Characterization of Biofilm Formation and Coating Degradation by Electrochemical Impedance Spectroscopy
Abstract
1. Introduction
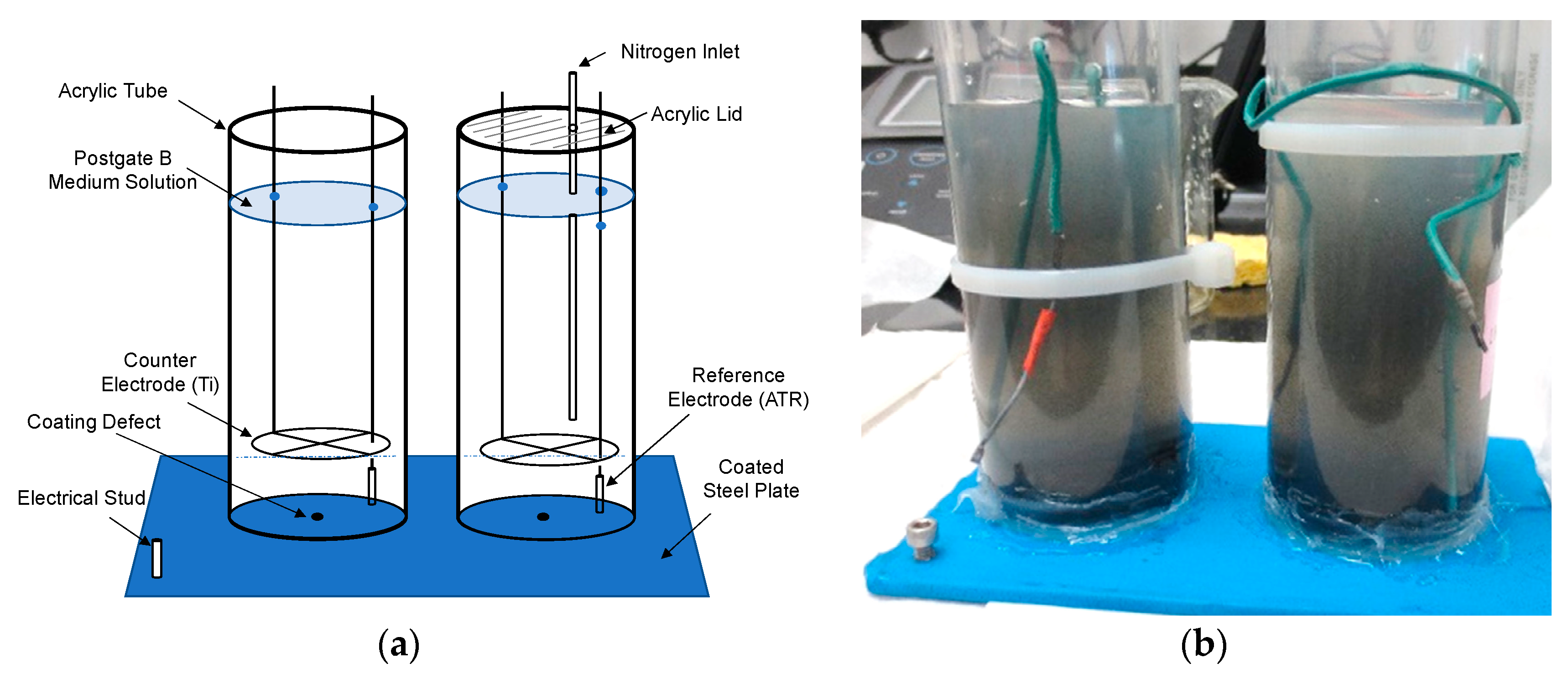
2. Methodology
3. Results and Discussion
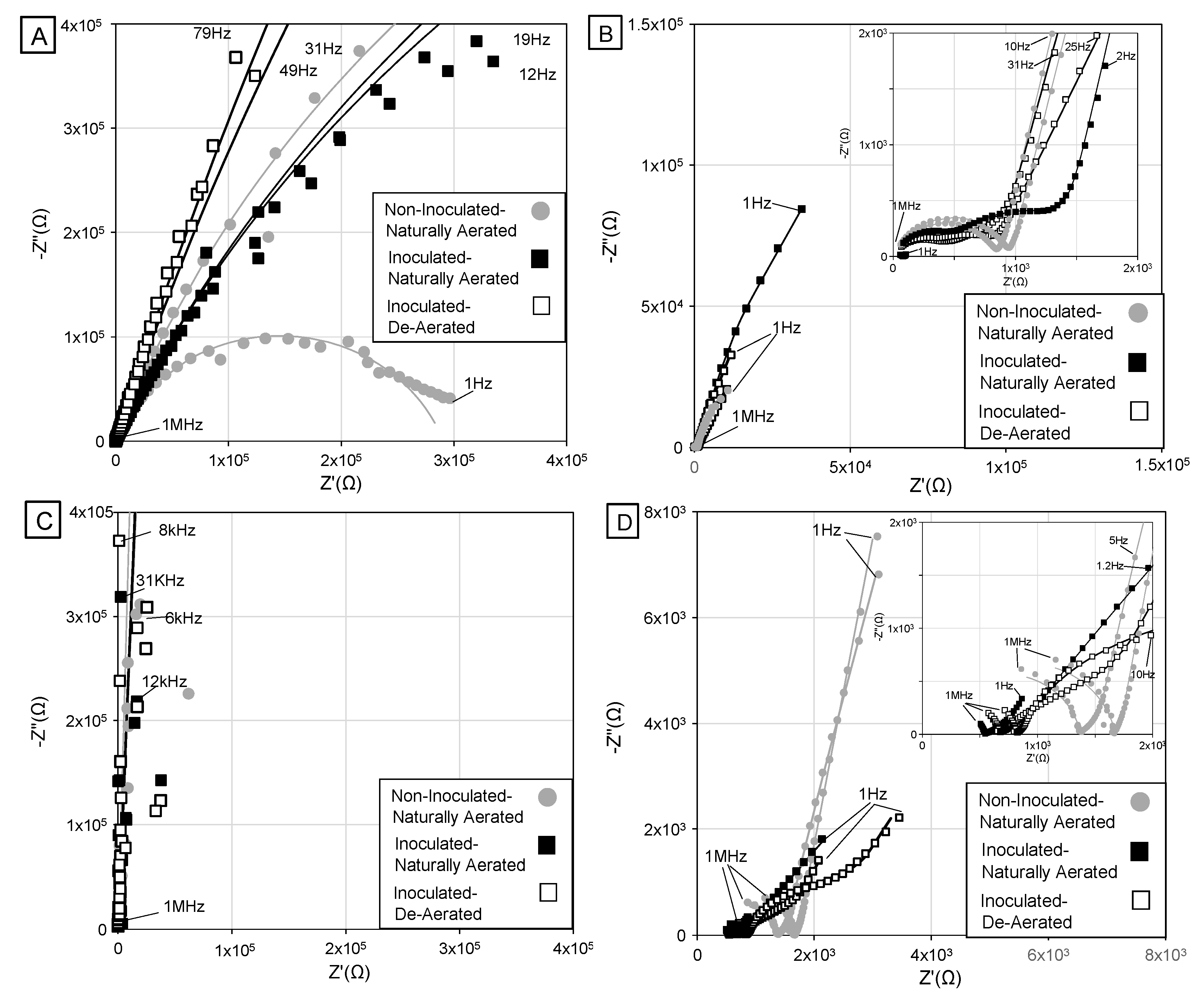
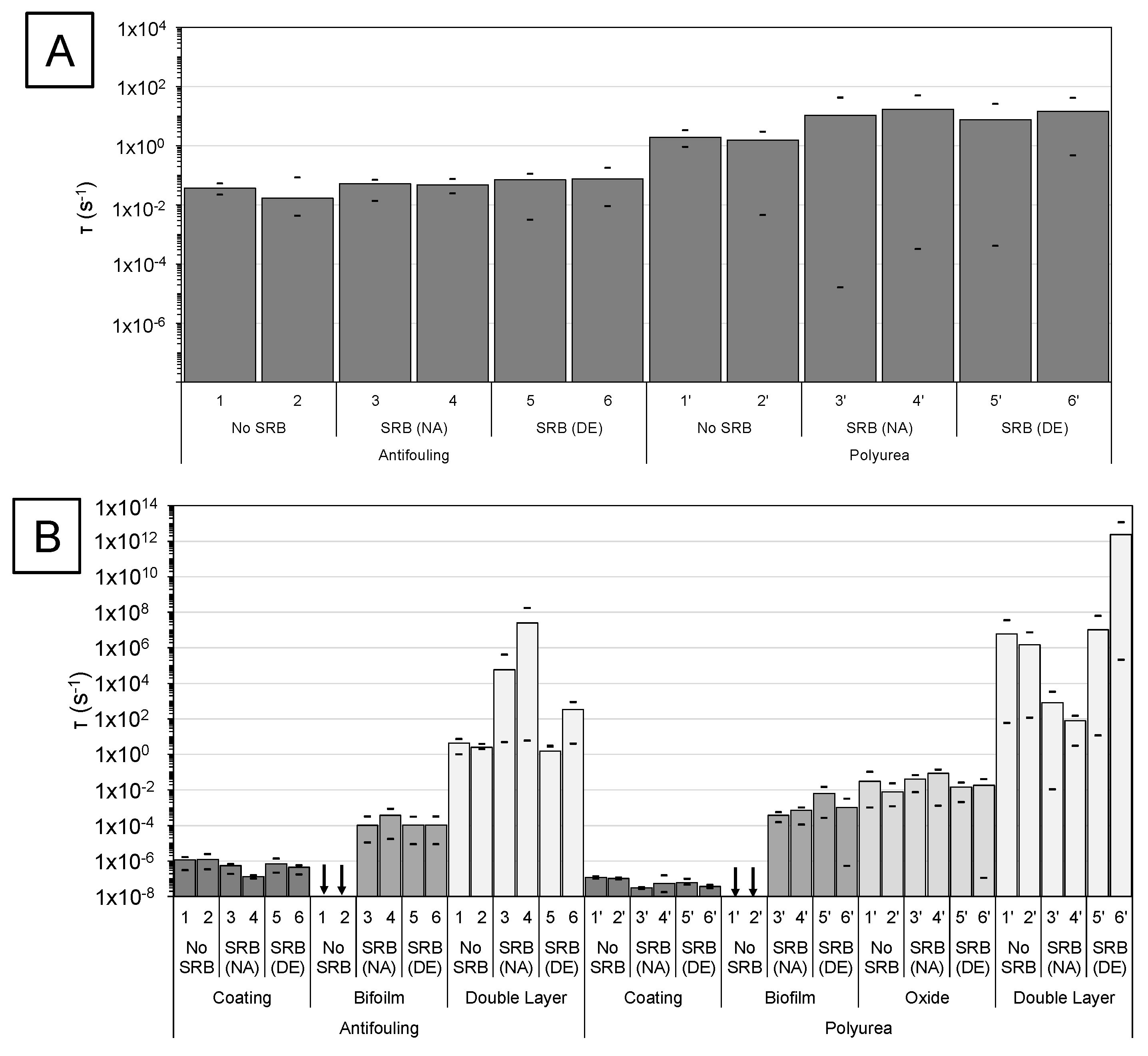
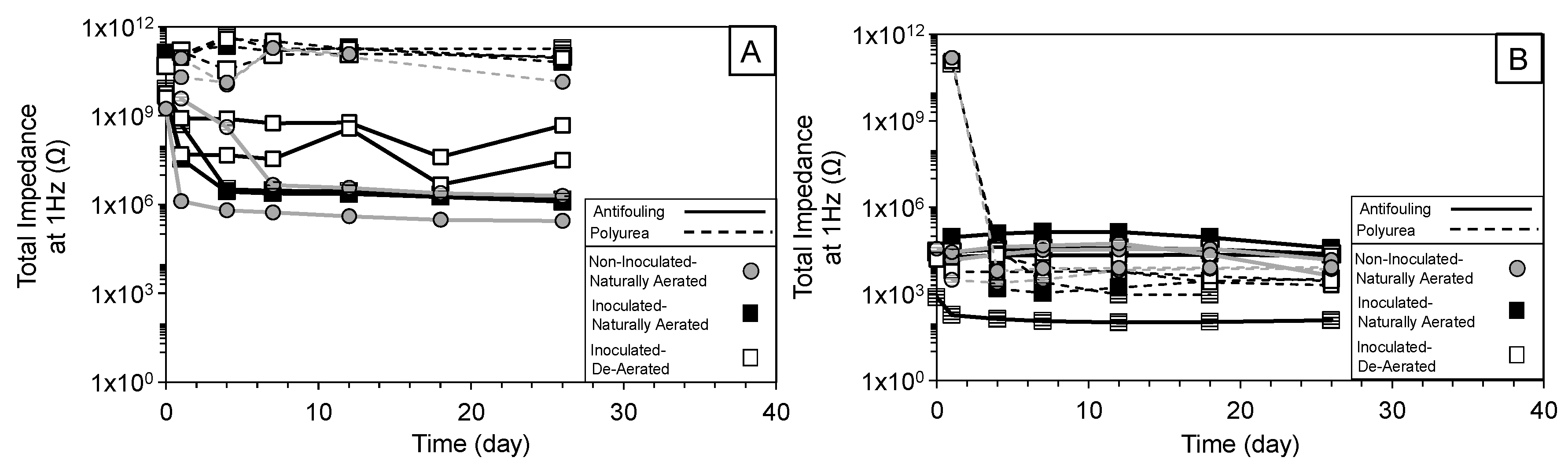
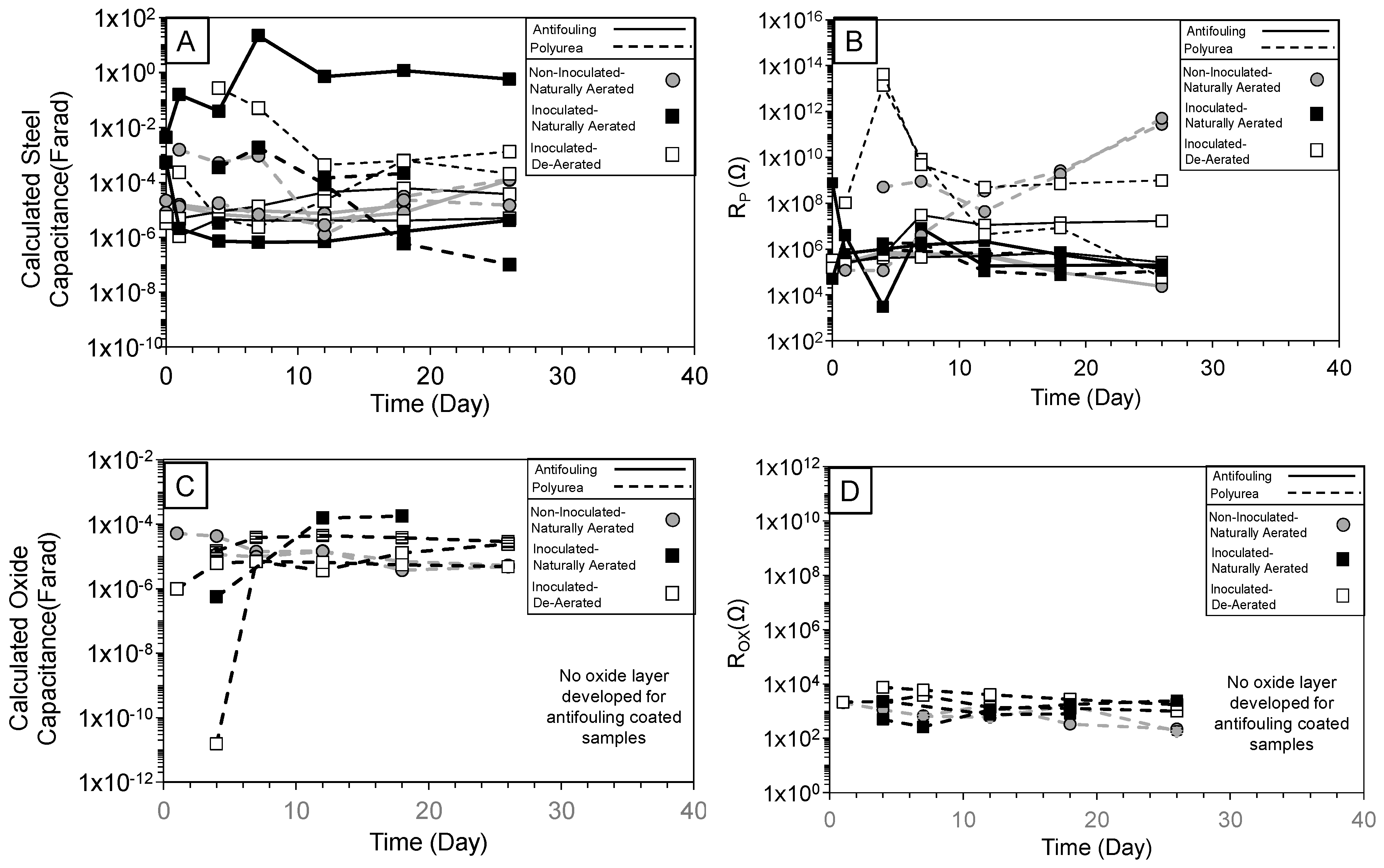
3.1. Overview of Impedance Response
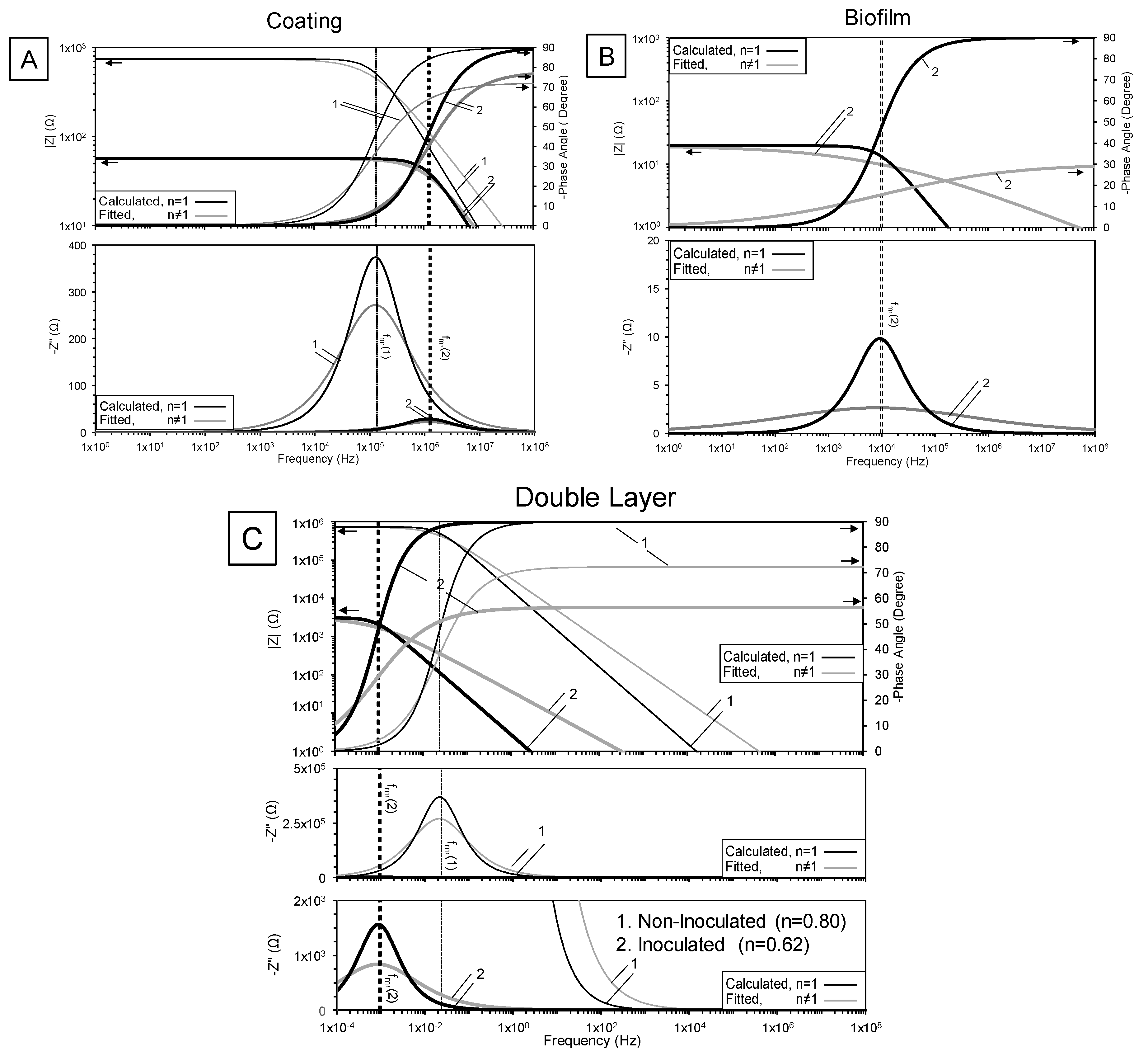
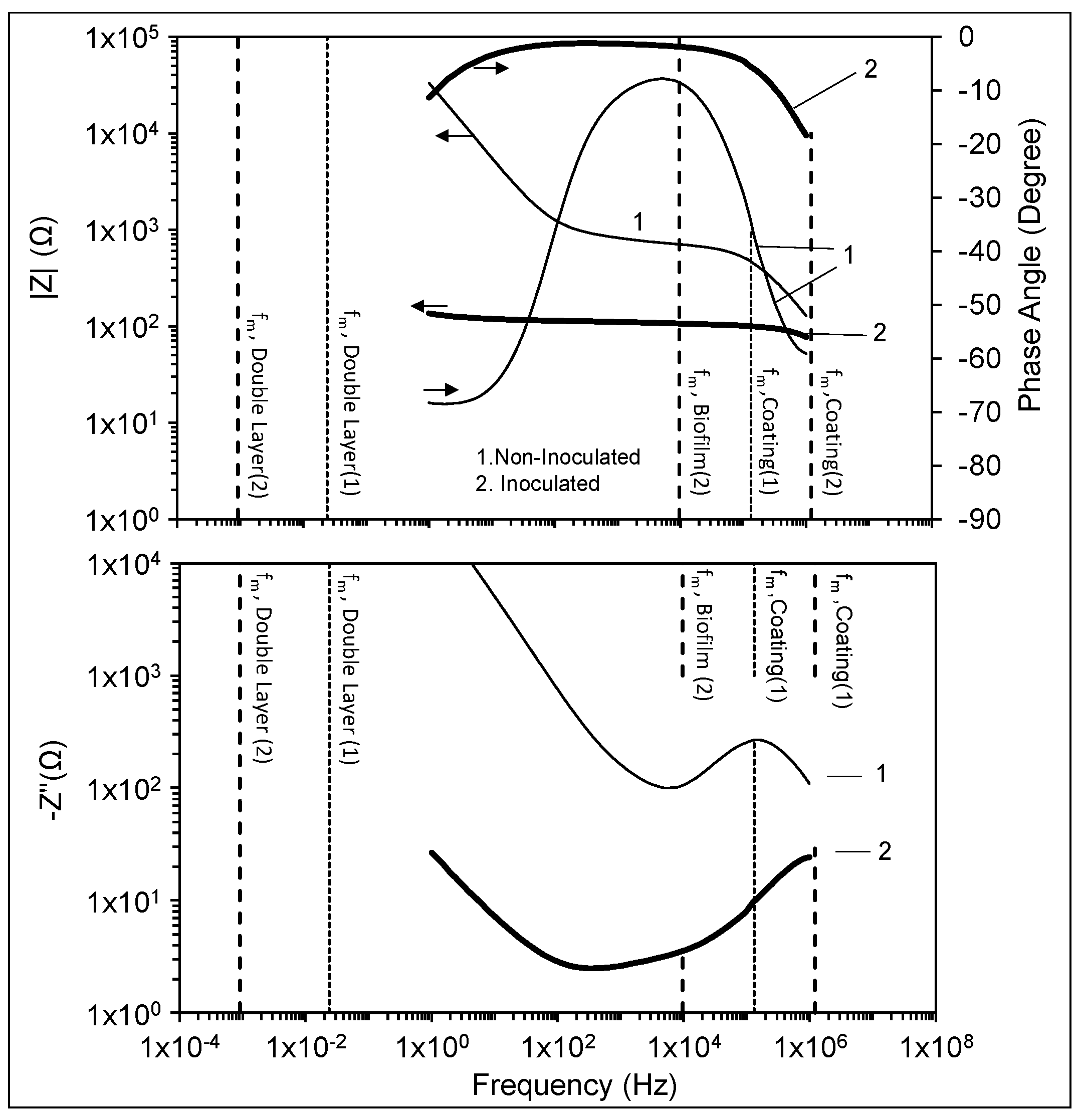
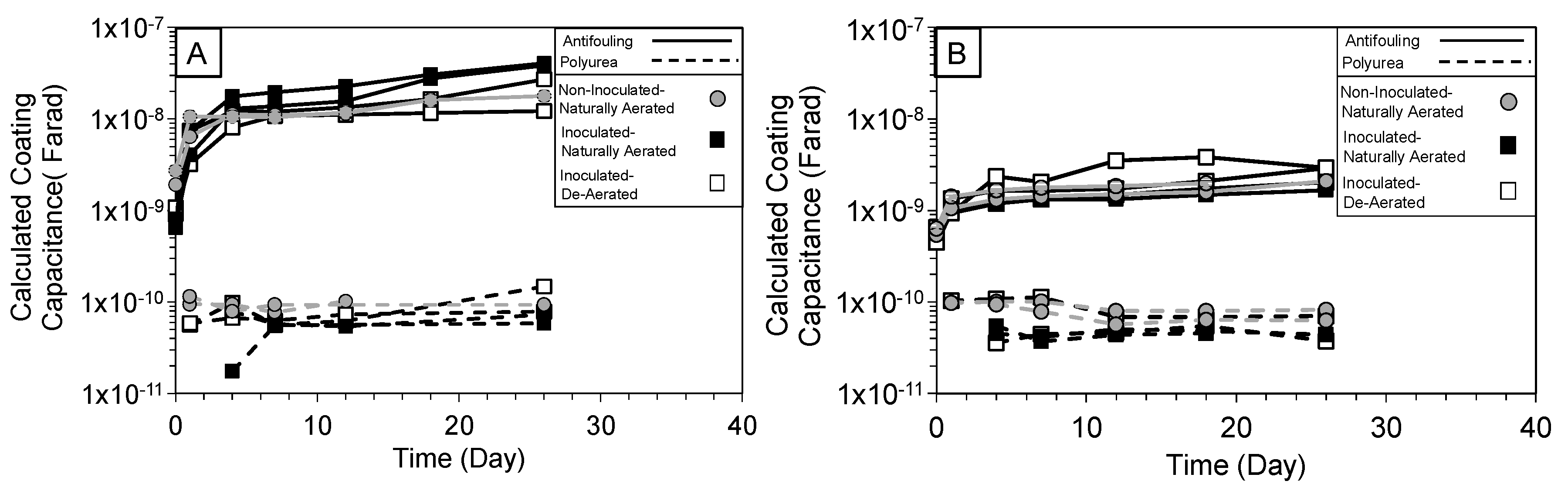
3.2. High-Frequency Impedance Behavior
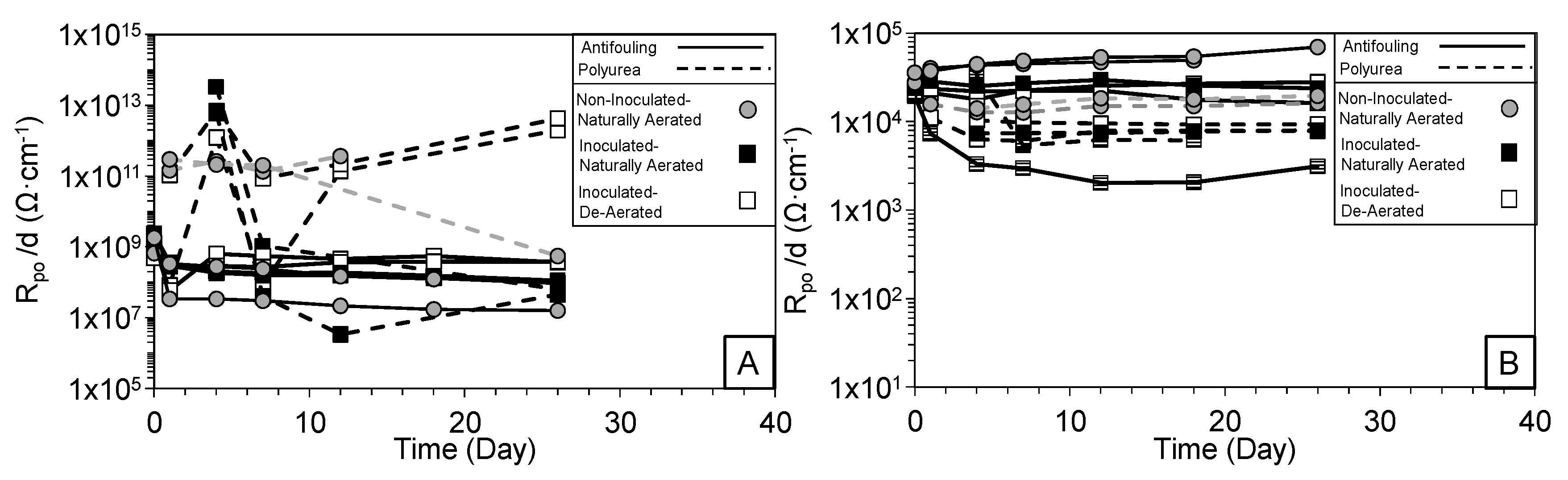
3.3. Intermediate Frequency and Biofilm Development
3.4. Low-Frequency Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Test | Day | Rs (Ω) | Coating 1 | Biofilm 2 | Oxide 2 | Double Layer 3 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rpo (Ω) | Yoc (nSs n) | nc | fm (Hz) | Cc (nF) | Rbf (Ω) | Yobf (µSs n) | nbf | fm (Hz) | Cbf (µF) | Rox (Ω) | Yoox (µSs n) | nox | fm (Hz) | Cox (µF) | Rp (kΩ) | Yodl (µSs n) | ndl | fm (mHz) | Cdl (µF) | |||
| AOCN | 0 | 0 0.45 | 1.2 × 107 3.1 × 107 | 2.9 3.5 | 0.89 0.91 | 78 2.1 | 1.9 2.7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 18 | 0 9.9 | 2.1 × 106 2.9 × 105 | 34 56 | 0.78 0.77 | 4.9 37 | 16 16 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| AOBN | 0 | 17.1 20.6 | 1.7 × 107 4.1 × 107 | 1.0 0.75 | 0.95 0.96 | 13 6.6 | 0.81 0.66 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 18 | 0 0 | 2.3 × 106 2.6 × 106 | 64 57 | 0.73 0.73 | 2.5 2.4 | 31 27 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| AOBD | 0 | 14.4 14.6 | 1.9 × 107 8.4 × 106 | 1.1 1.4 | 0.95 0.95 | 10 19 | 0.91 1.1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 18 | 8.2 10.9 | 9.6 × 106 6.6 × 106 | 17 26 | 0.82 0.81 | 1.6 1.6 | 12 12 | – | - | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| AXCN | 0 | 0 4.8 | 475 620 | 5.0 2.0 | 0.86 0.91 | 5.8 × 105 5.2 × 105 | 0.63 0.54 | – | – | – | – | – | – | – | – | – | – | 150 280 | 16 6.6 | 0.76 0.76 | 54 79 | 21 7.9 |
| 18 | 6.2 12 | 854 942 | 33 20 | 0.79 0.81 | 1.0 × 105 1.1 × 105 | 1.9 1.6 | – | – | – | – | – | – | – | – | – | – | 120 95 | 13 8.4 | 0.87 0.86 | 81 231 | 15 7.9 | |
| AXBN | 0 | 5 0.2 | 432 333 | 1.3 2.7 | 0.93 0.88 | 9.2 × 105 1.2 × 106 | 0.44 0.45 | – 78 | – 56 | – 0.69 | – 450 | – 4.9 | – | – | – | – | – | 7.6 × 105 52 | 9.2 574 | 0.68 0.62 | 0.004 0.76 | 545 4330 |
| 18 | 22.6 38 | 442 35 | 12.5 43.3 | 0.85 0.84 | 2.6 × 105 1.3 × 106 | 1.5 3.8 | 1100 29 | 0.52 5880 | 0.82 0.85 | 4100 180 | 0.4 31.4 | – | – | – | – | – | 570 190 | 1.6 9060 | 0.89 0.61 | 192 0.007 | 1.6 1.2 × 106 | |
| AXBD | 0 | 8 0 | 347 366 | 2.8 3.1 | 0.90 0.88 | 7.4 × 105 1.0 × 106 | 0.6 0.5 | 237 293 | 4.8 8.6 | 0.75 0.74 | 1500 570 | 0.5 1.0 | – | – | – | – | – | 342 151 | 3.2 5.8 | 0.83 0.88 | 155 205 | 3.2 5.6 |
| 18 | 0 22 | 463 305 | 133 16.2 | 0.70 0.85 | 1.8 × 105 3.3 × 105 | 2.1 1.7 | 700 550 | 2.7 0.7 | 0.74 0.71 | 840 1.9 × 104 | 0.3 0.03 | – | – | – | – | – | 702 1.4 × 104 | 3.6 11 | 0.88 0.74 | 63 0.2 | 4 61.4 | |
| POCN | 4 | 0 0 | 2.3 × 1010 1.9 × 1010 | 0.09 0.08 | 0.97 0.99 | 0.08 0.12 | 0.09 0.08 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 26 | – 0 | – 4.9 × 107 | – 0.09 | – 0.99 | – 38.5 | – 0.09 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| POBN | 4 | 0 0 | 5.3 × 1011 2.9 × 1012 | 0.07 0.02 | 0.97 0.99 | 0.004 0.004 | 0.08 0.02 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 26 | 0 0 | 4.1 × 106 5.5 × 106 | 0.1 0.02 | 0.95 0.99 | 600 520 | 0.07 0.06 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| POBD | 4 | 0 0 | 1.8 × 1011 6.1 × 1011 | 0.09 0.06 | 0.96 0.98 | 0.017 0.004 | 0.1 0.07 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 26 | 0 0 | 1.8 × 1011 3.7 × 1011 | 0.1 0.07 | 0.95 0.97 | 0.006 0.006 | 0.1 0.08 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| PXCN | 4 | 168 350 | 1134 1246 | 0.1 0.06 | 0.98 0.99 | 1.5 × 106 1.5 × 106 | 0.1 0.09 | – | – | – | – | – | 1104 657 | 147 26 | 0.59 0.83 | 3.5 22 | 42 22 | 116 5.1 × 105 | 113 10 | 0.59 0.83 | 2.9 0.03 | 513 17 |
| 26 | 3 3 | 1436 1735 | 0.2 0.2 | 0.92 0.94 | 1.5 × 106 1.6 × 106 | 0.08 0.06 | – | – | – | – | – | 217 225 | 14 14 | 0.83 0.85 | 160 140 | 4.8 5.3 | 2.8 × 108 5.1 × 108 | 16 10 | 0.83 0.85 | 5.0 × 10−6 0.02 | 131 15 | |
| PXBN | 4 | 0 0 | 649 2894 | 0.4 0.2 | 0.88 0.93 | 6.0 × 106 1.1 × 106 | 0.05 0.05 | 187 1365 | 85 3.9 | 0.53 0.58 | 400 1500 | 2.2 0.08 | 490 2353 | 53 0.8 | 0.74 0.94 | 23 130 | 15 0.5 | 1745 932 | 137 2.8 | 0.85 0.84 | 0.29 55 | 356 3.3 |
| 26 | 0 – | 708 – | 1.6 – | 0.79 – | 5.5 × 106 – | 0.04 – | 257 – | 32 – | 0.64 – | 290 – | 2.2 – | 2390 – | 75 – | 0.64 – | 2.5 – | 28 – | 110 – | 0.7 – | 0.57 – | 15 – | 0.1 – | |
| PXBD | 4 | 143 107 | 558 914 | 0.6 0.9 | 0.89 0.81 | 2.8 × 106 5.2 × 106 | 0.1 0.03 | 1545 2602 | 34 7.8 | 0.65 0.75 | 16 1.3 × 106 | 6.8 2.1 | 2271 7619 | 3.4 0.4 | 0.99 0.36 | 12 30 | 6 0.01 | 1 × 1010 4 × 1010 | 7.1 5.9 | 0.99 0.54 | 3 × 10−6 1 × 10−11 | 4.7 2.7 × 105 |
| 26 | 0 0 | 693 829 | 0.4 0.3 | 0.9 0.89 | 3.5 × 106 4.2 × 107 | 0.07 0.04 | 715 9.2 | 40 4.5 | 0.61 0.54 | 55 2 × 107 | 4.2 7 × 10−3 | 978 1719 | 112 15 | 0.6 0.76 | 7 20 | 24 4.8 | 57.2 9.9 × 105 | 157 204 | 0.87 0.79 | 17 0.02 | 208 1330 | |
References
- Munger, C.G. Corrosion Prevention by Protective Coatings; U.S. Department of Energy, Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1985.
- Crisp, D.J. Factors influencing the settlement of marine invertebrate larvae. Chemorecept. Mar. Org. 1974, 22, 177–265. [Google Scholar]
- Neville, A.; Hodgkiess, T. Comparative study of stainless steel and related alloy corrosion in natural sea water. Br. Corros. J. 1998, 33, 111–120. [Google Scholar] [CrossRef]
- Egan, S.; James, S.; Holmström, C.; Kjelleberg, S. Correlation between pigmentation and antifouling compounds produced by Pseudoalteromonas tunicata. Environ. Microbiol. 2002, 4, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Keough, M.J.; Raimondi, P.T. Responses of settling invertebrate larvae to bioorganic films: Effects of different types of films. J. Exp. Mar. Biol. Ecol. 1995, 185, 235–253. [Google Scholar] [CrossRef]
- Little, B.J.; Lee, J.S. Microbiologically Influenced Corrosion; John Wiley & Sons: Hoboken, NJ, USA, 2007; Volume 3. [Google Scholar]
- Borenstein, S. Microbiologically Influenced Corrosion Handbook; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Melchers, R.E.; Wells, T. Models for the anaerobic phases of marine immersion corrosion. Corros. Sci. 2006, 48, 1791–1811. [Google Scholar] [CrossRef]
- Permeh, S.; Boan, M.E.; Tansel, B.; Lau, K. Susceptibility of Bridge Steel and Concrete Components to Microbiological Influenced Corrosion (MIC) and Microbiological Influenced Deterioration (MID) in Florida; Final Report to Florida Department of Transportation, Contract No. BDV29-97726; Florida Department of Transportation: Tallahasse, FL, USA, 2019. [Google Scholar]
- Permeh, S.; Reid, C.; Boan, M.E.; Lau, K.; Tansel, B.; Duncan, M.; Lasa, I. Microbiological influenced corrosion (MIC) in Florida marine environment: A case study. In Proceedings of the Corrosion 2017, New Orleans, LA, USA, 26–30 March 2017; NACE International: Houston, TX, USA, 2017. [Google Scholar]
- Permeh, S.; Li, B.; Echeverría, M.; Tansel, B.; Lau, K.; Duncan, M. Microbially influenced steel corrosion with crevice conditions in natural water. In Proceedings of the Corrosion 2018, Phoenix, AZ, USA, 15–19 April 2018; NACE International: Houston, TX, USA, 2018. [Google Scholar]
- Permeh, S.; Boan, M.E.; Tansel, B.; Lau, K.; Duncan, M. Exploration of the influence of microbe availability on MIC of steel marine fouling environments. In Proceedings of the Corrosion 2019, Nashville, TN, USA, 24–28 March 2019; NACE International: Houston, TX, USA, 2019. [Google Scholar]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Mansfeld, F. Use of electrochemical impedance spectroscopy for the study of corrosion protection by polymer coatings. J. Appl. Electrochem. 1995, 25, 187–202. [Google Scholar] [CrossRef]
- Mansfeld, F. Electrochemical impedance spectroscopy. In Analytical Methods in Corrosion Science and Engineering; Mansfeld, F., Marcus, P., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 463–507. [Google Scholar]
- Hirayama, R.; Haruyama, S. Electrochemical impedance for degraded coated steel having pores. Corrosion 1991, 47, 952–958. [Google Scholar] [CrossRef]
- Mansfield, F.; Lee, C.C.; Han, L.T.; Zhang, G.; Little, B. The Impact of Microbiologically Influenced Corrosion on Protective Polymer Coatings; Department of Materials Science and Engineering, University of Southern California Los Angeles: Los Angeles, CA, USA, 1998. [Google Scholar]
- Kim, T.; Kang, J.; Lee, J.H.; Yoon, J. Influence of attached bacteria and biofilm on double-layer capacitance during biofilm monitoring by electrochemical impedance spectroscopy. Water Res. 2011, 45, 4615–4622. [Google Scholar] [CrossRef] [PubMed]
- Pérez, E.J.; Cabrera-Sierra, R.; González, I.; Ramírez-Vives, F. Influence of Desulfovibrio sp. biofilm on SAE 1018 carbon steel corrosion in synthetic marine medium. Corros. Sci. 2007, 49, 3580–3597. [Google Scholar] [CrossRef]
- Castaneda, H.; Benetton, X.D. SRB-biofilm influence in active corrosion sites formed at the steel-electrolyte interface when exposed to artificial seawater conditions. Corros. Sci. 2008, 50, 1169–1183. [Google Scholar] [CrossRef]
- Galicia, M.; Goujon, V.V.; Aguirre-Ramírez, M.; Castaneda, H. Interfacial and corrosion characterization of zinc rich-epoxy primers with carbon nanotubes exposed to marine bacteria. In Proceedings of the Corrosion 2017, New Orleans, LA, USA, 26–30 March 2017; NACE International: Houston, TX, USA, 2017. [Google Scholar]
- Downs, R.; Dean, J.; Downer, A.; Perry, J. Determination of the biocide Econea® in artificial seawater by solid phase extraction and high-performance liquid chromatography mass spectrometry. Separations 2017, 4, 34. [Google Scholar] [CrossRef]
- Postgate, J.R. The Sulphate-Reducing Bacteria; Cambridge University Press (CUP Archive): London, UK, 1979. [Google Scholar]
- TM0194-2014. Field Monitoring of Bacterial Growth in Oil and Gas Systems; NACE Standard: Houston, TX, USA, 2014. [Google Scholar]
- Hsu, C.H.; Mansfeld, F. Technical note: Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Mansfeld, F. Recording and analysis of AC impedance data for corrosion studies. Corrosion 1981, 37, 301–307. [Google Scholar] [CrossRef]
- Permeh, S.; Lau, K.; Duncan, M. Mitigation of microbiologically influenced corrosion by application of antifouling coatings: development of biofilm. Struct. Infrastruct. Eng. 2019. submitted. [Google Scholar]
- Hoog, N.A.; Mayer, M.J.J.; Miedema, H.; Olthuis, W.; Tomaszewska, A.A.; Paulitsch-Fuchs, A.H.; van den Berg, A. Online monitoring of biofouling using coaxial stub resonator technique. Sens. Biol. Sens. Res. 2015, 3, 79–91. [Google Scholar] [CrossRef]
- Liu, H.; Huang, L.; Huang, Z.; Zheng, J. Specification of sulfate reducing bacteria biofilms accumulation effects on corrosion initiation. Mater. Corros. 2007, 58, 44–48. [Google Scholar] [CrossRef]




| Constituents | Composition (%) |
|---|---|
| Potassium Phosphate (KH2PO4) | 0.05 |
| Ammonium Chloride (NH4Cl) | 0.1 |
| Sodium Sulfate (Na2SO4) | 0.1 |
| Sodium Chloride (NaCl) | 2.5 |
| Iron Sulfate (FeSO4·7H2O) | 0.05 |
| Sodium Lactate | 0.5 |
| Yeast extract | 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permeh, S.; Lau, K.; Duncan, M. Characterization of Biofilm Formation and Coating Degradation by Electrochemical Impedance Spectroscopy. Coatings 2019, 9, 518. https://doi.org/10.3390/coatings9080518
Permeh S, Lau K, Duncan M. Characterization of Biofilm Formation and Coating Degradation by Electrochemical Impedance Spectroscopy. Coatings. 2019; 9(8):518. https://doi.org/10.3390/coatings9080518
Chicago/Turabian StylePermeh, Samanbar, Kingsley Lau, and Matthew Duncan. 2019. "Characterization of Biofilm Formation and Coating Degradation by Electrochemical Impedance Spectroscopy" Coatings 9, no. 8: 518. https://doi.org/10.3390/coatings9080518
APA StylePermeh, S., Lau, K., & Duncan, M. (2019). Characterization of Biofilm Formation and Coating Degradation by Electrochemical Impedance Spectroscopy. Coatings, 9(8), 518. https://doi.org/10.3390/coatings9080518







