Abstract
Vascular disease in elderly patients is a growing health concern, with an estimated prevalence of 15–20% in patients above 70 years old. Current treatment for vascular diseases requires the use of a vascular graft (VG) to revascularize lower or upper extremities, create dialysis access, treat aortic aneurysms, and repair dissection. However, postoperative infection is a major complication associated with the use of these VG, often necessitating several operations to achieve complete or partial graft excision, vascular coverage, and extra-anatomical revascularization. There is also a high risk of morbidity, mortality, and limb loss. Therefore, it is important to develop a method to prevent or reduce the incidence of these infections. Numerous studies have investigated the efficacy of antibiotic- and antiseptic-impregnated grafts. In comparison to these traditional methods of creating antimicrobial grafts, nanotechnology enables researchers to design more efficient VG. Nanofibers and nanoparticles have a greater surface area compared to bulk materials, allowing for more efficient encapsulation of antibiotics and better control over their temporo-spatial release. The disruptive potential of nanofibers and nanoparticles is exceptional, and they could pave the way for a new generation of prosthetic VG. This review aims to discuss how nanotechnology is shaping the future of cardiovascular-related infection management.
1. Introduction
Vascular graft (VG) is the standard option for revascularization to treat several vascular diseases such as peripheral arterial disease (PAD), abdominal aortic aneurysm, and aortic dissection. Arteriovenous grafts are also required for hemodialysis access creation. With the number of Americans over the age of 60 beginning to increase, effective management of vascular diseases becomes important, because a significant portion of this age group will be affected by vascular diseases [1]. To treat such conditions, various strategies have been developed throughout the years, with vascular prosthesis becoming the gold standard in clinical practice. The number of patients requiring hemodialysis in the US is also rising, with expectations that the number of patients on hemodialysis will continue to grow by 4–8% yearly worldwide [2]. Due to the increasing number of individuals needing VG, the number of vascular graft infections (VGI) is rising as well, and their multifactorial etiology creates a concerning scenario as patients’ pre-existing conditions, environment, and surgical procedures all influence the risk of developing VGI [3].
1.1. Intracavitary Graft Infections
Intracavitary aortic graft infections have been shown to have an incidence rate as high as 5%, with extracavitary graft infection rates as high as 6% [4]. Despite the relatively low incidence of infection, the morbidity and mortality as a result of these infections is high. Aortic graft infections have a mortality rate between 24–75% [5]. VGI can occur in different cardiovascular regions including intracavitary locations such as the supra-aortic trunk (SAT), thoracic aorta, and abdominal aorta, as well as extracavitary infections in peripheral arteries, with different incidence rates according to each region [6,7,8,9].
The incidence of SAT VGI is extremely low, with only 140 cases reported over the last three decades [10]. Due to its rare occurrence, it is challenging to identify the etiology and develop a possible general therapeutic approach [11,12]. Thoracic aortic VGI incidence, however, can be up to 6%, with mortality rates that can reach up to 75% [13,14]. In recent years, an increase in thoracic aortic VGI has been reported, highlighting the need for more effective solutions [15]. The thoracic aorta is anatomically poorly exposed and visible signs of infection are difficult to detect, resulting in a high mortality rate [16]. The abdominal aortic VGI incidence rate is around 0.2% according to Vogel et al. [17], while Berger et al. reported a 1.6% incidence at 30 days, 3.6% at one year, and 4.5% after two years in a different study [18,19].
For intracavitary graft infections, the therapeutic approach includes excision of the graft, debridement of infected tissues, and extra-anatomic bypass along with antibiotic therapy [20]. When an infection has been detected, the first step is to control the sepsis process, followed by removal of all the infected materials/devices, and subsequent reconstruction in a clean environment, which can be complicated depending on the patient’s conditions and comorbidities (i.e., diabetes mellitus, chronic renal failure, chronic obstructive pulmonary disease, and obesity) [5,21]. Cryopreserved aortic allografts have shown good results for the replacement of infected thoracic VGI in terms of resistance to infection [22]. However, they are prone to degeneration, rupture, and bleeding when attacked by necrotizing organisms such as P. aeruginosa or C. albicans [23]. Another approach relies on polyethylene terephthalate (PET) rifampin-soaked and silver-coated synthetic VG, which have been used to decrease the risk of early infection. These treated PET VG have also shown promising results against the risk of re-infection, with an overall five-year survival around 53% in comparison with 12% for the standard grafts [24,25]. Among the treatment options, conservative therapy is considered only as a palliative strategy for patients who could not withstand open surgery. For all these individuals, long-term or lifelong antimicrobial therapy is the only possibility [26,27]. Complications that arise from these infections include the creation of aortoenteric and aortoesophageal fistulas as well as aortic stump blowout, requiring multiple surgical interventions if patients survive.
1.2. Extracavitary Graft Infections
PAD has an overall prevalence rate of 12% in adult Americans [28]. VGI incidence ranges from 2.5% in femorofemoral prosthetic bypasses [29] to 2.8% in femoropopliteal bypasses [30], with higher occurrence in patients with critical limb-threatening ischemia. The groin region is the most subject to VGI. Although the peripheral location could be perceived as less dangerous than thoracic or abdominal infections, conservative treatment is not an option for lower limb VGI. In fact, peripheral VGI is associated with high mortality, up to 45% at five years [31], as well as recurrent infection, anastomotic disruption, and active bleeding, with up to 40% of cases requiring limb amputation [4]. Risk of reinfection is high in partial excision; thus, total excision is preferred when it comes to peripheral graft infections. For arteriovenous graft infections, the preferred treatment option is the same as for peripheral grafts. Total excision of the graft is preferred as to reduce the occurrence of reinfection [4]. Since dialysis patients have high comorbidities and are dependent on hemodialysis, partial graft excision and preservation of dialysis access is a reasonable option. In situ reconstruction with autologous material such as great saphenous vein and femoral vein is the main strategy used by surgeons [32,33]. While treated prosthetic grafts have garnered a lot of interest due to their availability and reduced operation time, the associated risk of re-infection remains an open question, since the available data are not conclusive [34].
When VGI occurs in a patient that would not withstand a re-operation, there are few strategies available. In these cases, the best way to proceed is a combination of percutaneous drains and recurrent antibiotic administration to control the infection level [35]. Broad spectrum antibiotics are administered in the acute phase to control infection and sepsis. Once the infecting organism is identified, more appropriate therapeutic approaches are chosen. Although antibiotic administration is of paramount importance, no specific guidelines are provided on the length of the therapy. Usually, intravenous antibiotics are administered for at least 2–4 weeks, with good debridement if possible [36]. When the infection is under control, there is a general consensus that at least 4–6 weeks of parenteral antibiotic therapy is necessary [4]. In most cases requiring such graft preservation, patients are placed on lifelong suppressive therapy. While this is currently the only option to control infection, it can lead to development of antibiotic resistance or to antibiotic toxicity to the filtering organs (e.g., nephrotoxicity, ototoxicity) [20,24,37,38].
1.3. Preventing Graft Infections
Due to the high morbidity and mortality associated with current treatment options for VGI [4], the prevention of these infections remains a prime concern. In addition to preoperative precautions and prophylactic antibiotics, antimicrobial-impregnated VG have also emerged as a possible approach for reducing risk of infection. Two general methods are currently used to confer VG with antimicrobial properties: antibiotic and antiseptic impregnation of grafts. In the paragraphs below, we describe each method in greater detail.
Several antibiotics have been studied including rifampin, daptomycin, and vancomycin [39,40], for use in both Dacron and expanded polytetrafluoroethylene (ePTFE) grafts. In particular, rifampin-soaked Dacron grafts containing collagen or gelatin have demonstrated antibacterial activity with low rates of graft infections in both human and animal studies due to excellent biofilm penetration of rifampin [39,41]. According to Colburn et al., tests performed on dogs showed how the reinfection rate decreased from 100% to 62.5% using Dacron grafts soaked with rifampin and sealed with gelatin or collagen instead of untreated Dacron grafts [42]. Previous in vitro tests of this system demonstrated a duration of in vitro activity of 22 days, but in vivo experiments demonstrated that, at 10 and 12 days, the susceptibility of these rifampin/collagen-bonded grafts to a bacteremic challenge was similar to that of control grafts [41,43]. In a similar study, Almeida and colleagues investigated collagen implants impregnated with gentamicin (Collatamp) in the prevention of VGI. In 60 patients with lower limb ischemia who underwent femoropopliteal PTFE prosthetic bypass, the control group had a surgical site infection rate of 20% (6 of 30), whereas the implant group, which had Collatamp applied next to the prosthesis, had a surgical site infection rate of 0% (0 of 30) [44]. Graft infections caused by rifampin-resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA) are associated with poor outcomes. On the other hand, a Dacron graft coated with a biodegradable hydrogel providing sustained release of vancomycin significantly inhibited MRSA growth in vivo [40]. The fast release and clearance of impregnated drugs and inability to coat PTFE grafts are two major limitations of antibiotic-coated grafts.
In comparison to antibiotics, antiseptics typically have a broader spectrum of antimicrobial activity [45]. Several of these antiseptics have been extensively investigated for use in VG, including silver, triclosan, and chitosan. In fact, VG functionalized with silver have been in use for over a decade: as early as 2006, a multi-center study of InterGard Silver showed significantly lowered graft infection rates despite high incidences of nosocomial infections [46]. However, other in vivo studies in mouse models suggest that triclosan-coated vascular prostheses exhibit “potent” antimicrobial effects, while silver-coated ones do not [47]. In addition, an InterGard graft containing a silver and triclosan combination (IGSys) exhibited greater in vivo antimicrobial activity compared to a silver-only graft [48]. The IGSys graft also demonstrated more sustained antimicrobial activity compared to rifampin-soaked InterGard alternatives, which led to the emergence of rifampin-resistant mutants [48]. In addition to silver and triclosan, a photocrosslinkable chitosan hydrogel also demonstrated antibacterial activity against E. coli in vitro [49]. Again, the inability to coat PTFE grafts and lack of sustained release of antiseptic are the major limitations for this approach.
In addition to traditional antibiotic- and antiseptic-impregnation techniques, nanotechnology could help to reduce the rates of VGI. Nanotechnology can be exploited to develop a new generation of VG, where synthetic materials can be used together with endothelial cells, growth factors, and other active biomolecules to promote biocompatibility. Moreover, nanofabrication techniques allow the production of tailored solutions, recapitulating fundamental features of the extracellular matrix (ECM) such as interconnected porosity and architectural arrangement of the fibers [50,51,52]. Autologous grafts are considered the gold standard, due to their innate biocompatibility and mechanical properties. However, low availability, risk of comorbidities in the donor, and expensive costs for harvesting are critical limitations. Conversely, allografts are not limited in supply; however, they have the potential to cause an immune response and carry the risk of disease transfer, in addition to remaining an expensive solution due to harvesting and cryopreservation. Those limitations have pushed researchers towards the development of new strategies. In particular, nanofibers and nanoparticles, both nanotechnologies, are able to encapsulate and release different types of drugs. Moreover, they have shown promising results in terms of biocompatibility and infection prevention in different surgical approaches [51,52,53,54].
It has been broadly reported that nanofibrous mats, patches, and scaffolds, mainly fabricated by electrospinning, can imitate the nanostructure of natural ECM, thus improving cell adhesion and guiding phenotype differentiation as confirmed by more efficient vascular cell attachment and spreading [55,56,57]. Furthermore, nanofibers exhibit unique physicochemical properties that give them the ability to coat any surface area, even PTFE [46]. In nanoparticles, as compared to traditional metal-coated VG, the metal nanocomposites have greater specific surface area to volume ratios, due to their small size, and thus enhanced antimicrobial properties [58]. In particular, silver nanoparticles have drawn significant attention as a means to address implant-associated infections.
Despite the positive features of nanostructured grafts, some limitations need to be considered. Long term patency of the engineered VG and risk of triggering negative immune responses are drawbacks that still need to be overcome. Nanofibrous assemblies can potentially degrade over time, deteriorating the mechanical properties of the polymeric matrix and resulting in VG failure [59]. Moreover, the host immune response is something that must be considered carefully, since it has been demonstrated how it can severely affect the patency of VG in the long run.
In this regard, only autologous solutions do not trigger negative immunologic reaction. Allografts experience immunogenicity due to the interaction with the host body, and for this reason patients must be subjected to immunosuppressive therapies [60]. Immune rejection is considered one of the main causes of allograft failure and rupture in the long term [61]. VG are subjected to the same fate, even exacerbating the outcomes. Aggressive reaction from the host immune system leads to stenosis, thrombosis, and eventually failure of the implant. This aspect has been thoroughly studied by Hibino et al. using tissue engineered VG [62]. In a study performed in humans, they observed major failure of tissue engineered VG due to stenosis [63]. In order to investigate the reason for the failure, they examined the role of host immune function in excess neo-tissue formation in tissue engineered VG using an immunodeficient mouse model. Interestingly, grafts implanted in immunodeficient mice showed greater patency over time compared to those in an immunocompetent model. Sonography revealed stenosis of the grafts implanted in immunocompetent mice after 2 weeks, whereas the grafts of the immunodeficient group remained patent up to 10 weeks, suggesting a key role of the immune system in graft failure due to excessive formation of neo-intimal tissue. Despite the listed limitations, it is clear the enormous potential that nanocomposite materials bring in engineering novel solutions for the fabrication of more efficient VG.
This review aims to summarize the improvements achieved by exploiting nanofiber and nanoparticle technologies for VGI treatment, highlighting novel aspects and current challenges towards broader use in the clinical field.
2. Nanofibers
Nanofibers are an example of nanomaterials that are typically produced by the electrospinning technique. Electrospinning is a simple and cost-effective method that enables control over the fiber diameter and allows formation of fibers composed of a selected polymeric solution [54]. Briefly, the polymer solution is squeezed from a syringe into a drop in a high electrostatic field; this process allows for the formation of a jet, and while the solvent evaporates, the nano-sized fibers form (Figure 1). Nanofibers can be obtained from a wide range of both natural and synthetic polymers, according to the specific application [54,64,65,66]. Porosity as well as alignment of the nanofibers can be controlled with unprecedented detail [67]. Due to their nanometric dimensions, it is possible to exploit a greater surface area compared to bulk materials, allowing enhanced adhesion of cells or surface engrafting of proteins and drugs [68,69]. Moreover, mechanical properties of the nanofibrous constructs can be customized to a great extent, tuning key features such as flexibility and stiffness. Studies have investigated the feasibility of electrospinning as an innovative technique to generate nanofibrous constructs or coatings for premade grafts. Different approaches have been utilized, ranging from the fabrication of a de novo VG with materials that possess antimicrobial properties to the coating of premade grafts with nanofibrous coating of antibiotic (Figure 1).
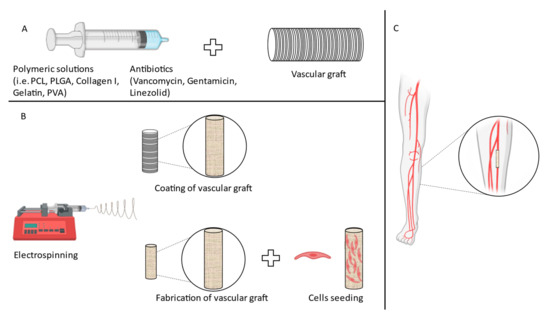
Figure 1.
Different approaches to fabricate VG. (A) Schematic of the coating of VG. (B) Different approaches to obtain VG with antimicrobial properties. (C) Schematic of PAD surgical procedure.
2.1. Nanofibrous Coating of Premade VG
One of the most effective ways of exploiting nanotechnology to confer antimicrobial properties to a prosthetic VG is through coating of a premade VG with nanofibers. Several research groups have investigated the effectiveness of this strategy, exploiting the characteristics of polymers easy to spin, such as poly(d,l)-lactide-co-glycolic acid (PLGA) or poly-ε-caprolactone (PCL), in combination with antimicrobial agents [2,70,71]. One study, performed by Liu et al., looked into electrospinning PLGA and vancomycin onto a VG and determining the release characteristics of vancomycin [70]. In this study, the in vitro release curves showed a continuous release of Vancomycin at a concentration of 2 µg/mL. Concentration levels above the 90% minimum inhibitory concentration (MIC90) were maintained for over 30 days. An initial burst period of 2 days was shown, which was followed by peaks between days 7 and 15. Finally, the concentration showed a gradual decrease. In vivo concentration profiles showed much lower vancomycin levels within the graft compared to outside of the graft. Because of this, the blood concentration of vancomycin was low throughout the time period. It was also shown that vancomycin levels were high at tissues beneath, 1 cm, and 2 cm surrounding the graft at 35 days. Limitations to the study were that the grafts were implanted into a subcutaneous pocket rather than being anastomosed to blood vessels and the grafts were not placed into an infected area. Another study investigated the effects of parameters such as solvent, voltage, and flow rate on electrospinning linezolid and PLGA [72]. The nanofibers were spun onto a flat sheet rather than a VG. Depending on the parameters used, controlled release of linezolid was observed for up to 28 days following an initial burst effect. In vitro antibacterial studies showed activity up to 16 days in some formulations. Nanofibers produced from polyvinyl alcohol (PVA), Pluronic F127 (Plur), and polyethyleneimine (PEI) solutions with titanium dioxide (TiO2) nanoparticles for use in topical antibacterial treatment also present significant potential for preventing post-implantation VGI, partially due to the biodegradability and complexing ability of the polymer. Inhibition zone studies of PVA-Plur-PEI/TiO2 nanofibers reveal that 0.03% TiO2 achieves significant infection control of pathogenic S. typhi and P. aeruginosa bacterial strains (44.7% and 21.6% respectively), providing encouraging results for the composite’s potential use in VG procedures [73]. This study shows an interesting approach that could be used to develop viable alternatives for the treatment of VGI, using a combination of nanofibers and nanoparticles, especially for small diameter VG. These results clearly prove the feasibility of these techniques, but the efficacy is still far from that of the current standard of care.
2.2. De Novo Nanofibrous VG
Another approach employing electrospinning is to engineer the VG itself with antimicrobial materials embedded. Researchers have started to investigate the feasibility of this de novo VG fabrication via electrospinning because of the problems of biocompatibility and patency encountered when using common PET and ePTFE VG [74,75]. In this way, it is possible to increase biocompatibility by tuning porosity and fiber alignment. Moreover, it is possible to recapitulate the hierarchical structure of natural ECM, leading to better cell attachment. Finally, drugs or bioactive molecules can be blended into the electrospinning solution and thus spun together with the polymer. An electrospinning technique has been developed to produce VG for hemodialysis access with collagen type I nanofibers on the luminal and adventitial sides with PCL as the medial layer [2]. Vancomycin and gentamicin can also be incorporated into the medial layer in order to achieve antimicrobial inhibition. These antimicrobial products were incorporated within the medial layer to obtain a slower release of drugs within the body. Shielding the medial layer with an inner and an outer nanofibrous level ensured a slower and prolonged release, due to reduced contact with biological fluids [69,76]. S. aureus and S. epidermidis were used in bacteria inhibition experiments to test the effects of the antibiotic-incorporated grafts, and inhibition zones were 14.5 mm and 20.5 mm on the first day, respectively. Over 28 days, the diameter of the inhibition zones remained unchanged for both bacteria. Electrospun PCL and gelatin loaded with an antibacterial plant extract, eugenol, also shows promise [71]. Different eugenol concentrations were used, namely 5, 10, 20 and 30 wt%, and all were found to have similar release patterns in vitro. Antibacterial activity was evaluated with an “immersion” method, where bacterial solutions of E. coli and S. aureus were added to wells containing discs of the electrospun membrane with different eugenol content. Approximately 25% of the eugenol was released within the first day. After this period, a sustained release was shown for up to 21 days. Finally, after this period, a slower rate of release was seen until a plateau was reached at 85% release of content. Antibacterial activity of eugenol was tested, and results showed growth inhibition rates of 71.6 ± 3.3% against E. coli and 78.6 ± 2.5% against S. aureus at the highest eugenol amount. Electrospinning has also been utilized to imbue antibacterial cetyltrimethylammonium bromide (CTAB) within polyvinylpyrrolidone (PVP) nanofibers, which exhibit a promising capability for infection control. Much like PVA-Plur-PEI composite, PVP’s biocompatibility and nontoxicity makes it an excellent nanofiber base for CTAB. Using an internationally standardized plate count methodology, 2.5 wt% was determined to be the minimum CTAB to PVP ratio for effective antibacterial activity against S. aureus, E. coli, and K. pneumonia [77]. Table 1 lists a summary of approaches to reduce VGI.

Table 1.
Nanofiber approaches to prevent VGI.
As shown in Table 1, a variety of in vitro tests were used among studies, such as growth inhibition and zones of inhibition. Because of these differences, it is difficult to compare the efficacy of the different nanofibers with each other. Nevertheless, the individual results of these studies suggest promising possibilities for the use of nanofibers to help reduce VGI. In vitro studies showed adequate antimicrobial activity over an adequate amount of time, ranging between 16 and 30 days depending on the study. One study investigated the in vivo release characteristics of vancomycin embedded within PLGA nanofibers [70]. However, its direct antimicrobial efficacy was not studied. Few studies have progressed to performing in vivo testing. Thus, further research is needed in order to investigate the antimicrobial properties of these nanofiber VG in vivo and determine their feasibility in reducing graft infections. Not only in vitro release and antibacterial activity, but also cytotoxicity of the nanofibers, needs to be evaluated.
Cytotoxicity has been proposed as one of the reasons for VG rupture in the medium to long term. In particular, aggressive strategies of infection prevention, involving high concentrations of drugs in impregnated VG, have led to necrosis at the anastomotic sites, eventually leading to graft failure [78]. For this reason, it is of vital importance to evaluate possible cytotoxic effects caused by not only the polymer but also drug concentration. Nanofibers and nanoparticles could solve the problem of drug-related cytotoxicity because a lower drug content can be used to avoid infection and drugs can be slowly released over time. A common way to evaluate cytotoxicity of electrospun constructs involves cell lines (e.g., murine fibroblasts, breast cancer cells, kidney epithelial cells) cultured and then exposed to different extracting solutions composed of dissolved electrospun matrix and antimicrobial agents such as vancomycin, eugenol, and gentamicin [2,70,71]. Polymer-related cytotoxicity can also potentially be avoided, because many of the polymers being used to create nanofibers have been previously known to be biocompatible, such as PLGA and PCL, and thus were found to have little to no toxicity. Among all these factors, host immune response is something that must be considered carefully [79]. Currently, only autologous solutions do not trigger a negative immunologic reaction. Allografts experience immunogenicity due to their interaction with the host body, and for this reason patients are normally treated with immunosuppressive therapies. Immune rejection is considered one of the main causes of allograft failure and rupture in the long term. Sadly, prosthetic VG are subject to the same fate, even exacerbating the outcomes. Aggressive reaction from the host immune system leads to stenosis, thrombosis, and eventually failure of the implant [80,81].
3. Nanoparticles
In addition to nanofibers, nanoparticles have also shown significant potential for use in preventing VGI. Nanoparticles are small particles that range from 1–100 nm in diameter [82,83]. Due to their nanoscale sizes and high surface to volume ratios, nanoparticles have attracted significant attention for their potential in drug delivery, imaging, and other stimuli-responsive applications [84,85,86]. For example, liposomal formulations are already used in the delivery of chemotherapeutics such as doxorubicin [84,87] and vincristine [84,88], while gold and iron oxide nanoparticles are used in computed tomography, magnetic resonance imaging, and positron emission tomography as contrast agents [86]. In the next few paragraphs, we will discuss several different types of nanoparticles that have been investigated for use in these applications. These consist of metal nanoparticles, metal nanoparticles with antiseptic polymers, and antibiotic-activated cyclodextrins.
3.1. Metal Nanoparticles
Several inorganic metals, including silver, copper, gold, and zinc have excellent broad-spectrum antiseptic properties [89,90,91]. As compared to traditional metal-coated VG, metal nanocomposites have larger specific surface areas; these larger exposed surfaces enable them to have greater antimicrobial activity per unit mass [92]. In particular, silver nanoparticles have drawn significant attention as a means to address implant-associated infections (Table 2). Existing literature suggests that silver ions can interact with bacterial wall sulfhydryl groups, thereby disrupting cell membranes, enzyme activities, respiratory chains, and cell proliferation. Silver nanoparticle-impregnated (0.1% w/w) PCL VG scaffolds have demonstrated antimicrobial properties against S. aureus and E. coli while being nontoxic to endothelial cells [93].

Table 2.
Nanoparticle approaches to prevent VG infections.
However, other studies suggest that silver nanoparticles are cytotoxic to a range of mammalian cells, including coronary endothelial cells [82] and umbilical vein endothelial cells [94]. Silver nanoparticles, as with other types of nanoparticles such as silica and tricalcium phosphate, have been found to cause significant hemolysis [95,96]. Gliga et al. have observed a size-dependent toxicity for silver nanoparticles. Using epithelial cells isolated from normal human bronchial epithelium (BEAS-2B) they noted that toxicity increased with nanoparticles with a diameter of 10 nm compared to 20 and 50 nm [97]. This can be explained, because when nanoparticles reach the blood system, they come into direct contact with blood cells, endothelial cells, and plasma proteins. The nanometric dimension of these nanoparticles can affect the intricate structure and critical functions of blood components. In fact, plasma proteins tend to adsorb to the surface of nanoparticles to form a protein corona that significantly influences their interaction with blood components and may even lead to increased cellular activation [98]. A pilot study by Sun et al. showed that 24 h exposure to ZnO NPs with a primary size of 45.3 nm was associated with significantly decreased mitochondrial activity in human cardiac microvascular endothelial cells, with a threshold as low as 5 μg/mL [99]. Likewise, Liang et al. showed that 24 h exposure to ZnO NP with a primary size of 70 nm at concentrations ≥15 μg/mL significantly induced cytotoxicity in human aortic endothelial cells as demonstrated by decreased mitochondrial activity, lactate dehydrogenase release, and apoptosis [100].
Sodium-triphosphate-capped silver nanoparticles embedded in polyurethane vascular scaffolds have been investigated as a more biocompatible approach to infection control; they have shown both biocompatibility and antimicrobial activity against S. aureus and E. coli in vitro [101]. This contrasts with regular silver nanoparticles embedded in polyurethane vascular scaffolds, which had both slightly greater antimicrobial activity and significantly greater cytotoxicity [101]. Scaffolds with sodium-triphosphate-capped silver nanoparticles were also able to maintain surgical artery patency and promote endothelialization at 30 days in in vivo mouse models [101].
3.2. Metal Nanoparticles with Antiseptic Polymers
One possible way to increase antimicrobial efficiency is to combine inorganic metal nanoparticles with polymeric compounds that possess antimicrobial properties (Figure 2) [102]. Two common polymeric compounds used for this purpose are chitosan and hyaluronic acid, which are biocompatible, antimicrobial, and able to form complexes with metals through chelating mechanisms (Table 2) [102].
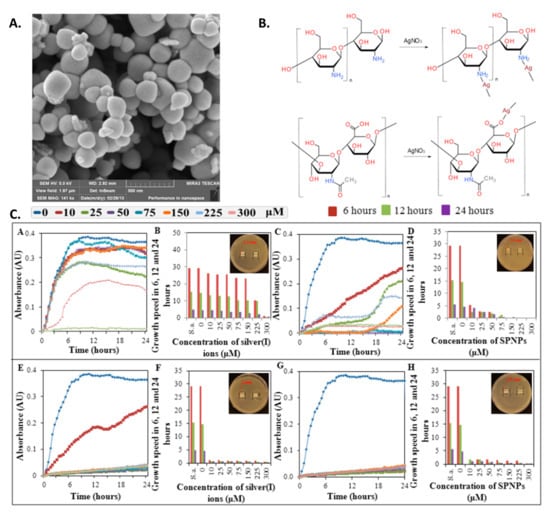
Figure 2.
Metal nanoparticles with antiseptic polymers (A) SEM image of silver phosphate nanoparticles. (B) Suggested structures of silver complexes. (C) Growth curves (subpanels (A,C,E,G)) as well as growth rate and inhibition zones (B,D,F,H) of S. aureus after application of (A,B) hyaluronic acid with AgNO3, (C,D) hyaluronic acid with silver phosphate nanoparticles (SPNP), (E,F) chitosan with AgNO3, and (G,H) chitosan with SPNP. Reprinted with permission from ref. [102]. Copyright 2013 MDPI.
While complexes of chitosan or hyaluronic acid with silver or silver phosphate nanoparticles all exhibited some amount of antimicrobial activity, chitosan and silver phosphate complexes showed significantly greater antibacterial effects against S. aureus in vitro compared to the other complexes [102].
3.3. Antibiotic-Activated Cyclodextrins
Cyclodextrins are cyclic oligosaccharides with nanoscale cavities that can be used to encapsulate hydrophobic compounds, including antibiotics, through a host–guest complexation mechanism (Figure 3). As a result, a higher concentration of ciprofloxacin, rifampin, and vancomycin is sorbed onto polyester vascular prostheses (PVP) functionalized with cyclodextrins (PVP-CD) as compared to regular polyester VG [103]. In vivo studies of PVP-CD with rifampin and vancomycin demonstrated greater growth inhibition against S. aureus, S. epidermidis, and MRSA compared to regular PVP [104] (Table 2). In addition, PVP-CD with ciprofloxacin also showed greater antimicrobial activity against E. coli, E. cloacae, and P. aeruginosa in vivo compared to regular PVP [104]. However, in previous in vitro studies of similar PVP-CD, only ciprofloxacin demonstrated greater antibacterial activity against S. aureus and E. coli when used in PVP-CD, while both rifampin and vancomycin showed similar antimicrobial activity in PVP-CD and PVP [103]. In addition, PVP-CD loaded with rifampin and ciprofloxacin resulted in reduced vitality and proliferation of human pulmonary microvascular endothelial cells; however, viability studies suggest that this toxicity was a result of the inherent cytotoxicity of these antibiotics, rather than the functionalization of the prostheses [103].
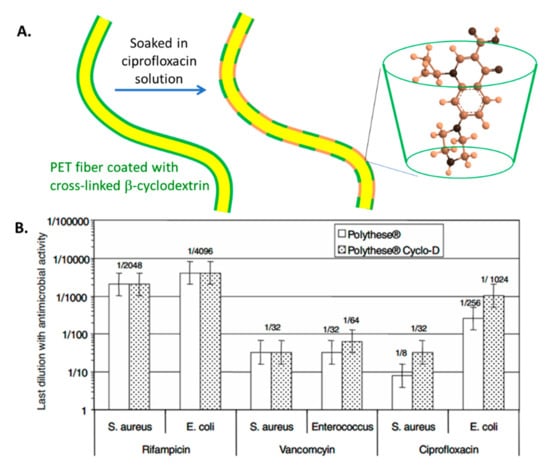
Figure 3.
Use of cyclodextrin polymer to encapsulate antibiotics. (A) Schematic of polyester prostheses coated with a cyclodextrin polymer and activated with ciprofloxacin. (B) Antimicrobial activity of polyester prostheses activated with antibiotics, with and without cyclodextrin. Reprinted with permission from [103] Copyright 2008.
3.4. Nanoparticles Used against Other Biofilm Infections
There has been extensive research on nanoparticle approaches to preventing biofilm infections. While much of this research was not done specifically on VG, it still provides possible future directions for VG research, and will thus be described briefly here. Metal oxide nanoparticles, such as zinc oxide [105] and iron oxide [106] nanoparticles, have demonstrated anti-biofilm properties. The photoactivation of gold [107] and graphene [108] nanoparticles can also lead to the thermal inactivation of bacteria.
Several polymeric nanoparticles have demonstrated anti-biofilm activity: ciprofloxacin-loaded PLGA nanoparticles have demonstrated activity against E. coli in vitro [109], while a dextran-block-poly((3-acrylamidopropyl) trimethylammonium chloride (AMPTMA)-co-butyl methacrylate (BMA)) block copolymer was able to remove preformed biofilms of various multidrug-resistant bacteria [71]. Nitric oxide-releasing silica nanoparticles have shown potent bactericidal effects against P. aeruginosa, E. coli, S. aureus, S. epidermidis, and C. albicans biofilms in vitro [110] and may be used to prevent VGI [111]. Moreover, Fernandez et al. have explored the efficacy of nitric oxide-releasing silica nanoparticles against MRSA, both in vitro and in vivo [112]. In addition, antibiotics encapsulated in lipid nanoparticles require a lower MIC compared to free antibiotics when used against biofilm organisms in vitro [113]. Fusogenic liposomes can also fuse with bacterial membranes, which enhances the bactericidal effect of its encapsulated antibiotic [113,114]. Further studies can help us better understand the feasibility of functionalizing VG with each of these nanoparticles, as well as the antimicrobial efficacy of these nanoparticles within a vascular setting.
4. Outlook
Metal and antibiotic-soaked grafts are already employed clinically to prevent VGI [14,24,25]. Nanotechnological approaches adopt many of the same basic mechanisms, only at the nanoscale. Furthermore, nanotechnologies have greater specific surface areas, and are thus likely to have greater antimicrobial activity and provide more sustained release of antimicrobial substances compared to non-nanoscale material grafts. Certain nanotechnology approaches, such as cyclodextrins, have also demonstrated greater antibiotic-loading capacity compared to traditional VG [102].
While these nanotechnologies show great promise, more information about their safety is needed. Current studies provide conflicting evidence on the cytotoxicity of silver nanoparticle-impregnated VG. While the silver nanoparticle-impregnated PCL VG designed by Madhavan et al. were said to be nontoxic to endothelial cells [93], Li et al.’s work suggests that silver nanoparticle-impregnated polyurethane vascular scaffolds possess some cytotoxicity to endothelial progenitor cells [101]. More studies are therefore needed to determine if silver nanoparticles can be used safely in clinical settings. Moreover, existing studies also suggest that some antibiotic-activated cyclodextrins are cytotoxic due to the toxicity of the antibiotics loaded [102]; additional research needs to be done to compare this cytotoxicity to that of traditional antibiotic-soaked VG and reaffirm that these nanoscale solutions are safe for use.
In this framework, 3D bioprinting is an attractive technology that has the potential to fabricate patient-specific grafts and could be very useful to overcome the challenges of growth potential, host-tissue integration, and anatomical differences [115]. Three-dimensional bioprinting could be an alternative to autologous or allogeneic tissue grafts for the replacement or treatment of damaged tissues [116]. Several categories of bioprinting methods have been developed in these years, but the most used is the extrusion bioprinting technique due to superior mechanical properties of the final products as compared to other bioprinting methods [117]. Three-dimensional bioprinting allows the possibility to print constructs in layers while controlling the spatial deposition of cell types. These unique features provide several advantages over other conventional processes. However, bioprinted grafts made from hydrogels are very fragile and have insufficient strength to withstand hemodynamic pressures in vivo [118].
Given the potential of these nanotechnologies, their efficacy warrants further investigation. As each of these studies employed different methods of quantifying antimicrobial efficacy, it is difficult to compare between studies. For example, while several studies quantified the minimum and total inhibitory concentration, others, such as Madhavan et al.’s, used largely non-quantitative zone of inhibition assays. Further studies using standardized, quantitative approaches should be done to identify the approaches that offer the most promise. Comparisons between nanostructured grafts and similar non-nanoscale grafts—such as silver nanoparticle grafts vs. silver grafts—can also help establish whether these nanotechnology approaches confer additional benefits compared to traditional grafts. In addition, while one of Jean-Baptiste et al.’s studies of antibiotic-activated cyclodextrins utilized an in vivo model, other studies have not progressed beyond in vitro studies despite promising results. Further in vivo studies of these nano-grafts could shed more light on their efficacy and provide a basis for potential clinical trials.
5. Conclusions
VGI are serious complications that are associated with high morbidity and mortality rates [20,35,119]. Antimicrobial nanofibers and nanoparticles can be particularly effective in preventing these infections due to their higher active surface area and lower toxicity compared to small-molecule antimicrobials. In this review, we have described a variety of nanotechnology-based approaches to preventing VGI. Recent research involving nanofibers has incorporated the use of electrospinning technology. Electrospinning allows for the creation of fibers down to the nanoscale using a wide variety of synthetic and natural polymers. Antibacterial materials such as antibiotics can also be incorporated along with polymers in order to create VG with antibacterial activity. By using electrospinning, it is possible to manipulate parameters to adjust the diameter, surface area, porosity, and other properties of the fibers. By manipulating these properties, it is possible to change the release rate of the antimicrobial substance within the graft, potentially extending infection control over a desired time period. These nanofiber-coated VG have shown antimicrobial properties such as sufficient inhibitory concentrations and release profiles over an adequate amount of time in several in vivo studies. In addition to nanofibers, metal, semimetal, and organic nanoparticles have all shown anti-biofilm activity in VG applications. Several other organic and lipid-based nanoparticles have also shown promising anti-biofilm activity in other implants and can be investigated for use in VG applications.
The need for antimicrobial VG is evident, and several commercially available grafts—such as the silver-coated InterGard Silver and B. Braun Silver Graft—have sought to address this need through traditional non-nanoscale approaches. This review suggests that there are several promising nanotechnology-based approaches to these antimicrobial grafts that may have advantages compared to existing conventional methods. Additional in vivo studies and potential trials of these nanotechnology-based approaches to addressing VGI are therefore warranted.
Author Contributions
For this review article M.R. and F.T. conceived the idea and edit the articles, E.H. and S.S. together with P.A. wrote the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by George and Angelina Kostas Research Center for Cardiovascular Nanomedicine.
Acknowledgments
The authors would like to thank Amanda Weiskoff (Academic Affairs, Houston Methodist Academic Institute) for scientific writing assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levin, S.R.; Farber, A.; Cheng, T.W.; Arinze, N.; Jones, D.W.; Kalish, J.A.; Rybin, D.; Siracuse, J.J. Risk assessment of significant upper extremity arteriovenous graft infection in the vascular quality initiative. J. Vasc. Surg. 2020, 71, 913–919. [Google Scholar] [CrossRef]
- Radakovic, D.; Reboredo, J.; Helm, M.; Weigel, T.; Schürlein, S.; Kupczyk, E.; Leyh, R.G.; Walles, H.; Hansmann, J. A multilayered electrospun graft as vascular access for hemodialysis. PLoS ONE 2017, 12, e0185916. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Gharamti, A.; Kanafani, Z.A. Vascular graft infections: An update. Infect. Dis. Clin. 2018, 32, 789–809. [Google Scholar] [CrossRef] [PubMed]
- Legout, L.; Sarraz-Bournet, B.; D’Elia, P.V.; Devos, P.; Pasquet, A.; Caillaux, M.; Wallet, F.; Yazdanpanah, Y.; Senneville, E.; Haulon, S.; et al. Characteristics and prognosis in patients with prosthetic vascular graft infection: A prospective observational cohort study. Clin. Microbiol. Infect. 2012, 18, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, R.; Astore, D.; Frigerio, S.; Garriboli, L.; Piccolo, G.; Castellano, R.; Scalamogna, M.; Odero, A.; Pirrelli, S.; Biasi, G.; et al. Vascular prosthetic graft infection: Epidemiology, bacteriology, pathogenesis and treatment. Acta Chir. Belg. 2002, 102, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.D.; Nasim, A.; London, N.J.; Sayers, R.D.; Barrie, W.W.; Bell, P.R.; Naylor, A.R. In situ replacement of infected aortic grafts with rifampicin-bonded prostheses: The Leicester experience (1992 to 1998). J. Vasc. Surg. 1999, 30, 92–98. [Google Scholar] [CrossRef]
- Swain, T.W., III; Calligaro, K.D.; Dougherty, M.D. Management of infected aortic prosthetic grafts. Vasc. Endovasc. Surg. 2004, 38, 75–82. [Google Scholar] [CrossRef]
- Baddour, L.M.; Bettmann, M.A.; Bolger, A.F.; Epstein, A.E.; Ferrieri, P.; Gerber, M.A.; Gewitz, M.H.; Jacobs, A.K.; Levison, M.E.; Newburger, J.W.; et al. Nonvalvular cardiovascular device–related infections. Circulation 2003, 108, 2015–2031. [Google Scholar] [CrossRef]
- Knight, B.C.; Tait, W.F. Dacron patch infection following carotid endarterectomy: A systematic review of the literature. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Asciutto, G.; Geier, B.; Marpe, B.; Hummel, T.; Mumme, A. Dacron patch infection after carotid angioplasty. A report of 6 cases. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.R.; Payne, D.; London, N.J.M.; Thompson, M.M.; Dennis, M.S.; Sayers, R.D.; Bell, P.R.F. Prosthetic patch infection after carotid endarterectomy. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Erb, S.; Sidler, J.A.; Elzi, L.; Gurke, L.; Battegay, M.; Widmer, A.F.; Weisser, M. Surgical and antimicrobial treatment of prosthetic vascular graft infections at different surgical sites: A retrospective study of treatment outcomes. PLoS ONE 2014, 9, e112947. [Google Scholar] [CrossRef]
- Kahlberg, A.; Melissano, G.; Mascia, D.; Loschi, D.; Grandi, A.; Chiesa, R. How to best treat infectious complications of open and endovascular thoracic aortic repairs. in Seminars in vascular surgery. Semin. Vasc. Surg. 2017, 30, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sekar, N. Primary aortic infections and infected aneurysms. Ann. Vasc. Dis. 2010, 3, 24–27. [Google Scholar] [CrossRef]
- Kahlberg, A.; Grandi, A.; Loschi, D.; Vermassen, F.; Moreels, N.; Chakfe, N. Infection of descending thoracic aortic graft and endograft: A systematic review. J. Vasc. Surg. 2019, 69, 1941. [Google Scholar] [CrossRef]
- Vogel, T.R.; Symons, R.; Flum, D.R. The incidence and factors associated with graft infection after aortic aneurysm repair. J. Vasc. Surg. 2008, 47, 264–269. [Google Scholar] [CrossRef]
- Berger, P.; De Borst, G.J.; Moll, F.L. Current opinions about diagnosis and treatment strategy for aortic graft infections in The Netherlands. J. Cardiovasc. Surg. 2015, 56, e76. [Google Scholar]
- Argyriou, C.; Georgiadis, G.S.; Lazarides, M.K.; Georgakarakos, E.; Antoniou, G.A. Endograft infection after endovascular abdominal aortic aneurysm repair: A systematic review and meta-analysis. J. Endovasc. Ther. 2017, 24, 688–697. [Google Scholar] [CrossRef]
- Wilson, W.R.; Bower, T.C.; Creager, M.A.; Amin-Hanjani, S.; O’Gara, P.T.; Lockhart, P.B.; Darouiche, R.O.; Ramlawi, B.; Derdeyn, C.P.; Bolger, A.F.; et al. Vascular graft infections, mycotic aneurysms, and endovascular infections: A scientific statement from the American heart association. Circulation 2016, 134, e412–e460. [Google Scholar] [CrossRef] [PubMed]
- Chaar, C.I.O.; Zafar, M.A.; Velasquez, C.; Saeyeldin, A.; Elefteriades, J.A. Complex two-stage open surgical repair of an aortoesophageal fistula after thoracic endovascular aortic repair. J. Vasc. Surg. Cases Innov. Tech. 2019, 5, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Vogt, P.R.; Pfammatter, T.; Schlumpf, R.; Genoni, M.; Künzli, A.; Candinas, D.; Zünd, G.; Turina, M. In situ repair of aortobronchial, aortoesophageal, and aortoenteric fistulae with cryopreserved aortic homografts. J. Vasc. Surg. 1997, 26, 11–17. [Google Scholar] [CrossRef][Green Version]
- Chiesa, R.; Tshomba, Y.; Kahlberg, A.; Marone, E.M.; Civilini, E.; Coppi, G.; Psacharopulo, D.; Melissano, G. Management of thoracic endograft infection. J. Cardiovasc. Surg. 2010, 51, 15. [Google Scholar]
- Spiliotopoulos, K.; Preventza, O.; Green, S.Y.; Price, M.D.; Amarasekara, H.S.; Davis, B.M.; Kim, I.; LeMaire, S.A.; Coselli, J.S. Open descending thoracic or thoracoabdominal aortic approaches for complications of endovascular aortic procedures: 19-year experience. J. Thorac. Cardiovasc. Surg. 2018, 155, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Falk, V.; Kuntze, T.; Borger, M.A.; Schmidt, A.; Scheinert, D.; Mohr, F.W. Secondary surgical procedures after endovascular stent grafting of the thoracic aorta: Successful approaches to a challenging clinical problem. J. Thorac. Cardiovasc. Surg. 2008, 136, 1289–1294. [Google Scholar] [CrossRef]
- Oderich, G.S.; Bower, T.C.; Cherry, K.J., Jr.; Panneton, J.M.; Sullivan, T.M.; Noel, A.A.; Carmo, M.; Cha, S.; Kalra, M.; Gloviczki, P. Evolution from axillofemoral to in situ prosthetic reconstruction for the treatment of aortic graft infections at a single center. J. Vasc. Surg. 2006, 43, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Bandyk, D.F.; Novotney, M.L.; Back, M.R.; Johnson, B.L.; Schmacht, D.C. Expanded application of in situ replacement for prosthetic graft infection. J. Vasc. Surg. 2001, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Dua, A.; Lee, C.J. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech. Vasc. Interv. Radiol. 2016, 19, 91–95. [Google Scholar] [CrossRef]
- Eiberg, J.P.; Røder, O.; Stahl-Madsen, M.; Eldrup, N.; Qvarfordt, P.; Laursen, A.; Greve, M.; Flörenes, T.; Nielsen, O.M.; Seidelin, C.; et al. Fluoropolymer-coated dacron versus PTFE grafts for femorofemoral crossover bypass: Randomised trial. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 431–438. [Google Scholar] [CrossRef]
- Exton, R.J.; Galland, R.B. Major groin complications following the use of synthetic grafts. Eur. J. Vasc. Endovasc. Surg. 2007, 34, 188–190. [Google Scholar] [CrossRef]
- Saleem, B.R.; Meerwaldt, R.; Tielliu, I.F.; Verhoeven, E.L.; van den Dungen, J.J.; Zeebregts, C.J. Conservative treatment of vascular prosthetic graft infection is associated with high mortality. Am. J. Surg. 2010, 200, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Siracuse, J.J.; Nandivada, P.; Giles, K.A.; Hamdan, A.D.; Wyers, M.C.; Chaikof, E.L.; Pomposelli, F.B.; Schermerhorn, M.L. Prosthetic graft infections involving the femoral artery. J. Vasc. Surg. 2013, 57, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, O.; Gibbons, C.P. A 10-year experience of using femoro-popliteal vein for re-vascularisation in graft and arterial infections. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 172–179. [Google Scholar] [CrossRef]
- Töpel, I.; Audebert, F.; Betz, T.; Steinbauer, M.G. Microbial spectrum and primary resistance to rifampicin in infectious complications in vascular surgery: Limits to the use of rifampicin-bonded prosthetic grafts. Angiology 2010, 61, 423–426. [Google Scholar] [CrossRef]
- Hasse, B.; Husmann, L.; Zinkernagel, A.; Weber, R.; Lachat, M.; Mayer, D. Vascular graft infections. Swiss Med. Wkly. 2013, 143, w13754. [Google Scholar] [CrossRef][Green Version]
- Calligaro, K.D.; Veith, F.J.; Yuan, J.G.; Gargiulo, N.J.; Dougherty, M.J. Intra-abdominal aortic graft infection: Complete or partial graft preservation in patients at very high risk. J. Vasc. Surg. 2003, 38, 1199–1204. [Google Scholar] [CrossRef]
- Young, M.H.; Upchurch, G.R.; Malani, P.N. Vascular graft infections. Infect. Dis. Clin. 2012, 26, 41–56. [Google Scholar] [CrossRef]
- Bandyk, D.F.; Novotney, M.L.; Johnson, B.L.; Back, M.R.; Roth, S.R. Use of rifampin-soaked gelatin-sealed polyester grafts for in situ treatment of primary aortic and vascular prosthetic infections. J. Surg. Res. 2001, 95, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Lew, W.; Moore, W. Antibiotic-impregnated grafts for aortic reconstruction. Semin. Vasc. Surg. 2011, 24, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Morishima, M.; Marui, A.; Yanagi, S.; Nomura, T.; Nakajima, N.; Hyon, S.H.; Ikeda, T.; Sakata, R. Sustained release of vancomycin from a new biodegradable glue to prevent methicillin-resistant Staphylococcus aureus graft infection. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 52–55. [Google Scholar] [CrossRef][Green Version]
- Goëau-Brissonnière, O.; Mercier, F.; Nicolas, M.H.; Bacourt, F.; Coggia, M.; Lebrault, C.; Pechère, J.C. Treatment of vascular graft infection by in situ replacement with a rifampin-bonded gelatin-sealed Dacron graft. J. Vasc. Surg. 1994, 19, 739–744. [Google Scholar] [CrossRef][Green Version]
- Colburn, M.D.; Moore, W.S.; Chvapil, M.; Gelabert, H.A.; Quioñones-Baldrich, W.J. Use of an antibiotic-bonded graft for in situ reconstruction after prosthetic graft infections. J. Vasc. Surg. 1992, 16, 651–660. [Google Scholar] [CrossRef][Green Version]
- Chervu, A.; Moore, W.S.; Chvapil, M.; Henderson, T. Efficacy and duration of antistaphylococcal activity comparing three antibiotics bonded to Dacron vascular grafts with a collagen release system. J. Vasc. Surg. 1991, 13, 897–901. [Google Scholar] [CrossRef][Green Version]
- Almeida, C.E.P.C.; Reis, L.; Carvalho, L.; Almeida, C.M.C. Collagen implant with gentamicin sulphate reduces surgical site infection in vascular surgery: A prospective cohort study. Int. J. Surg. 2014, 12, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. Available online: https://pubmed.ncbi.nlm.nih.gov/9880479/ (accessed on 15 June 2021). [CrossRef] [PubMed]
- Ricco, J.-B.; Assadian, O. Antimicrobial silver grafts for prevention and treatment of vascular graft infection. Semin. Vasc. Surg. 2011, 24, 234–241. [Google Scholar] [CrossRef]
- Hernandez-Richter, T.; Schardey, H.M.; Wittmann, F.; Mayr, S.; Schmitt-Sody, M.; Blasenbreu, S.; Heiss, M.M.; Gabka, C.; Angele, M.K. Rifampin and Triclosan but not silver is effective in preventing bacterial infection of vascular dacron graft material. Eur. J. Vasc. Endovasc. Surg. 2003, 26, 550–557. [Google Scholar] [CrossRef]
- Berard, X.; Stecken, L.; Pinaquy, J.B.; Cazanave, C.; Puges, M.; Pereyre, S.; Bordenave, L.; M’Zali, F. Comparison of the antimicrobial properties of silver impregnated vascular grafts with and without triclosan. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 285–292. [Google Scholar] [CrossRef]
- Fujita, M.; Kinoshita, M.; Ishihara, M.; Kanatani, Y.; Morimoto, Y.; Simizu, M.; Ishizuka, T.; Saito, Y.; Yura, H.; Matsui, T.; et al. Inhibition of vascular prosthetic graft infection using a photocrosslinkable chitosan hydrogel. J. Surg. Res. 2004, 121, 135–140. [Google Scholar] [CrossRef]
- Bondar, B.; Fuchs, S.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. Functionality of endothelial cells on silk fibroin nets: Comparative study of micro-and nanometric fibre size. Biomaterials 2008, 29, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoo, J.J.; Lim, G.J.; Atala, A.; Stitzel, J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 83, 999–1008. [Google Scholar] [CrossRef]
- He, W.; Yong, T.; Teo, W.E.; Ma, Z.; Ramakrishna, S. Fabrication and endothelialization of collagen-blended biodegradable polymer nanofibers: Potential vascular graft for blood vessel tissue engineering. Tissue Eng. 2005, 11, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Goëau-Brissonnière, O.A.; Fabre, D.; Leflon-Guibout, V.; Di Centa, I.; Nicolas-Chanoine, M.H.; Coggia, M. Comparison of the resistance to infection of rifampin-bonded gelatin-sealed and silver/collagen-coated polyester prostheses. J. Vasc. Surg. 2002, 35, 1260–1263. [Google Scholar] [CrossRef]
- Venugopal, J.; Ramakrishna, S. Applications of polymer nanofibers in biomedicine and biotechnology. Appl. Biochem. Biotechnol. 2005, 125, 147–157. [Google Scholar] [CrossRef]
- Alsberg, E.; Feinstein, E.; Joy, M.P.; Prentiss, M.; Ingber, D.E. Magnetically-guided self-assembly of fibrin matrices with ordered nano-scale structure for tissue engineering. Tissue Eng. 2006, 12, 3247–3256. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Bhattacharyya, S.; Bender, J.D.; Greish, Y.E.; Brown, P.W.; Allcock, H.R.; Laurencin, C.T. Fabrication and optimization of methylphenoxy substituted polyphosphazene nanofibers for biomedical applications. Biomacromolecules 2004, 5, 2212–2220. [Google Scholar] [CrossRef]
- Pandolfi, L.; Furman, N.T.; Wang, X.; Lupo, C.; Martinez, J.O.; Mohamed, M.; Taraballi, F.; Tasciotti, E. A nanofibrous electrospun patch to maintain human mesenchymal cell stemness. J. Mater. Sci. Mater. Med. 2017, 28, 44. [Google Scholar] [CrossRef]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef]
- Brossollet, L.J. Mechanical issues in vascular grafting: A review. Int. J. Artif. Organs 1992, 15, 579–584. [Google Scholar] [CrossRef]
- Katsimpoulas, M.; Morticelli, L.; Gontika, I.; Kouvaka, A.; Mallis, P.; Dipresa, D.; Böer, U.; Soudah, B.; Haverich, A.; Michalopoulos, E.; et al. Biocompatibility and immunogenicity of decellularized allogeneic aorta in the orthotopic rat model. Tissue Eng. Part A 2019, 25, 399–415. [Google Scholar] [CrossRef]
- Lesèche, G.; Castier, Y.; Petit, M.D.; Bertrand, P.; Kitzis, M.; Mussot, S.; Besnard, M.; Cerceau, O. Long-term results of cryopreserved arterial allograft reconstruction in infected prosthetic grafts and mycotic aneurysms of the abdominal aorta. J. Vasc. Surg. 2001, 34, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Hibino, N.; Mejias, D.; Pietris, N.; Dean, E.; Yi, T.; Best, C.; Shinoka, T.; Breuer, C. The innate immune system contributes to tissue-engineered vascular graft performance. FASEB J. 2015, 29, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Hibino, N.; McGillicuddy, E.; Matsumura, G.; Ichihara, Y.; Naito, Y.; Breuer, C.; Shinoka, T. Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 2010, 139, 431–436.e2. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Gunn, J.; Zhang, M. Polyblend nanofibers for biomedical applications: Perspectives and challenges. Trends Biotechnol. 2010, 28, 189–197. [Google Scholar] [CrossRef]
- Liu, H.; Ding, X.; Zhou, G.; Li, P.; Wei, X.; Fan, Y. Electrospinning of nanofibers for tissue engineering applications. J. Nanomater. 2013, 2013, 3. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; John, K.; Shoji, T.; Shinoka, T. The evolution of tissue engineered vascular graft technologies: From preclinical trials to advancing patient care. Appl. Sci. 2019, 9, 1274. [Google Scholar] [CrossRef] [PubMed]
- Miroshnichenko, S.; Timofeeva, V.; Permyakova, E.; Ershov, S.; Kiryukhantsev-Korneev, P.; Dvořaková, E.; Shtansky, D.V.; Zajíčková, L.; Solovieva, A.; Manakhov, A. Plasma-coated polycaprolactone nanofibers with covalently bonded platelet-rich plasma enhance adhesion and growth of human fibroblasts. Nanomaterials 2019, 9, 637. [Google Scholar] [CrossRef]
- Sirc, J.; Kubinova, S.; Hobzova, R.; Stranska, D.; Kozlik, P.; Bosakova, Z.; Marekova, D.; Holan, V.; Sykova, E.; Michalek, J. Controlled gentamicin release from multi-layered electrospun nanofibrous structures of various thicknesses. Int. J. Nanomed. 2012, 7, 5315. [Google Scholar] [CrossRef]
- Liu, K.S.; Lee, C.H.; Wang, Y.C.; Liu, S.J. Sustained release of vancomycin from novel biodegradable nanofiber-loaded vascular prosthetic grafts: In vitro and in vivo study. Int. J. Nanomed. 2015, 10, 885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.; Zhou, P.; Zhou, F.; Zhao, Y.; Ren, L.; Yuan, X. Antimicrobial eugenol-loaded electrospun membranes of poly (ε-caprolactone)/gelatin incorporated with REDV for vascular graft applications. Colloids Surf. B Biointerfaces 2018, 162, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Eren Boncu, T.; Ozdemir, N.; Uskudar Guclu, A. Electrospinning of linezolid loaded PLGA nanofibers: Effect of solvents on its spinnability, drug delivery, mechanical properties, and antibacterial activities. Drug Dev. Ind. Pharm. 2020, 46, 109–121. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; El Fawal, G.F.; El-Deeb, N.M.; Hassan, H.S.; Mo, X. Electrospun Polyvinyl Alcohol/Pluronic F127 Blended Nanofibers Containing Titanium Dioxide for Antibacterial Wound Dressing. Appl. Biochem. Biotechnol. 2016, 178, 1488–1502. Available online: https://pubmed.ncbi.nlm.nih.gov/26686499/ (accessed on 15 June 2021). [CrossRef] [PubMed]
- Davoudi, P.; Assadpour, S.; Derakhshan, M.A.; Ai, J.; Solouk, A.; Ghanbari, H. Biomimetic modification of polyurethane-based nanofibrous vascular grafts: A promising approach towards stable endothelial lining. Mater. Sci. Eng. C 2017, 80, 213–221. [Google Scholar] [CrossRef]
- Montini-Ballarin, F.; Calvo, D.; Caracciolo, P.C.; Rojo, F.; Frontini, P.M.; Abraham, G.A.; Guinea, G.V. Mechanical behavior of bilayered small-diameter nanofibrous structures as biomimetic vascular grafts. J. Mech. Behav. Biomed. Mater. 2016, 60, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Yoon, H.; Park, Y. Drug release from various thicknesses of layered mats consisting of electrospun polycaprolactone and polyethylene oxide micro/nanofibers. Appl. Phys. A 2010, 100, 1197–1204. [Google Scholar] [CrossRef]
- Uykun, N.; Ergal, İ.; Kurt, H.; Gökçeören, A.T.; Göcek, İ.; Kayaoğlu, B.K.; Akarsubaşı, A.T.; Sarac, A.S. Electrospun antibacterial nanofibrous polyvinylpyrrolidone/cetyltrimethylammonium bromide membranes for biomedical applications. J. Bioact. Compat. Polym. 2014, 29, 382–397. [Google Scholar] [CrossRef]
- Schneider, F.; O’Connor, S.; Becquemin, J.P. Efficacy of collagen silver-coated polyester and rifampin-soaked vascular grafts to resist infection from MRSA and Escherichia coli in a dog model. Ann. Vasc. Surg. 2008, 22, 815–821. [Google Scholar] [CrossRef]
- Taraballi, F.; Sushnitha, M.; Tsao, C.; Bauza, G.; Liverani, C.; Shi, A.; Tasciotti, E. Biomimetic tissue engineering: Tuning the immune and inflammatory response to implantable biomaterials. Adv. Healthc. Mater. 2018, 7, 1800490. [Google Scholar] [CrossRef] [PubMed]
- de Valence, S.; Tille, J.C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Möller, M.; Walpoth, B.H. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 2012, 33, 38–47. [Google Scholar] [CrossRef]
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomatrials 2009, 30, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2013, 47, 013001. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Deitcher, S.R. Marqibo®(vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2010, 2, 544–568. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.S.N.; Gunasekara, T.D.C.P.; Holton, J. Antimicrobial Nanoparticles: Applications and mechanisms of action. Sri Lankan J. Infect. Dis. 2018, 8, 2–11. [Google Scholar] [CrossRef]
- Panayala, N.R.; Pena-Mendez, M.E.; Havel, J. Havel, Silver or silver nanoparticles. J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef]
- Madhavan, R.V.; Rosemary, M.J.; Nandkumar, M.A.; Krishnan, K.V.; Krishnan, L.K. Silver nanoparticle impregnated poly (ɛ-Caprolactone) scaffolds: Optimization of antimicrobial and noncytotoxic concentrations. Tissue Eng. Part A 2011, 17, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, J.; Boudreau, M.; Meng, J.; Yin, J.J.; Liu, J.; Xu, H. Intravenous administration of silver nanoparticles causes organ toxicity through intracellular ROS-related loss of inter-endothelial junction. Part. Fibre Toxicol. 2015, 13, 1–13. [Google Scholar] [CrossRef]
- Mocan, T.J.B. Hemolysis as expression of nanoparticles-induced cytotoxicity in red blood cells. BMBN 2013, 1, 7–12. [Google Scholar]
- de la Harpe, K.M.; Kondiah, P.P.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The hemocompatibility of nanoparticles: A review of cell–nanoparticle interactions and hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 1–17. [Google Scholar] [CrossRef]
- Ritz, S.; Schöttler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein corona of nanoparticles: Distinct proteins regulate the cellular uptake. Biomacromolecules 2015, 16, 1311–1321. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.; Zhao, D.; Hun, F.H.; Weng, L.; Liu, H. Cytotoxicity, permeability, and inflammation of metal oxide nanoparticles in human cardiac microvascular endothelial cells. Cell Biol. Toxicol. 2011, 27, 333–342. [Google Scholar] [CrossRef]
- Liang, S.; Sun, K.; Wang, Y.; Dong, S.; Wang, C.; Liu, L.; Wu, Y. Role of Cyt-C/caspases-9, 3, Bax/Bcl-2 and the FAS death receptor pathway in apoptosis induced by zinc oxide nanoparticles in human aortic endothelial cells and the protective effect by alpha-lipoic acid. Chem.-Biol. Interact. 2016, 258, 40–51. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Mo, H.; Zhang, J.; Jiang, X.; Zhang, L.; Yang, L.; Fu, L.; He, L.; Zhao, Y.; et al. Sodium triphosphate–capped silver nanoparticles on a decellularized scaffold-based polyurethane vascular patch for bacterial infection inhibition and rapid endothelialization. J. Bioact. Compat. Polym. 2019, 34, 357–372. [Google Scholar] [CrossRef]
- Chudobova, D.; Nejdl, L.; Gumulec, J.; Krystofova, O.; Rodrigo, M.A.M.; Kynicky, J.; Ruttkay-Nedecky, B.; Kopel, P.; Babula, P.; Adam, V.; et al. Complexes of silver (I) ions and silver phosphate nanoparticles with hyaluronic acid and/or chitosan as promising antimicrobial agents for vascular grafts. Int. J. Mol. Sci. 2013, 14, 13592–13614. [Google Scholar] [CrossRef]
- Blanchemain, N.; Laurent, T.; Chai, F.; Neut, C.; Haulon, S.; Krump-konvalinkova, V.; Morcellet, M.; Martel, B.; Kirkpatrick, C.J.; Hildebrand, H.F. Polyester vascular prostheses coated with a cyclodextrin polymer and activated with antibiotics: Cytotoxicity and microbiological evaluation. Acta Biomater. 2008, 4, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Jean-Baptiste, E.; Blanchemain, N.; Neut, C.; Chai, F.; Maton, M.; Martel, B.; Hildebrand, H.; Haulon, S. Evaluation of the anti-infectious properties of polyester vascular prostheses functionalised with cyclodextrin. J. Infect. 2014, 68, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Thukkaram, M.; Sitaram, S.; Subbiahdoss, G. Antibacterial efficacy of iron-oxide nanoparticles against biofilms on different biomaterial surfaces. Int. J. Biomater. 2014, 2014, 716080. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.; Lee, J.H.; Lee, J. Potent antimicrobial and antibiofilm activities of bacteriogenically synthesized gold–silver nanoparticles against pathogenic bacteria and their physiochemical characterizations. J. Biomater. Appl. 2016, 31, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Deokar, A.R.; Liao, J.H.; Shih, P.Y.; Ling, Y.C. Graphene-based photothermal agent for rapid and effective killing of bacteria. ACS Nano 2013, 7, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Chang, M.W.; Hadinoto, K. Antibacterial efficacy of inhalable antibiotic-encapsulated biodegradable polymeric nanoparticles against E. coli biofilm cells. J. Biomed. Nanotechnol. 2010, 6, 391–403. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009, 30, 2782–2789. [Google Scholar] [CrossRef]
- Shin, J.H.; Schoenfisch, M.H. Inorganic/organic hybrid silica nanoparticles as a nitric oxide delivery scaffold. Chem. Mater. 2008, 20, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moure, J.S.; Van Eps, J.L.; Scherba, J.C.; Haddix, S.; Livingston, M.; Bryan, N.S.; Cantu, C.; Valson, C.; Taraballi, F.; Kaplan, L.J.; et al. Polyester mesh functionalization with nitric oxide-releasing silica nanoparticles reduces early methicillin-resistant staphylococcus aureus contamination. Surg. Infect. 2021, 2014, 716080. [Google Scholar] [CrossRef]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Beaulac, C.; Clement-Major, S.; Hawari, J.; Lagace, J. Eradication of mucoid Pseudomonas aeruginosa with fluid liposome-encapsulated tobramycin in an animal model of chronic pulmonary infection. Antimicrob. Agents Chemother. 1996, 40, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, A.J.; Hibino, N.; Best, C.A.; Yi, T.; Lee, Y.U.; Kraynak, C.A.; Kimerer, L.K.; Krieger, A.; Kim, P.; Breuer, C.K.; et al. 3D-printed biodegradable polymeric vascular grafts. Adv. Healthc. Mater. 2016, 5, 319–325. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An introduction to 3D bioprinting: Possibilities, challenges and future aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, A.J.; Fisher, J.P. Bioprinting of blood vessels. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 337–350. [Google Scholar]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The tissue-engineered vascular graft—Past, present, and future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).