonal Hematite (a-Fe2O3) Flakes Using Pluronic F127-Gelatin Template for Adsorption and Photodegradation of Ibuprofen. Materials 2021, 14, 6779. [Google Scholar] [CrossRef] [PubMed]
- Rahman, L.; Bhattacharjee, S.; Islam, S.; Zahan, F.; Biswas, B.; Sharmin, N. A study on the preparation and characterization of maghemite (γ-Fe2O3) particles from iron-containing waste materials. J. Asian Ceram. Soc. 2020, 8, 1083–1094. [Google Scholar] [CrossRef]
- Guedidi, H.; Reinert, L.; Soneda, Y.; Bellakhal, N.; Duclaux, L. Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths. Arab. J. Chem. 2017, 10, S3584–S3594. [Google Scholar] [CrossRef] [Green Version]
- Faraji, M.; Shirani, M.; Rashidi-Nodeh, H. The recent advances in magnetic sorbents and their applications. Trends Anal. Chem. 2021, 141, 116302. [Google Scholar] [CrossRef]
| Analyte | Amount Add (mg mL−1) | Intra-Day | Inter-Day | ||
|---|---|---|---|---|---|
| Accuracy (BIAS%) | Precision (RSD%) | Accuracy (BIAS%) | Precision (RSD%) | ||
| Furprofen | 0.005 | −2.15 | 3.12 | −3.60 | 2.70 |
| 0.010 | 2.73 | 2.73 | 1.90 | 3.24 | |
| 0.75 | 7.40 | 1.25 | 3.20 | 1.66 | |
| 15.0 | −3.95 | 3.50 | −1.99 | 4.31 | |
| Indoprofen | 0.005 | 3.19 | 2.67 | 3.00 | 4.39 |
| 0.010 | 0.94 | 0.71 | 2.10 | 2.20 | |
| 0.75 | 2.15 | 3.44 | 2.58 | 0.99 | |
| 15.0 | −1.10 | 2.67 | −3.55 | 4.09 | |
| Ketoprofen | 0.005 | −2.34 | 7.49 | −3.70 | 3.98 |
| 0.010 | 4.05 | 2.79 | 2.15 | 3.45 | |
| 0.75 | −1.98 | 3.99 | −1.15 | 3.80 | |
| 15.0 | 5.90 | 5.06 | 3.85 | 1.03 | |
| Flurbiprofen | 0.005 | −6.57 | 3.07 | −5.10 | 0.50 |
| 0.010 | 3.10 | 2.67 | 1.50 | 2.28 | |
| 0.75 | 4.51 | 4.17 | 4.04 | 2.19 | |
| 15.0 | 1.99 | 4.95 | 1.54 | 4.06 | |
| Indomethacin | 0.005 | 1.34 | 5.45 | 5.13 | 5.05 |
| 0.010 | −3.34 | 3.93 | −1.56 | 4.17 | |
| 0.75 | 1.98 | 2.20 | 1.09 | 2.40 | |
| 15.0 | 2.21 | 3.36 | 1.34 | 5.10 | |
| Sample | Time Analysis | Instrumentation | Limit of Detection (µg/mL) | Limit of Quantification (µg/mL) | Sample Preparation | Elution Solvent (µL) | Ref. |
|---|---|---|---|---|---|---|---|
| Plasma | 20 min | HPLC–UV | 0.030 | 0.100 | MEPS | 250 µL | [31] |
| Dialyzed | 5 min | UHPLC–UV | 0.008 | 0.025 | MEPS | 250 µL | [12] |
| Urine | 15 min | CE–UV | 0.07 | 0.2 | SDME | 50 µL | [32] |
| Wastewater | 12 min | HPLC–UV | 0.0003 | 0.001 | SBSE | 100 µL | [33] |
| Water sample | 15 min | HPLC–FLD | 0.006 | 0.017 | IL-DLLME | 300 µL | [34] |
| Plasma and urine | 5 min | UHPLC–PDA | 0.0007 | 0.002 | MSPE | 500 µL | [35] |
| Plasma | 4.5 min | UHPLC–PDA | 0.001 | 0.005 | MSPE | 500 µL | Presented work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrone, V.; Bruni, P.; Canale, V.; Sbrascini, L.; Nobili, F.; Carlucci, G.; Ferrari, S. Simple Synthesis of Fe3O4@-Activated Carbon from Wastepaper for Dispersive Magnetic Solid-Phase Extraction of Non-Steroidal Anti-Inflammatory Drugs and Their UHPLC–PDA Determination in Human Plasma. Fibers 2022, 10, 58. https://doi.org/10.3390/fib10070058
Ferrone V, Bruni P, Canale V, Sbrascini L, Nobili F, Carlucci G, Ferrari S. Simple Synthesis of Fe3O4@-Activated Carbon from Wastepaper for Dispersive Magnetic Solid-Phase Extraction of Non-Steroidal Anti-Inflammatory Drugs and Their UHPLC–PDA Determination in Human Plasma. Fibers. 2022; 10(7):58. https://doi.org/10.3390/fib10070058
Chicago/Turabian StyleFerrone, Vincenzo, Pantaleone Bruni, Valentino Canale, Leonardo Sbrascini, Francesco Nobili, Giuseppe Carlucci, and Stefania Ferrari. 2022. "Simple Synthesis of Fe3O4@-Activated Carbon from Wastepaper for Dispersive Magnetic Solid-Phase Extraction of Non-Steroidal Anti-Inflammatory Drugs and Their UHPLC–PDA Determination in Human Plasma" Fibers 10, no. 7: 58. https://doi.org/10.3390/fib10070058
APA StyleFerrone, V., Bruni, P., Canale, V., Sbrascini, L., Nobili, F., Carlucci, G., & Ferrari, S. (2022). Simple Synthesis of Fe3O4@-Activated Carbon from Wastepaper for Dispersive Magnetic Solid-Phase Extraction of Non-Steroidal Anti-Inflammatory Drugs and Their UHPLC–PDA Determination in Human Plasma. Fibers, 10(7), 58. https://doi.org/10.3390/fib10070058







