Mechanical Properties and Oxidation Behavior of Cr–Si–N Coatings
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
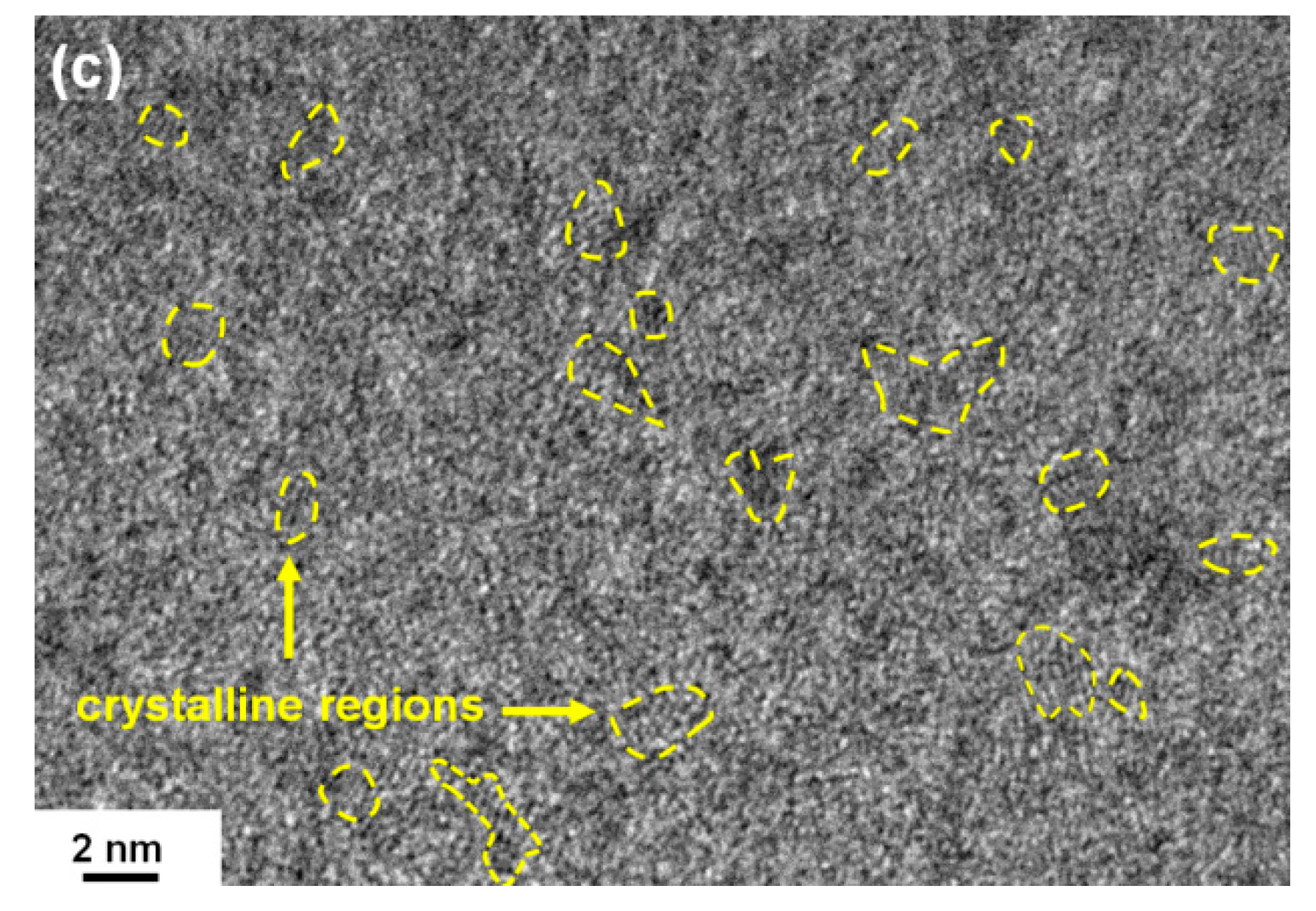
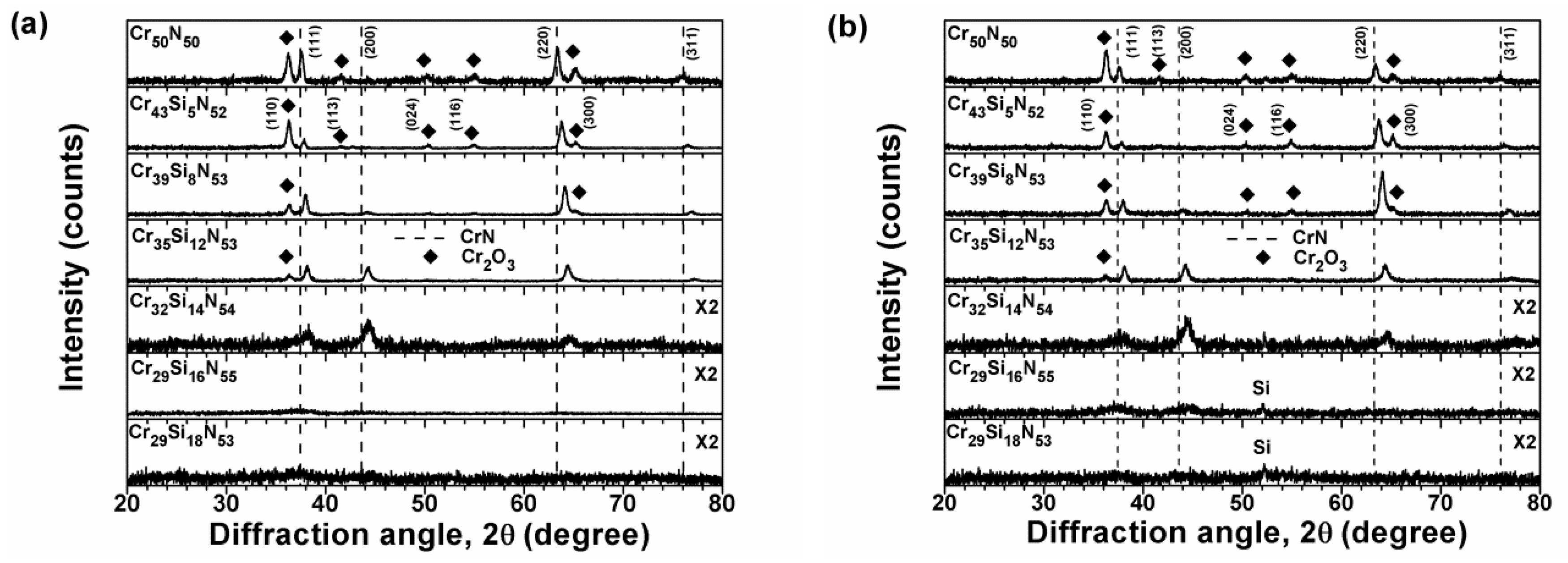
3.1. Chemical Compositions and Microstructure of As-Deposited Cr–Si–N Coatings
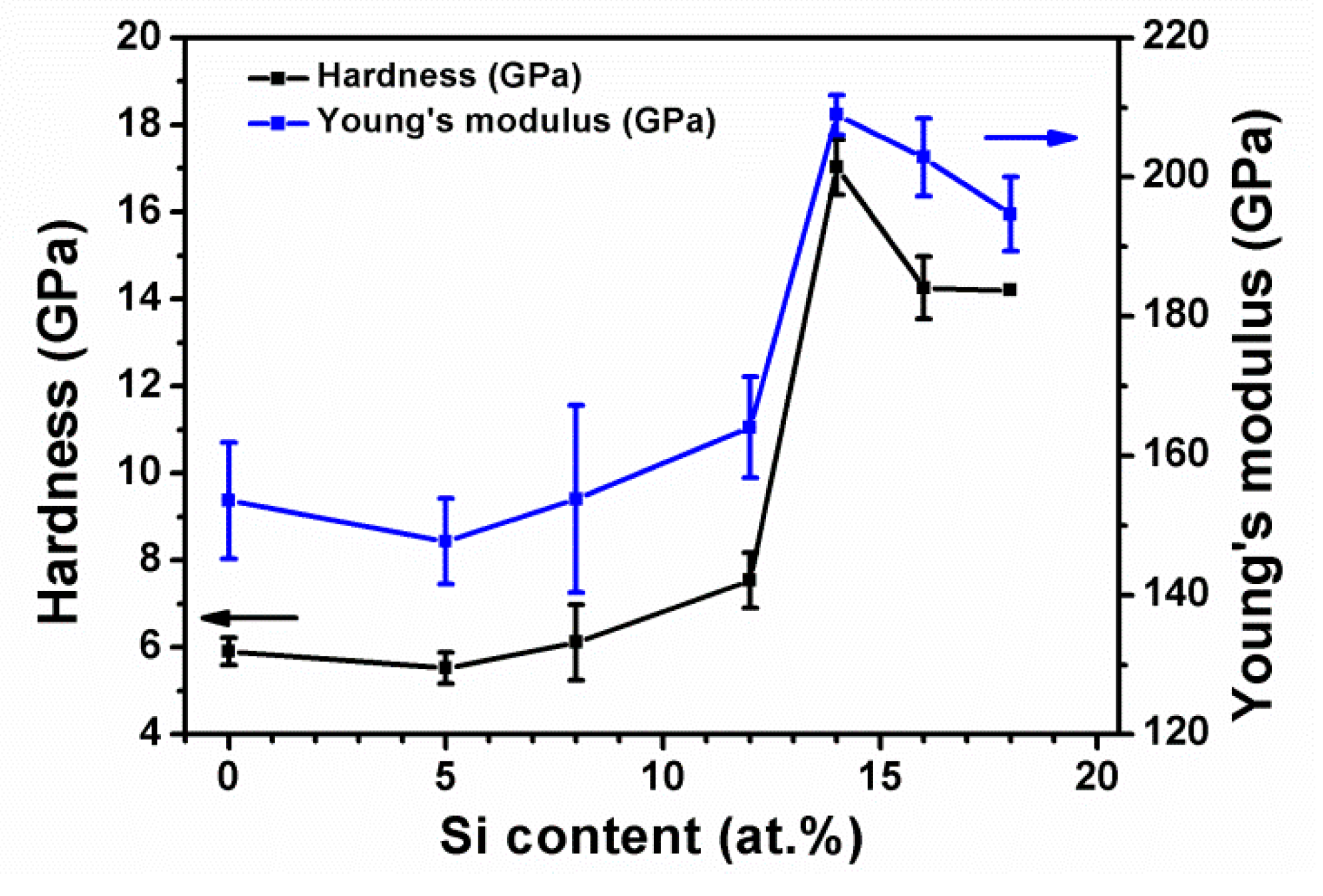
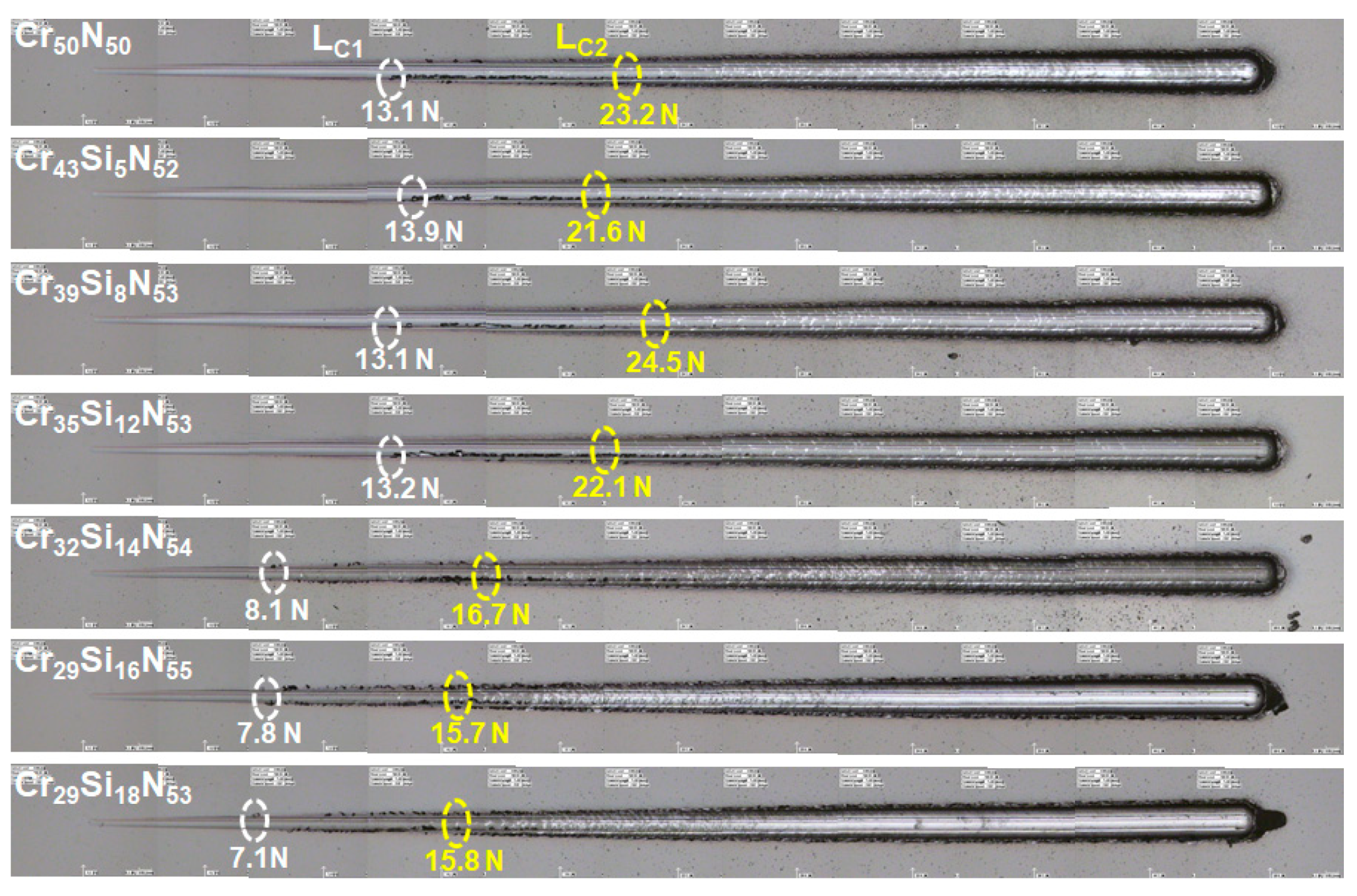
3.2. Mechanical Properties of As-Deposited Cr–Si–N Coatings
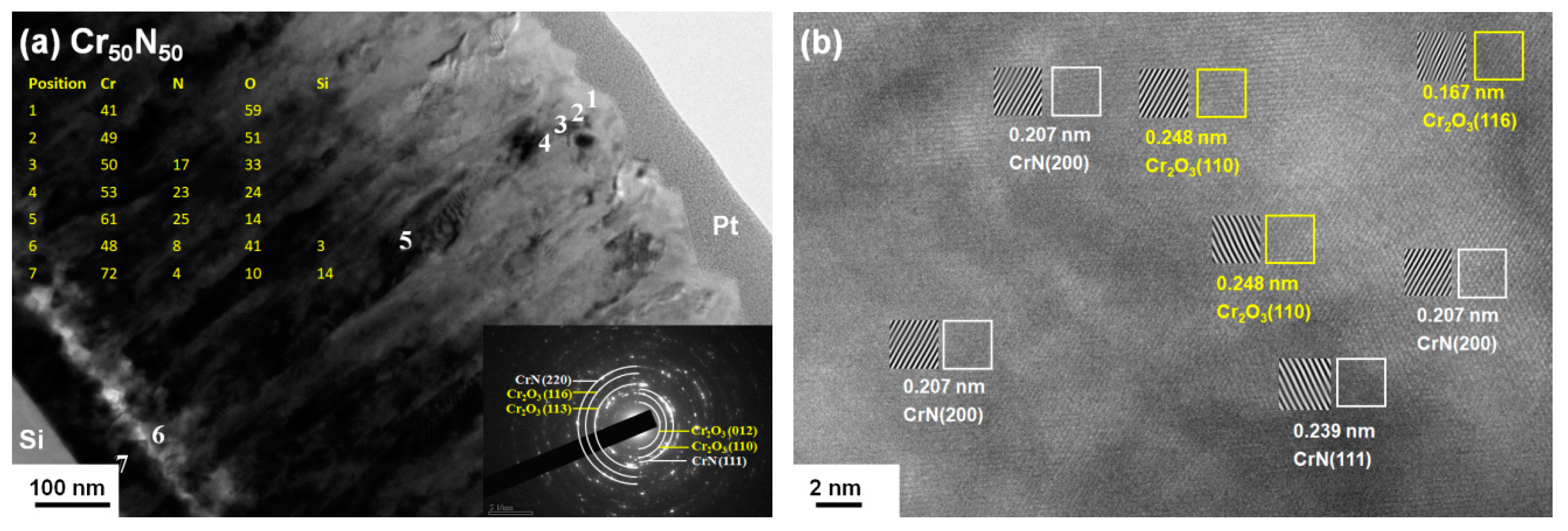
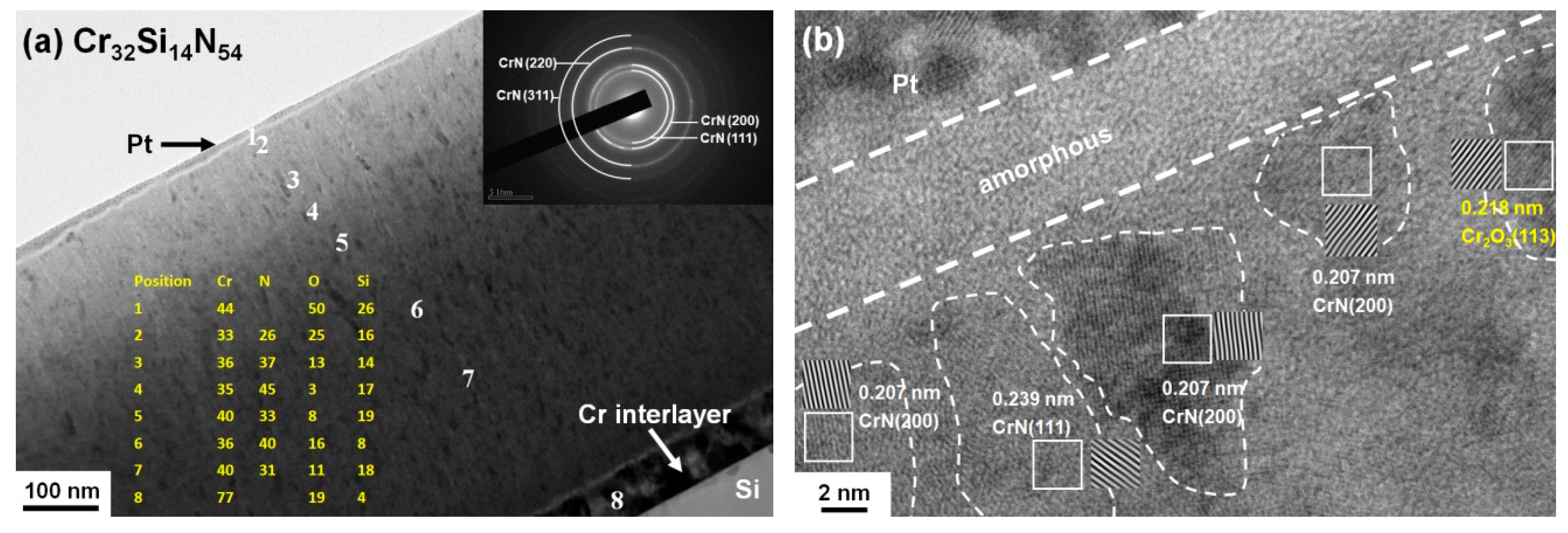
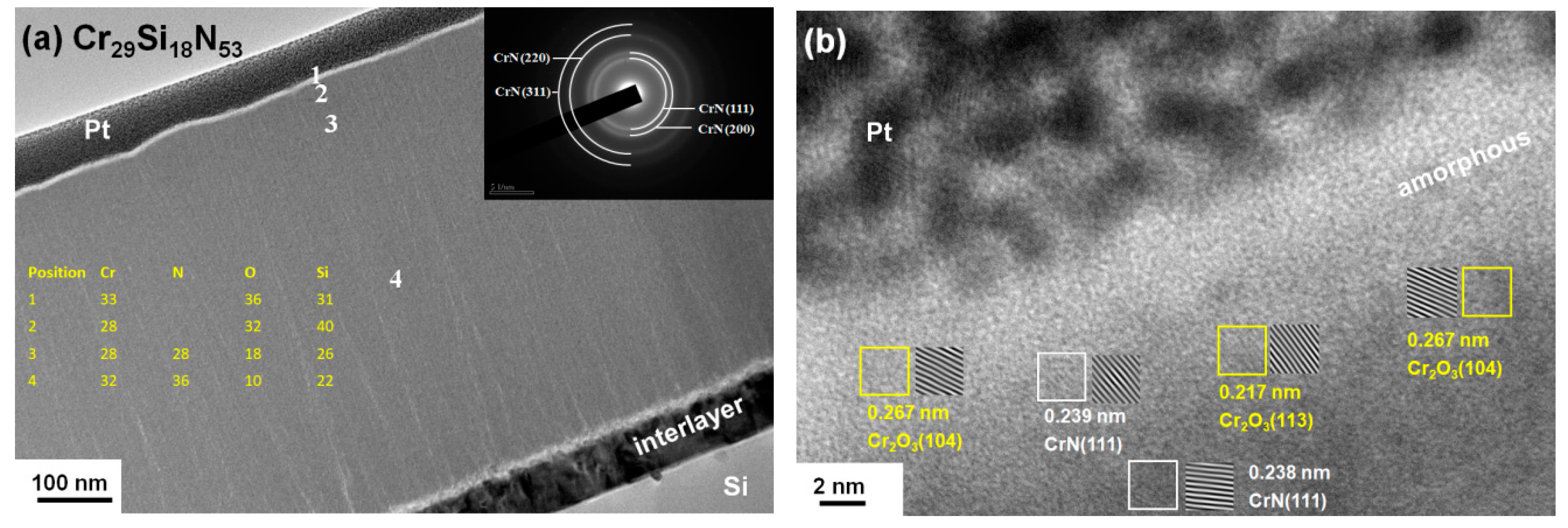
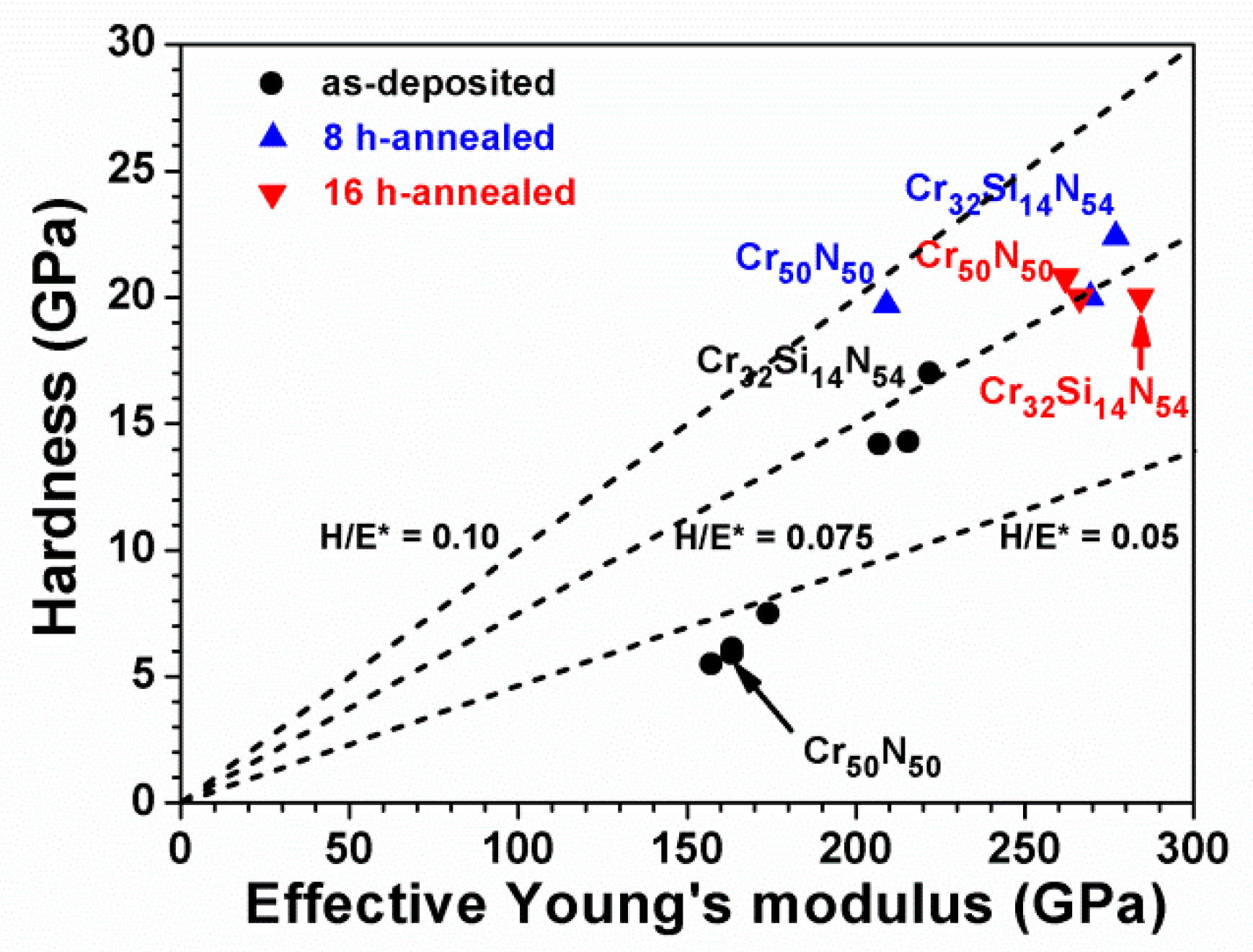
3.3. Oxidation Behavior of Cr–Si–N Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Navinšek, B.; Panjan, P.; Mološev, I. Industrial applications of CrN (PVD) coatings, deposited at high and low temperatures. Surf. Coat. Technol. 1997, 97, 182–191. [Google Scholar] [CrossRef]
- Hones, P.; Sanjines, R.; Lévy, F. Characterization of sputter-deposited chromium nitride thin films for hard coatings. Surf. Coat. Technol. 1997, 94–95, 398–402. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Mitterer, C.; Musil, J. Structure–property relationships in single- and dual-phase nanocrystalline hard coatings. Surf. Coat. Technol. 2003, 174–175, 725–731. [Google Scholar] [CrossRef]
- Lee, J.W.; Kuo, Y.C.; Wang, C.J.; Chang, L.C.; Liu, K.T. Effects of substrate bias frequencies on the characteristics of chromium nitride coatings deposited by pulsed DC reactive magnetron sputtering. Surf. Coat. Technol. 2008, 203, 721–725. [Google Scholar] [CrossRef]
- Shan, L.; Wang, Y.; Li, J.; Jiang, X.; Chen, J. Improving tribological performance of CrN coatings in seawater by structure design. Tribol. Int. 2015, 82, 78–88. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Matthews, A. EIS comparison on corrosion performance of PVD TiN and CrN coated mild steel in 0.5 N NaCl aqueous solution. Corros. Sci. 2001, 43, 1953–1961. [Google Scholar] [CrossRef]
- Navinšek, B.; Panjan, P. Oxidation resistance of PVD Cr, Cr–N and Cr–N–O hard coatings. Surf. Coat. Technol. 1993, 59, 244–248. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Willmann, H.; Mitterer, C. Oxidation kinetics of sputtered Cr–N hard coatings. Surf. Coat. Technol. 2001, 146–147, 222–228. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lu, F.H. Oxidation behavior of chromium nitride films. Thin Solid Films 2006, 515, 2179–2184. [Google Scholar] [CrossRef]
- Meng, C.; Yang, L.; Wu, Y.; Tan, J.; Dang, W.; He, X.; Ma, X. Study of the oxidation behavior of CrN coating on Zr alloy in air. J. Nucl. Mater. 2019, 515, 354–369. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Selvakumar, N.; Deepthi, B.; Rajam, K.S. A comparative study of reactive direct current magnetron sputtered CrAlN and CrN coatings. Surf. Coat. Technol. 2006, 201, 2193–2201. [Google Scholar] [CrossRef]
- Lin, J.; Mishra, B.; Moore, J.J.; Sproul, W.D. A study of the oxidation behavior of CrN and CrAlN thin films in air using DSC and TGA analyses. Surf. Coat. Technol. 2008, 202, 3272–3283. [Google Scholar] [CrossRef]
- Martinez, E.; Sanjinés, R.; Banakh, O.; Lévy, F. Electrical, optical and mechanical properties of sputtered CrNy and Cr1−xSixN1.02 thin films. Thin Solid Films 2004, 447–448, 332–336. [Google Scholar] [CrossRef]
- Hones, P.; Consiglio, R.; Randall, N.; Lévy, F. Mechanical properties of hard chromium tungsten nitride coatings. Surf. Coat. Technol. 2000, 125, 179–184. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G.; Yau, B.S. Processing of chromium tungsten nitride hard coatings for glass molding. Surf. Coat. Technol. 2006, 201, 1316–1322. [Google Scholar] [CrossRef]
- Kim, G.S.; Kim, B.S.; Lee, S.Y.; Hahn, J.H. Structure and mechanical properties of Cr–Zr–N films synthesized by closed field unbalanced magnetron sputtering with vertical magnetron sources. Surf. Coat. Technol. 2005, 200, 1669–1675. [Google Scholar] [CrossRef]
- Park, J.H.; Chung, W.S.; Cho, Y.R.; Kim, K.H. Synthesis and mechanical properties of Cr–Si–N coatings deposited by a hybrid system of arc ion plating and sputtering techniques. Surf. Coat. Technol. 2004, 188–189, 425–430. [Google Scholar] [CrossRef]
- Thobor-Keck, A.; Lapostolle, F.; Dehlinger, A.S.; Pilloud, D.; Pierson, J.F.; Coddet, C. Influence of silicon addition on the oxidation resistance of CrN coatings. Surf. Coat. Technol. 2005, 200, 264–268. [Google Scholar] [CrossRef]
- Mercs, D.; Bonasso, N.; Naamane, S.; Bordes, J.M.; Coddet, C. Mechanical and tribological properties of Cr–N and Cr–SI–N coatings reactively sputter deposited. Surf. Coat. Technol. 2005, 200, 403–407. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jung, W.S.; Han, J.G.; Seo, S.M.; Kim, J.H.; Bae, Y.H. The synthesis of CrSiN film deposited using magnetron sputtering system. Surf. Coat. Technol. 2005, 200, 1026–1030. [Google Scholar] [CrossRef]
- Sandu, C.S.; Sanjinés, R.; Benkahoul, M.; Medjani, F.; Lévy, F. Formation of composite ternary nitride thin films by magnetron sputtering co-deposition. Surf. Coat. Technol. 2006, 201, 4083–4089. [Google Scholar] [CrossRef]
- Lee, J.W.; Chang, Y.C. A study on the microstructures and mechanical properties of pulsed DC reactive magnetron sputtered Cr–Si–N nanocomposite coatings. Surf. Coat. Technol. 2007, 202, 831–836. [Google Scholar] [CrossRef]
- Benkahoul, M.; Robin, P.; Gujrathi, S.C.; Martinu, L.; Klemberg-Sapieha, J.E. Microstructure and mechanical properties of Cr–Si–N coatings prepared by pulsed reactive dual magnetron sputtering. Surf. Coat. Technol. 2008, 202, 3975–3980. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, M.W.; Kang, M.C.; Kim, K.H.; Kim, K.H.; Kwon, D.H.; Kim, J.S. Cutting performance of CrN and Cr–Si–N coated end-mill deposited by hybrid coating system for ultra-high speed micro machining. Surf. Coat. Technol. 2008, 202, 5613–5616. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, L.; Wang, S.C.; Yan, P.; Xue, Q. Structure and mechanical properties of reactive sputtering CrSiN films. Appl. Surf. Sci. 2009, 255, 4425–4429. [Google Scholar] [CrossRef]
- Lin, J.; Wang, B.; Ou, Y.; Sproul, W.D.; Dahan, I.; Moore, J.J. Structure and properties of CrSiN nanocomposite coatings deposited by hybrid modulated pulsed power and pulsed dc magnetron sputtering. Surf. Coat. Technol. 2013, 216, 251–258. [Google Scholar] [CrossRef]
- Veprek, S.; Veprek-Heijman, M.G.J.; Karvankova, P.; Prochazka, J. Different approaches to superhard coatings and nanocomposites. Thin Solid Films 2005, 476, 1–29. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, S.R.; Yoon, S.Y. Superhard Ti–Si–N coatings by a hybrid system of arc ion plating and sputtering techniques. Surf. Coat. Technol. 2002, 298, 243–248. [Google Scholar] [CrossRef]
- Tien, S.K.; Duh, J.G.; Lee, J.W. Oxidation behavior of sputtered CrN/AlN multilayer coatings during heat treatment. Surf. Coat. Technol. 2007, 201, 5138–5142. [Google Scholar] [CrossRef]
- Castaldi, L.; Kurapov, D.; Reiter, A.; Shklover, V.; Schwaller, P.; Patscheider, J. High temperature phase changes and oxidation behavior of Cr–Si–N coatings. Surf. Coat. Technol. 2007, 202, 781–785. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Rapaud, O.; Bonasso, N.; Mercs, D.; Dong, C.; Coddet, C. Control of microstructures and properties of dc magnetron sputtering deposited chromium nitride films. Vacuum 2008, 82, 501–509. [Google Scholar] [CrossRef]
- Liu, S.C.; Chen, Y.I.; Tsai, H.Y.; Lin, K.C.; Chen, Y.H. Thermal stability of Ir–Re coatings annealed in oxygen-containing atmospheres. Surf. Coat. Technol. 2013, 237, 105–111. [Google Scholar] [CrossRef]
- Chang, L.C.; Liu, B.W.; Chen, Y.I. Mechanical Properties and Oxidation Behavior of Multilayered Hf–Si–N Coatings. Coatings 2018, 8, 354. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Olaya, J.J.; Wei, G.; Rodil, S.E.; Muhl, S.; Bhushan, B. Influence of the ion–atom flux ratio on the mechanical properties of chromium nitride thin films. Vacuum 2007, 81, 610–618. [Google Scholar] [CrossRef]
- Janssen, G.C.A.M.; Abdalla, M.M.; van Keulen, F.; Pujada, B.R.; van Venrooy, B. Celebrating the 100th anniversary of the Stoney equation for film stress: Developments from polycrystalline steel strips to single crystal silicon wafers. Thin Solid Films 2009, 517, 1858–1867. [Google Scholar] [CrossRef]
- Lee, J.W.; Tien, S.K.; Kuo, Y.C.; Chen, C.M. The mechanical properties evaluation of the CrN coatings deposited by the pulsed DC reactive magnetron sputtering. Surf. Coat. Technol. 2006, 200, 3330–3335. [Google Scholar] [CrossRef]
- Čekada, M.; Panjan, P.; Navinšek, B.; Cvelbar, F. Characterization of (Cr,Ta)N hard coatings reactively sputtered at low temperature. Vacuum 1999, 52, 461–467. [Google Scholar] [CrossRef]
- Choi, E.Y.; Kang, M.C.; Kwon, D.H.; Shin, D.W.; Kim, K.H. Comparative studies on microstructure and mechanical properties of CrN, Cr–C–N and Cr–Mo–N coatings. J. Mater. Process. Technol. 2007, 187–188, 566–570. [Google Scholar] [CrossRef]
- Musil, J. Hard nanocomposite coatings: Thermal stability, oxidation resistance and toughness. Surf. Coat. Technol. 2012, 207, 50–65. [Google Scholar] [CrossRef]
- Odén, M.; Ericsson, C.; Håkansson, G.; Ljungcrantz, H. Microstructure and mechanical behavior of arc-evaporated Cr–N coatings. Surf. Coat. Technol. 1999, 114, 39–51. [Google Scholar] [CrossRef]
- Hones, P.; Diserens, M.; Lévy, F. Characterization of sputter-deposited chromium oxide thin films. Surf. Coat. Technol. 1999, 120–121, 277–283. [Google Scholar] [CrossRef]
- Ji, A.L.; Wang, W.; Song, G.H.; Wang, Q.M.; Sun, C.; Wen, L.S. Microstructures and mechanical properties of chromium oxide films by arc ion plating. Mater. Lett. 2004, 58, 1993–1998. [Google Scholar] [CrossRef]
- Eklund, P.; Sridharan, M.; Sillassen, M.; Bøttiger, J. α-Cr2O3 template-texture effect on α-Al2O3 thin-film growth. Thin Solid Films 2008, 516, 7447–17450. [Google Scholar] [CrossRef]
- Jeong, H.; Cho, J. Fabrication and evaluation of protective SiOx layers using plasma-enhanced chemical vapor deposition. Surf. Coat. Technol. 2017, 330, 71–76. [Google Scholar] [CrossRef]
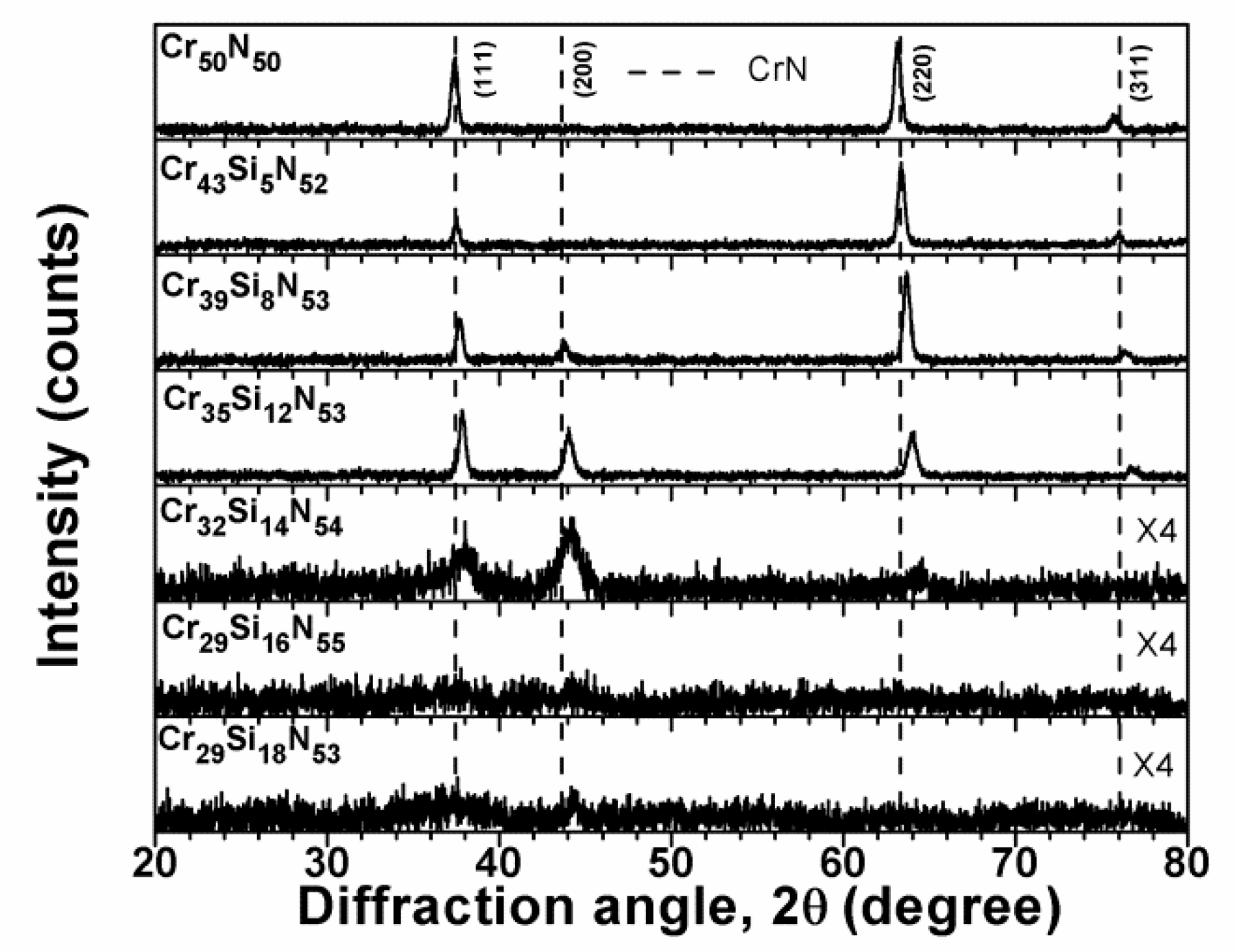
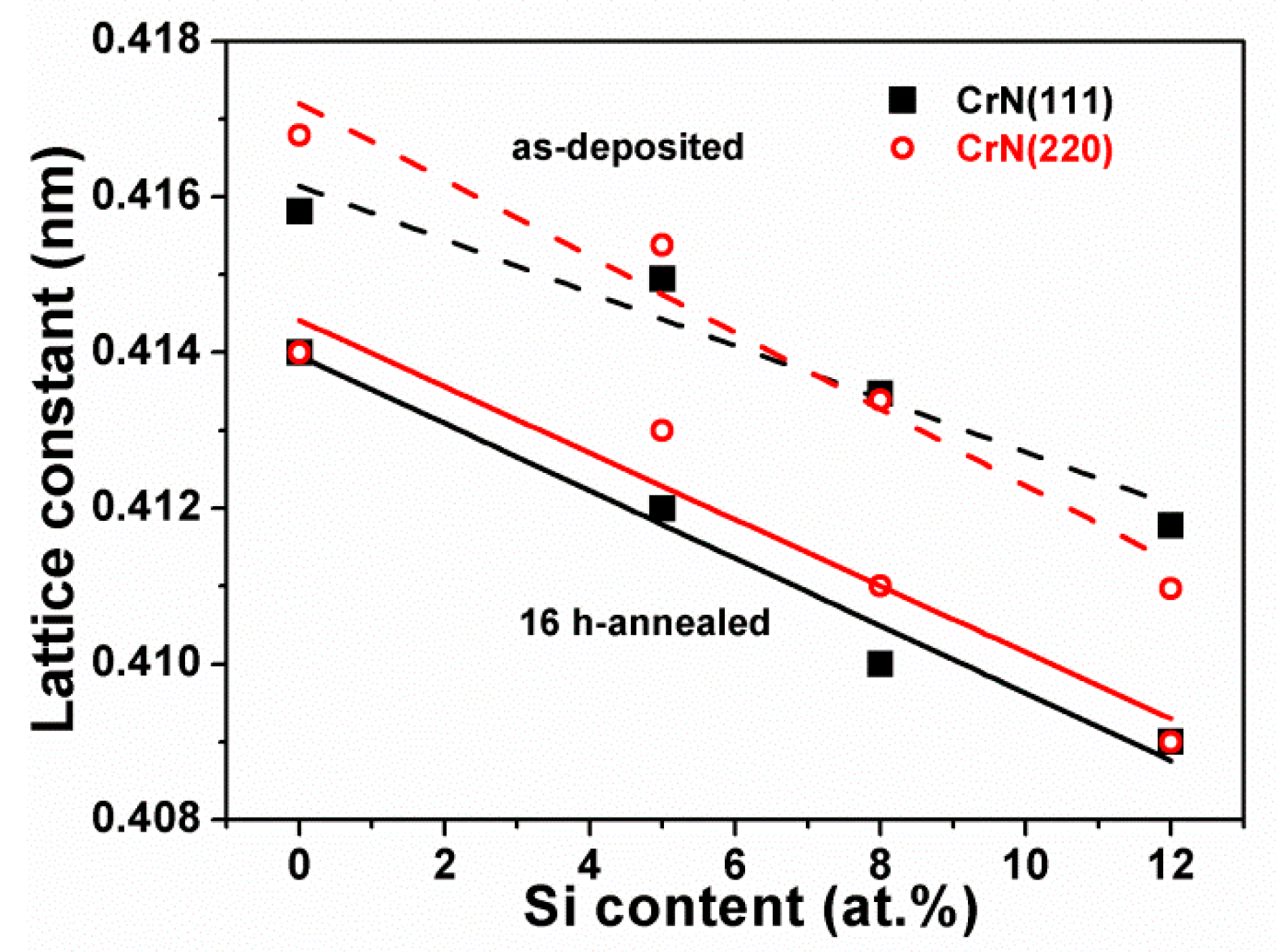

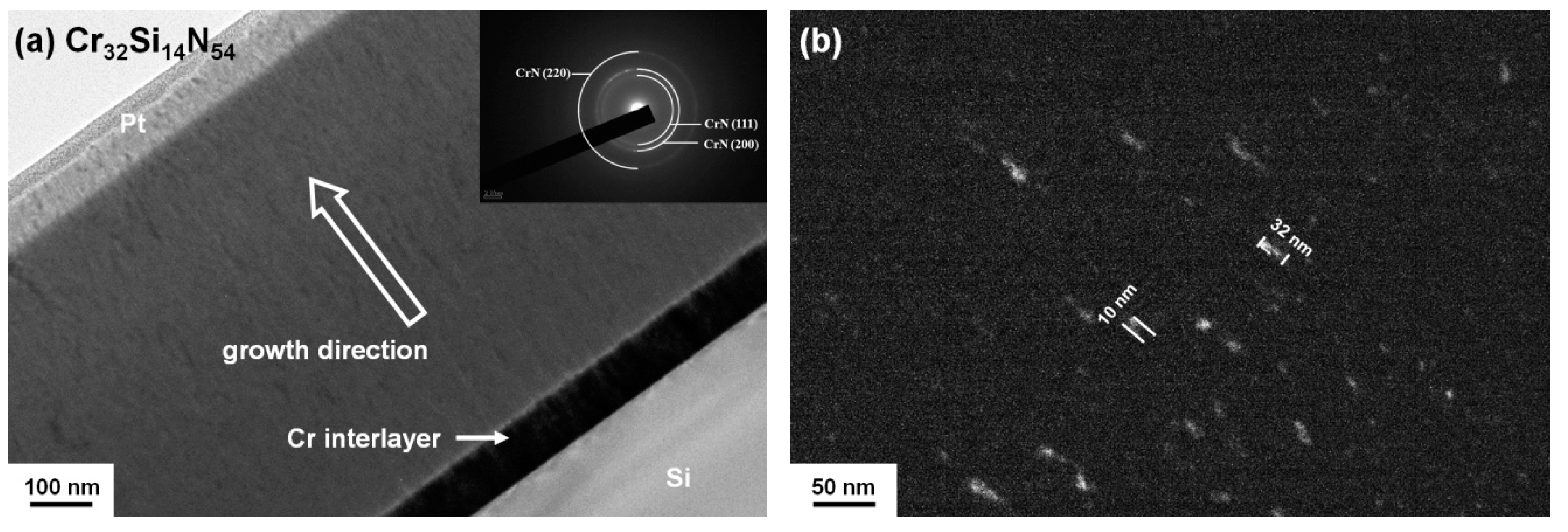
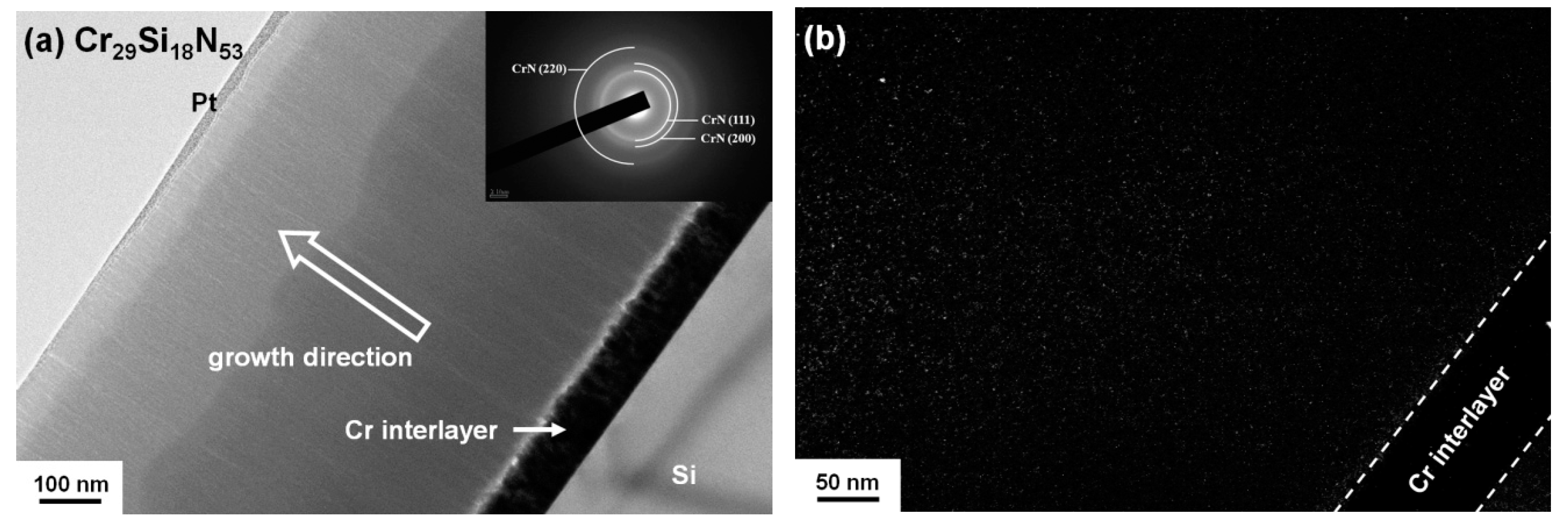
| Sample | Power (W) | Chemical Composition (at %) | D 1 (nm/min) | Ra 2 (nm) | ||||
|---|---|---|---|---|---|---|---|---|
| Cr | Si | Cr | Si | N | O | |||
| Cr50N50 | 100 | 0 | 48.7 ± 0.4 | – | 49.3 ± 0.3 | 2.0 ± 0.2 | 5.9 | 6.2 ± 0.1 |
| Cr43Si5N52 | 100 | 25 | 42.1 ± 0.8 | 4.4 ± 0.1 | 50.5 ± 0.9 | 3.0 ± 0.3 | 6.6 | 6.8 ± 0.6 |
| Cr39Si8N53 | 100 | 50 | 37.7 ± 0.3 | 8.0 ± 0.1 | 50.7 ± 0.4 | 3.6 ± 0.1 | 7.8 | 2.4 ± 0.1 |
| Cr35Si12N53 | 100 | 75 | 33.7 ± 0.4 | 11.0 ± 0.1 | 50.7 ± 0.4 | 4.6 ± 0.3 | 8.3 | 2.7 ± 0.2 |
| Cr32Si14N54 | 100 | 100 | 30.0 ± 0.4 | 14.5 ± 0.1 | 51.5 ± 0.5 | 5.0 ± 0.1 | 8.1 | 2.3 ± 0.5 |
| Cr29Si16N55 | 100 | 125 | 28.1 ± 0.2 | 16.1 ± 0.1 | 53.7 ± 0.4 | 2.1 ± 0.2 | 8.9 | 0.9 ± 0.2 |
| Cr29Si18N53 | 100 | 150 | 28.4 ± 0.3 | 17.9 ± 0.1 | 51.3 ± 0.4 | 2.4 ± 0.1 | 9.8 | 0.9 ± 0.2 |
| Sample | H1 | E2 | H/E* | We3 | Stress |
|---|---|---|---|---|---|
| (GPa) | (GPa) | % | (GPa) | ||
| Cr50N50 | 5.9 ± 0.3 | 154 ± 8 | 0.036 | 37 | 0.4 ± 0.1 |
| Cr43Si5N52 | 5.5 ± 0.6 | 148 ± 6 | 0.035 | 35 | 0.2 ± 0.1 |
| Cr39Si8N53 | 6.1 ± 0.3 | 154 ± 13 | 0.037 | 34 | 0.4 ± 0.1 |
| Cr35Si12N53 | 7.5 ± 0.6 | 164 ± 7 | 0.043 | 40 | 0.5 ± 0.1 |
| Cr32Si14N54 | 17.0 ± 0.6 | 209 ± 3 | 0.077 | 55 | 0.7 ± 0.1 |
| Cr29Si16N55 | 14.3 ± 0.7 | 203 ± 6 | 0.066 | 50 | 0.9 ± 0.2 |
| Cr29Si18N53 | 14.2 ± 0.7 | 195 ± 5 | 0.069 | 53 | 0.6 ± 0.1 |
| Sample | Time 1 | Chemical Composition (at %) | Ra 2 | Stress | H3 | E4 | H/E* | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (h) | Cr | Si | N | O | (nm) | (GPa) | (GPa) | (GPa) | ||
| Cr50N50 | 0 | 48.7 ± 0.4 | – | 49.3 ± 0.3 | 2.0 ± 0.2 | 6.2 ± 0.1 | 0.4 ± 0.1 | 5.9 ± 0.3 | 154 ± 8 | 0.036 |
| 8 | 42.9 ± 0.2 | – | 26.3 ± 0.5 | 30.8 ± 0.6 | 4.7 ± 0.1 | 1.1 ± 0.0 | 19.7 ± 1.4 | 197 ± 6 | 0.094 | |
| 16 | 42.4 ± 0.2 | – | 27.7 ± 0.2 | 29.9 ± 0.1 | 5.3 ± 0.1 | 1.0 ± 0.0 | 20.8 ± 1.4 | 247 ± 9 | 0.079 | |
| Cr32Si14N54 | 0 | 30.0 ± 0.4 | 14.5 ± 0.1 | 51.5 ± 0.5 | 5.0 ± 0.1 | 2.3 ± 0.5 | 0.7 ± 0.1 | 17.0 ± 0.6 | 209 ± 3 | 0.077 |
| 8 | 29.4 ± 0.2 | 13.1 ± 0.1 | 47.6 ± 0.2 | 9.9 ± 0.3 | 2.6 ± 0.1 | 1.3 ± 0.0 | 22.4 ± 1.0 | 261 ± 14 | 0.081 | |
| 16 | 30.8 ± 0.3 | 13.6 ± 0.1 | 45.5 ± 0.4 | 10.1 ± 0.0 | 2.7 ± 0.0 | 2.4 ± 0.1 | 20.0 ± 1.4 | 268 ± 12 | 0.070 | |
| Cr29Si18N53 | 0 | 28.4 ± 0.3 | 17.9 ± 0.1 | 51.3 ± 0.4 | 2.4 ± 0.1 | 0.9 ± 0.2 | 0.6 ± 0.1 | 14.2 ± 0.7 | 195 ± 5 | 0.069 |
| 8 | 27.8 ± 0.1 | 17.3 ± 0.1 | 50.3 ± 0.3 | 4.6 ± 0.2 | 1.9 ± 0.1 | 1.4 ± 0.0 | 20.0 ± 1.7 | 254 ± 15 | 0.074 | |
| 16 | 27.4 ± 0.4 | 17.2 ± 0.3 | 52.2 ± 0.4 | 3.2 ± 0.1 | 2.6 ± 0.0 | 1.4 ± 0.3 | 20.0 ± 1.0 | 251 ± 10 | 0.075 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.-C.; Liu, Y.-H.; Chen, Y.-I. Mechanical Properties and Oxidation Behavior of Cr–Si–N Coatings. Coatings 2019, 9, 528. https://doi.org/10.3390/coatings9080528
Chang L-C, Liu Y-H, Chen Y-I. Mechanical Properties and Oxidation Behavior of Cr–Si–N Coatings. Coatings. 2019; 9(8):528. https://doi.org/10.3390/coatings9080528
Chicago/Turabian StyleChang, Li-Chun, Yu-Heng Liu, and Yung-I Chen. 2019. "Mechanical Properties and Oxidation Behavior of Cr–Si–N Coatings" Coatings 9, no. 8: 528. https://doi.org/10.3390/coatings9080528
APA StyleChang, L.-C., Liu, Y.-H., & Chen, Y.-I. (2019). Mechanical Properties and Oxidation Behavior of Cr–Si–N Coatings. Coatings, 9(8), 528. https://doi.org/10.3390/coatings9080528




