4.1. Characterization of Sucrose Tetraaldehyde Solution
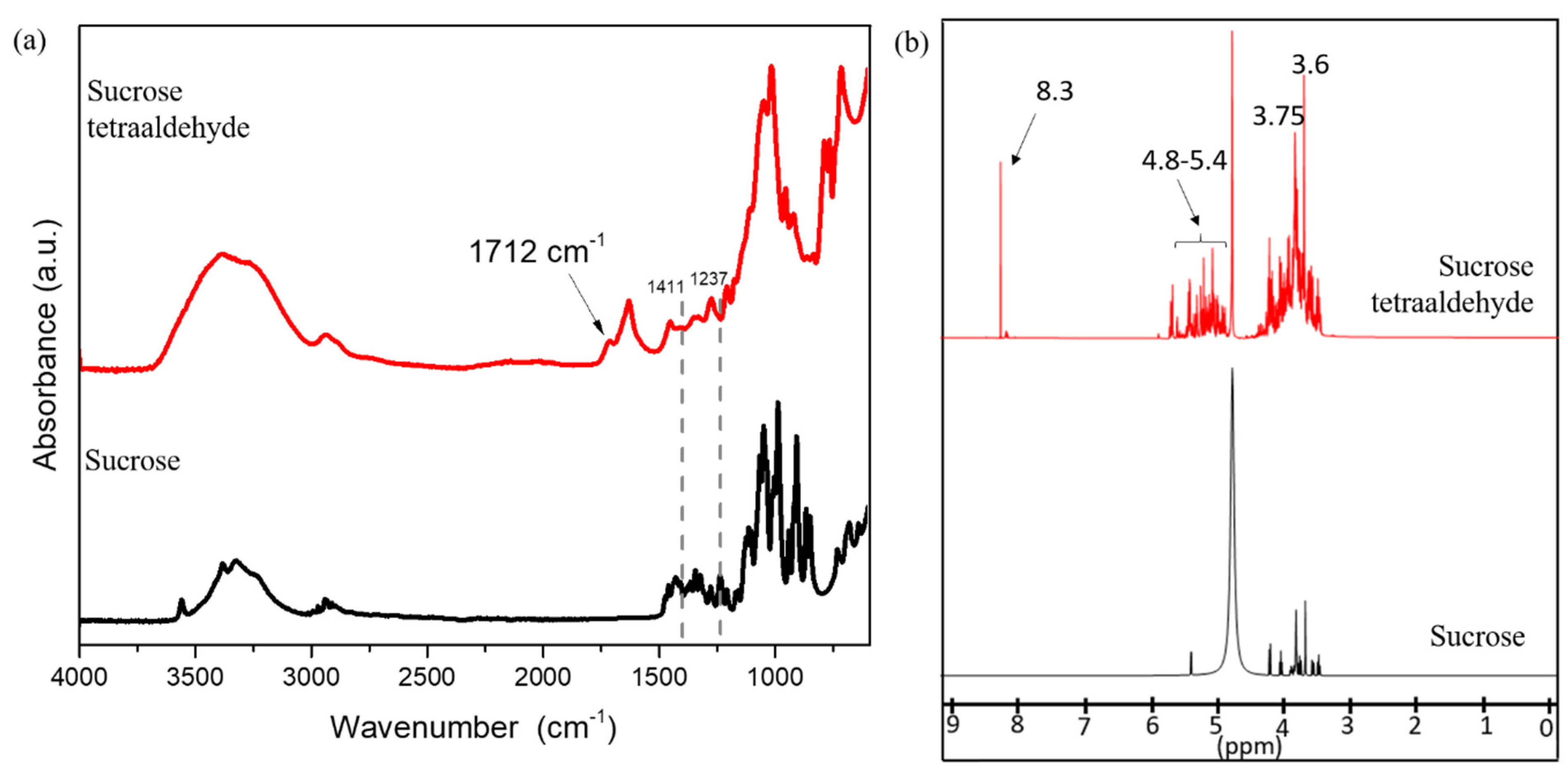
Figure 1 shows ATR-FTIR and
1H-NMR spectra of sucrose and the prepared ST solution. As can be seen in
Figure 1a, the ATR-FTIR spectrum for sucrose shows unique peaks associated with sucrose in the fingerprint region between 1500 cm
−1 and 700 cm
−1 wavenumbers. The peak observed at 907 cm
−1 corresponds to the C-H bending in sucrose. The peaks at 1049 cm
−1 and 1237 cm
−1 correspond to the C-O stretch and C-C stretch in sucrose from C-OH groups, respectively, while the peak at 1003 cm
−1 corresponds to the stretching of the C-O band of the C-O-C linkage [
18]. The peaks at 1322 cm
−1 and 1411 cm
−1 are due to O-H bending of C-OH groups in sucrose. As seen in the spectrum of ST in
Figure 1a, the peak at 1237 cm
−1 disappeared, and the intensity of the peak at 1411 cm
−1 reduced. Both these peaks correspond to the C-OH bending in sucrose, and the reduction in the peak intensity is the result of the oxidation of hydroxyls to aldehyde groups. The ATR-FTIR spectra of ST showed an additional peak at 1712 cm
−1. This new peak at 1712 cm
−1 lies in the carbonyl region and corresponds to the aldehyde groups resulting from sucrose oxidation [
18,
19]. NaIO
4 has been shown to cleave the vicinal hydroxyl groups showed by red color bonds in
Scheme 1 and attach IO
4− onto the hydroxyl groups [
20,
21,
22]. The lone pair on OH then attacks the iodine and initiates the proton transfer. At the same time, the lone pair from the adjacent hydroxyl group attacks the same iodine, leading to two proton transfers, which causes the oxidation of hydroxyl groups to aldehyde groups. As seen from the structure of sucrose in
Scheme 1, fructose has only two vicinal hydroxyl groups that IO
4− can attach to and initiate proton transfer from two hydroxyl groups oxidizing them to two aldehyde groups and leaving NaIO
3− and H
2O [
20]. Glucose has three such vicinal hydroxyl groups, and IO
4− cleaves one pair of hydroxyl groups shown in red color bonds and initiates proton transfer. Another IO
4− attaches to the second red color bond shown in
Scheme 1 and initiates the proton transfer. The middle hydroxyl group shown in blue color is released as formaldehyde, as shown in
Scheme 1, leaving behind 2NaIO
3− and H
2O. Thus, overall, five secondary hydroxyl groups that lie in the vicinity of each other result in four aldehyde groups creating sucrose tetraaldehyde (ST). This confirms earlier results obtained by Xu et al. [
22]. A similar peak at 1718 cm
−1 was also seen by Jalaja and James after oxidizing sucrose using NaIO
4 [
20]. In both spectra obtained for sucrose and the ST solution, the peak observed around 3360 cm
−1 corresponds to hydroxyl groups in sucrose, and the peaks between 2900 and 2700 cm
−1 correspond to the C-H stretch. The additional peak at 1630 cm
−1 in ST is from the hydroxyl groups of water.
Figure 1b shows
1H-NMR spectra for sucrose and ST. The protons associated with sucrose were seen at 5.4 ppm and between 4.2 ppm and 3.2 ppm. The proton shift seen at 4.7 ppm in both spectra is a result of the D
2O used as a solvent. The proton shifts at 4 ppm and 4.2 ppm in the sucrose spectrum were due to the protons from secondary hydroxyl groups present on the fructose ring in sucrose. The three proton shifts associated with the three secondary hydroxyl groups on the glucose molecule are seen between 3.5 and 3.75 ppm in the sucrose spectrum. Fructose and glucose have two and three vicinal hydroxyl groups or secondary hydroxyl groups, respectively, which are cleaved by NaIO
4 and oxidized to form two aldehyde groups on each fructose and glucose (as discussed above and in reaction
Scheme 1). Comparing the intensities of the two strong proton shifts at 3.6 and 3.75 in sucrose and ST, the intensity of the proton shift at 3.75 reduced significantly relative to the intensity of proton shift at 3.6 ppm in sucrose tetraaldehyde. While both proton shifts at 3.6 and 3.75 are due to the secondary protons, the proton shift at 3.75 ppm corresponds to the proton attached to the carbon present at the center of the three hydroxyl groups in glucose (shown in blue color in
Scheme 1). The lower intensity of proton shift at 3.75 ppm relative to the proton shift at 3.6 ppm, indeed, shows that the NaIO
4 successfully cleaved the vicinal hydroxyl groups and the proton attached to the center carbon of the two bonds shown in red color in
Scheme 1 is lost due to the attack. The aldehyde group formation can be clearly seen from the
1H-NMR of ST, where an additional proton shift was observed at 8.3 ppm due to the protons associated with the aldehyde groups created by oxidation [
19]. The additional proton shifts between 4.8 and 5.5 ppm indicate the intermolecular acetal protons due to the hemiacetal formation [
19,
20,
21,
22]. These results confirm earlier results by de Witt et al., who observed sucrose tetraaldehyde formation using the HPLC method [
23].
4.2. Characterization of Hair
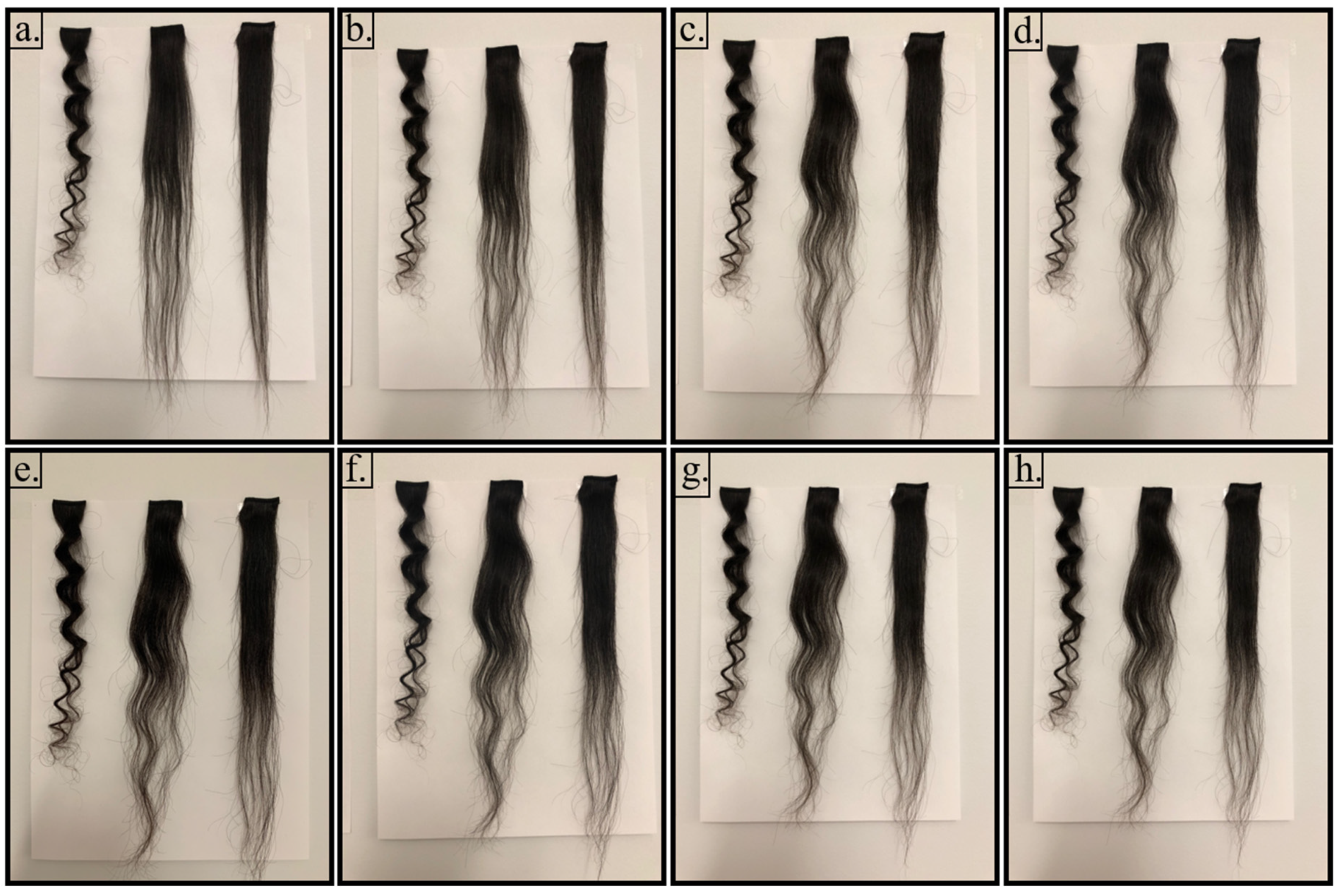
Figure 2 shows pictures of control (C), flat ironed (FI), and ST solution-treated (STA) hair swatches, from left to right, respectively, taken from day 0 to day 15 exposure in the conditioning room after the treatments. As seen in
Figure 2a, the control hair swatch showed five curls (each curl is counted as a ‘z’ from top to bottom). It is clear that flat ironing helps to straighten the curls, as seen in
Figure 2a (0 day), and so does the STA treatment. As can be seen in
Figure 2b, however, the FI hair showed at least one curl at the bottom of the swatch after just 1 day in the conditioning room at 21 °C and 65% RH, while the STA hair maintained its full straightness.
Figure 2c shows pictures of hair after 2 days in the conditioning room. The curl formed after 1 day on the FI hair is seen to be enhanced, and further, it is accompanied by two additional distinct curls. The STA hair, however, retained most of its straightness except for the very bottom tip of the swatch after 2 days in the conditioning room. Because of their unequal lengths, the number of hairs near the bottom of the swatch are fewer and, hence, slip through the flat iron gap. As a result, the hairs at the bottom of the swatch do not receive adequate treatment as at the top and middle of the swatch.
Figure 2d–f shows the pictures of hair swatches after 3, 5, and 7 days, respectively. It can be observed that FI hair became fluffed-up, showing at least three distinct curls, while only the bottom tip of STA hair showed signs of curling. Most of the top hair in the STA hair swatch maintained perfect straightness. A similar effect was observed at the end of 15 days in the conditioning room (
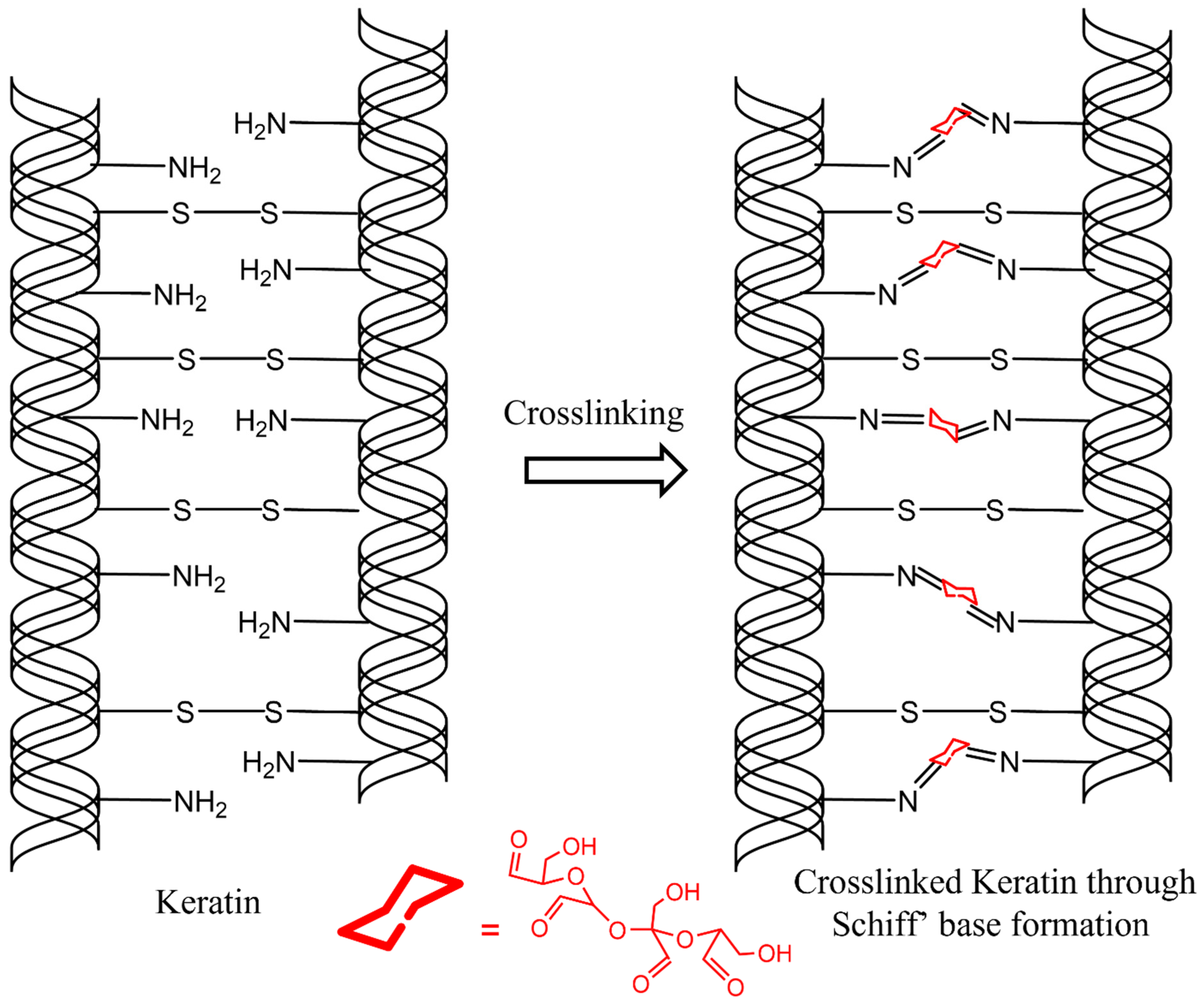
Figure 2h). As mentioned earlier, the STA hair was well crosslinked due to the reaction of aldehyde groups from the ST solution and the amine groups from the keratin in the hair. As discussed earlier, amine groups from hair and aldehyde groups from ST react to form covalent bonds via imine (Schiff base) formation, as shown in
Scheme 2 [
18]. Since the crosslinked molecules are unable to move, it provides the hair significant stabilization against the humidity. The FI hair, on the other hand, is just straightened due to the mechanical force and the heat applied through flat ironing, which involves rearranging hydrogen bonds. However, the newly formed hydrogen bonds from using a flat iron (heat and mechanical treatment) are susceptible to humid conditions, and the hair reverts back to its original state over time [
24]. Moisture (65% RH) present in the conditioning room is known to easily plasticize the FI hair, causing them to curl and fluff-up [
25]. Four aldehyde groups present on ST significantly increase the potential for crosslinking with many amine groups present in the keratin as compared to formaldehyde or glutaraldehyde, which consist of one and two aldehyde groups, respectively. Multiple intra- and interfibrillar crosslinks formed between cortical cells, as well as within the cortex, after ST treatment allow forming a 3D network that stabilizes the hair and helps retain its desired shape. This would be true even when not all of the aldehyde groups present on ST are able to react. Commercial hair straightening products, on the other hand, contain alkali-based relaxers that break the disulfide bond in the hair. The disulfide covalent bonds provide structural integrity to the hair, and, as a result, alkaline relaxation carried out using commercial products can weaken the hair. However, most commercial hair straightening products rely on breaking the disulfide bonds and further oxidizing or crosslinking them. A two-step process similar to that used by commercial products was used in this research to compare the effect of reduction in hair. Glutathione (GSH), commonly used to break the disulfide bonds in proteins, was used to break the disulfide bonds in keratin as the 1st step. In the second step, hair was treated with ST to crosslink the hair. The reduction step, using GSH, did not increase the hair straightening efficiency. It also did not reduce the number of passes or the temperature of the flat iron. Thus, breaking of disulfide bonds was deemed unnecessary for obtaining straightening of hair using ST, which can be a significant benefit.
The C, FI, and STA hair swatches were repeatedly washed using a warm 2% shampoo solution for 5 min and rinsed thoroughly under running tap water at RT until no lather was present. The wet hair swatches were placed on paper and hung on a wall in the conditioning room (21 °C and 65% RH) for 2 days before washing again.
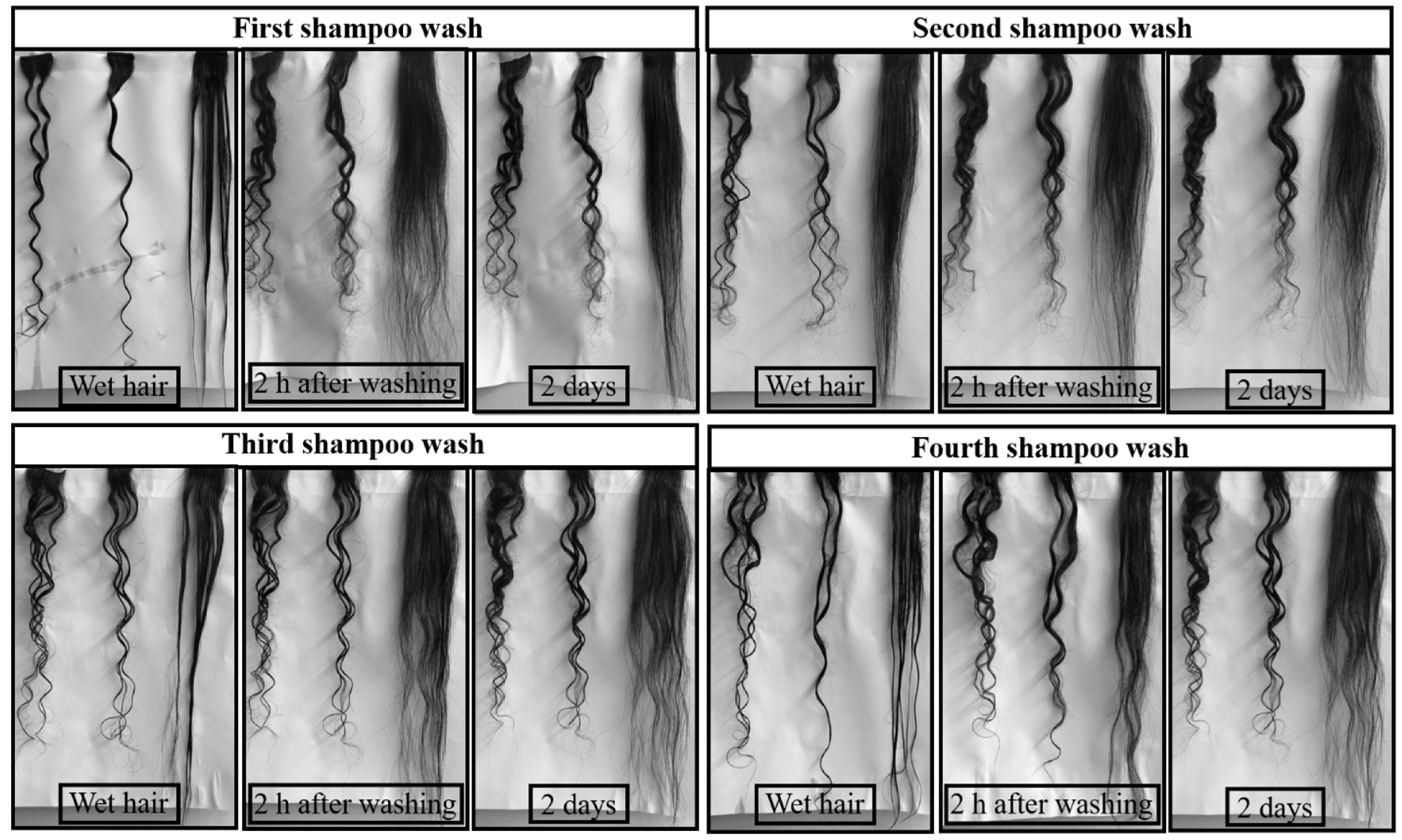
Figure 3 shows pictures of C, FI, and STA hair swatches after four shampoo washes. Pictures of wet hair were taken immediately after washing. The second set of pictures of hair swatches was taken after 2 h of drying in the conditioning room after shampoo washes and the third set after 2 days in the conditioning room. As can be seen in
Figure 3, the control wet hair showed five distinct curls when washed with shampoo. Upon drying for 2 h in the conditioning room, the control hair fluffed up, causing a reduction in the length of the hair swatch and enhancing the curvature of the curls. The FI hair also curled-up immediately during the first shampoo wash, while the STA hair remained straight after the first shampoo wash. Upon drying in the conditioning room after the shampoo wash, the wet FI hair fluffed up, and the curvature of the curls increased. As a result, the length of the FI swatch was also reduced. The FI hair looked close to the C hair, as it regained all the original curls after the first shampoo wash. As mentioned earlier, flat ironing only straightens the hair temporarily as it only involves alterations of hydrogen bonds and salt linkages [
24]. As the FI hair regained most of its original curls, the length of the swatch decreased and became close to that of control hair. STA hair, however, maintained its straightness for two shampoo washes as a result of the covalent crosslinks formed, which are more permanent. As mentioned earlier, the 3D network formed within the cortex after crosslinking restricts the movement or slippage of the keratin molecules and helps retain their shape. The STA hair retained its straightness even after the third shampoo wash. It, however, started to curl around the bottom part of the swatch while drying in the conditioning room. As mentioned earlier, the bottom part of the swatch has fewer hairs that do not come in complete contact with the flat iron plates and reflect inadequate crosslinking treatment. Because of the insufficient crosslinking, the moisture in the conditioning room causes the bottom of the STA hair to begin curling. At least one curl, with low curvature, was observed after the third and fourth shampoo wash while drying with negligible reduction in the overall length of the hair swatch.
The straightening efficiency (SE) values were calculated for FI and STA hair swatches for up to 45 days of exposure to 65% RH and for 21 °C, as well as FI and STA hair swatches after up to 4 shampoo washes, using Equation (1).
Figure 4a shows the SE of FI and STA hair as a function of the days the hair swatches were hung on a wall in the conditioning room at 65% RH for up to 45 days. As seen in
Figure 4a, the SE of FI and STA hair was 100% on day 0. After 1 day in the conditioning room, the SE of the FI hair reduced to 67%, while the SE of STA hair was still 100%. The change in SE was a result of at least one curl observed in the FI hair after 1 day at 65% RH, while the STA hair was fully straight (SE 100%). This can be clearly seen in
Figure 2. The SE of FI hair further reduced to 60% after 2 days in the conditioning room at 65% RH as two additional curls developed on the FI swatch, while a slight curl in the bottom part of the STA hair was observed, bringing down the SE of the STA hair to 87%. The reduction in SE for FI hair to 65% was found to be statistically significant using an unpaired t-test at a significance level of 0.05. The SE of the FI hair was further reduced to 58.3% and 57.6% as the intensity of the curvature of the curls and the number of curls increased after 3 and 5 days in the conditioning room, respectively. No additional curls, however, were observed on STA hair, and its SE remained constant at 87% for up to 5 days in the conditioning room. The SE of the FI hair further reduced to 57% after 7 days in the conditioning room and remained constant at 57% over time for up to 45 days. The SE of STA hair, on the other hand, was found to be 87% after 7 days in the conditioning room, after which it remained unchanged for up to 45 days. The change in SE of the STA hair after 2 days in the conditioning room was not found to be statistically different for up to 45 days in the conditioning room using an unpaired t-test at a significance level of 0.05. Overall, the results indicate that the SE of the STA hair was significantly higher and more permanent compared to the FI hair.
Figure 4b shows the SE values of C, FI, and STA hair as a function of the number of shampoo washes. It should be noted that each shampoo wash was accompanied by a 2-day exposure to 65% RH at 21 °C. As can be seen from
Figure 4b, the SE of FI hair reduced to 22.4% after the very first shampoo wash. This implies that FI hair regained most of its curls upon washing with shampoo. This is quite expected because flat ironing is only a temporary way of hair straightening. Hair straightening using a flat iron only involved breaking and reforming of a few hydrogen bonds in keratin, which are weak and easily disrupted by water. As a result, FI hair almost reverted back to its original curly state after washing [
6]. The SE of FI hair was found to decrease further to 12.8% after the second and third shampoo washes and down to just 8% after the fourth shampoo wash. This implies that FI hair gets very close to its original state or C hair after washing with shampoo. It was also found that shampoo washing of the C hair introduces some additional curls onto the swatch, reducing their length. Thus, the SE of the C hair was negative. It was found to be −4.2% after two shampoo washes, after which it remained constant at −13.6% after the third and fourth shampoo washes. The SE of STA hair was maintained at 100% for up to two shampoo washes, after which it reduced to 87% after the third and fourth shampoo washings. Overall, the data in
Figure 4b indicate that the SE of FI hair reduces significantly (very close to the control) as the hair regains most of its original curls after washing, while the SE of STA only reduces to 87% after four shampoo washes. This further confirms that the STA treatment is durable and can be used to attain permanent hair styling.
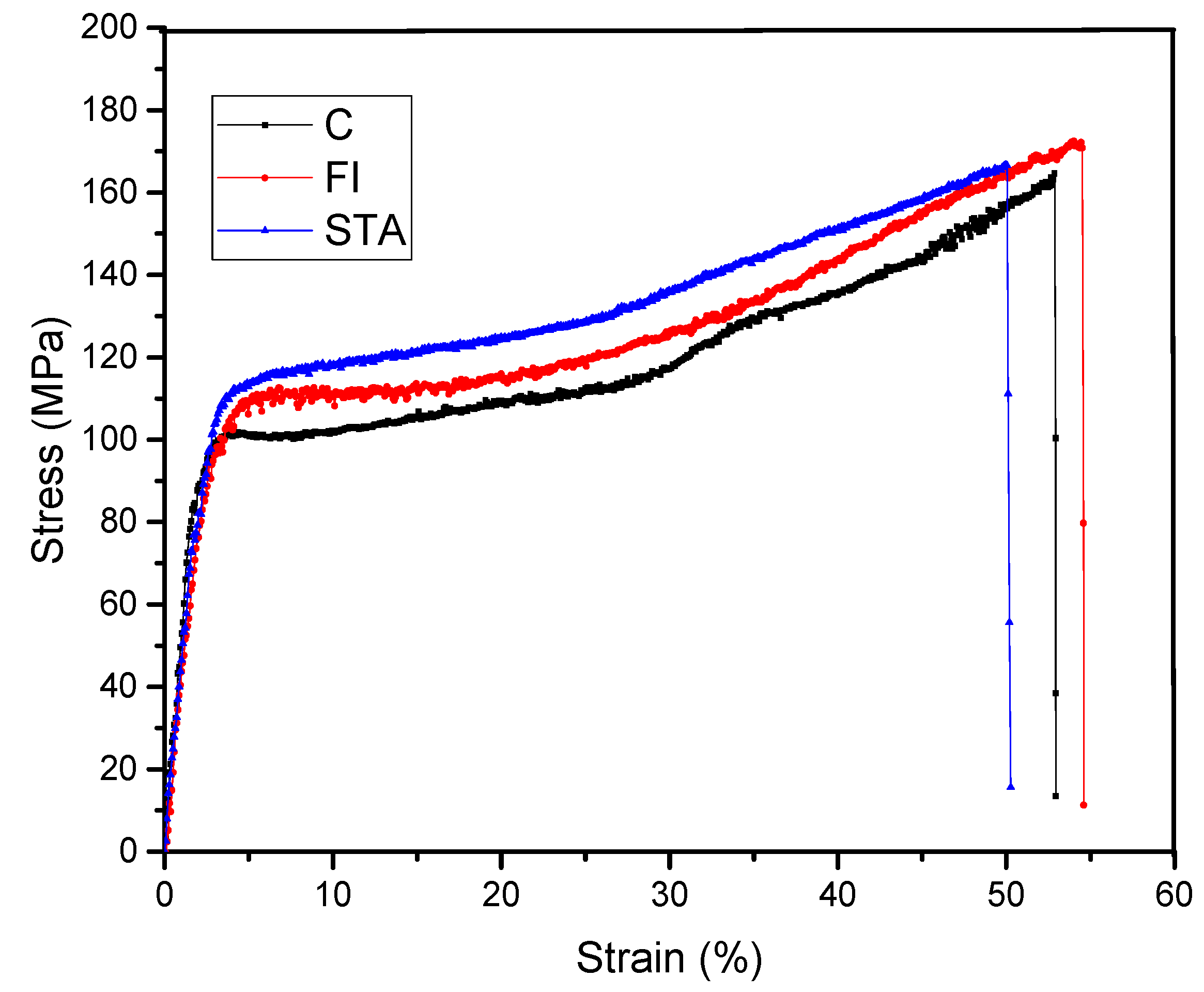
Figure 5 shows typical stress–strain plots for C, FI, and STA hair specimens. The tensile properties of C, FI, and STA individual hair specimens are summarized in
Table 1. As seen in
Figure 5, stress–strain plots for all hair show three distinct regions: initial Hookean region, yield region, and post-yield region [
26]. The Hookean region lies between 0% and 5% tensile strain. The average tensile stress value for the C hair specimen at the end of the Hookean region was 103 MPa. In comparison, the average tensile stress values of the FI and STA hair specimens were observed to be 99.8 MPa and 99.7 MPa, respectively. These values are not significantly different compared to C hair and indicate that properties within the Hookean region remained unchanged after treatments. The tensile strains of C, FI, and STA hair specimens in the Hookean region were found to be 4.1%, 4.7%, and 4.3%, respectively. This suggests that the initial elastic characteristics of the FI and STA hair also remained unchanged after the treatments. The strain in the Hookean region comes from the extension of weaker bonds, such as hydrogen bonding within the amino acids, Van der Waals forces, and coulombic interactions [
19]. Immediately following the Hookean region, the hair showed yielding behavior where a large amount of strain was associated with a very small rise in stress, signifying very low modulus or high compliance. This yield region extended from 5% to 30% strain for all hair specimens. The yielding involves the unfolding of the α-keratin to β-keratin conformation, which allows a larger deformation in the hair before breaking [
19,
26]. Strain-hardening phenomenon was observed beyond the yield region for all fibers [
19,
26]. The strain-hardening region extends from 30% to 50% strain and ends when the hair ruptures. The breaking or ultimate fracture stress values for C, FI, and STA hair specimens were found to be 164 MPa, 171 MPa, and 166 MPa, respectively. The differences in these fracture stress values were found to be statistically insignificant, using an unpaired t-test, at a significance level of 0.05. This implies that the tensile stress of the hair strands was unaffected after flat ironing performed in this study, as well as the sucrose ST. It is known that the keratin in hair is susceptible to thermal degradation during flat ironing, which results in the weakening of the hair strands [
6]. Continual heat treatments, such as flat ironing, particularly in wet conditions, increase hair damage [
27]. In the present study, flat ironing of hair in a wet state was intentionally avoided, and ST solution was allowed to dry before flat ironing of hair. While the pH of most commercial hair straightening products is highly alkaline (above 10), the pH of sucrose ST was adjusted to neutral pH of 7 to avoid alkaline denaturation of keratin protein and retain tensile properties. Overall, the tensile results suggest that the tensile properties of hair remain unchanged after the ST treatment and, hence, could be a viable, healthy alternative to currently used toxic hair crosslinkers. It is also clear from the data presented in
Table 1 that the FI treatment reduced the initial modulus (Young’s modulus) to 2.3 GPa from 2.6 Gpa obtained for C hair. However, after the ST treatment, the modulus recovered back to 2.6 Gpa, as a result of crosslinking. The crosslinked network formed by ST, however, does not seem too highly crosslinked, and overall, the ST treatment results in the least damage to hair.
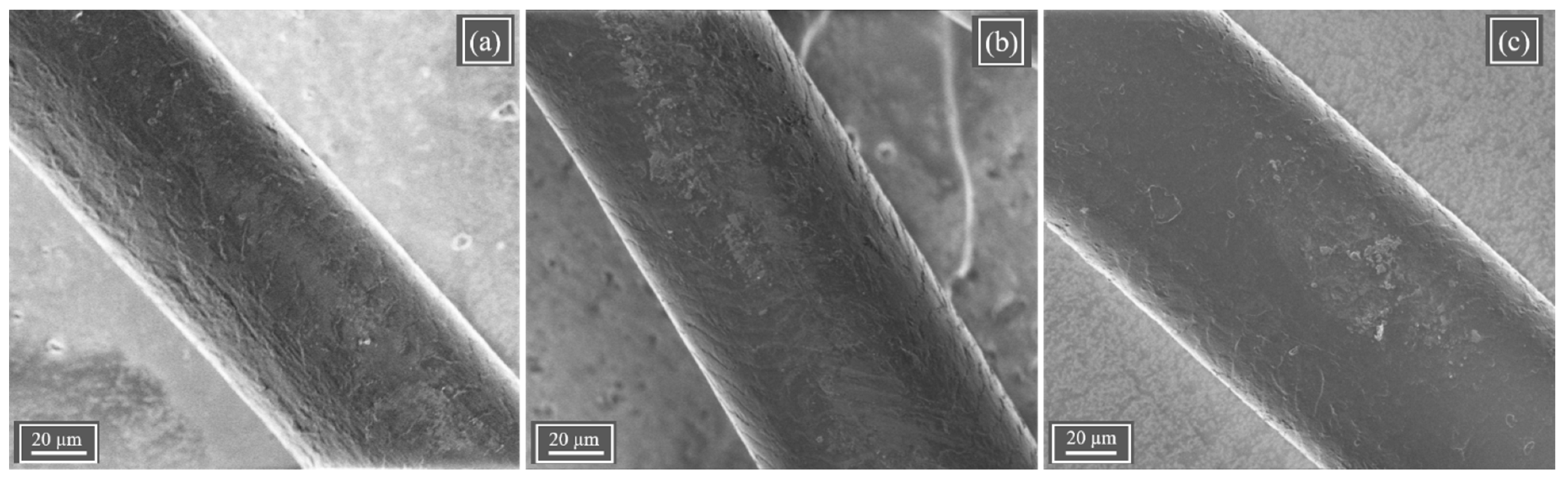
Figure 6 shows typical SEM images of C, FI, and STA hair specimens. As seen in
Figure 6a, the control hair showed typical scales on the surface. No visible change or damage to the scales on the surface was observed after FI or STA treatments on the hair. Since the excess heat of the flat iron can damage the hair, especially the surface scales, which are in contact with the flat iron, if carried out in the wet condition, care was taken to ensure that the hair was dry after treating with STA (by leaving them to air dry at RT for 10 min) before flat ironing. Most of the keratin crosslinking by ST solution occurs inside the hair as it is a small molecule and can penetrate the hair easily, leaving the surface of the hair undamaged after STA treatment.