Antimicrobial Efficacy of Low Concentration PVP-Silver Nanoparticles Deposited on DBD Plasma-Treated Polyamide 6,6 Fabric
Abstract
:1. Introduction
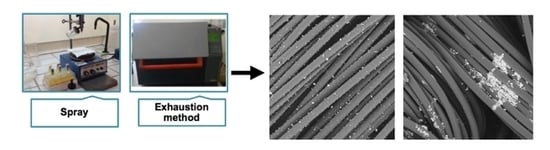
2. Materials and Methods
3. Results
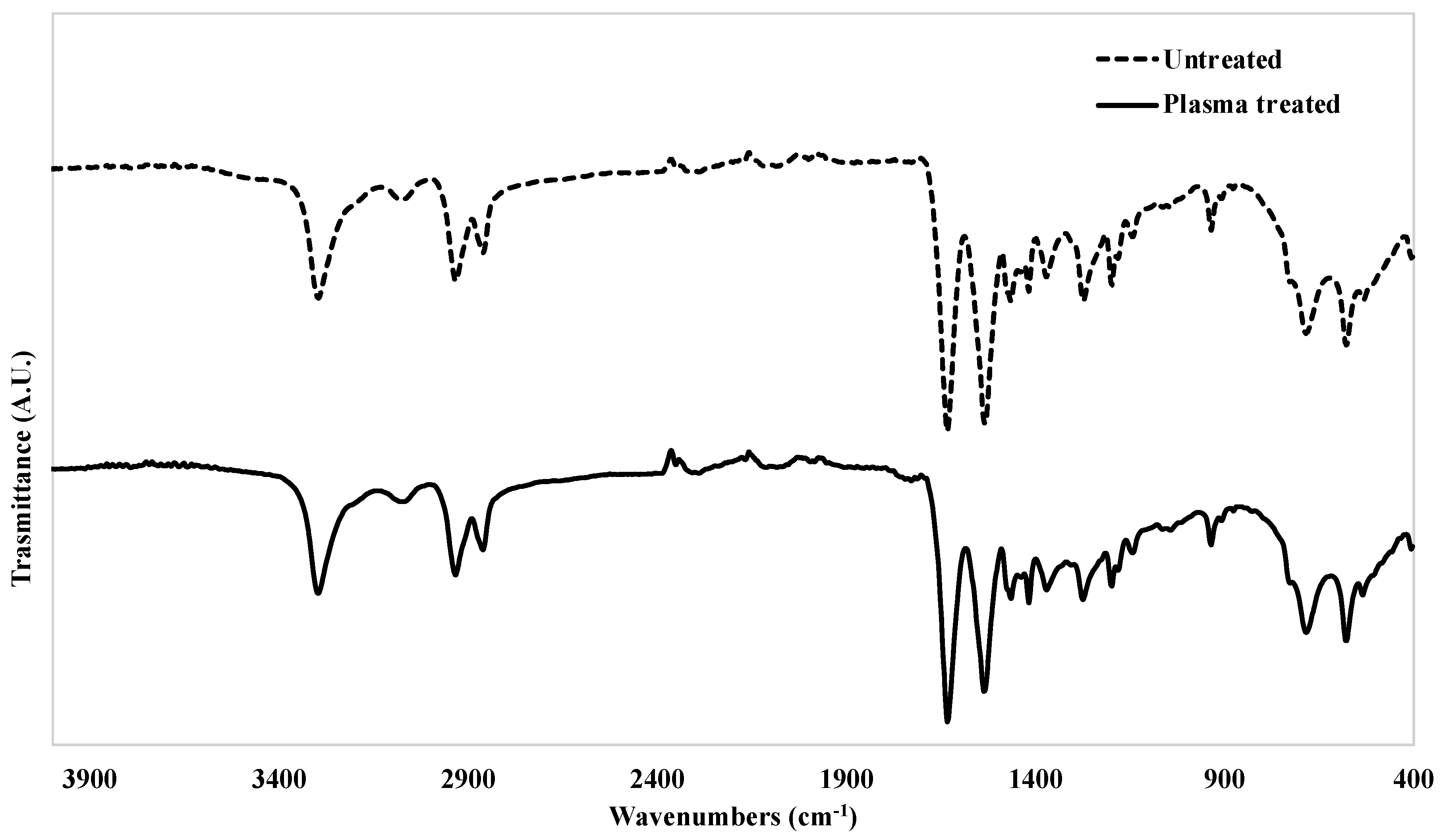
3.1. FTIR–ATR Analyses of Untreated and Plasma-Treated PA66 Samples
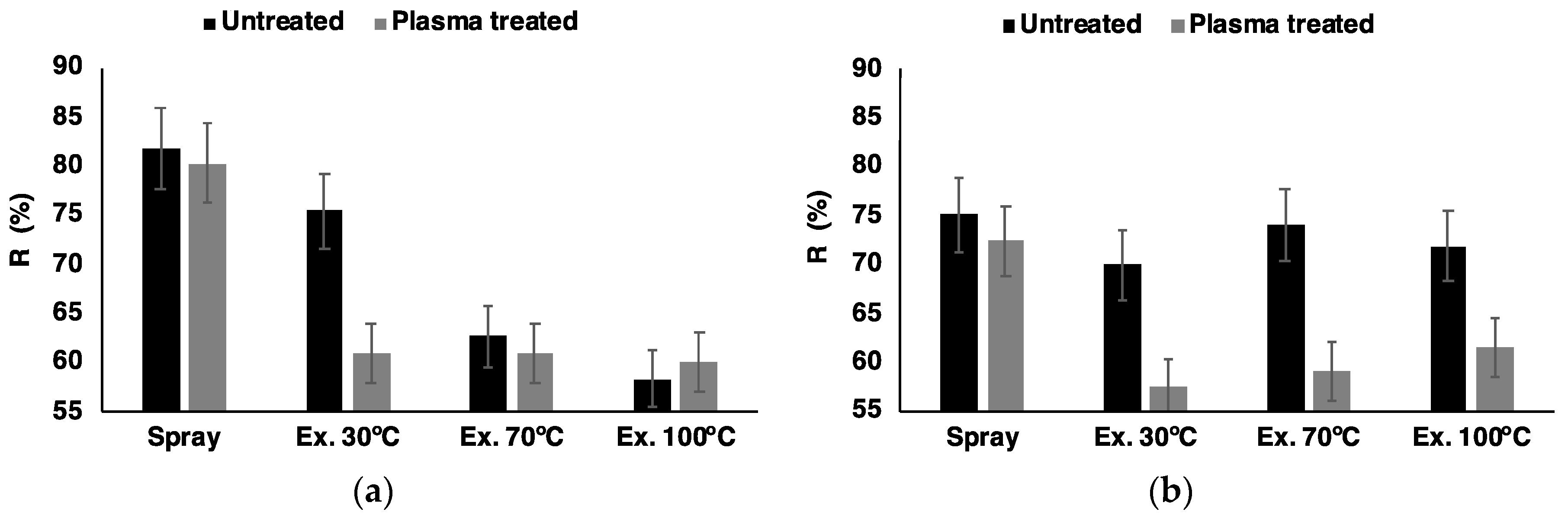
3.2. Reflectance Spectroscopy
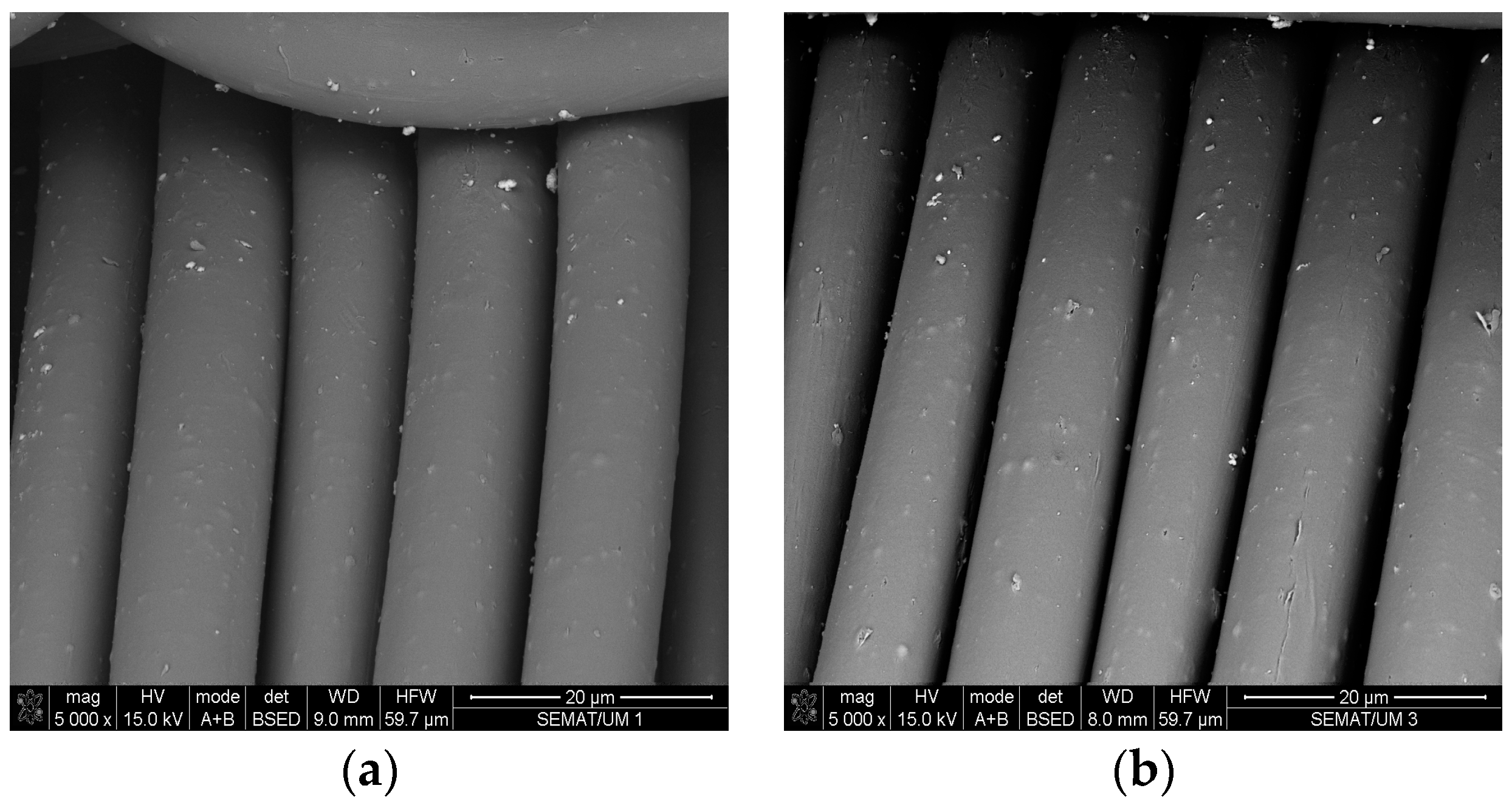
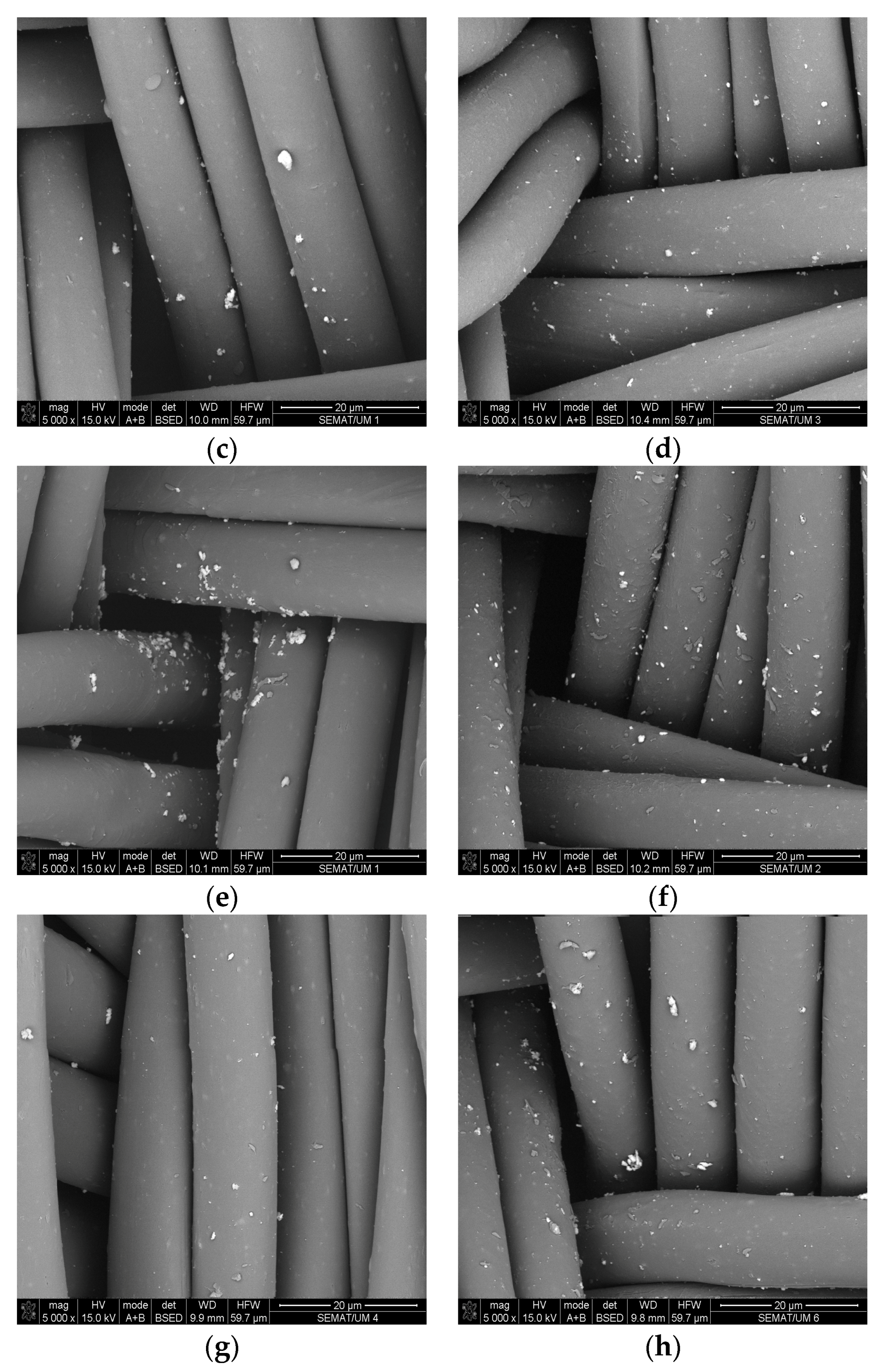
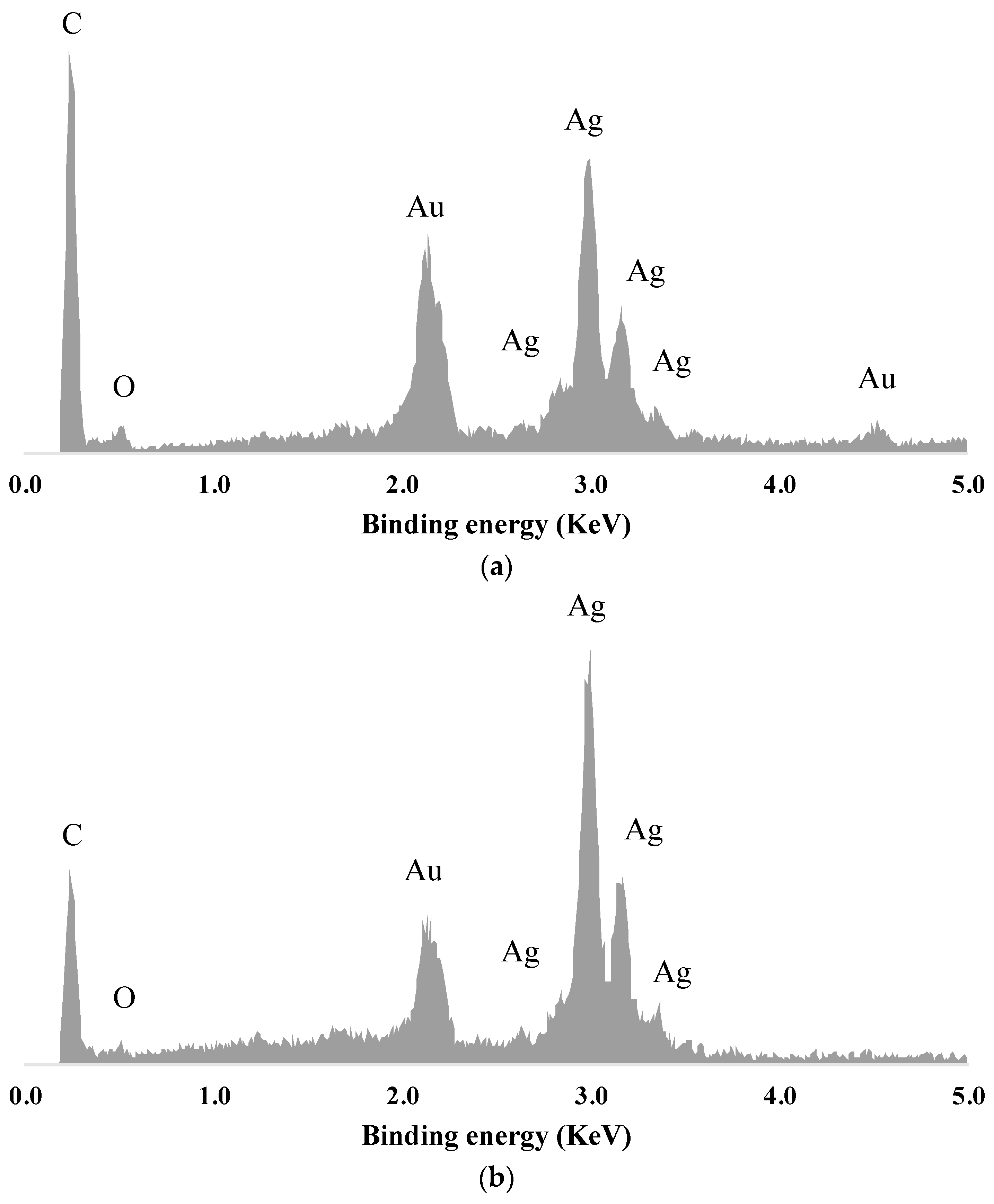
3.3. SEM and EDS Analysis
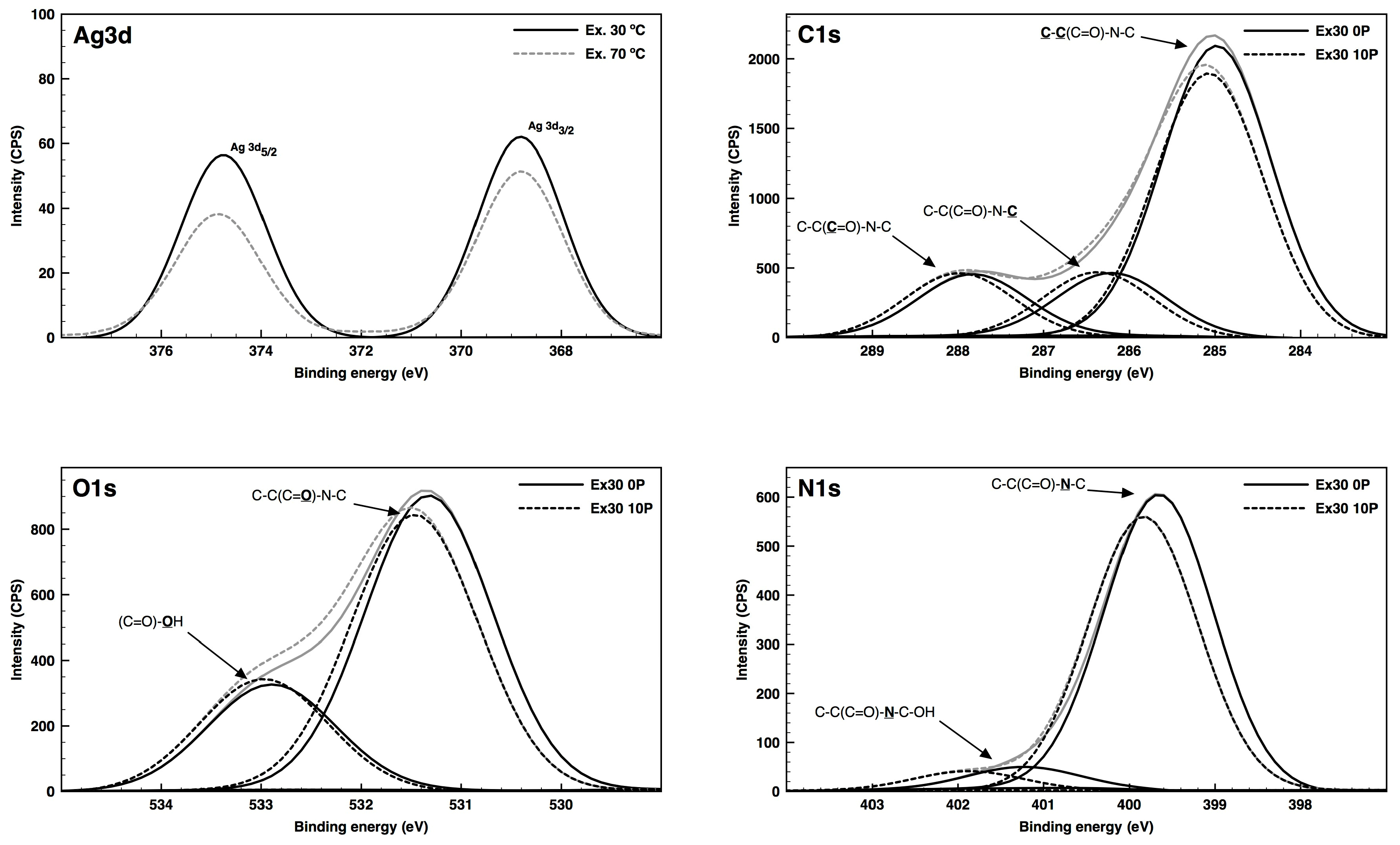
3.4. XPS Analysis
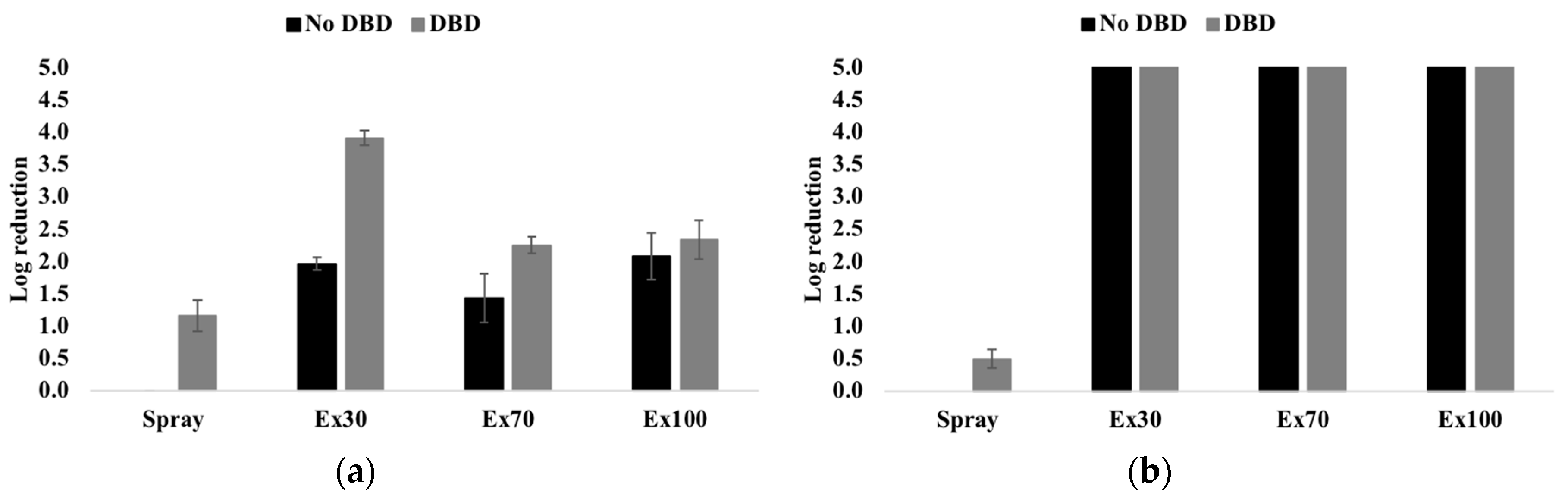
3.5. Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Surwade, P.; Ghildyal, C.; Weikel, C.; Luxton, T.; Peloquin, D.; Fan, X.; Shah, V. Augmented antibacterial activity of ampicillin with silver nanoparticles against methicillin-resistant Staphylococcus aureus (MRSA). J. Antibiot. 2018, 72, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Zhang, J.; Xing, T.; Chen, G.; Tao, R.; Choo, K.-H. Green synthesis of silver nanoparticles using grape seed extract and their application for reductive catalysis of direct orange 26. J. Ind. Eng. Chem. 2018, 58, 74–79. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Jeon, H.-J.; Ahn, C.W. Synthesis of silver nanoparticles in an eco-friendly way using phyllanthus amarus leaf extract: Antimicrobial and catalytic activity. Adv. Powder Technol. 2018, 29, 86–93. [Google Scholar] [CrossRef]
- Vidanapathirana, A.K.; Thompson, L.C.; Herco, M.; Odom, J.; Sumner, S.J.; Fennell, T.R.; Brown, J.M.; Wingard, C.J. Acute intravenous exposure to silver nanoparticles during pregnancy induces particle size and vehicle dependent changes in vascular tissue contractility in sprague dawley rats. Reprod. Toxicol. 2018, 75, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Veisi, H.; Azizi, S.; Mohammadi, P. Green synthesis of the silver nanoparticles mediated by thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. J. Clean. Prod. 2018, 170, 1536–1543. [Google Scholar] [CrossRef]
- Filipak Neto, F.; Cardoso da Silva, L.; Liebel, S.; Voigt, C.L.; Oliveira Ribeiro, C.A.D. Responses of human hepatoma HepG2 cells to silver nanoparticles and polycyclic aromatic hydrocarbons. Toxicol. Mech. Methods 2017, 28, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Pansara, C.; Chan, W.Y.; Parikh, A.; Trott, D.J.; Mehta, T.; Mishra, R.; Garg, S. Formulation optimization of chitosan-stabilized silver nanoparticles using in vitro antimicrobial assay. J. Pharm. Sci. 2019, 108, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Eklöf, J.; Gschneidtner, T.; Lara-Avila, S.; Nygård, K.; Moth-Poulsen, K. Controlling deposition of nanoparticles by tuning surface charge of SiO2 by surface modifications. RSC Adv. 2016, 6, 104246–104253. [Google Scholar] [CrossRef]
- Perelshtein, I.; Lipovsky, A.; Perkas, N.; Tzanov, T.; Gedanken, A. Sonochemical co-deposition of antibacterial nanoparticles and dyes on textiles. Beilstein J. Nanotechnol. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Parham, S.; Nemati, M.; Sadir, S.; Bagherbaigi, S.; Wicaksono, D.H.B.; Nur, H. In situ synthesis of silver nanoparticles for Ag-NP/cotton nanocomposite and its bactericidal effect. J. Chin. Chem. Soc. 2017, 64, 1286–1293. [Google Scholar] [CrossRef]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- El-Zahry, M.R. A comparative study of sterically and electro-statically stabilized silver nanoparticles for the determination of muscle relaxant tizanidine: Insights of localized surface plasmon resonance, surface enhanced raman spectroscopy and electrocatalytic activity. Talanta 2018, 186, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, A.; Deng, X.; Xiong, Q.; Cvelbar, U.; DeGeyter, N.; Morent, R.; Leys, C. Non-thermal plasma technology for the development of antimicrobial surfaces: A review. J. Phys. D Appl. Phys. 2016, 49, 204002. [Google Scholar] [CrossRef]
- Petlin, D.G.; Tverdokhlebov, S.I.; Anissimov, Y.G. Plasma treatment as an efficient tool for controlled drug release from polymeric materials: A review. J. Control. Release 2017, 266, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Zille, A.; Oliveira, F.R.; Souto, A.P. Plasma treatment in textile industry. Plasma Process. Polym. 2015, 12, 98–131. [Google Scholar] [CrossRef]
- Gorjanc, M.; Gorensek, M.; Jovancic, P.; Mozetic, M. Multifunctional textiles—Modification by plasma, dyeing and nanoparticles. In Eco-Friendly Textile Dyeing and Finishing; InTech Open: London, UK, 2013. [Google Scholar]
- Szulc, J.; Urbaniak-Domagała, W.; Machnowski, W.; Wrzosek, H.; Łącka, K.; Gutarowska, B. Low temperature plasma for textiles disinfection. Int. Biodeterior. Biodegrad. 2018, 131, 97–106. [Google Scholar] [CrossRef]
- Tseng, K.-H.; Chou, C.-J.; Liu, T.-C.; Tien, D.-C.; Wu, T.-C.; Stobinski, L. Interactive relationship between silver ions and silver nanoparticles with PVA prepared by the submerged arc discharge method. Adv. Mater. Sci. Eng. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kang, S.K.; Kwon, H.C.; Lee, H.W.; Lee, J.K. Gas temperature effect on reactive species generation from the atmospheric pressure air plasma. Plasma Process. Polym. 2013, 10, 686–697. [Google Scholar] [CrossRef]
- Napartovich, A.P. Overview of atmospheric pressure discharges producing nonthermal plasma. Plasma Polym. 2001, 6, 1–14. [Google Scholar] [CrossRef]
- ISO 15797:2017 Textiles-Industrial Washing and Finishing Procedures for Testing of Workwear; ISO-International Organization for Standardization: Geneve, Swiss, 2017.
- ASTM E2149-01 Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents Under Dynamic Contact Conditions; ASTM International: West Conshohocken, PA, USA, 2001.
- Ma, Y.; Zhou, T.; Su, G.; Li, Y.; Zhang, A. Understanding the crystallization behavior of polyamide 6/polyamide 66 alloys from the perspective of hydrogen bonds: Projection moving-window 2D correlation ftir spectroscopy and the enthalpy. RSC Adv. 2016, 6, 87405–87415. [Google Scholar] [CrossRef]
- Zille, A.; Fernandes, M.M.; Francesko, A.; Tzanov, T.; Fernandes, M.; Oliveira, F.R.; Almeida, L.; Amorim, T.; Carneiro, N.; Esteves, M.F.; et al. Size and aging effects on antimicrobial efficiency of silver nanoparticles coated on polyamide fabrics activated by atmospheric DBD plasma. ACS Appl. Mater. Interfaces 2015, 7, 13731–13744. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, B.; Thundat, T.; Fleck, B.A.; Sadrzadeh, M. A novel approach toward fabrication of high performance thin film composite polyamide membranes. Sci. Rep. 2016, 6, 22069. [Google Scholar] [CrossRef] [PubMed]
- Menchaca, C.; Alvarez-Castillo, A.; Martinez-Barrera, G.; Lopez-Valdivia, H.; Carrasco, H.; Castano, V.M. Mechanisms for the modification of nylon 6,12 by gamma irradiation. Int. J. Mater. Prod. Technol. 2003, 19, 521–529. [Google Scholar] [CrossRef]
- Navarro-Pardo, F.; Martínez-Barrera, G.; Martínez-Hernández, A.; Castaño, V.; Rivera-Armenta, J.; Medellín-Rodríguez, F.; Velasco-Santos, C. Effects on the thermo-mechanical and crystallinity properties of nylon 6,6 electrospun fibres reinforced with one dimensional (1D) and two dimensional (2D) carbon. Materials 2013, 6, 3494–3513. [Google Scholar] [CrossRef] [PubMed]
- Montazer, M.; Shamei, A.; Alimohammadi, F. Synthesis of nanosilver on polyamide fabric using silver/ammonia complex. Mater. Sci. Eng. C 2014, 38, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.R.; Zille, A.; Souto, A.P. Dyeing mechanism and optimization of polyamide 6,6 functionalized with double barrier discharge (DBD) plasma in air. Appl. Surf. Sci. 2014, 293, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.-A.; Sun, G. Durable antimicrobial nylon 66 fabrics: Ionic interactions with quaternary ammonium salts. J. Appl. Polym. Sci. 2003, 90, 2194–2199. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, P.; Qiu, Y.; Yu, Q.; Ma, J.; Wu, H.; Luo, G.; Xu, M.; Yao, H. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem. Eng. J. 2015, 263, 1–8. [Google Scholar] [CrossRef]
- Pamreddy, A.; Skácelová, D.; Haničinec, M.; Sťahel, P.; Stupavská, M.; Černák, M.; Havel, J. Plasma cleaning and activation of silicon surface in dielectric coplanar surface barrier discharge. Surf. Coat. Technol. 2013, 236, 326–331. [Google Scholar] [CrossRef]
- Boroumand, M.N.; Montazer, M.; Simon, F.; Liesiene, J.; Šaponjic, Z.; Dutschk, V. Novel method for synthesis of silver nanoparticles and their application on wool. Appl. Surf. Sci. 2015, 346, 477–483. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, C.; Qiu, Y. Influence of the amount of absorbed moisture in nylon fibers on atmospheric pressure plasma processing. Surf. Coat. Technol. 2007, 201, 7453–7461. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, K.; Loo, L.S. Investigation of surface properties of plasma-modified polyamide 6 and polyamide 6/layered silicate nanocomposites. J. Mater. Sci. 2010, 46, 3084–3093. [Google Scholar] [CrossRef]
- Upadhyay, D.J.; Cui, N.-Y.; Anderson, C.A.; Brown, N.M.D. A comparative study of the surface activation of polyamides using an air dielectric barrier discharge. Colloids Surf. A Physicochem. Eng. Asp. 2004, 248, 47–56. [Google Scholar] [CrossRef]
- Zanna, S.; Saulou, C.; Mercier-Bonin, M.; Despax, B.; Raynaud, P.; Seyeux, A.; Marcus, P. Ageing of plasma-mediated coatings with embedded silver nanoparticles on stainless steel: An XPS and ToF-SIMS investigation. Appl. Surf. Sci. 2010, 256, 6499–6505. [Google Scholar] [CrossRef]
- Wang, X.; Somsen, C.; Grundmeier, G. Ageing of thin Ag/fluorocarbon plasma polymer nanocomposite films exposed to water-based electrolytes. Acta Mater. 2008, 56, 762–773. [Google Scholar] [CrossRef]
- Liu, W.; Li, B.; Cao, R.; Jiang, Z.; Yu, S.; Liu, G.; Wu, H. Enhanced pervaporation performance of poly (dimethyl siloxane) membrane by incorporating titania microspheres with high silver ion loading. J. Membr. Sci. 2011, 378, 382–392. [Google Scholar] [CrossRef]
- Itani, H.; Keil, P.; Lützenkirchen-Hecht, D.; Haake, U.; Bongard, H.; Dreier, A.; Lehmann, C.W.; Grundmeier, G. XANES studies of the formation of Ag-nanoparticles in LBL deposited polyelectrolyte thin films. Surf. Coat. Technol. 2010, 205, 2113–2119. [Google Scholar] [CrossRef]
- Vu, N.K.; Zille, A.; Oliveira, F.R.; Carneiro, N.; Souto, A.P. Effect of particle size on silver nanoparticle deposition onto dielectric barrier discharge (DBD) plasma functionalized polyamide fabric. Plasma Process. Polym. 2013, 10, 285–296. [Google Scholar] [CrossRef]
- Navarro Gallón, S.M.; Alpaslan, E.; Wang, M.; Larese-Casanova, P.; Londoño, M.E.; Atehortúa, L.; Pavón, J.J.; Webster, T.J. Characterization and study of the antibacterial mechanisms of silver nanoparticles prepared with microalgal exopolysaccharides. Mater. Sci. Eng. C 2019, 99, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Kulthong, K.; Srisung, S.; Boonpavanitchakul, K.; Kangwansupamonkon, W.; Maniratanachote, R. Determination of silver nanoparticle release from antibacterial fabrics into artificial sweat. Part. Fibre Toxicol. 2010, 7, 8. [Google Scholar] [CrossRef] [PubMed]
| Samples | C (at %) | O (at %) | N (at %) | Ag (at %) | O/C Ratio | N/C Ratio |
|---|---|---|---|---|---|---|
| Spray | 80.5 | 11.0 | 8.5 | ND | 0.14 | 0.11 |
| Spray DBD | 79.5 | 13.0 | 7.5 | ND | 0.16 | 0.09 |
| Ex. 30 | 77.9 | 11.8 | 10.3 | ND | 0.15 | 0.13 |
| Ex. 30 DBD | 77.0 | 12.5 | 9.0 | 1.5 | 0.16 | 0.12 |
| Ex. 70 | 80.1 | 11.0 | 8.9 | ND | 0.14 | 0.11 |
| Ex. 70 DBD | 79.9 | 11.6 | 8.2 | 0.3 | 0.15 | 0.10 |
| Samples | Ag3d | C1s | O1s | N1s | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 367.8 eV | 374.6 eV | 285.0 eV | 286.1 eV | 286.4 eV | 287.8 eV | 288.0 eV | 531.2 eV | 531.4 eV | 532.9 eV | 399.7 eV | 401.6 eV | |
| Spray No DBD | – | – | 71.5 | 13.4 | – | 15.0 | – | 74.3 | – | 25.7 | 95.6 | 4.4 |
| Spray DBD | – | – | 67.6 | – | 16.9 | 15.5 | – | – | 55.9 | 44.1 | 91.4 | 8.6 |
| Ex. 30 No DBD | – | – | 70.0 | 15.1 | – | 14.9 | – | 73.6 | – | 26.3 | 93.3 | 6.7 |
| Ex. 30 DBD | 52.2 | 47.8 | 67.2 | – | 16.5 | – | 16.3 | – | 71.4 | 28.6 | 93.4 | 6.6 |
| Ex. 70 No DBD | – | – | 71.4 | 13.5 | – | 15.1 | – | 73.9 | – | 26.1 | 88.9 | 11.1 |
| Ex. 70 DBD | 57.7 | 42.3 | 68.9 | – | 15.4 | – | 15.7 | – | 71.7 | 28.3 | 89.5 | 10.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.I.; Senturk, D.; Silva, K.K.; Modic, M.; Cvelbar, U.; Dinescu, G.; Mitu, B.; Nikiforov, A.; Leys, C.; Kuchakova, I.; et al. Antimicrobial Efficacy of Low Concentration PVP-Silver Nanoparticles Deposited on DBD Plasma-Treated Polyamide 6,6 Fabric. Coatings 2019, 9, 581. https://doi.org/10.3390/coatings9090581
Ribeiro AI, Senturk D, Silva KK, Modic M, Cvelbar U, Dinescu G, Mitu B, Nikiforov A, Leys C, Kuchakova I, et al. Antimicrobial Efficacy of Low Concentration PVP-Silver Nanoparticles Deposited on DBD Plasma-Treated Polyamide 6,6 Fabric. Coatings. 2019; 9(9):581. https://doi.org/10.3390/coatings9090581
Chicago/Turabian StyleRibeiro, Ana Isabel, Dilara Senturk, Késia Karina Silva, Martina Modic, Uros Cvelbar, Gheorghe Dinescu, Bogdana Mitu, Anton Nikiforov, Christophe Leys, Irina Kuchakova, and et al. 2019. "Antimicrobial Efficacy of Low Concentration PVP-Silver Nanoparticles Deposited on DBD Plasma-Treated Polyamide 6,6 Fabric" Coatings 9, no. 9: 581. https://doi.org/10.3390/coatings9090581