Design of Scorodite@Fe3O4 Core–Shell Materials and the Fe3O4 Shell Prevents Leaching of Arsenic from Scorodite in Neutral and Alkaline Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Scorodite Synthesis
2.3. Scorodite@Fe3O4 Synthesis
2.4. Characterization
2.5. Stability Evaluation of Products
3. Results and Discussion
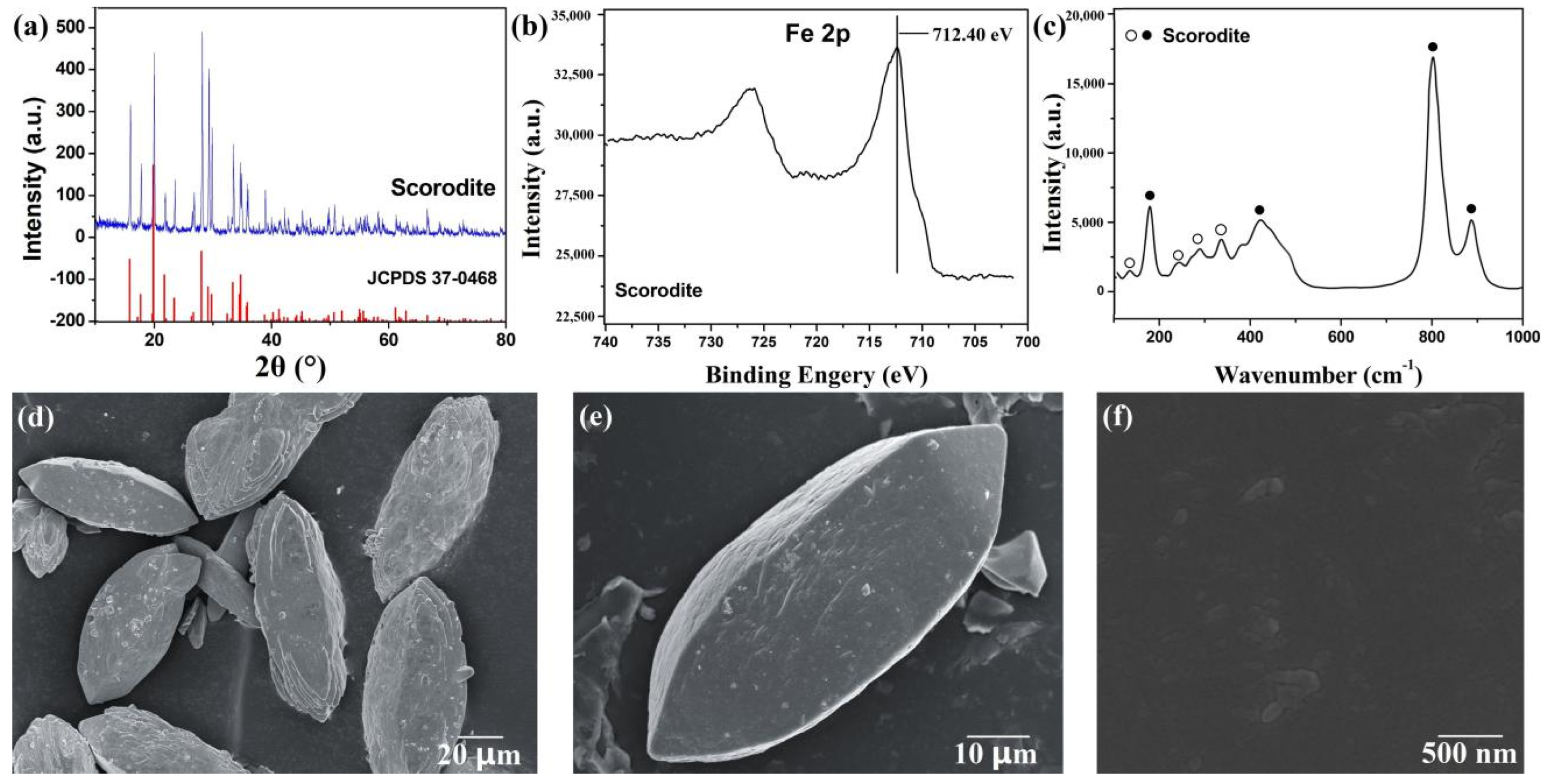
3.1. Characterization of the Scorodite
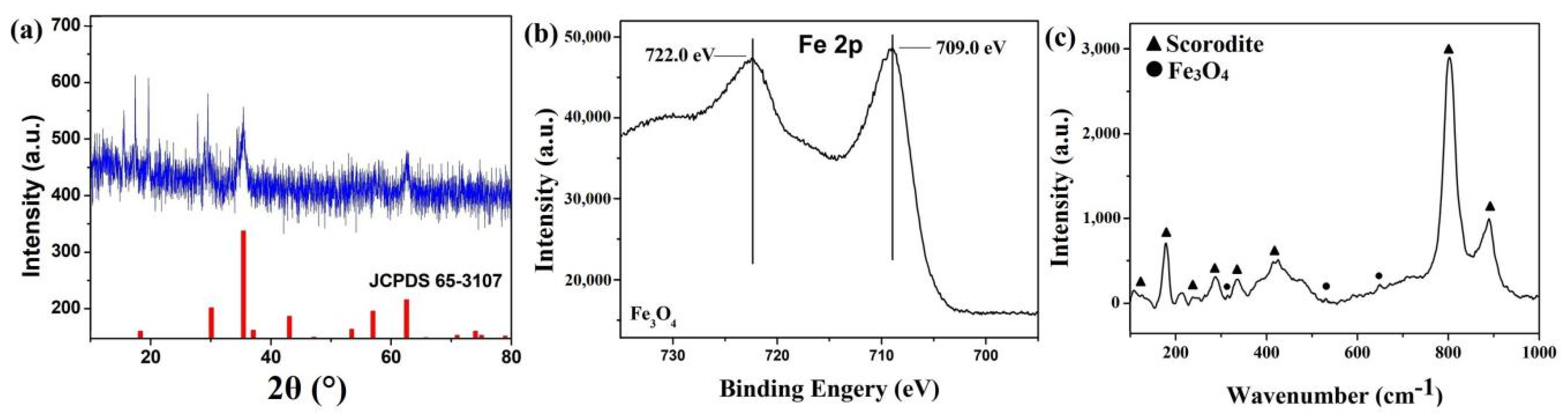
3.2. Characterization of Scorodite@Fe3O4
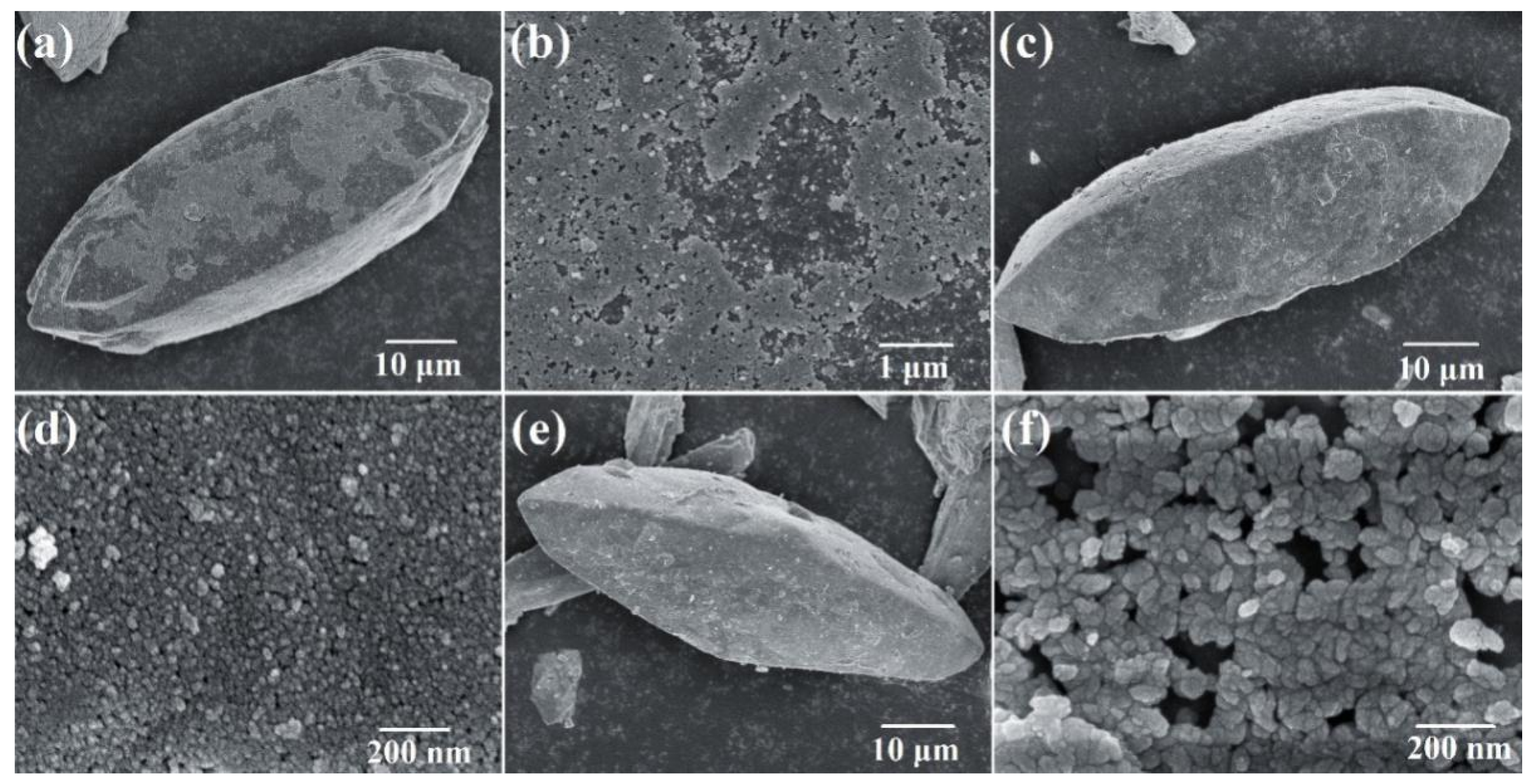
3.3. Morphology of Scorodite@Fe3O4
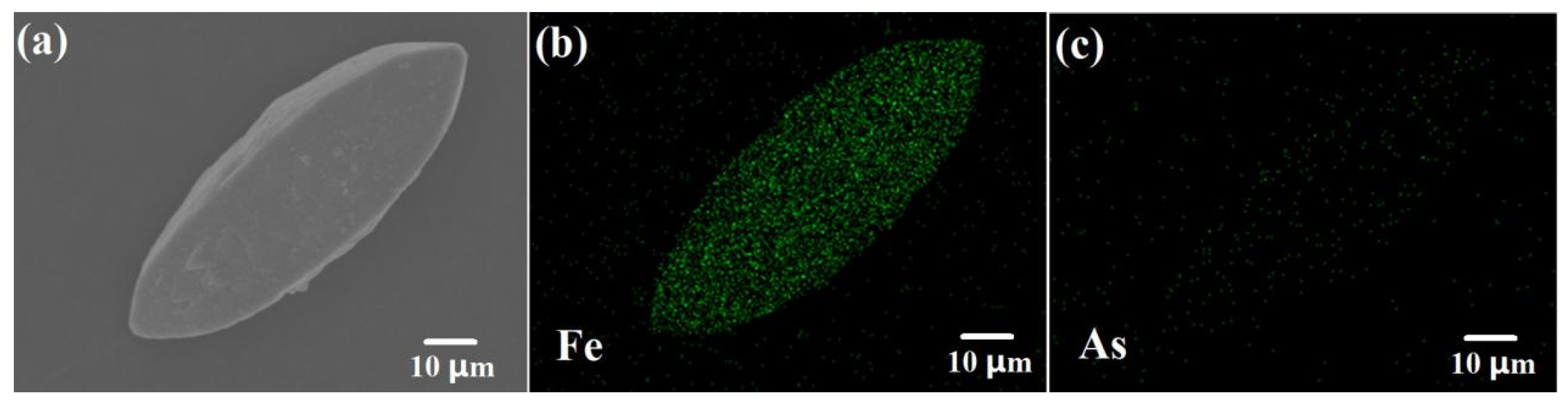
3.4. EDS Mapping Analysis of Scorodite@Fe3O4
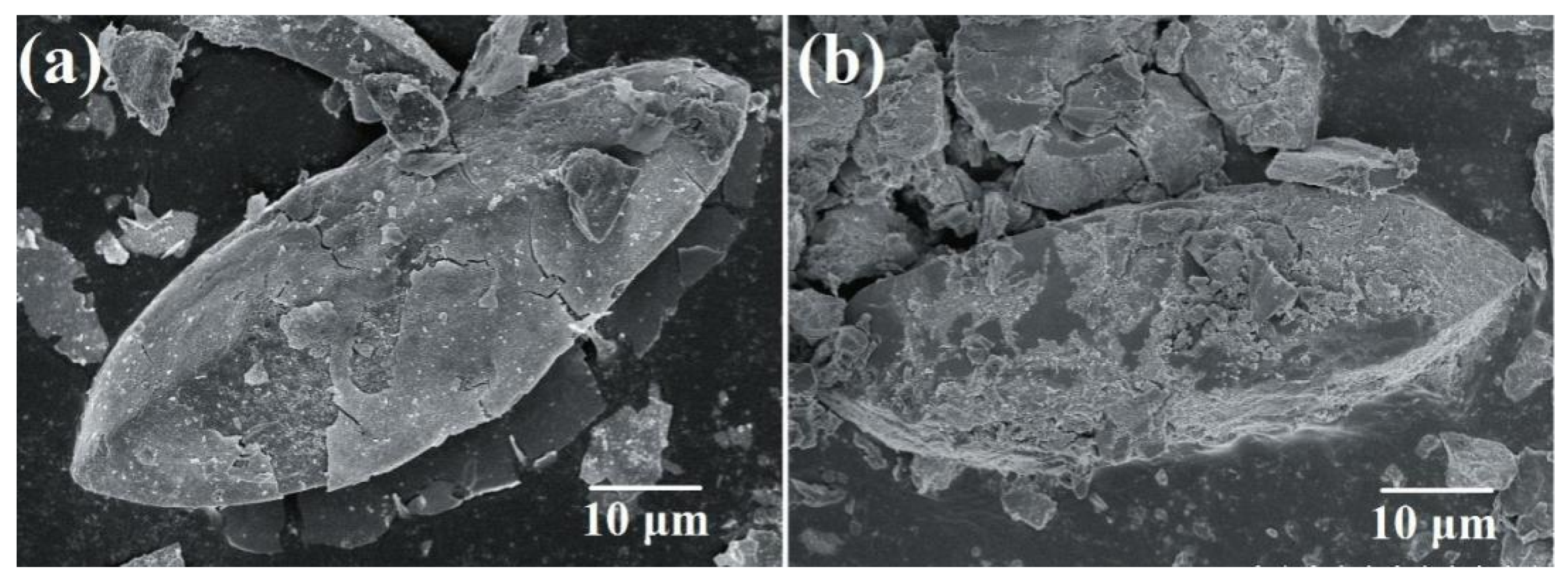
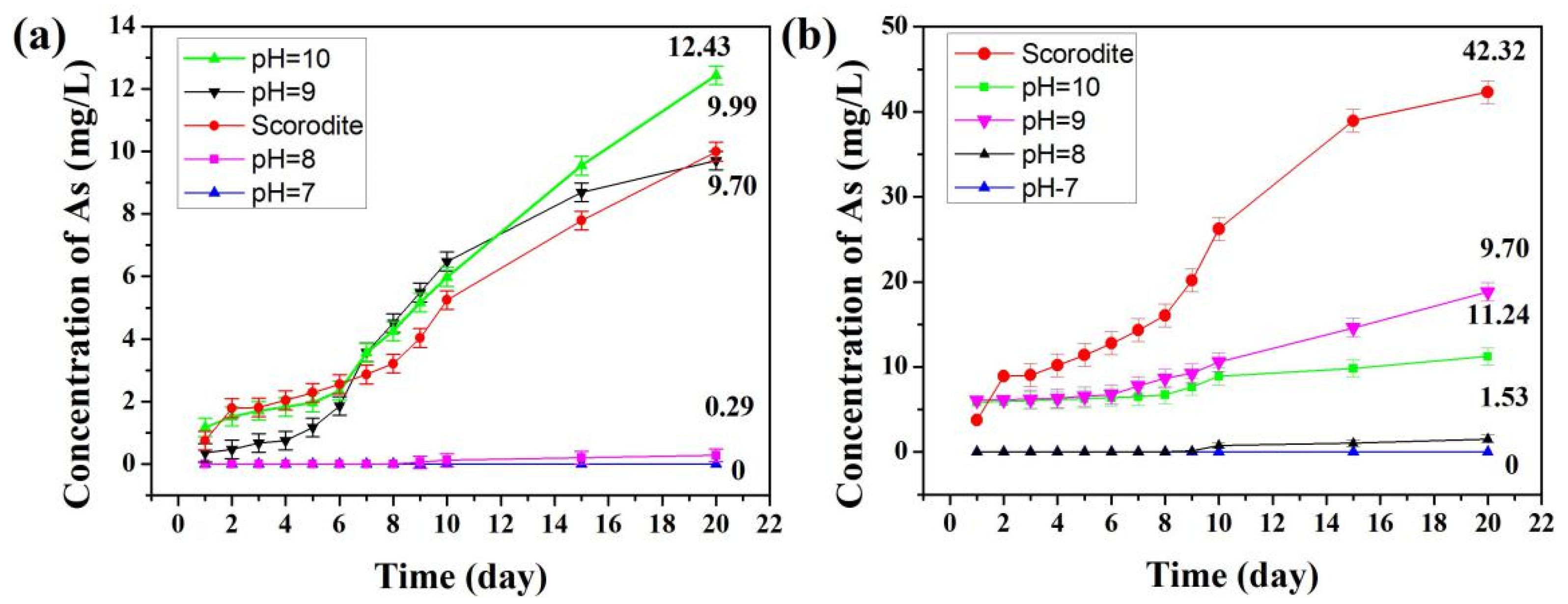
3.5. Stability Evaluation Analysis of Scorodite@Fe3O4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fakhri, Y.; Bjørklund, G.; Bandpei, A.M.; Chirumbolo, S.; Keramati, H.; Pouya, R.H.; Asadi, A.; Amanidaz, N.; Sarafraz, M.; Sheikhmohammad, A. Concentrations of arsenic and lead in rice (Oryza sativa L.) in Iran: A systematic review and carcinogenic risk assessment. Food Chem. Toxicol. 2018, 113, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Khan, S.; Ibrahim, M.; Sun, T.-R.; Tang, J.-F.; Cotner, J.B.; Xu, Y.-Y. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J. Hazard. Mater. 2018, 348, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Neff, J.M. Ecotoxicology of arsenic in the marine environment. Environ. Toxicol. Chem. 1997, 16, 917–927. [Google Scholar] [CrossRef]
- Bretzler, A.; Lalanne, F.; Nikiema, J.; Podgorski, J.; Pfenninger, N.; Berg, M.; Schirmer, M. Groundwater arsenic contamination in Burkina Faso, West Africa: Predicting and verifying regions at risk. Sci. Total Environ. 2017, 584, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution: A Global Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 28. [Google Scholar]
- Vadahanambi, S.; Lee, S.-H.; Kim, W.-J.; Oh, I.-K. Arsenic removal from contaminated water using three-dimensional graphene-carbon nanotube-iron oxide nanostructures. Environ. Sci. Technol. 2013, 47, 10510–10517. [Google Scholar] [CrossRef] [PubMed]
- Leonard, R.L.; Whellock, J.G. Extraction or Recovery of Non-Ferrous Metal Values from Arsenic-Containing Materials. U.S. Patent 5,482,534A, 9 January 1996. [Google Scholar]
- Dudka, S.; Adriano, D.C. Environmental impacts of metal ore mining and processing: A review. J. Environ. Qual. 1997, 26, 590–602. [Google Scholar] [CrossRef]
- Agrawal, A.; Sahu, K.; Pandey, B. Solid waste management in non-ferrous industries in India. Resour. Conserv. Recycl. 2004, 42, 99–120. [Google Scholar] [CrossRef]
- Wei, C.; Jiang, Q.; Luo, T.; Huang, M. Arsenic removal and recovery in heavy metals smelting process. Min. Res. Dev. 2003, 2, 1. [Google Scholar]
- Mukherjee, A.B. Arsenic flows in the environment of the European Union: A synoptic review. Trace Met. Other Contam. Environ. 2007, 9, 527–547. [Google Scholar]
- Ferguson, J.F.; Gavis, J. A review of the arsenic cycle in natural waters. Water Res. 1972, 6, 1259–1274. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.B.; Guo, X.; Su, Y.B.; Wang, G. Risk assessment of heavy metals in soils and vegetables around non-ferrous metals mining and smelting sites, Baiyin, China. J. Environ. Sci. 2006, 18, 1124–1134. [Google Scholar]
- Leist, M.; Casey, R.; Caridi, D. The management of arsenic wastes: Problems and prospects. J. Hazard. Mater. 2000, 76, 125–138. [Google Scholar] [CrossRef]
- Sullivan, C.; Tyrer, M.; Cheeseman, C.R.; Graham, N.J. Disposal of water treatment wastes containing arsenic—A review. Sci. Total Environ. 2010, 408, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Li, J.; Xie, B.; Xu, Z. Recycling arsenic from gallium arsenide scraps through sulfurizing thermal treatment. ACS Sustain. Chem. Eng. 2017, 5, 3179–3185. [Google Scholar] [CrossRef]
- Bothe, J.V.; Brown, P.W. Arsenic immobilization by calcium arsenate formation. Environ. Sci. Technol. 1999, 33, 3806–3811. [Google Scholar] [CrossRef]
- Coussy, S.; Benzaazoua, M.; Blanc, D.; Moszkowicz, P.; Bussière, B. Assessment of arsenic immobilization in synthetically prepared cemented paste backfill specimens. J. Environ. Manag. 2012, 93, 10–21. [Google Scholar] [CrossRef]
- Palfy, P.; Vircikova, E.; Molnar, L. Processing of arsenic waste by precipitation and solidification. Waste Manag. 1999, 19, 55–59. [Google Scholar] [CrossRef]
- Ahoranta, S.H.; Kokko, M.E.; Papirio, S.; Özkaya, B.; Puhakka, J.A. Arsenic removal from acidic solutions with biogenic ferric precipitates. J. Hazard. Mater. 2016, 306, 124–132. [Google Scholar] [CrossRef]
- Lim, K.; Shukor, M.; Wasoh, H. Physical, chemical, and biological methods for the removal of arsenic compounds. BioMed Res. Int. 2014, 2014, 503784. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X. On the potential of biological treatment for arsenic contaminated soils and groundwater. J. Environ. Manag. 2009, 90, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Bluteau, M.-C.; Becze, L.; Demopoulos, G.P. The dissolution of scorodite in gypsum-saturated waters: Evidence of Ca–Fe–AsO4 mineral formation and its impact on arsenic retention. Hydrometallurgy 2009, 97, 221–227. [Google Scholar] [CrossRef]
- Nikolaidis, N.P.; Dobbs, G.M.; Lackovic, J.A. Arsenic removal by zero-valent iron: Field, laboratory and modeling studies. Water Res. 2003, 37, 1417–1425. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Paktunc, D.; Bruggeman, K. Solubility of nanocrystalline scorodite and amorphous ferric arsenate: Implications for stabilization of arsenic in mine wastes. Appl. Geochem. 2010, 25, 674–683. [Google Scholar] [CrossRef]
- Fujita, T.; Fujieda, S.; Shinoda, K.; Suzuki, S. Environmental leaching characteristics of scorodite synthesized with Fe (II) ions. Hydrometallurgy 2012, 111, 87–102. [Google Scholar] [CrossRef]
- Le Berre, J.; Gauvin, R.; Demopoulos, G. A study of the crystallization kinetics of scorodite via the transformation of poorly crystalline ferric arsenate in weakly acidic solution. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 117–129. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonavita, A.; Bonyani, M.; Leonardi, S.G.; Neri, G. Synthesis, characterization and gas sensing properties of Ag@α-Fe2O3 core–shell nanocomposites. Nanomaterials 2015, 5, 737–749. [Google Scholar] [CrossRef]
- Ke, P.-C.; Liu, Z.-H. Synthesis, in-situ coating and characterization of scorodite with high leaching stability. Trans. Nonferrous Met. Soc. China 2019, 29, 876–892. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of amine-modified magnetite-electrochemically reduced graphene oxide nanocomposite modified glassy carbon electrode for sensitive dopamine determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, X.; Kaneti, Y.V.; Yu, A. Design and construction of polymerized-glucose coated Fe3O4 magnetic nanoparticles for delivery of aspirin. Powder Technol. 2013, 236, 157–163. [Google Scholar] [CrossRef]
- Kendall, D.S. Toxicity characteristic leaching procedure and iron treatment of brass foundry waste. Environ. Sci. Technol. 2003, 37, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Baghurst, D.R.; Barrett, J.; Coleyshaw, E.E.; Griffith, W.P.; Mingos, D.M.P. Microwave techniques for the synthesis and deuteration of minerals, with particular reference to scorodite, FeAsO4.2H2O. Mineral. Mag. 1996, 60, 821–828. [Google Scholar] [CrossRef]
- Frau, F.; Addari, D.; Atzei, D.; Biddau, R.; Cidu, R.; Rossi, A. Influence of major anions on As (V) adsorption by synthetic 2-line ferrihydrite. Kinetic investigation and XPS study of the competitive effect of bicarbonate. Water Air Soil Pollut. 2010, 205, 25–41. [Google Scholar] [CrossRef]
- Coleyshaw, E.E.; Griffith, W.P.; Bowell, R.J. Fourier-transform Raman spectroscopy of minerals. Spectrochim. Acta Part A Mol. Spectrosc. 1994, 50, 1909–1918. [Google Scholar] [CrossRef]
- Fujita, T.; Taguchi, R.; Abumiya, M.; Matsumoto, M.; Shibata, E.; Nakamura, T. Effect of pH on atmospheric scorodite synthesis by oxidation of ferrous ions: Physical properties and stability of the scorodite. Hydrometallurgy 2009, 96, 189–198. [Google Scholar] [CrossRef]
- Das, S.; Hendry, M.J. Application of Raman spectroscopy to identify iron minerals commonly found in mine wastes. Chem. Geol. 2011, 290, 101–108. [Google Scholar] [CrossRef]
- Tong, G.; Wu, W.; Guan, J.; Qian, H.; Yuan, J.; Li, W. Synthesis and characterization of nanosized urchin-like α-Fe2O3 and Fe3O4: Microwave electromagnetic and absorbing properties. J. Alloy. Compd. 2011, 509, 4320–4326. [Google Scholar] [CrossRef]
- Hu, C.; Gao, Z.; Yang, X. Fabrication and magnetic properties of Fe3O4 octahedra. Chem. Phys. Lett. 2006, 429, 513–517. [Google Scholar] [CrossRef]
- Muraliganth, T.; Murugan, A.V.; Manthiram, A. Facile synthesis of carbon-decorated single-crystalline Fe3O4 nanowires and their application as high performance anode in lithium ion batteries. Chem. Commun. 2009, 47, 7360–7362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Merkel, B. The dissolution and solubility of scorodite, FeAsO4 2H2O: Evaluation and simulation with PHREEQC2. Wiss. Mitt. Inst. Geol. Tech. Univ. Bergakad. Freib. 2001, 18, 72–87. [Google Scholar]
- Paktunc, D.; Dutrizac, J.; Gertsman, V. Synthesis and phase transformations involving scorodite, ferric arsenate and arsenical ferrihydrite: Implications for arsenic mobility. Geochim. Cosmochim. Acta 2008, 72, 2649–2672. [Google Scholar] [CrossRef]
| Elements | As | K | Al | Zn | Na |
|---|---|---|---|---|---|
| Dust (wt %) | 42.3 ± 1.0 | 0.6 ± 0.1 | 0.1 ± 0.0 | 0.5 ± 0.1 | 0.3 ± 0.1 |
| Leach liquid (g·L–1) | 22.5 ± 2.2 | 0.6 ± 0.2 | 0.01 ± 0.0 | 0.01 ± 0.0 | 0.2 ± 0.1 |
| Sample | Initial Fe Ions/Scorodite Molar Ratio (%) | Reaction Time (min) | Initial Reaction pH | Concentration of As (TCLP 1; mg·L−1) | Concentration of As (TCLP 2; mg·L−1) | Quality of Fe3O4 Shell (1–3 Stars) |
|---|---|---|---|---|---|---|
| Scorodite | – | – | – | 0.671 ± 0.012 | 3.754 ± 0.125 | – |
| 1 | 1 | 15 | 7 | 0 | 0.994 ± 0.009 | 2 |
| 2 | 5 | 15 | 7 | 0.004 ± 0.001 | 0.136 ± 0.011 | 2 |
| 3 | 9 | 15 | 7 | 0 | 0 | 3 |
| 4 | 1 | 15 | 8 | 0 | 0.955 ± 0.012 | 2 |
| 5 | 5 | 15 | 8 | 0 | 0.624 ± 0.010 | 2 |
| 6 | 9 | 15 | 8 | 0 | 0 | 3 |
| 7 | 9 | 25 | 7 | 0 | 0 | 3 |
| 8 | 9 | 35 | 7 | 0 | 0 | 3 |
| 9 | 9 | 25 | 8 | 0 | 0 | 3 |
| 10 | 9 | 35 | 8 | 0 | 0 | 3 |
| 11 | 9 | 15 | 9 | 0.354 ± 0.016 | 6.091 ± 0.172 | 1 |
| 12 | 9 | 15 | 10 | 0.967 ± 0.021 | 3.194 ± 0.046 | 2 |
| 13 | 9 | 25 | 10 | 0 | 1.873 ± 0.104 | 2 |
| 14 | 9 | 35 | 10 | 0 | 1.592 ± 0.120 | 2 |
| 15 | 9 | 15 | 11 | 1.258 ± 0.033 | 7.031 ± 0.163 | 1 |
| 16 | 9 | 15 | 12 | 5.174 ± 0.106 | 12.68 ± 0.118 | 1 |
| 17 | 1 | 15 | 9 | 0.549 ± 0.028 | 7.183 ± 0.049 | 1 |
| 18 | 5 | 15 | 9 | 0.487 ± 0.021 | 6.627 ± 0.094 | 1 |
| 19 | 9 | 25 | 9 | 0.138 ± 0.014 | 3.734 ± 0.043 | 2 |
| 20 | 9 | 35 | 9 | 0 | 0.982 ± 0.011 | 2 |
| 21 | 1 | 15 | 10 | 1.691 ± 0.026 | 8.735 ± 0.114 | 1 |
| 22 | 5 | 15 | 10 | 1.172 ± 0.035 | 5.549 ± 0.073 | 1 |
| 23 | 1 | 15 | 11 | 2.054 ± 0.013 | 9.723 ± 0.047 | 1 |
| 24 | 5 | 15 | 11 | 1.813 ± 0.091 | 7.871 ± 0.083 | 1 |
| 25 | 9 | 25 | 11 | 0.749 ± 0.010 | 4.312 ± 0.144 | 1 |
| 26 | 9 | 35 | 11 | 0.498 ± 0.033 | 3.165 ± 0.075 | 1 |
| 27 | 1 | 15 | 12 | 7.682 ± 0.072 | 15.36 ± 0.148 | 1 |
| 28 | 5 | 15 | 12 | 6.235 ± 0.084 | 13.44 ± 0.162 | 1 |
| 29 | 9 | 25 | 12 | 4.058 ± 0.103 | 11.78 ± 0.092 | 1 |
| 30 | 9 | 35 | 12 | 3.262 ± 0.062 | 10.24 ± 0.136 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Rong, Z.; Tang, X.; Cao, S. Design of Scorodite@Fe3O4 Core–Shell Materials and the Fe3O4 Shell Prevents Leaching of Arsenic from Scorodite in Neutral and Alkaline Environments. Coatings 2019, 9, 523. https://doi.org/10.3390/coatings9080523
Wang Y, Rong Z, Tang X, Cao S. Design of Scorodite@Fe3O4 Core–Shell Materials and the Fe3O4 Shell Prevents Leaching of Arsenic from Scorodite in Neutral and Alkaline Environments. Coatings. 2019; 9(8):523. https://doi.org/10.3390/coatings9080523
Chicago/Turabian StyleWang, Yang, Zhihao Rong, Xincun Tang, and Shan Cao. 2019. "Design of Scorodite@Fe3O4 Core–Shell Materials and the Fe3O4 Shell Prevents Leaching of Arsenic from Scorodite in Neutral and Alkaline Environments" Coatings 9, no. 8: 523. https://doi.org/10.3390/coatings9080523
APA StyleWang, Y., Rong, Z., Tang, X., & Cao, S. (2019). Design of Scorodite@Fe3O4 Core–Shell Materials and the Fe3O4 Shell Prevents Leaching of Arsenic from Scorodite in Neutral and Alkaline Environments. Coatings, 9(8), 523. https://doi.org/10.3390/coatings9080523





