Antimicrobial Activity of Lignin-Derived Polyurethane Coatings Prepared from Unmodified and Demethylated Lignins
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Kraft Lignin (KL) and Organosolv Lignin (OL)
2.2. Synthesis of Demethylated Kraft Lignin
2.3. Size Exclusion Chromatography
2.4. Determination of Hydroxyl Groups
2.5. Antibacterial Activity of Lignin
2.6. Hemmhoff Test
2.7. Synthesis of Lignin-Based Polyurethane Coatings
2.8. Antimicrobial Activity of the LPU Coatings
2.9. Thermogravimetric Analysis
2.10. Optical Contact Angle
2.11. Scanning Electron Microscopy
3. Results and Discussion
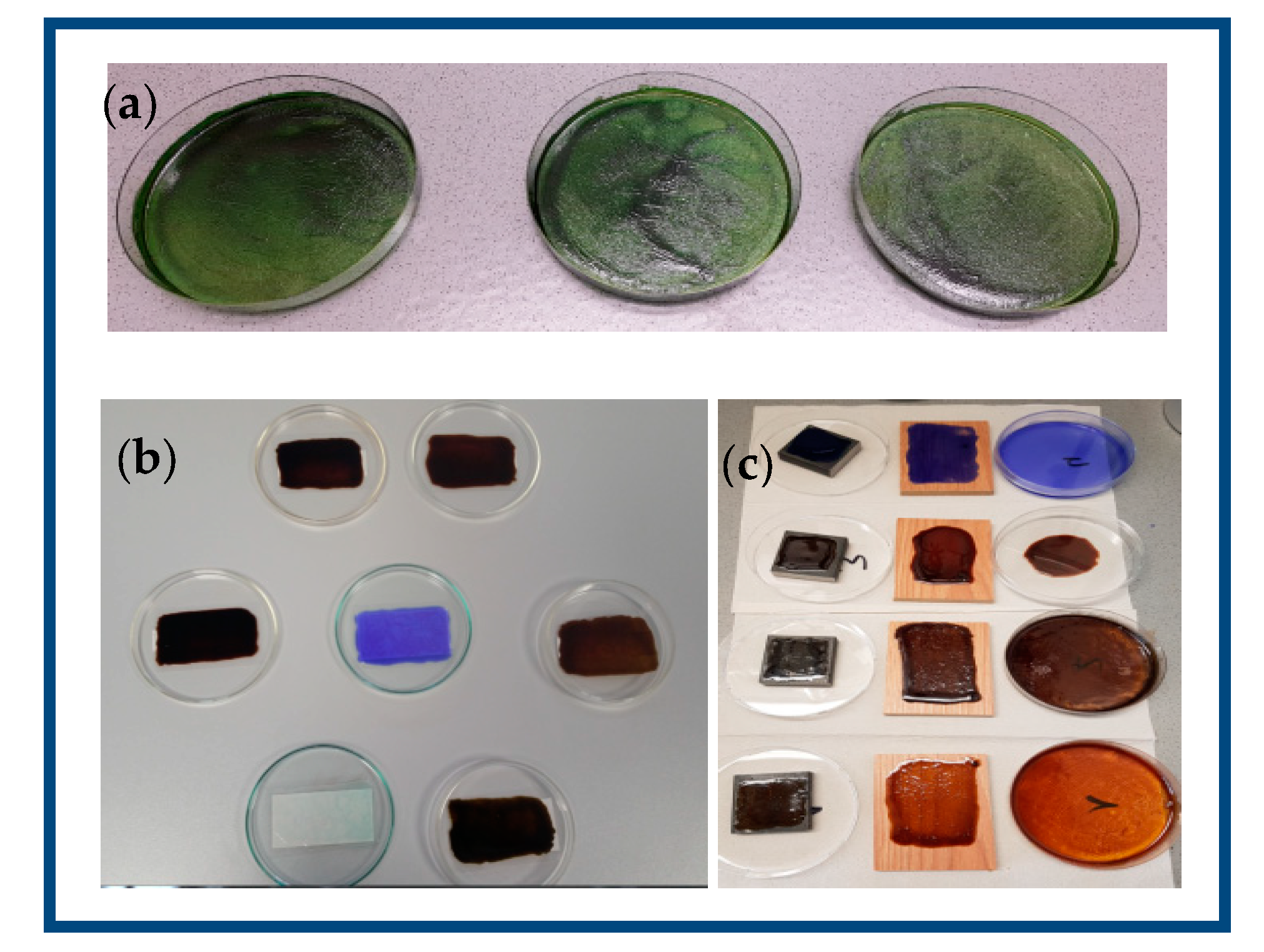
3.1. Antibacterial Activity of Kraft Lignin
3.2. Antibacterial Activity of LPU Coatings
3.3. Thermal Properties (TGA)
3.4. Contact Angle of LPU Coatings
3.5. Morphology of the LPU Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the way for lignin valorisation: Recent advances in bioengineering, biorefining and catalysis. Angew. Chem. Int. Ed. 2016, 55, 2–54. [Google Scholar] [CrossRef] [PubMed]
- Alzagameem, A.; El Khaldi-Hansen, B.; Kamm, B.; Schulze, M. Lignocellulosic biomass for energy, biofuels, biomaterials, and chemicals. In Biomass and Green Chemistry, 1st ed.; Vaz, S., Jr., Ed.; Springer International Publishing: Basel, Switzerland, 2018; pp. 95–132. [Google Scholar]
- Hansen, B.; Kamm, B.; Schulze, M. Qualitative and quantitative analysis of lignins from different sources and isolation methods for an application as a biobased chemical resource and polymeric material. In Analytical Techniques and Methods for Biomass Products; Vaz, S., Jr., Seidl, P., Eds.; Springer: Berlin, Germany, 2017; pp. 15–44. [Google Scholar]
- Naseem, A.; Tabasum, S.; Zia, K.M.; Zuber, M.; Ali, M.; Noreen, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.K.; Goudarzi, A.; Lin, L.-T.; Li, Y.; Kadla, J.F. Lignin-based composite carbon nanofibers. In Lignin in Polymer Composites, 1st ed.; Faruk, O., Sain, M., Eds.; Elsevier B.V: Amsterdam, The Netherlands, 2016; pp. 167–194. [Google Scholar]
- Ponomarenko, J.; Dizhbite, T.; Lauberts, M.; Viksna, A.; Dobele, G.; Bikovens, O.; Telysheva, G. Characterization of softwood and hardwood lignoboost kraft lignins with emphasis on their antioxidant activity. Bioresources 2014, 9, 2051–2068. [Google Scholar] [CrossRef]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity, 1st ed.; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 77–106. [Google Scholar]
- Alzagameem, A.; El Khaldi-Hansen, B.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic biomass as source for lignin-based environmentally benign antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef] [PubMed]
- Bergs, M.; Völkering, G.; Kraska, T.; Do, X.; Monakhova, Y.; Konow, C.; Pude, R.; Schulze, M. Miscanthus x giganteus stem versus leave-derived lignins differing in monolignol ratio and linkage. Int. J. Mol. Sci. 2019, 20, 1200. [Google Scholar] [CrossRef]
- Hansen, B.; Kamm, B.; Schulze, M. Qualitative and quantitative analysis of lignin produced from beech wood by different conditions of the Organosolv process. J. Polym. Environ. 2016, 24, 85. [Google Scholar] [CrossRef]
- Richter, A.P.; Brown, J.S.; Bharti, B.; Wang, A.; Gangwal, S.; Houck, K.; Cohen Hubal, E.A.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat. Nanotechnol. 2015, 10, 817–824. [Google Scholar] [CrossRef]
- Gregorova, A.; Redik, S.; Sedlarik, V.; Stelzer, F. Lignin-containing polyethylene films with antibacterial activity. In Proceedings of the 3rd International Conference on Thomson Reuters of NANOCON, Brno, Czech Republic, 21–23 September 2011; Available online: http://konference.tanger.cz/data/nanocon2011/sbornik/lists/papers/1366.pdf (accessed on 5 May 2019).
- Dai, L.; Liu, R.; Hu, L.-Q.; Zou, Z.-F.; Si, C.-L. Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Lu, S.; Lao, S.; Qiu, X. Modified lignin with anionic surfactant and its application in controlled release of avermectin. J. Agric. Food Chem. 2018, 66, 3457–3464. [Google Scholar] [CrossRef]
- Pang, Y.; Li, X.; Wang, S.; Qiu, X.; Yang, D.; Lou, H. Lignin-polyurea microcapsules with anti-photolysis and sustained-release performances synthesized via pickering emulsion template. React. Funct. Polym. 2018, 123, 115–121. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.; Wang, J.; Zhang, X. Ultrasonic-assisted fabrication of montmorillonite-lignin hybrid hydrogel: Highly efficient swelling behaviors and super-sorbent for dye removal from wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 903–913. [Google Scholar] [CrossRef]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose–lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C 2012, 32, 452–463. [Google Scholar] [CrossRef]
- Kosikova, B.; Labaj, J. Lignin-stimulated protection of polypropylene films and DNA in cells of mice against oxidation damage. Bioresources 2009, 4, 805–815. [Google Scholar]
- Gao, Y.; Cranston, R. Recent advances in antimicrobial treatments of textiles. Text. Res. J. 2008, 78, 60–72. [Google Scholar] [CrossRef]
- Bshena, O.; Heunis, T.D.; Dicks, L.M.; Klumperman, B. Antimicrobial fibers: Therapeutic possibilities and recent advances. Future Med. Chem. 2011, 3, 1821–1847. [Google Scholar] [CrossRef]
- Gordts, S.C.; Férir, G.; D’huys, T.; Petrova, M.I.; Lebeer, S.; Snoeck, R.; Andrei, G.; Schols, D. The low-cost compound lignosulfonic acid (LA) exhibits broad-spectrum anti-HIV and anti-HSV activity and has potential for microbicidal applications. PLoS ONE 2015, 10, e0131219. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, Q.; Chu, Y.; Yuan, Z.; Song, H.; Chen, Z.; Wu, Z. Lignosulfonic acid exhibits broadly anti-HIV-1activity-potential as a microbicide candidate for the prevention of HIV-1 sexual transmission. PLoS ONE 2012, 7, e35906. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Lora, J.H. Lignin: A platform for renewable aromatic polymeric materials. In Quality Living Through Chemurgy and Green Chemistry. Green Chemistry and Sustainable Technology; Lora, J.H. Springer: Berlin/Heidelberg, Germany, 2016; pp. 221–263. [Google Scholar]
- Ten, E.; Vermerris, W. Recent developments in polymers derived from industrial lignin. J. Appl. Polym. Sci. 2015, 132, 1–13. [Google Scholar] [CrossRef]
- Jia, Z.; Lu, C.; Zhou, P.; Wang, L. Preparation and characterization of high boiling solvent lignin-based polyurethane film with lignin as the only hydroxyl group provider. RSC Adv. 2015, 5, 53949–53955. [Google Scholar] [CrossRef]
- Griffini, G.; Passoni, V.; Suriano, R.; Levi, M.; Turri, S. Polyurethane coatings based on chemically unmodified fractionated lignin. ACS Sustain. Chem. Eng. 2015, 3, 1145–1154. [Google Scholar] [CrossRef]
- Klein, S.E.; Rumpf, J.; Kusch, P.; Albach, R.; Rehahn, M.; Witzleben, S.; Schulze, M. Utilization of unmodified kraft lignin for the preparation of highly flexible and transparent polyurethane coatings. RSC Adv. 2018, 8, 40765. [Google Scholar] [CrossRef]
- Klein, S.E.; Rumpf, J.; Rehahn, M.; Witzleben, S.; Schulze, M. Biobased flexible polyurethane coatings prepared from kraft lignin: One-pot synthesis and antioxidant activity. J. Coat. Technol. Res. 2019. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Q.; Lee, D.-J. Kraft lignin biorefinery: A proposal. Bioresour. Technol. 2017, 247, 1181–1183. [Google Scholar] [CrossRef]
- Sain, M.; Faruk, O. Lignin in Polymer Composites, 1st ed.; Elsevier: Kidlington, UK, 2016. [Google Scholar]
- Li, J.; Wang, W.; Shifeng, Z.; Qiang, G.; Zhang, W.; Li, J. Preparation and characterization of lignin demethylated at atmospheric pressure and its application in fast curing biobased phenolic resins. RSC Adv. 2016, 6, 67435–67443. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Chung, H.; Washburn, N.R. Improved lignin polyurethane properties with Lewis acid treatment. ACS Appl. Mater. Interfaces 2012, 4, 2840–2846. [Google Scholar] [CrossRef]
- Zou, L.; Ross, B.M.; Hutchison, L.J.; Christopher, L.P.; Dekker, R.F.; Malek, L. Fungal demethylation of Kraft lignin. Enzyme Microb. Technol. 2015, 73–74, 44–50. [Google Scholar] [CrossRef]
- Ibrahim, V.; Mendoza, L.; Mamo, G.; Hatti-Kaul, R. Blue laccase from Galerina sp.: Properties and potential for Kraft lignin demethylation. Process Biochem. 2011, 46, 379–384. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Yan, N.; Zhang, R.; Li, J. Demethylation of wheat straw alkali lignin for application in phenol formaldehyde adhesives. Polymers 2016, 8, 209. [Google Scholar] [CrossRef]
- An, X.; Schroeder, H.A.; Thompson, G.E. Demethylated kraft lignin as a substitute for phenol in wood adhesive. Chem. Ind. For. Prod. 1995, 15, 36–42. [Google Scholar]
- Ferhan, M.; Sain, M.; Yan, N. A new method for demethylation of lignin from woody biomass using biophysical methods. J. Chem. Eng. Process. Technol. 2013, 4, 160. [Google Scholar] [CrossRef]
- Podschun, J.; Saake, B.; Lehnen, R. Catalytic demethylation of organosolv lignin in aqueous medium using indium triflate under microwave irradiation. React. Funct. Polym. 2017, 119, 82–86. [Google Scholar] [CrossRef]
- Webb, C.H.S. A note on the value of brilliant green as an antiseptic. Br. Med. J. 1917, 1, 870. [Google Scholar] [CrossRef][Green Version]
- Sneader, W. Drug Discovery: A history; John Wiley and Sons Ltd.: Chichester, UK, 2005; p. 468. [Google Scholar]
- Schirmer, R.H.; Coulibaly, B.; Stich, A.; Scheiwein, M.; Merkle, H.; Eubel, J.; Becker, K.; Becher, H.; Müller, O.; Zich, T.; et al. Methylene blue as an antimalarial agent. Redox Rep. 2003, 8, 272–275. [Google Scholar] [CrossRef]
- Boulos, R. Bacterial Mechanosensitive Channels as Novel Targets for Antibacterial Agents. Ph.D. Thesis, The University of Western Australia, Perth, Australia, December 2011. [Google Scholar]
- Boulos, R.A. Antimicrobial Compounds. U.S. Patent 20120329871 A1, 27 December 2012. [Google Scholar]
- Boulos, R.A.; Eroglu, E.; Chen, X.; Scaffidi, A.; Edwards, B.R.; Toster, J.; Raston, C.L. Unravelling the structure and function of human hair. Green Chem. 2013, 15, 1268–1273. [Google Scholar] [CrossRef]
- Vilela, S.F.G.; Junqueira, J.C.; Barbosa, J.O.; Majewski, M.; Munin, E.; Jorge, A.O.C. Photodynamic inactivation of Staphylococcus aureus and Escherichia coli biofilms by malachite green and phenothiazine dyes: An in vitro study. Arch. Oral Biol. 2012, 57, 704–710. [Google Scholar] [CrossRef]
- Noimark, S.; Allan, E.; Parkin, I.P. Light-activated antimicrobial surfaces with enhanced efficacy induced by a dark-activated mechanism. Chem. Sci. 2014, 5, 2216. [Google Scholar] [CrossRef]
- Bartoszewicz, L. Antimicrobial Photo-Stable Coating Composition. WO2009015476A1, 5 February 2009. [Google Scholar]
- Santos, M.R.E.; Fonseca, A.C.; Mendonça, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent developments in antimicrobial polymers: A review. Materials 2016, 9, 599. [Google Scholar] [CrossRef]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; Schulze, M. Antimicrobial activity of lignin and lignin-derived cellulose and chitosan composites against selected pathoge nic and spoilage microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016. [Google Scholar] [CrossRef]
- Kaur, R.; Uppal, S.K.; Sharma, P. Antioxidant and antibacterial activities of sugarcane bagasse lignin and chemically modified lignins. Sugar Tech. 2017, 19, 675–680. [Google Scholar] [CrossRef]
- Witzler, M.; Alzagameem, A.; Bergs, M.; El Khaldi-Hansen, B.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-derived biomaterials for drug release and tissue engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef]
- Monakhova, Y.; Diehl, B.W.K.; Do, X.T.; Witzleben, S.; Schulze, M. Novel method for the determination of average molecular weight of natural polymers based on 2D DOSY NMR and chemometrics: Example of heparin. J. Pharm. Biomed. Anal. 2018, 149, 128–132. [Google Scholar] [CrossRef]
- Alzagameem, A.; Bergs, M.; Do, X.T.; Klein, S.E.; Rumpf, J.; Larkins, M.; Monakhova, Y.; Pude, R.; Schulze, M. Low-input crops as lignocellulosic feedstock for second generation biorefineries and the potential of chemometrics in biomass quality control. Appl. Sci. 2019, 9, 2252. [Google Scholar] [CrossRef]
- Garcia, A.; Toledano, A.; Serrano, A.; Egüés, I.; González, M.; Marín, F.; Labidi, J. Characterization of lignins obtained by selective precipitation. Sep. Purif. Technol. 2009, 68, 193–198. [Google Scholar] [CrossRef]
- Japanese Industrial Standard. Z 2801:2000. ICS 07.100.10; 11.100 Descriptors: Bacteriocide-Activity Determination, Microbiological-Resistance Tests, Biological Hazards, Culture Techniques. Available online: http://lotusyapi.com.tr/Antibacterial/JIS%20Z%202801%202000.pdf (accessed on 5 May 2019).
- Pan, X.; Saddler, J.N. Effect of replacing polyol by organosolv and kraft lignin on the property and structure of rigid polyurethane foam. Biotechnol. Biofuels 2013, 6, 12–21. [Google Scholar] [CrossRef]
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-based polyurethane prepared from Kraft lignin and modified castor oil. eXPRESS Pol. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Dohlen, S.; Braun, C.; Brodkorb, F.; Fischer, B.; Ilg, Y.; Kalbfleisch, K.; Kreyenschmidt, M.; Lorenz, R.; Kreyenschmidt, J. Effect of different packaging materials containing poly-[2-(tert-butylamino) methylstyrene] on the growth of spoilage and pathogenic bacteria on fresh meat. Int. J. Food Microbiol. 2017, 257, 91–100. [Google Scholar] [CrossRef]
- Dohlen, S.; Braun, C.; Brodkorb, F.; Fischer, B.; Ilg, Y.; Kalbfleisch, K.; Kreyenschmidt, M.; Lorenz, R.; Robers, O.; Kreyenschmidt, J. Potential of the polymer poly-[2-(tert-butylamino) methylstyrene] as antimicrobial packaging material for meat products. J. Appl. Microbiol. 2016, 4, 1059–1070. [Google Scholar] [CrossRef]
- Hüwe, C.; Schmeichel, J.; Brodkorb, F.; Dohlen, S.; Kalbfleisch, K.; Kreyenschmidt, M.; Lorenz, R.; Kreyenschmidt, J. Potential of antimicrobial treatment of linear low-density polyethylene with poly((tert-butyl-amino)-methyl-styrene) to reduce biofilm Formation in the Food industry. Biofouling 2018, 34, 378–387. [Google Scholar] [CrossRef]
- Braun, C.; Dohlen, S.; Ilg, Y.; Brodkorb, F.; Fischer, B.; Heindirk, P.; Kalbfleisch, K.; Richter, T.; Robers, O.; Kreyenschmidt, M. Antimicrobial activity of intrinsic antimicrobial polymers based on poly((tertbutyl-amino)-methyl-styrene) against selected pathogenic and spoilage microorganisms relevant in meat processing facilities. J. Antimicrob Agents 2017, 3, 1000136. [Google Scholar] [CrossRef]
- Song, W.; Ge, S. Application of antimicrobial nanoparticles in dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef]
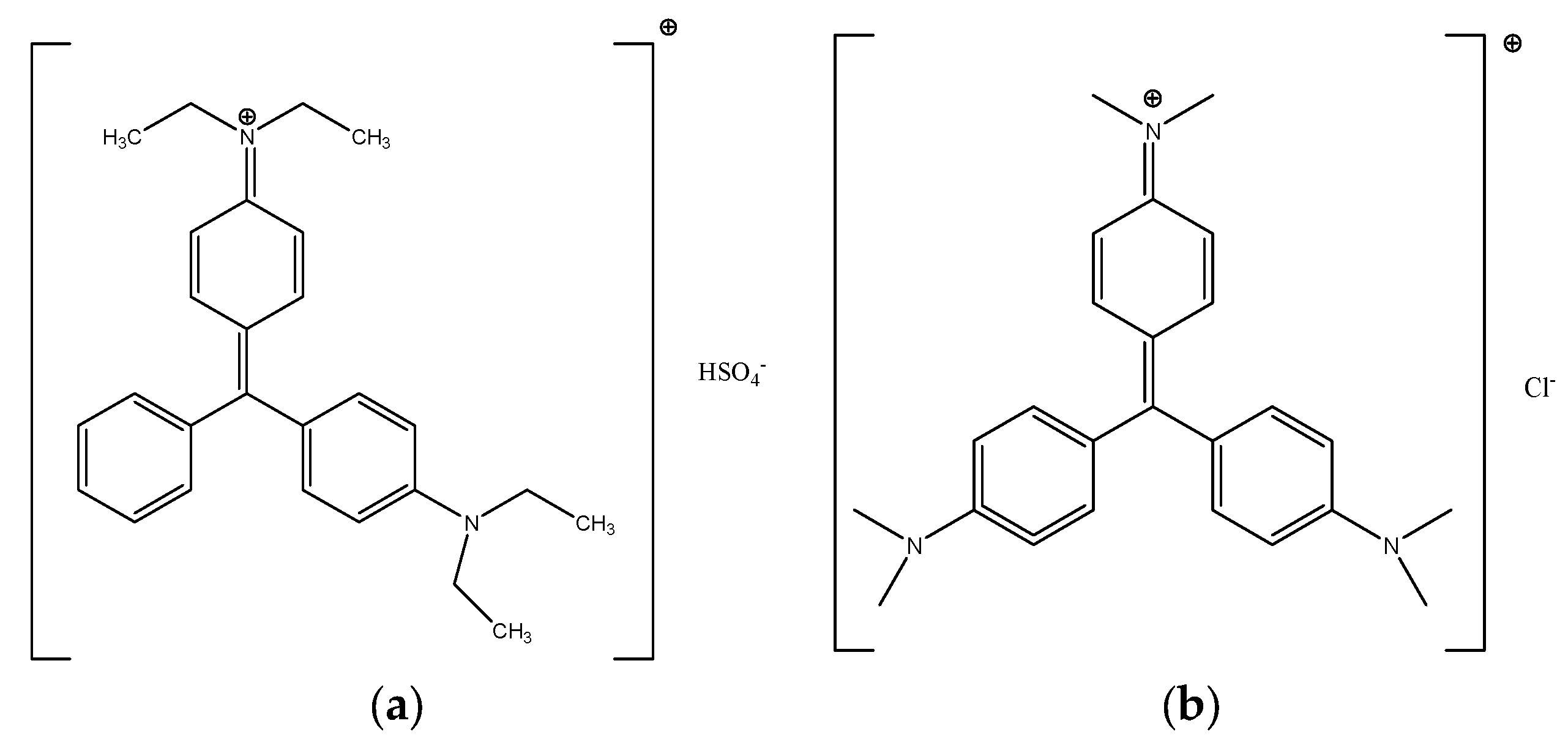


| Sample Composition | Studied Activity (Antibacterial, Antifungal) | Microorganisms (Bacteria, Fungi) | Results | References |
|---|---|---|---|---|
| Triphenylmethane (TPM) dyes | Mechanistic studies of the antimicrobial effects of triphenylmethanes (crystal violet, methylene blue, malachite green, brilliant green). | Various gram positive and gram negative bacteria | Evidence to link the antimicrobial properties of TPM dyes, especially brilliant green, to the activity of mechanosensitive ion channel (MIC) of large conductance, known to be highly specific/ubiquitous in various bacterial species. | Bolous et al. [45,46,47] |
| TPM dyes (i.e., methylene blue, malachite green) used as photosensitizer for acrylic resins | Microbial reduction of biofilms. | S. aureus | Best microbial reduction with 3000 µM malachite green with microbial reduction of 1.6–4.0 log10. | Vilela et al. [48] |
| TPM-based antimicrobial surfaces | Antimicrobial effects of crystal violet and methylene blue. | S. epidermidis (RP62a) and E. coli (NCTC 25522) | Light-activated antimicrobial surfaces with enhanced efficacy induced by a dark-activated mechanism. | Noimark et al. [49] |
| TPM-based coating additives (i.e., brilliant green, crystal violet) for polymeric substrates including PU | Antimicrobial activity of photo-stable composition used for coating a variety of medical materials. | Not specified | The coating composition comprising silver and TPM dyes (malachite green) provided photostability to the silver ions and antimicrobial activity. | Bartoszewicz et al. WO 2009/015476 Al [50] |
| Antimicrobial polymers | Bioactive polymers including biocidal activity, antifungal and antibacterial capacity. | Various gram positive and gram negative bacteria | Comprehensive review discussing different mechanisms regarding antimicrobial effects in polymer materials. | Santos et al. 2016 [51] |
| Lignin/HPMC and HPMC/lignin/chitosan composites | Antibacterial effects. | E. coli and S. aureus, B. thermosphacta and P. fluorescens | Testing the films against spoilage bacteria that grow at low temperatures revealed the activity of the 30% addition on HPMC/lignin against B. thermosphacta and P. fluorescens. HPMC/lignin/chitosan films (5% lignin) showed activity against both B. thermosphacta and P. fluorescens. | Alzagameem et al. 2019 [52] |
| Cellulose and lignin effects on disintegration, antimicrobial and antioxidant properties of PLA active films | Antimicrobial, antioxidant and disintegrability activities | Gram negative bacteria: Xanthomonas axonopodis pv. vesicatoria and Xanthomonas arboricola pv. pruni | Inhibition capacity for Gram negative bacteria (Xanthomonas axonopodis pv. vesicatoria and Xanthomonas arboricola pv. pruni) for lignin-modified PLA films. | Yang et al. 2016 [53] |
| Lignin derivatives (epoxides, esters, ether) | Antimicrobial activity of chemically modified lignins (by acetylation, epoxidation and hydroxymethylation reactions). | Bacillus aryabhattai and Klebsiella | Epoxy/lignin was found to be the most effective antibacterial among modified lignin with minimum inhibitory concentration of 90 and 200 µg/disc. | Kaur et al. 2017 [54] |
| Lignin for benign encapsulation | Antimicrobial activity of nanoparticles coated with LignoBoostTM softwood Kraft lignin. | E. coli and Pseudomonas aeruginosa | Nanoparticle flash precipitation with subsequent silver ion infusion and polyelectrolyte coating including lignin. | Richter et al. 2015 [12] |
| Antibacterial lignin–polyethylene (PE) | Lignin nanoparticles embedded in polyethylene films (Björkman lignin from beech wood flour). | E. coli and S. aureus | Lignin particles exhibit antibacterial effect against E. coli and S. aureus in the same order of magnitude as other antibacterial agents such as Bronopol® and Chlorohexidine®. | Gregorova et al. 2011 [13] |
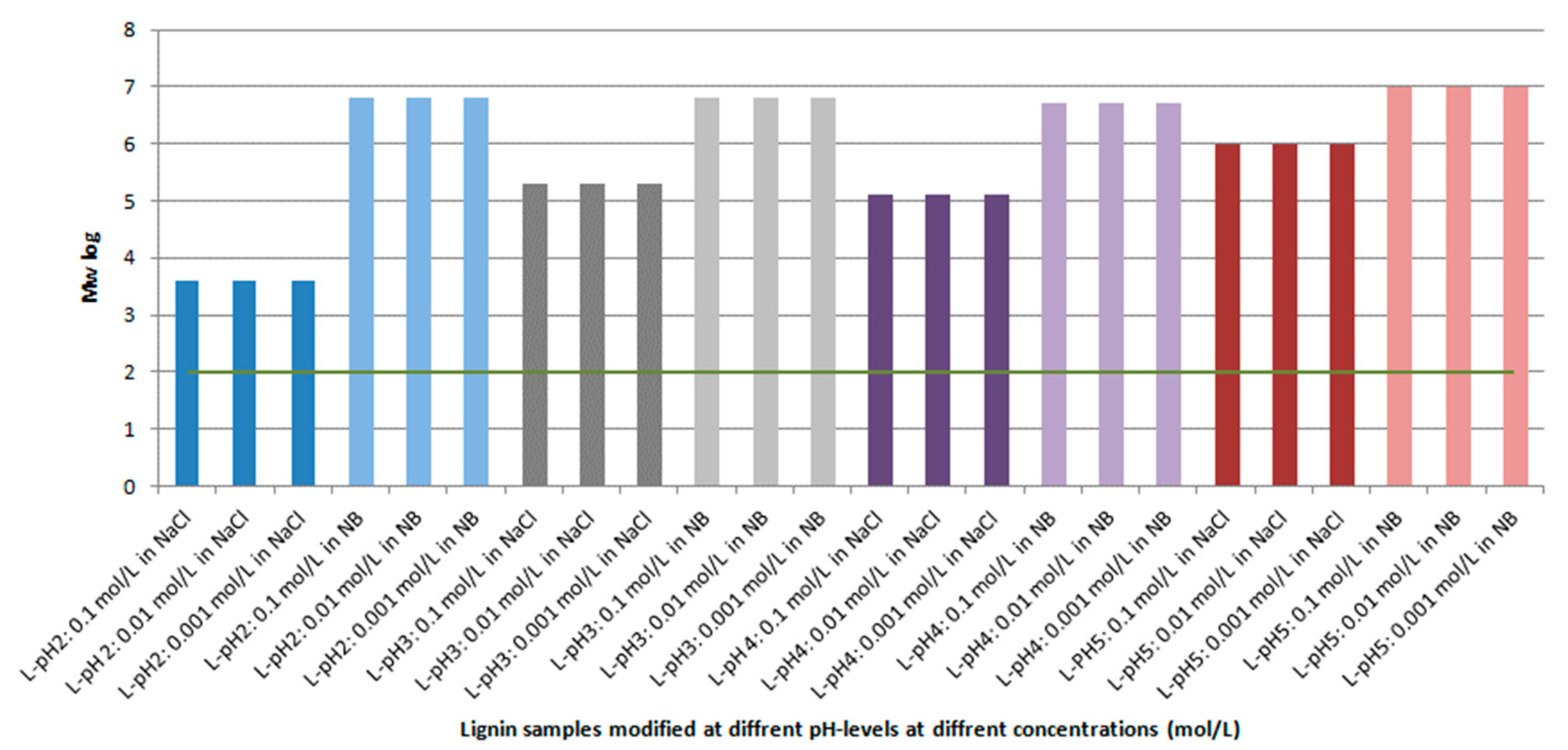
| Lignin | Mw (g/mol) | Mn (g/mol) | PDI | OH content (ISO 14900) | Reference | |
|---|---|---|---|---|---|---|
| (mmol∙g−1) | (mg KOH) g−1 | |||||
| pH2 | 1879 | 574 | 3.3 | 2.67 | 150 | [29] |
| pH3 | 1732 | 538 | 3.2 | 4.48 | 251 | [29] |
| pH4 | 1570 | 441 | 3.6 | 5.02 | 282 | [29] |
| pH5 | 1502 | 490 | 3.0 | 5.34 | 300 | [29] |
| DL-pH2 | 5417 | 1299 | 4.2 | 4.75 | 266 | – |
| DL-pH3 | 5461 | 1318 | 4.1 | 4.00 | 224 | – |
| DL-pH4 | 5522 | 1335 | 4.1 | 5.51 | 309 | – |
| DL-pH5 | 5610 | 1347 | 4.2 | 4.80 | 269 | – |
| Reference Systems (blank) | Kbe mL−1 | Ø log Kbe mL−1 |
|---|---|---|
| Petri dish | 1.18 × 105 | 5.05 |
| Glass | 1.07 × 107 | 6.71 |
| Plastic dish (PP *) | 4.07 × 106 | 6.48 |
| Transparencies (PS **) | 9.62 × 106 | 6.94 |
| Stainless steel | 2.60 × 101 | 1.41 |
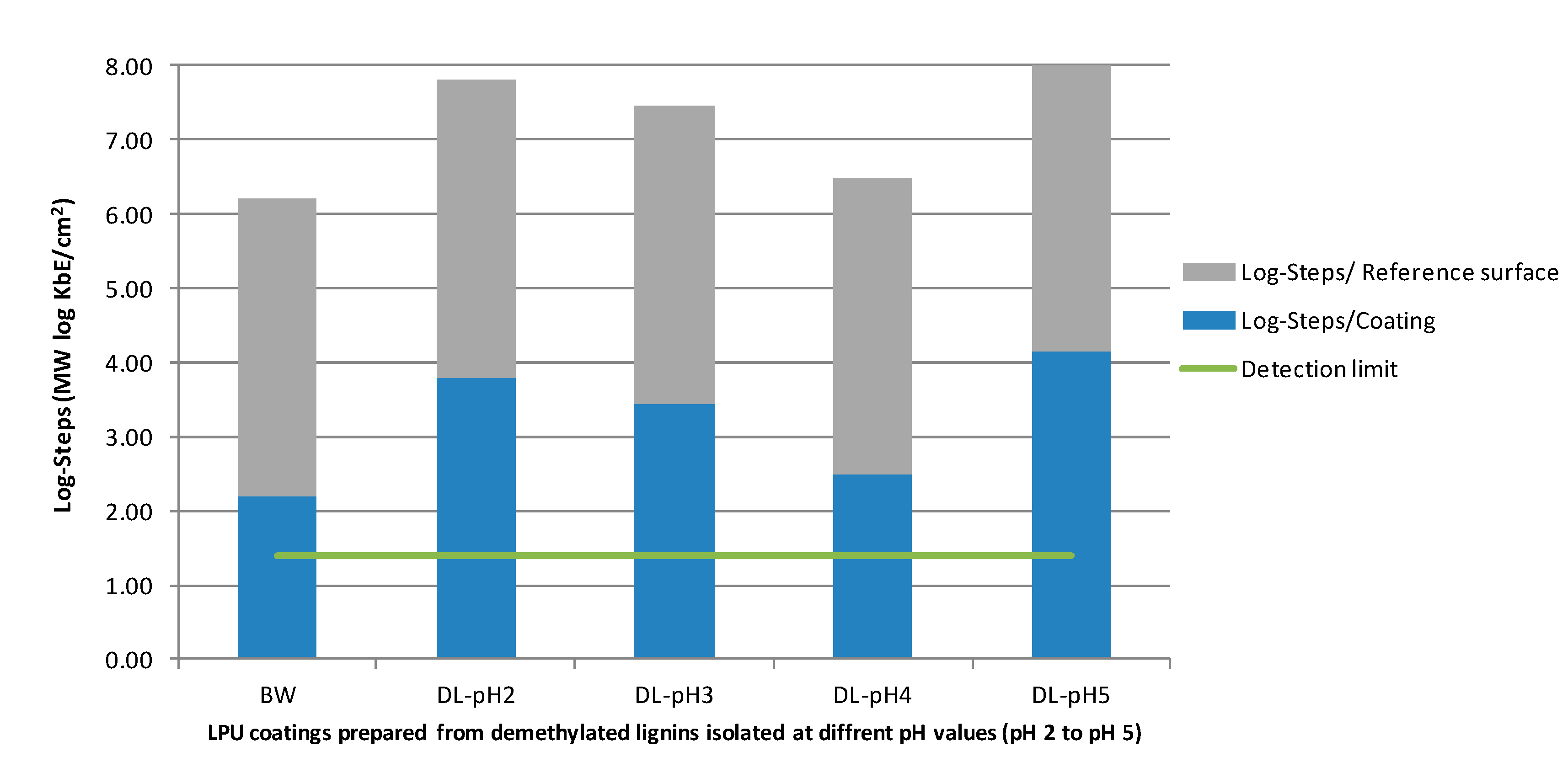
| Lignin–PU Coatings | Kbe/cm2 | Ø log Kbe/cm2 |
|---|---|---|
| DL-pH2-060718 | 2.07 × 103 | 3.03 |
| DL-pH3-060718 | 1.17 × 103 | 2.51 |
| DL-pH4-060718 | 3.09 × 101 | 2.36 |
| DL-pH5-060718 | 6.25 × 10−1 | 0 |
| Antimicrobial Activity | Lignin (Isolated at pH 5) | LPU Coating (DL-pH5) | LPU Coating (KL-pH5) | LPU with 0.8% (w/v) BG | LPU with 0.8% (w/v) CV | HPMC/lignin (15 wt.% L1) [52] |
|---|---|---|---|---|---|---|
| Log10 reduction | 7.00 | 4.12 | 2.62 | 8.31 | 8.60 | 2.50 |
| Lignin Coatings | Temperature (°C) | Δm (%) | Residual Mass (%) |
|---|---|---|---|
| Blank (PU coating without lignin) | 250 | −4.75% | 0.39 |
| LPU-pH 5 | 166 | −3.31% | 35.99 |
| Lignin coating CV | 165 | −4.05% | 20.80 |
| Lignin coating BG | 146 | −4.49% | 21.43 |
| Lignin coating organosolv | 143 | −4.03% | 8.00 |
| Lignin-DLPU coating | 153 | −4.55% | 21.43 |
| Sample | Contact Angle (°) |
|---|---|
| PU-KL-pH 2 | 92.28 ± 0.49 |
| PU-KL-pH 3 | 80.49 ± 1.03 |
| PU-KL-pH 4 | 83.28 ± 0.24 |
| PU-KL-pH 5 | 86.01 ± 0.22 |
| PU-OL | 61,59 ± 0.69 |
| PU-KL-Demethylated | 84.22 ± 0.51 |
| LPU Coatings with TPM dyes | |
| PU-BV-BG | 62.93 ± 0.34 |
| PU-BV-CV | 80.19 ± 0.28 |
| PU-KL-pH2-BG | 87.36± 0.15 |
| PU-KL-pH2-CV | 81.11 ± 0.18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, S.E.; Alzagameem, A.; Rumpf, J.; Korte, I.; Kreyenschmidt, J.; Schulze, M. Antimicrobial Activity of Lignin-Derived Polyurethane Coatings Prepared from Unmodified and Demethylated Lignins. Coatings 2019, 9, 494. https://doi.org/10.3390/coatings9080494
Klein SE, Alzagameem A, Rumpf J, Korte I, Kreyenschmidt J, Schulze M. Antimicrobial Activity of Lignin-Derived Polyurethane Coatings Prepared from Unmodified and Demethylated Lignins. Coatings. 2019; 9(8):494. https://doi.org/10.3390/coatings9080494
Chicago/Turabian StyleKlein, Stephanie Elisabeth, Abla Alzagameem, Jessica Rumpf, Imke Korte, Judith Kreyenschmidt, and Margit Schulze. 2019. "Antimicrobial Activity of Lignin-Derived Polyurethane Coatings Prepared from Unmodified and Demethylated Lignins" Coatings 9, no. 8: 494. https://doi.org/10.3390/coatings9080494
APA StyleKlein, S. E., Alzagameem, A., Rumpf, J., Korte, I., Kreyenschmidt, J., & Schulze, M. (2019). Antimicrobial Activity of Lignin-Derived Polyurethane Coatings Prepared from Unmodified and Demethylated Lignins. Coatings, 9(8), 494. https://doi.org/10.3390/coatings9080494