The Influence of Electrophoretic Deposition Parameters and Heat Treatment on the Microstructure and Tribological Properties of Nanocomposite Si3N4/PEEK 708 Coatings on Titanium Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
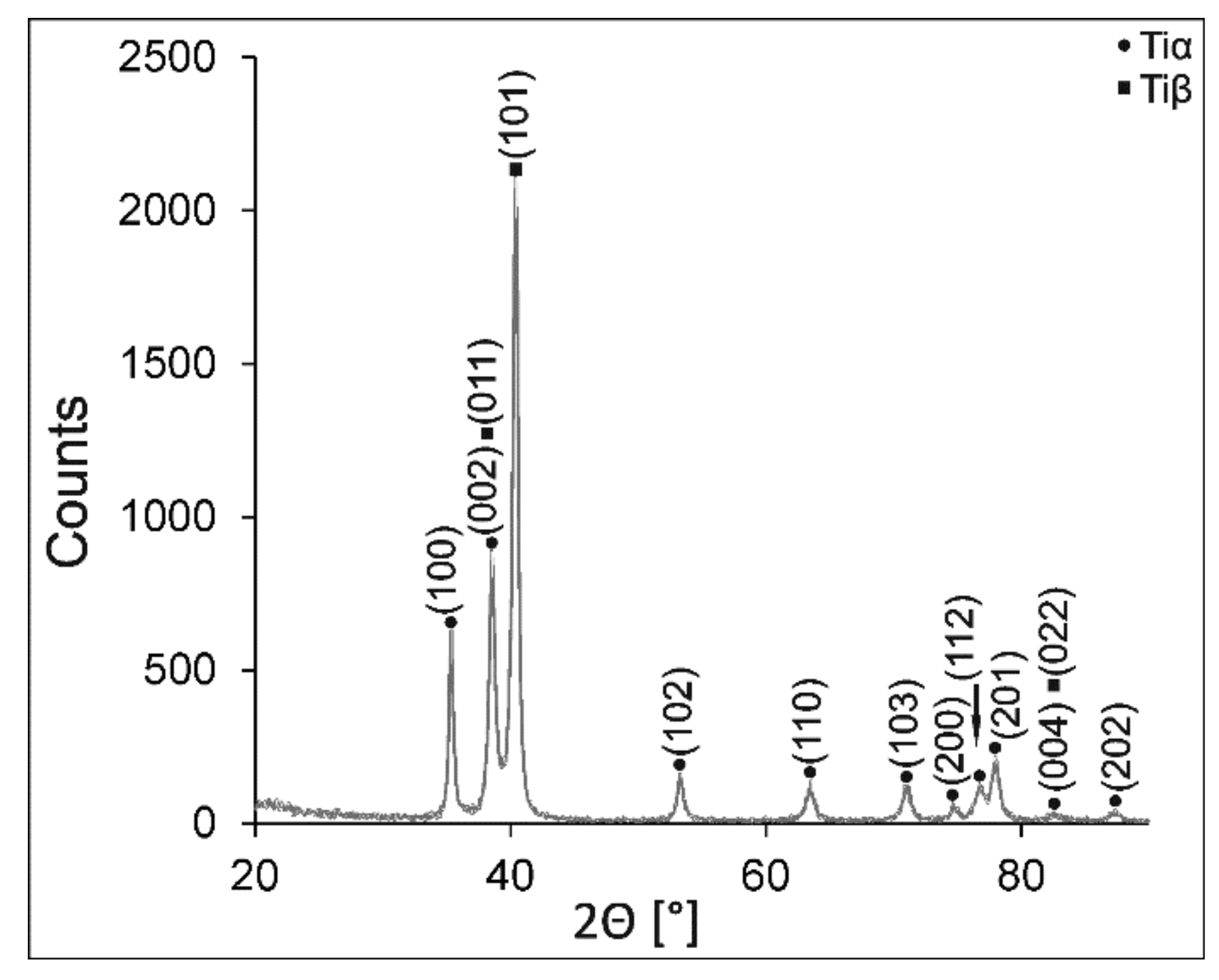
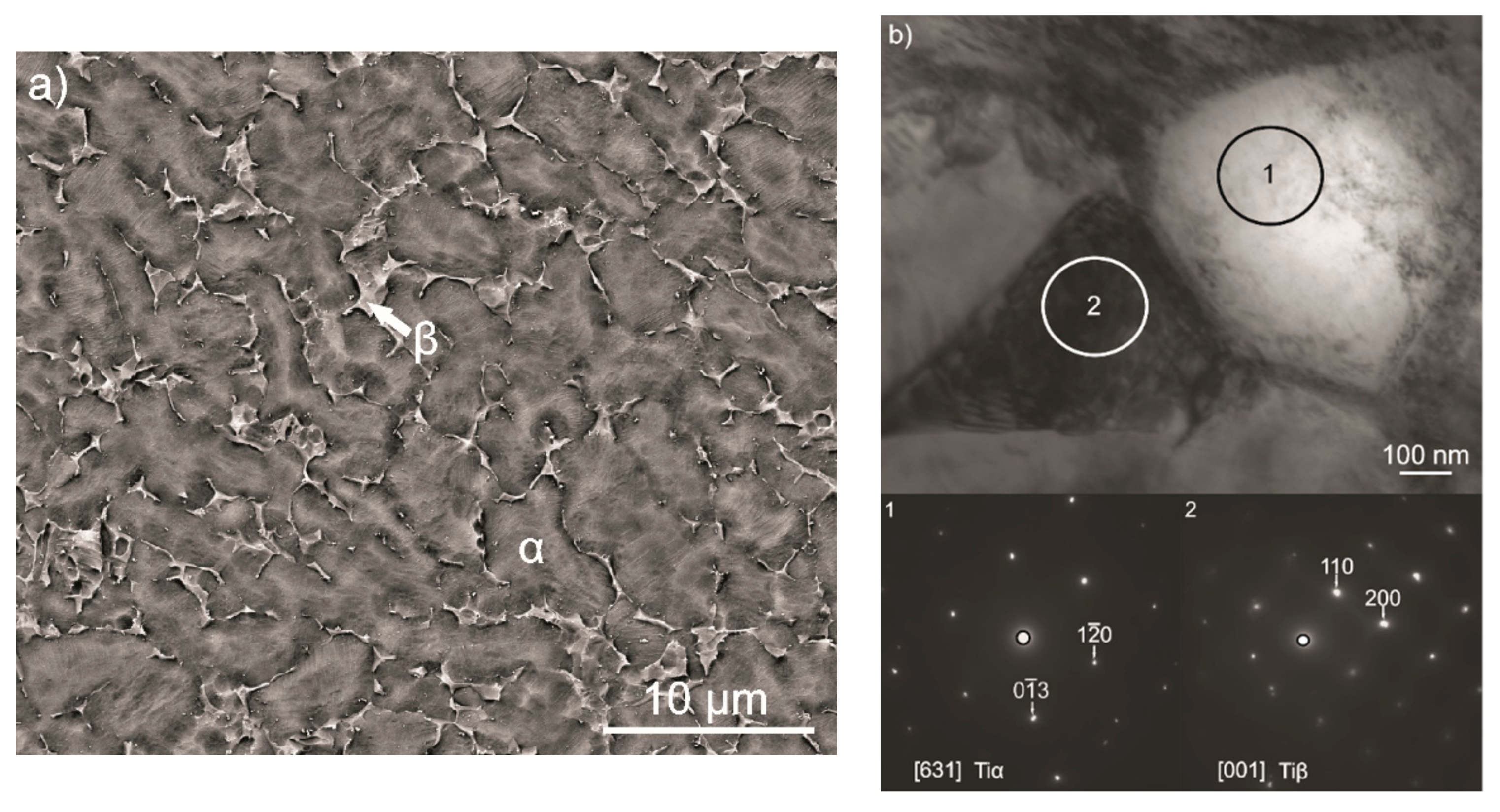
3.1. Microstructure of the Ti-6Al-4V Alloy
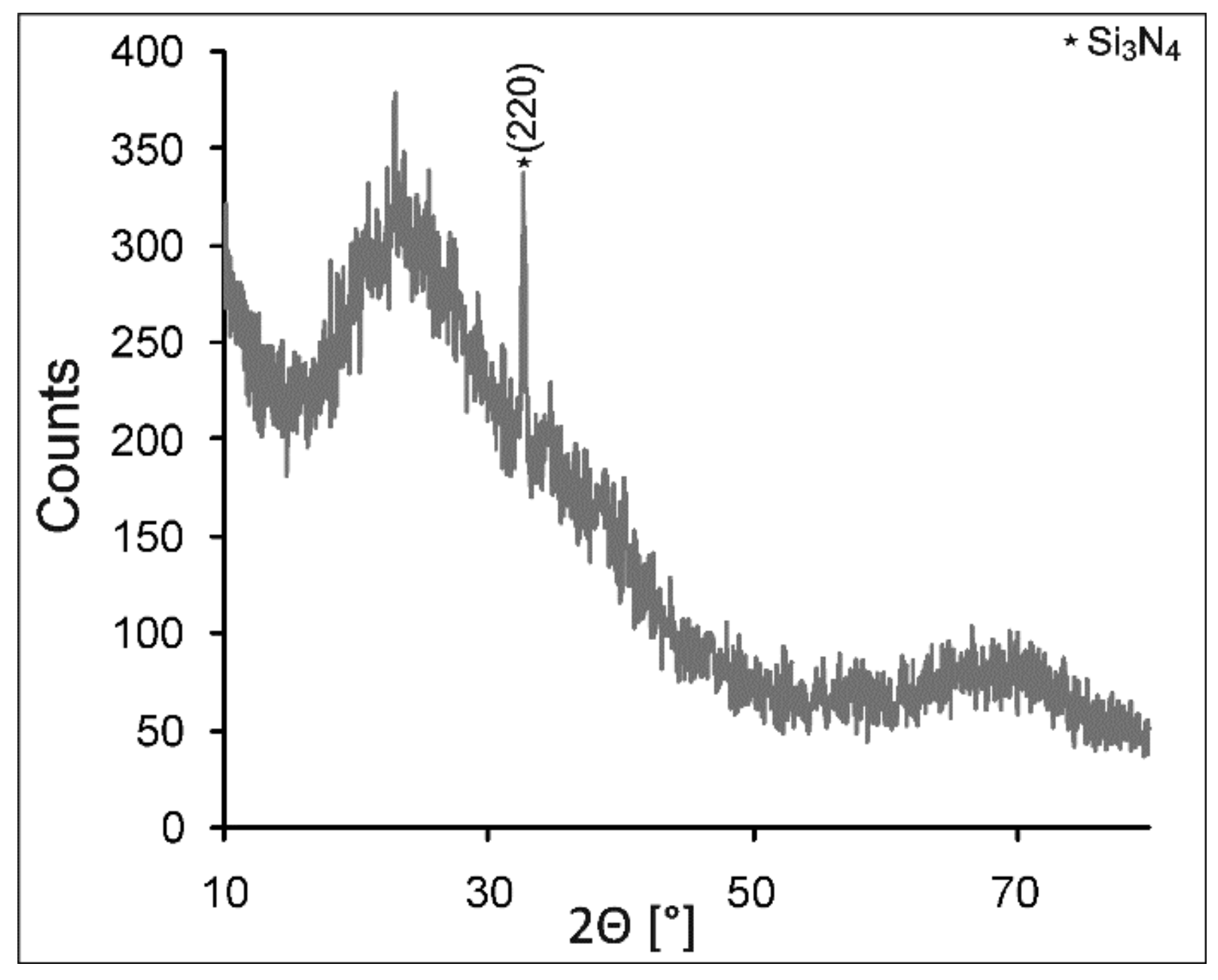
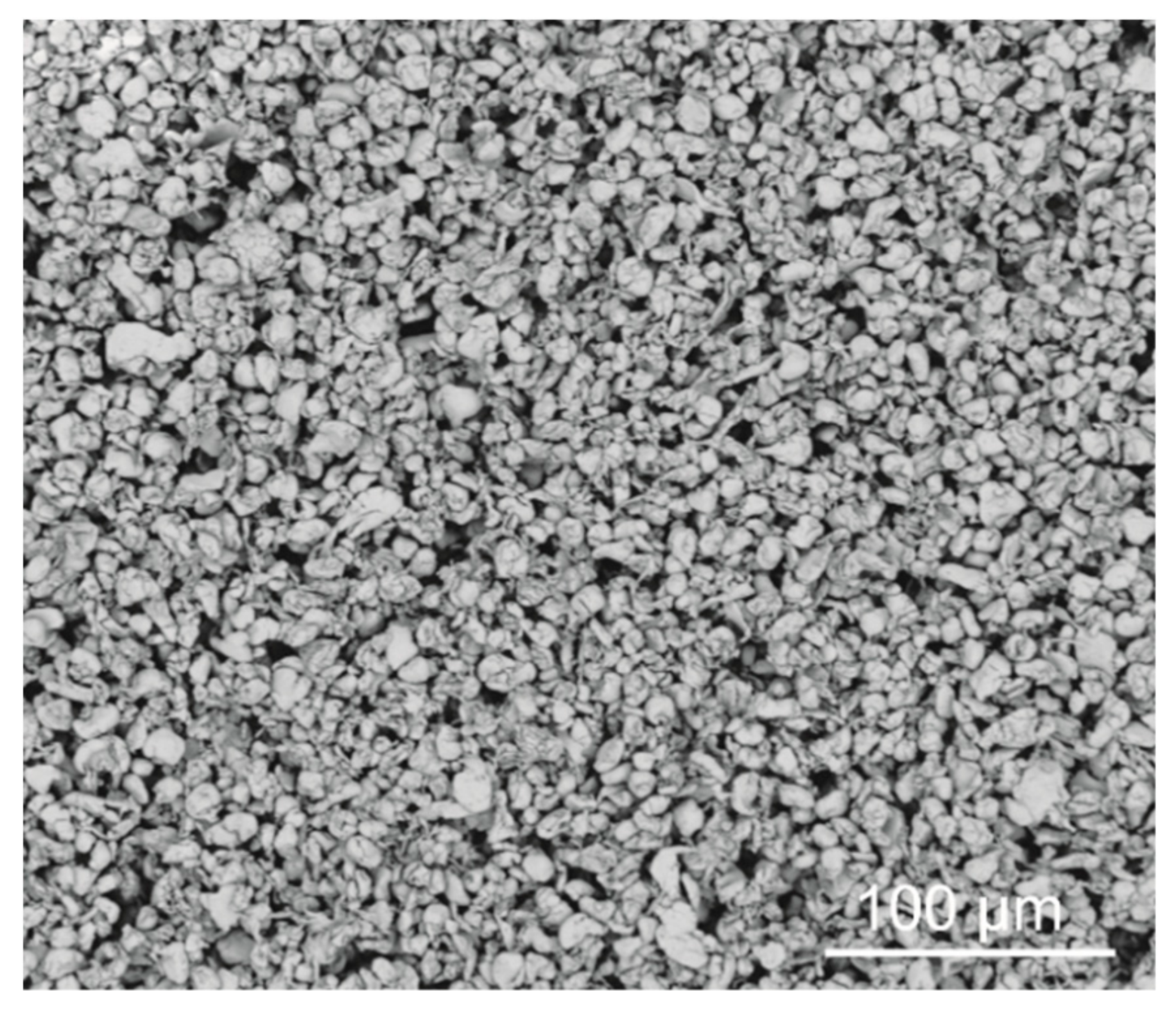
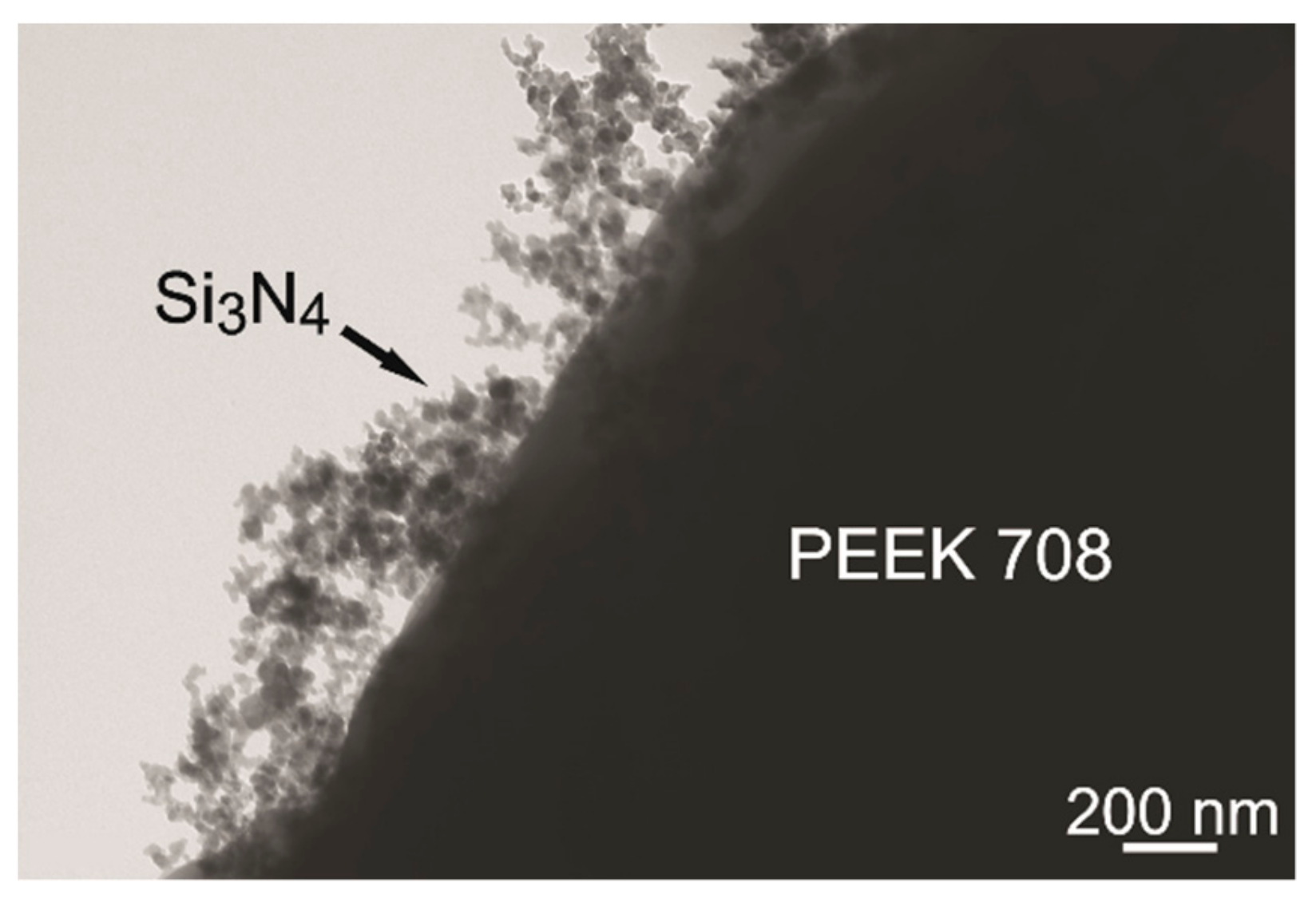
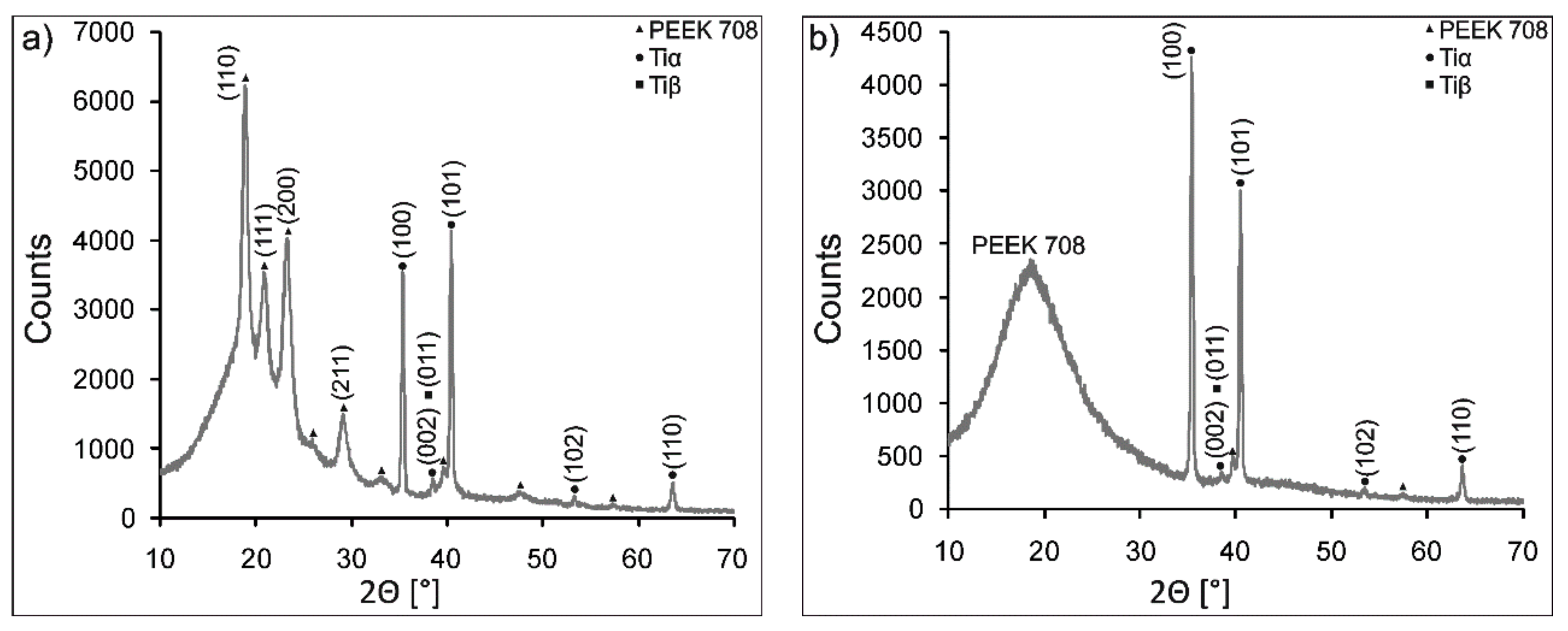
3.2. Characterization of Coating Components
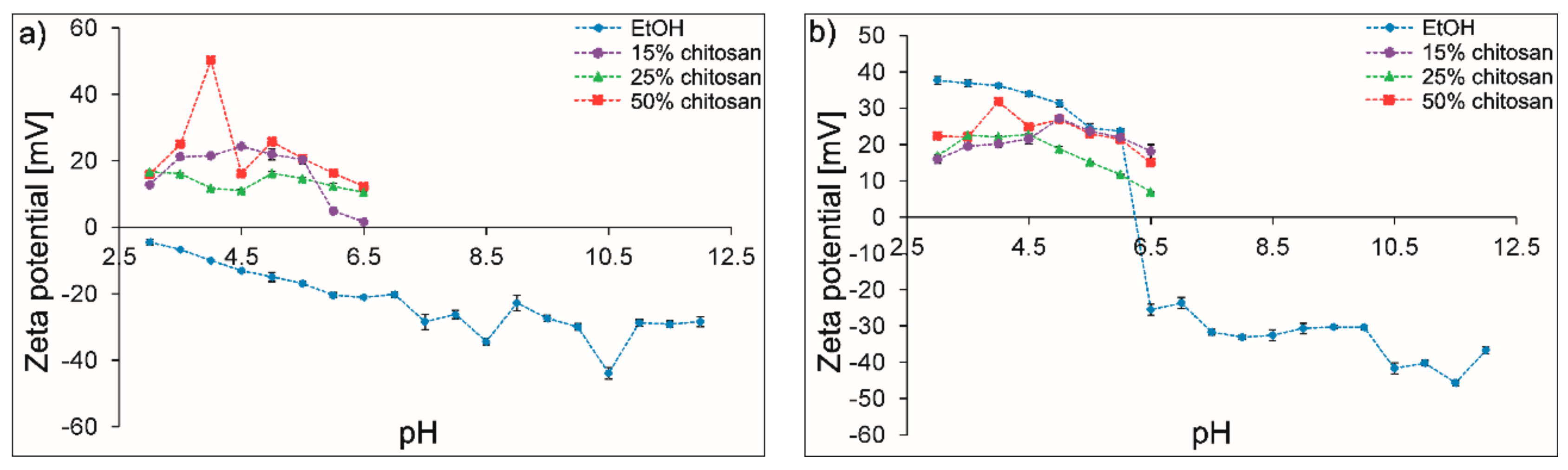
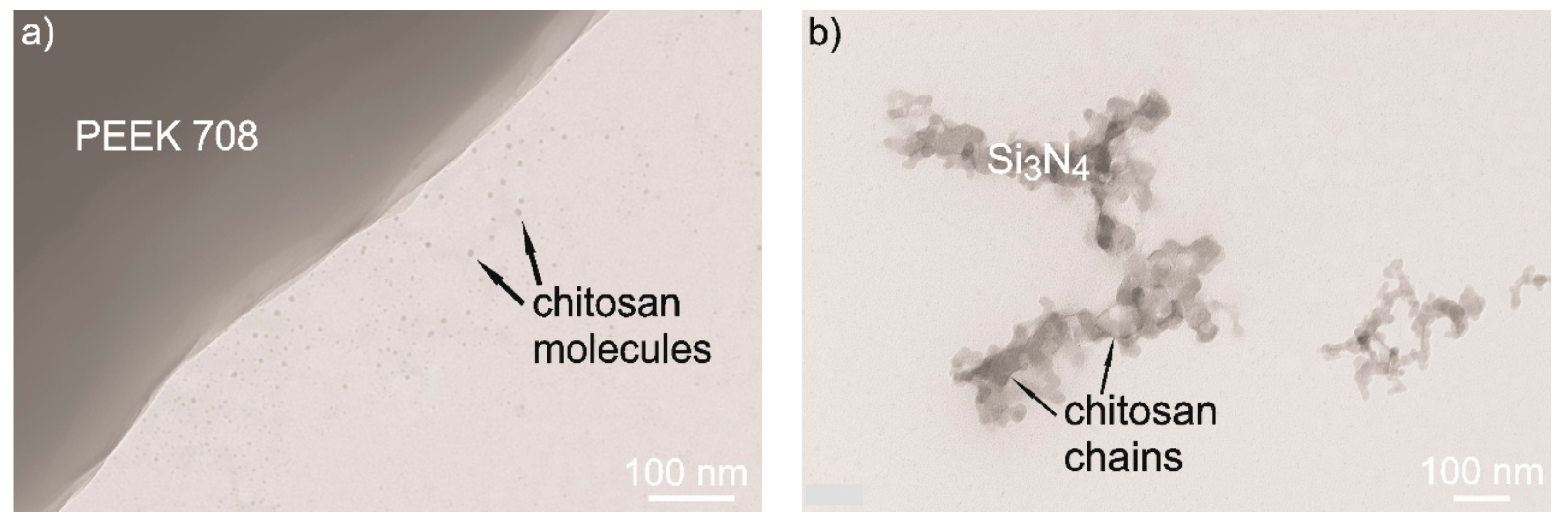
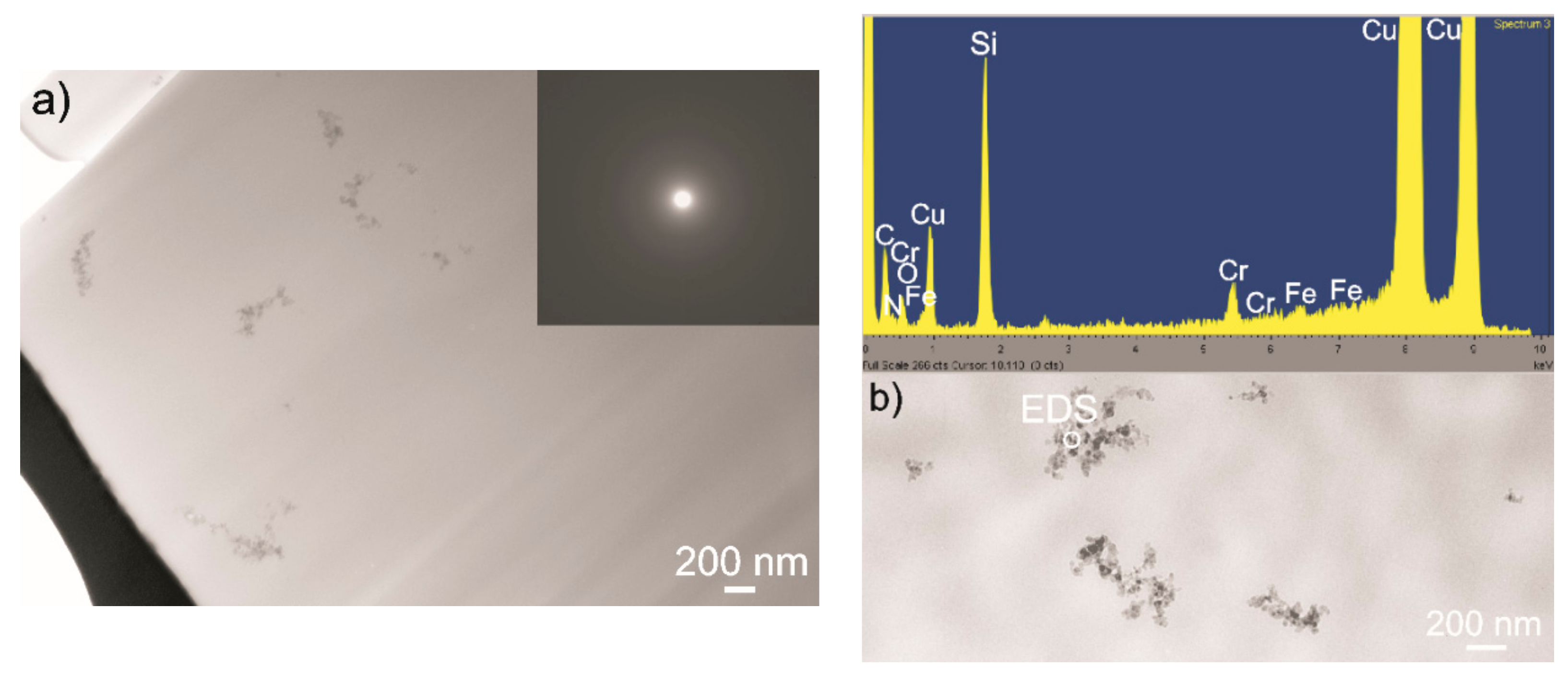
3.3. Elaboration of Suspensions for EPD
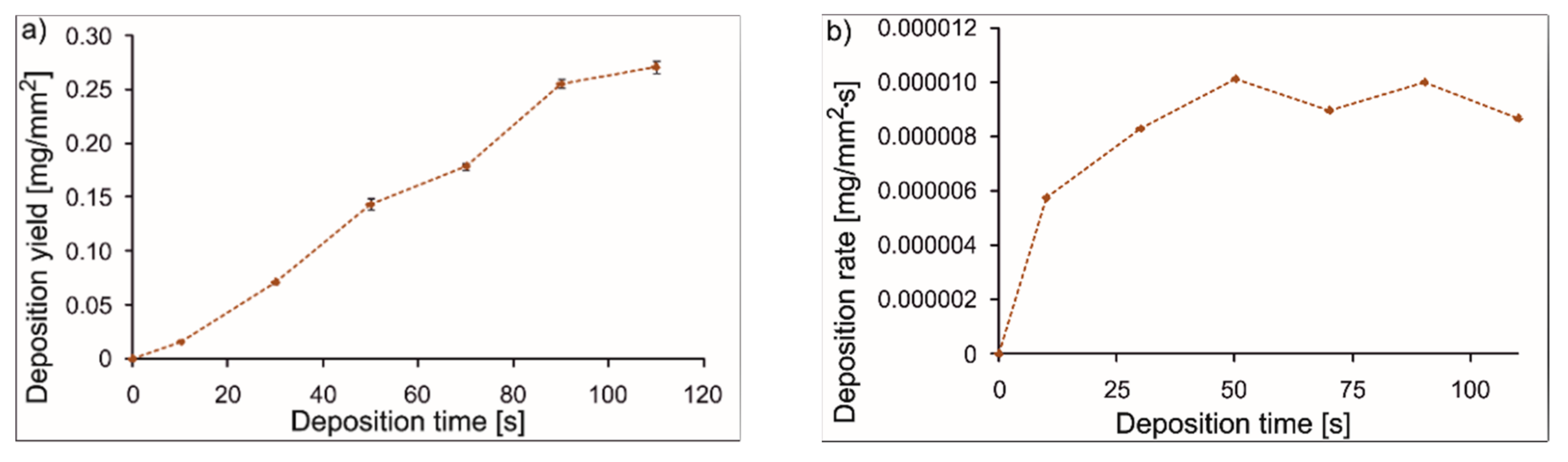
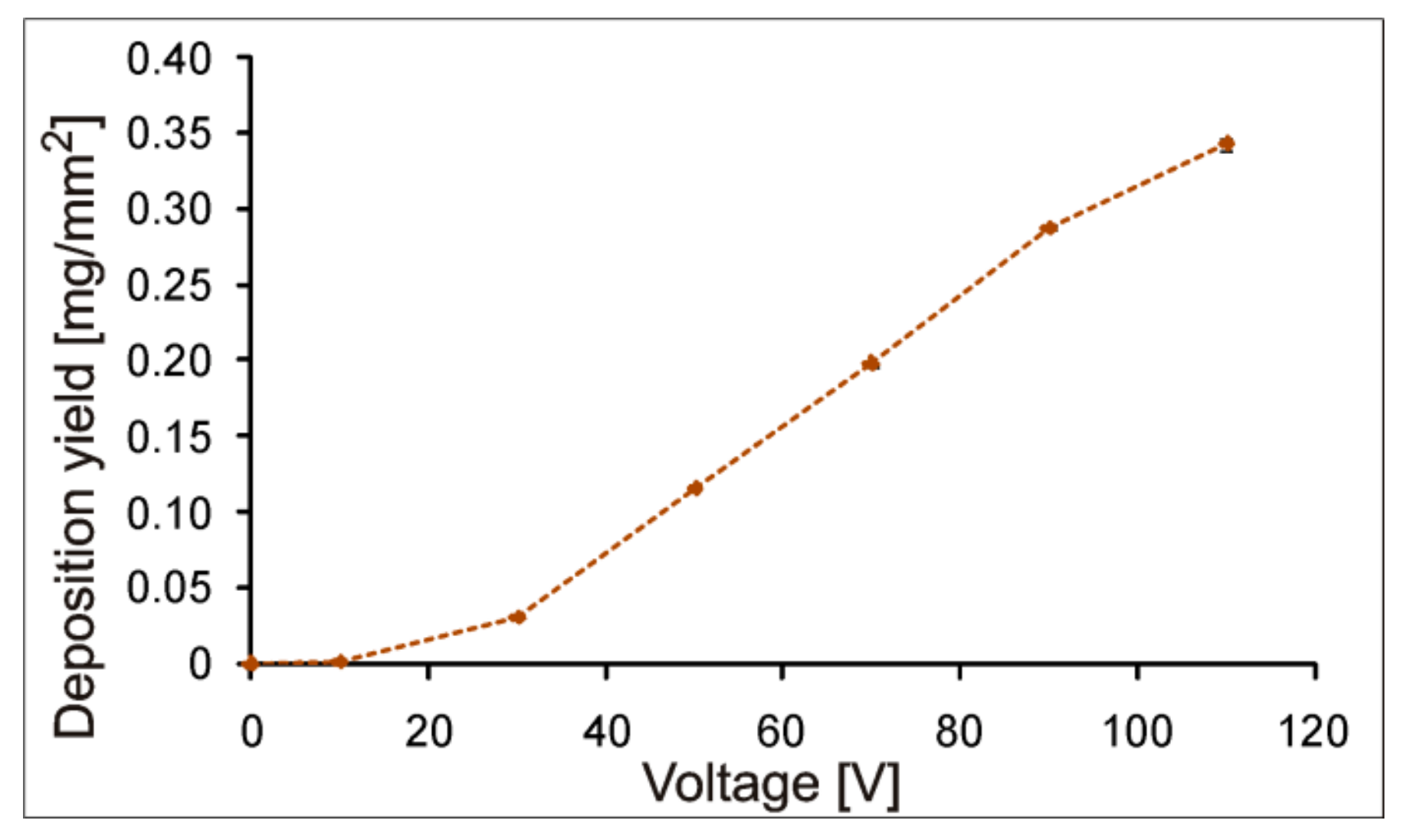
3.4. EPD of Coatings
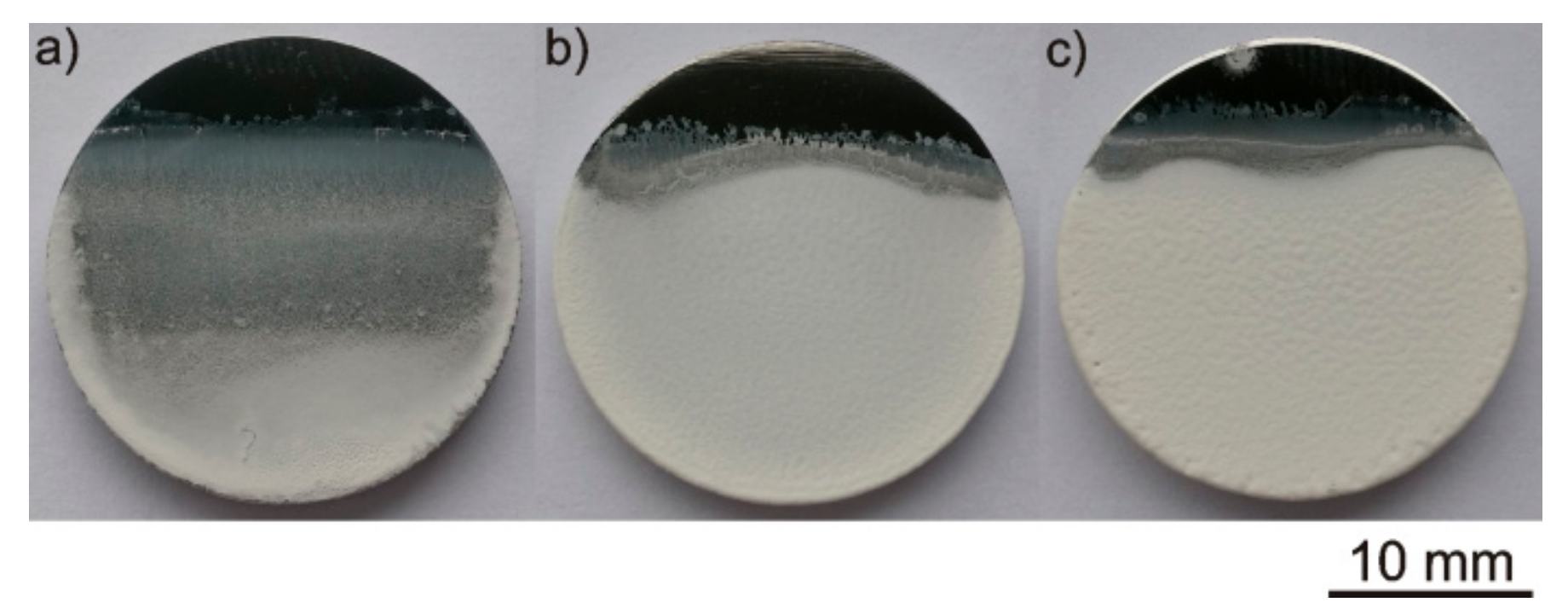
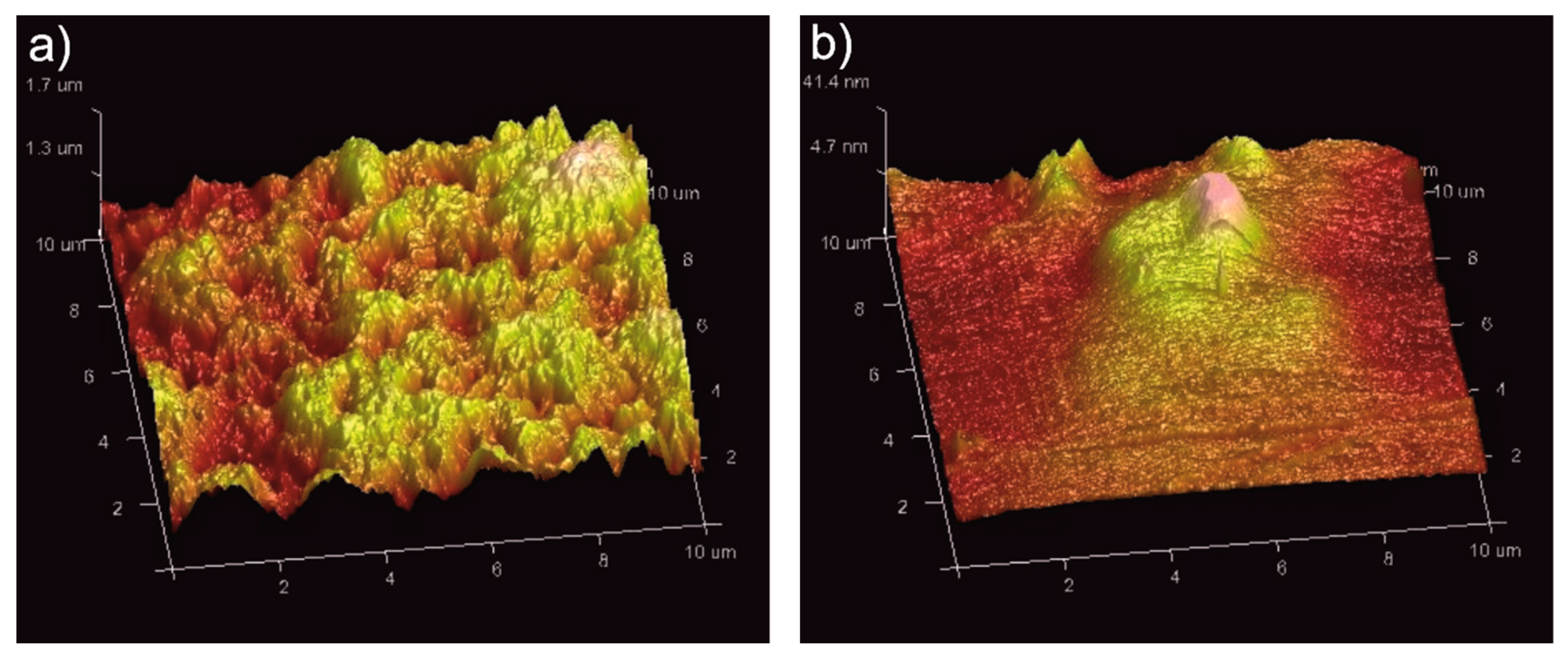
3.5. Influence of Heat Treatment on Coating Microstructure and Surface Topography
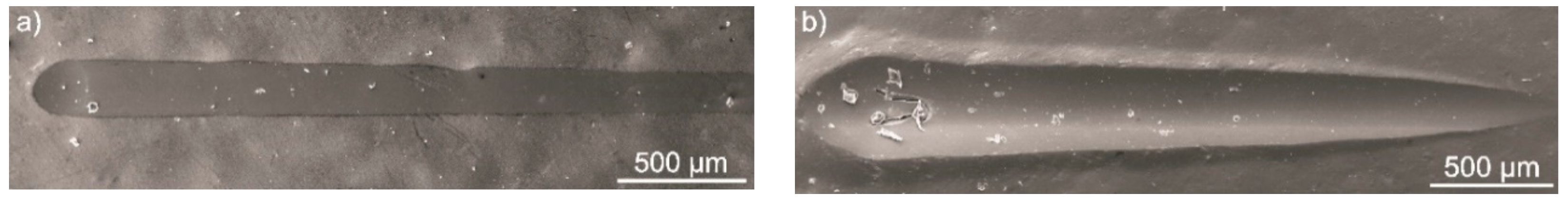
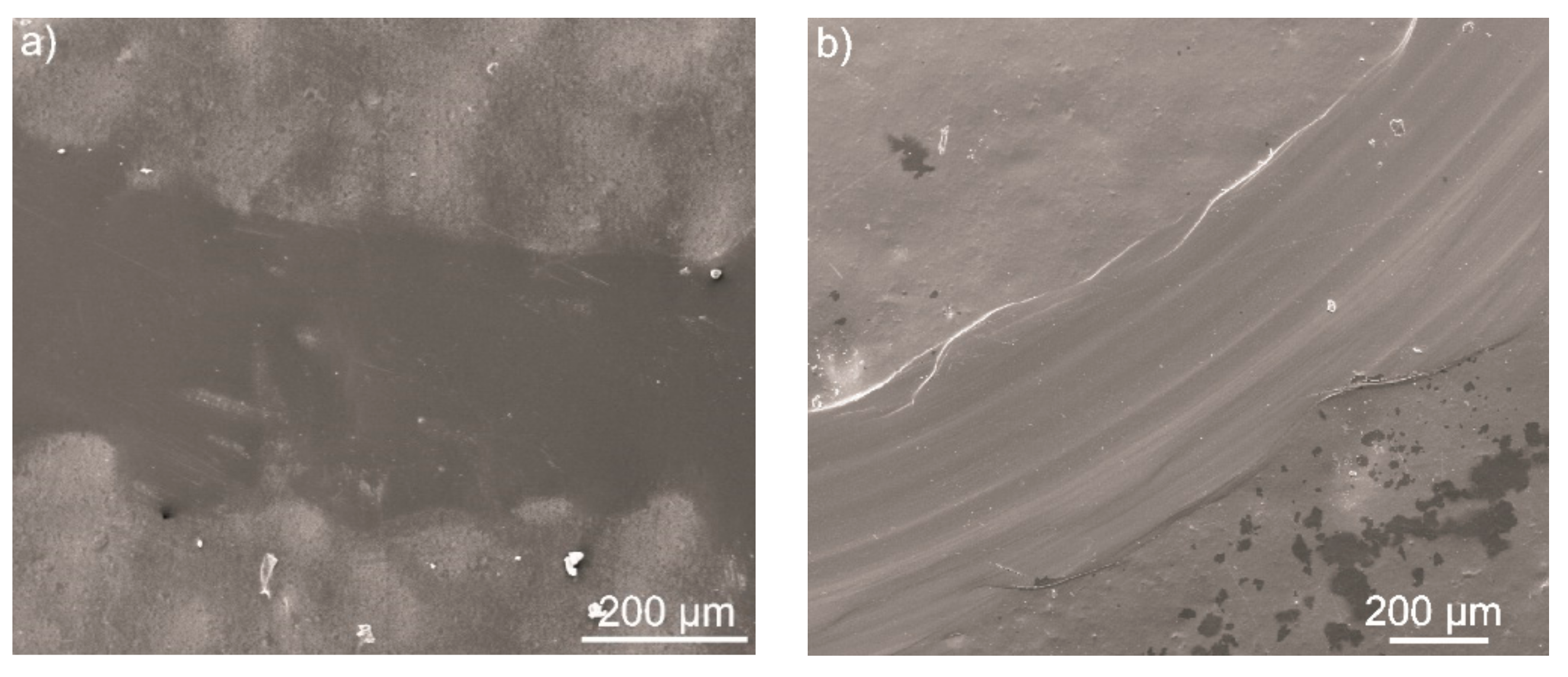
3.6. Micromechanical and Tribological Properties of the Coatings
4. Conclusions
- The addition of chitosan polyelectrolyte changed the surface potential of the PEEK 708 particles and increased the stability of the suspension. Coatings with the highest homogeneity were obtained from a suspension containing 25 vol % of chitosan polyelectrolyte. Zeta potential measurements and TEM observations clearly indicate the mechanisms of interactions between the ceramic nanoparticles and the polymeric micro-particles. The electrostatic interaction between Si3N4 and PEEK particles was indicated in pure ethanol. The steric stabilization of both types of particles by chitosan molecules and chains were indicated in suspensions containing chitosan polyelectrolyte.
- Appropriate selection of the EPD parameters allows uniform coatings to be obtained with relatively even distributions of polymeric particles and ceramic particles adsorbed to them. Macroscopically homogeneous coatings were obtained using a constant voltage of 90 V and a deposition time of 90 s.
- The post EPD heat treatment caused the formation of a dense polymer matrix as a result of heating above the PEEK 708 melting point. The coating structure was semi-crystalline or amorphous depending on the cooling rate after heating, with a furnace and in water at RT, respectively.
- The semi-crystalline coatings exhibited higher roughness and higher surface development compared to the amorphous ones, due to the longer presence at elevated temperature during heat treatment and PEEK crystallization.
- Both hardness and elastic modulus of the semi-crystalline coating were higher compared to the amorphous one. However, both coatings revealed high scratch resistance and good adhesion to the substrate, despite the presence of open porosity.
- Both coatings increased the wear resistance and decreased the COF of the Ti-6Al-4V alloy. However, this improvement was more significant in the case of the semi-crystalline coating as the amorphous coating exhibited significantly poorer tribological properties.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oh, I.-H.; Nomura, N.; Masahashi, N.; Hanada, S. Mechanical properties of porous titanium compacts prepared by powder sintering. Scr. Mater. 2003, 49, 1197–1202. [Google Scholar] [CrossRef]
- Muhammad, M.; Masoomi, M.; Torries, B.; Shamsaei, N.; Haghshenas, M. Depth-sensing time-dependent response of additively manufactured Ti-6Al-4V alloy. Addit. Manuf. 2018, 24, 37–46. [Google Scholar] [CrossRef]
- Niu, Q.L.; Zheng, X.H.; Ming, W.W.; Chen, M. Friction and Wear Performance of Titanium Alloys against Tungsten Carbide under Dry Sliding and Water Lubrication. Tribol. Trans. 2013, 56, 101–108. [Google Scholar] [CrossRef]
- Peters, M.; Hemptenmacher, J.; Kumpfert, J.; Leyens, C. Structure and Properties of Titanium and Titanium Alloys. In Titanium and Titanium Alloys, Fundamentals and Applications; Leyens, C., Peters, M., Eds.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Wendler, B.; Zimowski, S.; Dubiel, B.; Czyrska-Filemonowicz, A. Microstructure, micro-mechanical and tribological properties of the nc-WC/a-C nanocomposite coatings magnetron sputtered on non-hardened and oxygen hardened Ti-6Al-4V alloy. Surf. Coat. Technol. 2010, 205, 2668–2677. [Google Scholar] [CrossRef]
- Amanov, A.; Pyun, Y.S. Local heat treatment with and without ultrasonic nanocrystal surface modification of Ti-6Al-4V alloy: Mechanical and tribological properties. Surf. Coat. Technol. 2017, 326, 343–354. [Google Scholar] [CrossRef]
- Samanta, A.; Bhattacharya, M.; Ratha, I.; Chakraborty, H.; Datta, S.; Ghosh, J.; Bysakh, S.; Sreemany, M.; Rane, R.; Joseph, A.; et al. Nano- and micro-tribological behaviours of plasma nitrided Ti6Al4V alloys. J. Mech. Behav. Biomed. Mater. 2018, 77, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Han, Y.; Lu, W.; Mao, J.; Wang, L.; Zhang, D. Effect of solid carburization on surface microstructure and hardness of Ti-6Al-4V alloy and (TiB+La2O3)/Ti-6Al-4V composite. Trans. Nonferr. Met. Soc. China (Engl. Ed.) 2016, 26, 1871–1877. [Google Scholar] [CrossRef]
- Movassagh-Alanagh, F.; Abdollah-zadeh, A.; Aliofkhazraei, M.; Abedi, M. Improving the wear and corrosion resistance of Ti–6Al–4V alloy by deposition of TiSiN nanocomposite coating with pulsed-DC PACVD. Wear 2017, 390–391, 93–103. [Google Scholar] [CrossRef]
- Rahmati, B.; Sarhan Ahmed, A.D.; Zalnezhad, E.; Kamiab, Z.; Dabbagh, A.; Choudhury, D.; Abas, W.A.B.W. Development of tantalum oxide (Ta-O) thin film coating on biomedical Ti-6Al-4V alloy to enhance mechanical properties and biocompatibility. Ceram. Int. 2016, 42, 466–480. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, X.; Zhitomirsky, I. Electrophoretic deposition of chiral polymers and composites. Colloids Surf. B Biointerfaces 2011, 87, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, J.; Cheng, W. Formation of polyetheretherketone polymer coating by electrophoretic deposition method. Surf. Coat. Technol. 2003, 173, 271–275. [Google Scholar] [CrossRef]
- Narkevica, I.; Stradina, L.; Stipniece, L.; Jakobsons, E.; Ozolins, J. Electrophoretic deposition of nanocrystalline TiO2 particles on porous TiO2-x ceramic scaffolds for biomedical applications. J. Eur. Ceram. Soc. 2017, 37, 3185–3193. [Google Scholar] [CrossRef]
- Charlot, A.; Deschanels, X.; Toquer, G. Submicron coating of SiO2 nanoparticles from electrophoretic deposition. Thin Solid Film. 2014, 553, 148–152. [Google Scholar] [CrossRef]
- Fraczek-Szczypta, A.; Jantas, D.; Ciepiela, F.; Grzonka, J.; Bernasik, A.; Marzec, M. Carbon nanomaterials coatings—Properties and influence on nerve cells response. Diam. Relat. Mater. 2018, 84, 127–140. [Google Scholar] [CrossRef]
- Jeun, J.H.; Kim, W.S.; Hong, S.H. Electrophoretic deposition of carbon nanoparticles on dendritic Sn foams fabricated by electrodeposition. Mater. Lett. 2013, 112, 109–112. [Google Scholar] [CrossRef]
- Bartmanski, M.; Cieslik, B.; Glodowska, J.; Kalka, P.; Pawlowski, L.; Pieper, M.; Zielinski, A. Electrophoretic deposition (EPD) of nanohydroxyapatite - nanosilver coatings on Ti13Zr13Nb alloy. Ceram. Int. 2017, 43, 11820–11829. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Simsiriwong, J.; Shrestha, R.; Shamsaei, N.; Lugo, M.; Moser, R.D. Effects of microstructural inclusions on fatigue life of polyether ether ketone (PEEK). J. Mech. Behav. Biomed. Mater. 2015, 51, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Dandy, L.O.; Oliveux, G.; Wood, J.; Jenkins, M.J.; Leeke, G.A. Accelerated degradation of Polyetheretherketone (PEEK) composite materials for recycling applications. Polym. Degrad. Stab. 2015, 112, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Corni, I.; Neumann, N.; Eifler, D.; Boccaccini, A.R. Polyetheretherketone (PEEK) coatings on stainless steel by electrophoretic deposition. Adv. Eng. Mater. 2008, 10, 559–564. [Google Scholar] [CrossRef]
- Clavijo, S.; Membrives, F.; Boccaccini, A.R.; Santillán, M.J. Characterization of polyetheretherketone particle suspensions for electrophoretic deposition. J. Appl. Polym. Sci. 2014, 131, 2–7. [Google Scholar] [CrossRef]
- Li, E.Z.; Guo, W.L.; Wang, H.D.; Xu, B.S.; Liu, X.T. Research on tribological behavior of PEEK and glass fiber reinforced PEEK composite. Phys. Procedia 2013, 50, 453–460. [Google Scholar] [CrossRef]
- Regis, M.; Bellare, A.; Pascolini, T.; Bracco, P. Characterization of thermally annealed PEEK and CFR-PEEK composites: Structure-properties relationships. Polym. Degrad. Stab. 2017, 136, 121–130. [Google Scholar] [CrossRef]
- Lu, Z.P.; Friedrich, K. On sliding friction and wear of PEEK and its composites. Wear 1995, 183, 624–631. [Google Scholar] [CrossRef]
- Zhang, G.; Liao, H.; Coddet, C. Friction and wear behavior of PEEK and its composite coatings. Tribol. Interface Eng. Ser. 2008, 55, 458–482. [Google Scholar] [CrossRef]
- Vail, J.R.; Krick, B.A.; Marchman, K.R.; Sawyer, W.G. Polytetrafluoroethylene (PTFE) fiber reinforced polyetheretherketone (PEEK) composites. Wear 2011, 270, 737–741. [Google Scholar] [CrossRef]
- Baştan, F.E.; Atiq Ur Rehman, M.; Avcu, Y.Y.; Avcu, E.; Üstel, F.; Boccaccini, A.R. Electrophoretic co-deposition of PEEK-hydroxyapatite composite coatings for biomedical applications. Colloids Surf. B. 2018, 169, 176–182. [Google Scholar] [CrossRef]
- Balaji, V.; Tiwari, A.N.; Goyal, R.K. Study on High-Performance Poly(etheretherketone)/Si3N4Nanocomposites: New Electronic Substrate Materials. Polym. Eng. Sci. 2011, 51, 509–517. [Google Scholar] [CrossRef]
- Qihua, W.; Jinfen, X.; Weichang, S.; Weimin, L. An investigation of the friction and wear properties of nanometer Si3N4 filled PEEK. Wear 1996, 196, 82–86. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Zych, A.; Kruk, A.; Kopia, A.; Zimowski, S.; Sitarz, M.; Cieniek, Ł. Electrophoretic deposition and microstructure development of Si3N4/polyetheretherketone coatings on titanium alloy. Surf. Coat. Technol. 2018, 350, 633–647. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W. Microstructural, surface and electrochemical properties of pulse electrodeposited Ni-W/Si3N4 nanocomposite coating. Ceram. Int. 2018, 44, 19907–19918. [Google Scholar] [CrossRef]
- Xia, R.; Zhang, Y.; Zhu, Q.; Qian, J.; Dong, Q.; Li, F. Surface Modification of Nano-Sized Silicon Nitride with BA-MAA-AN Tercopolymer. J. Appl. Polym. Sci. 2008, 107, 562–570. [Google Scholar] [CrossRef]
- Liu, X.; Yi, X.; Guo, R.; Li, Q.; Nomura, T. Formation mechanisms of Si3N4 microstructures during silicon powder nitridation. Ceram. Int. 2017, 43, 16773–16779. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Scharnagl, N.; Kainer, K.U. Influence of incorporating Si3N4 particles into the oxide layer produced by plasma electrolytic oxidation on AM50 Mg alloy on coating morphology and corrosion properties. J. Magnes. Alloy. 2013, 1, 267–274. [Google Scholar] [CrossRef]
- Golla, B.R.; Ko, J.W.; Kim, J.M.; Kim, H.D. Effect of particle size and oxygen content of Si on processing, microstructure and thermal conductivity of sintered reaction bonded Si3N4. J. Alloy. Compd. 2015, 595, 60–66. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Z.; Yun, J.; Yan, J.; You, T. Synthesis of α-Si3N4crystallon by a solvothermal method at a low temperature of 180 °C. Phys. B Condens. Matter. 2013, 428, 10–13. [Google Scholar] [CrossRef]
- Hao, W.; Yang, W.; Cai, H.; Huang, Y. Non-isothermal crystallization kinetics of polypropylene/silicon nitride nanocomposites. Polym. Test. 2010, 29, 527–533. [Google Scholar] [CrossRef]
- Deepthi, M.V.; Sailaja, R.R.N.; Sampathkumaran, P.; Seetharamu, S.; Vynatheya, S. High density polyethylene and silane treated silicon nitride nanocomposites using high-density polyethylene functionalized with maleate ester: Mechanical, tribological and thermal properties. Mater. Des. 2014, 56, 685–695. [Google Scholar] [CrossRef]
- Kusunose, T.; Yagi, T.; Firoz, S.H.; Sekino, T. Fabrication of epoxy/silicon nitride nanowire composites and evaluation of their thermal conductivity. J. Mater. Chem. A 2013, 1, 3440–3445. [Google Scholar] [CrossRef]
- Nsib, F.; Ayed, N.; Chevalier, Y. Selection of dispersants for the dispersion of carbon black in organic medium. Prog. Org. Coat. 2006, 55, 303–310. [Google Scholar] [CrossRef]
- Cheng, G.; Qian, J.; Tang, Z.; Ding, G.; Zhu, J. Dispersion stability of Si3N4 nano-particles modified by γ-methacryloxypropyltrimethoxysilane (MAPTMS) in organic solvent. Ceram. Int. 2015, 41, 1879–1884. [Google Scholar] [CrossRef]
- Su, F.; Zhang, S. Tribological properties of polyimide coatings filled with PTFE and surface-modified nano-Si3N4. J. Appl. Polym. Sci. 2014, 131, 1–11. [Google Scholar] [CrossRef]
- Sun, N.; Meng, X.; Xiao, Z. Functionalized Si3N4 nanoparticles modified with hydrophobic polymer chains by surface-initiated atom transfer radical polymerization (ATRP). Ceram. Int. 2015, 41, 13830–13835. [Google Scholar] [CrossRef]
- Tai, Y.; Miao, J.; Qian, J.; Xia, R.; Zhang, Y. An effective way to stabilize silicon nitride nanoparticles dispersed in rubber matrix by a one-step process. Mater. Chem. Phys. 2008, 112, 659–667. [Google Scholar] [CrossRef]
- Kruk, A.; Zimowski, S.; Łukaszczyk, A.; Cieniek, Ł.; Moskalewicz, T. The influence of heat treatment on the microstructure, surface topography and selected properties of PEEK coatings electrophoretically deposited on the Ti-6Al-4V alloy. Prog. Org. Coat. 2019, 133, 180–190. [Google Scholar] [CrossRef]
- Hang, J.; Shi, L.; Feng, X.; Xiao, L. Electrostatic and electrosteric stabilization of aqueous suspensions of barite nanoparticles. Powder Technol. 2009, 192, 166–170. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Kruk, A.; Sitarz, M.; Kopia, A. Effect of the Processing and Heat Treatment Route on the Microstructure of MoS2/Polyetheretherketone Coatings Obtained by Electrophoretic Deposition. J. Electrochem. Soc. 2019, 166, 151–161. [Google Scholar] [CrossRef]
- Chung, Y.C.; Tsai, C.F.; Li, C.F. Preparation and characterization of water-soluble chitosan produced by Maillard reaction. Fish. Sci. 2006, 72, 1096–1103. [Google Scholar] [CrossRef]
- Luo, D.; Zhitomirsky, I. Electrophoretic Deposition of Polyetheretherketone Composites, Containing Huntite and Alumina Platelets. J. Electrochem. Soc. 2015, 162, 3057–3062. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Oraby, S.E.; Alaskari, A.M. Atomic Force Microscopy (AFM) Topographical Surface Characterization of Multilayer-Coated and Uncoated Carbide Inserts. Int. J. Mech. Aerosp. Ind. Mechatron. Manuf. Eng. 2010, 4, 977–988. [Google Scholar] [CrossRef]
- Raposo, M.; Ferreira, Q.; Ribeiro, P.A. A Guide for Atomic Force Microscopy Analysis of Soft-Condensed Matter. Mod. Res. Educ. Top. Microsc. 2006, 38, 679–681. [Google Scholar]




| Element | Content (wt %) | |
|---|---|---|
| Minimum | Maximum | |
| Al | 6.12 | 6.15 |
| V | 3.90 | 4.00 |
| Fe | 0.17 | 0.18 |
| N | 0.01 | 0.01 |
| C | 0.03 | 0.03 |
| O | 0.11 | 0.12 |
| H | 0.004 | 0.005 |
| Y | 0.0020 | 0.0021 |
| Ti | Balance | |
| Parameter | Semi-Crystalline Coating | Amorphous Coating |
|---|---|---|
| Value | ||
| Rq | 125.5 ± 26.6 | 15.4 ± 6.2 |
| Ra | 100.7 ± 20.5 | 11.9 ± 4.5 |
| Rmax | 829.1 ± 264.6 | 125.3 ± 45.1 |
| ISAD | 16.9 ± 5.1 | 0.06 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiołek, A.; Zimowski, S.; Kopia, A.; Moskalewicz, T. The Influence of Electrophoretic Deposition Parameters and Heat Treatment on the Microstructure and Tribological Properties of Nanocomposite Si3N4/PEEK 708 Coatings on Titanium Alloy. Coatings 2019, 9, 530. https://doi.org/10.3390/coatings9090530
Fiołek A, Zimowski S, Kopia A, Moskalewicz T. The Influence of Electrophoretic Deposition Parameters and Heat Treatment on the Microstructure and Tribological Properties of Nanocomposite Si3N4/PEEK 708 Coatings on Titanium Alloy. Coatings. 2019; 9(9):530. https://doi.org/10.3390/coatings9090530
Chicago/Turabian StyleFiołek, Aleksandra, Sławomir Zimowski, Agnieszka Kopia, and Tomasz Moskalewicz. 2019. "The Influence of Electrophoretic Deposition Parameters and Heat Treatment on the Microstructure and Tribological Properties of Nanocomposite Si3N4/PEEK 708 Coatings on Titanium Alloy" Coatings 9, no. 9: 530. https://doi.org/10.3390/coatings9090530
APA StyleFiołek, A., Zimowski, S., Kopia, A., & Moskalewicz, T. (2019). The Influence of Electrophoretic Deposition Parameters and Heat Treatment on the Microstructure and Tribological Properties of Nanocomposite Si3N4/PEEK 708 Coatings on Titanium Alloy. Coatings, 9(9), 530. https://doi.org/10.3390/coatings9090530





